Evaluation of Antioxidant Properties of Choloroform Extract of Chasmanthera dependens Roots †
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Study Animals
2.3. Chemicals and Reagents
2.4. Extraction Procedure
2.5. Acute Toxicity Study of CECDR
2.5.1. Qualitative and Quantitative Phytochemical Analysis of CECDR
2.5.2. Quantitative Diphenylpicryl Hydrazyl (DPPH) Radical Scavenging Assay
- A0 = absorbance of control
- As = absorbance of CECDR
2.5.3. Ferric Reducing Antioxidant Power (FRAP) Assay
2.5.4. Hydrogen Peroxide (H2O2) Assay
2.5.5. Total Antioxidant Capacity (TAC) Assay
- A0 = absorbance of control
- As = absorbance of CECDR
2.5.6. Evaluation of Endogenous Antioxidant
2.6. Experimental Animals
2.7. Statistical Analysis
3. Results
3.1. Acute Toxicity and Lethality Study of CECDR
3.2. Qualitative Phytochemical Analysis of Chloroform Extract of Chasmanthera Dependens
3.3. Quantitative Phytochemical Analysis of CECDR
3.4. Effect of CECDR on DPPH Radical Scavenging Activity
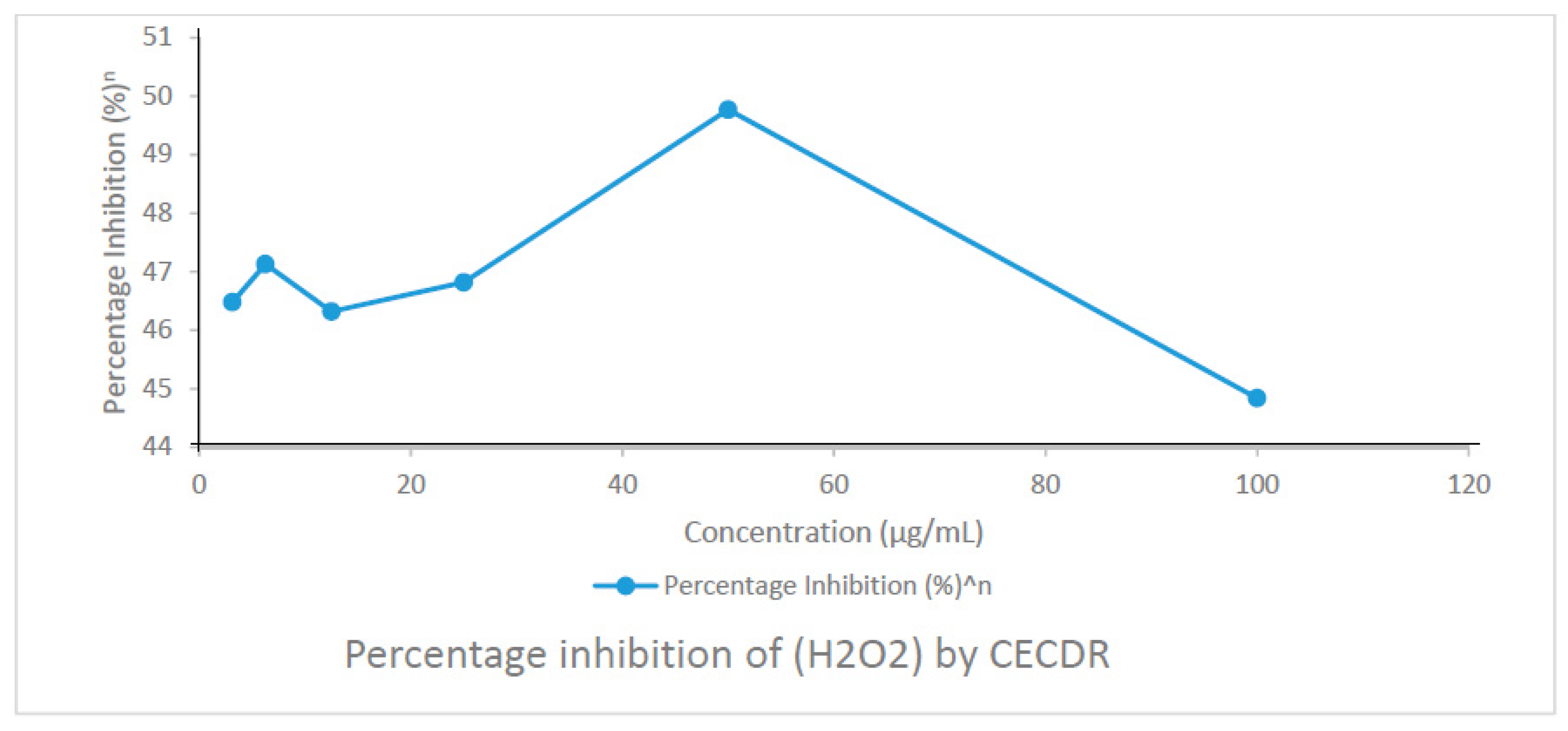
3.5. Effect of CECDR on Hydrogen Peroxide (H2O2)
3.6. Effect of Chloroform Extract of C. dependens on Ferric Reducing Antioxidant Power (FRAP)
3.7. Effect of Chloroform Extract of Chasmanthera dependens on Total Antioxidant Capacity (TAC)
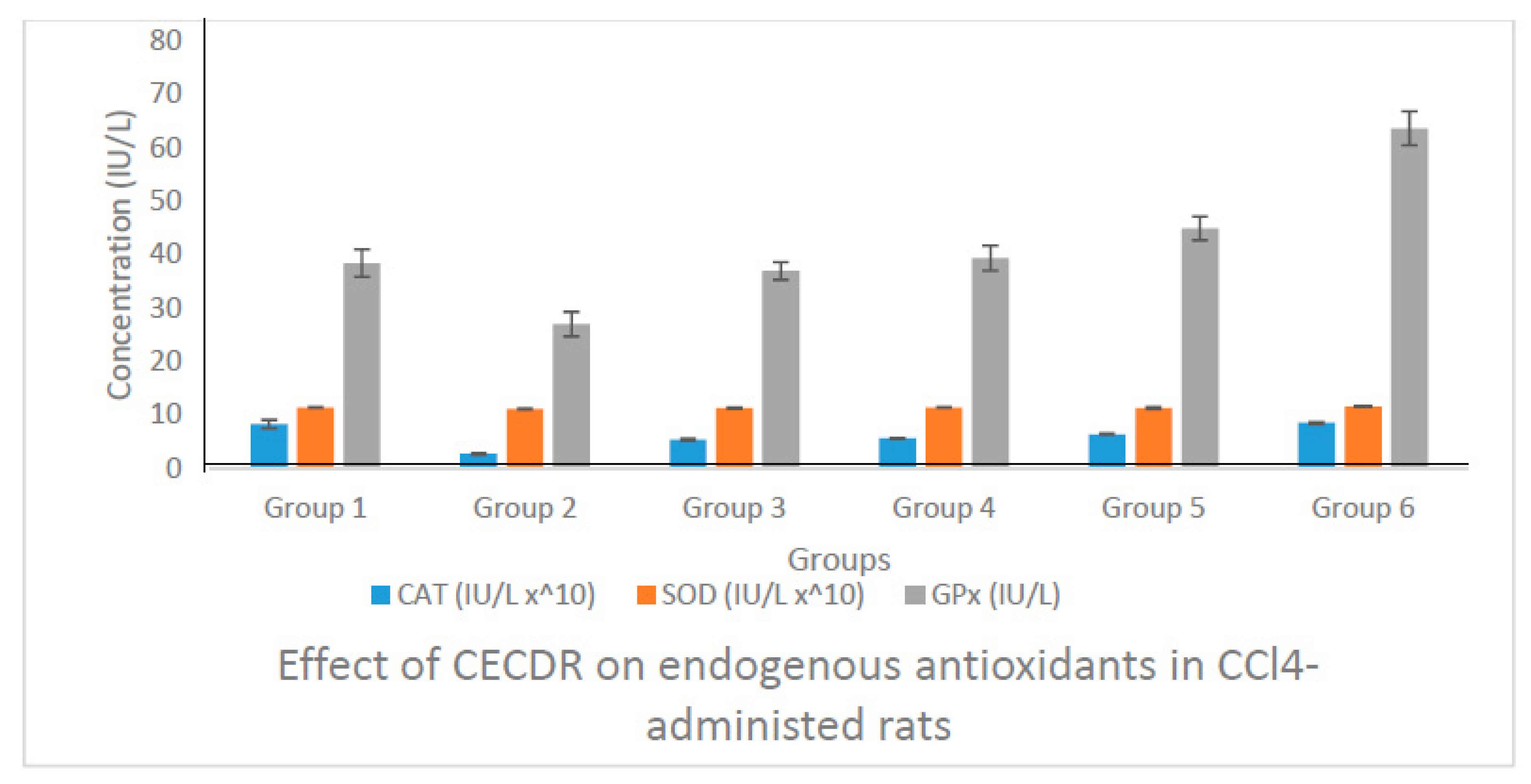
3.8. Result of the Biochemical Analysis
3.8.1. Catalase (CAT) Activity of Rats Administered with CECDR
3.8.2. Superoxide Dismutase (SOD) Activity of Rats Administered with CECDR
3.8.3. Glutathione Peroxidase (GPx) Activity of Rats Administered with CECDR
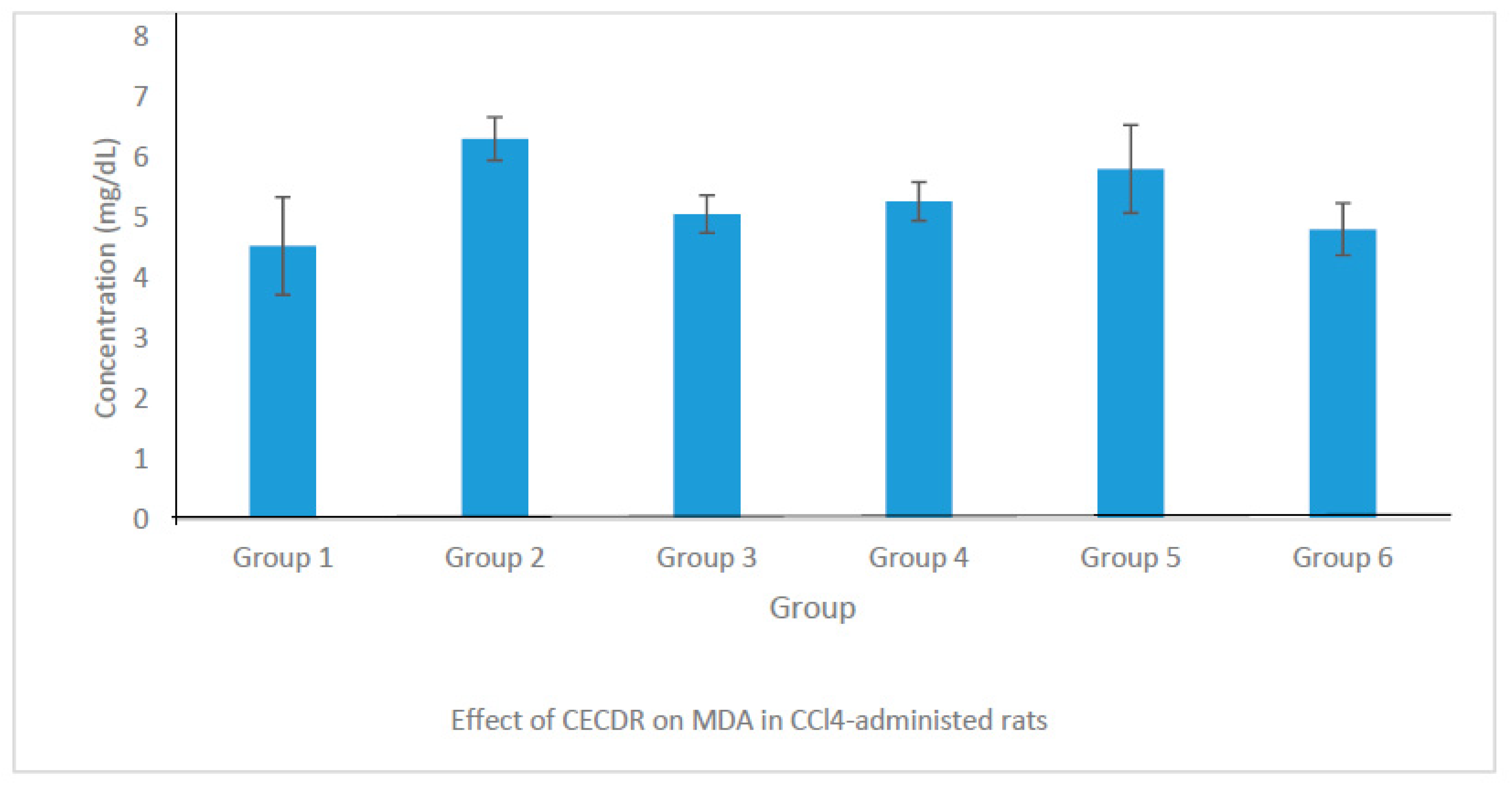
3.8.4. Concentration of Malondialdehyde (MDA)
4. Discusion
5. Conclusions
Institutional Review Board Statement
Informed Consent Statement
References
- Ogunlesi, M.; Okiei, W.; Ademoye, M.; Osibote, A.E. Analysis of essential oil from the stem of Chasmanthera dependens. J. Nat. Prod. 2009, 3, 47–53. [Google Scholar]
- Shrikant, S.S.; Gupta, B.L. Antimicrobial activity of medicinal plants on urinary tract pathogens. Int. J. Pharm. Pharm. 2012, 4, 1. [Google Scholar]
- Monon, K.; Karamoko, O.; Goueh, G.; Abuo, O.; Adama, C. Study on ethnopharmacological and phytochemical screening of some plants involved in the treatment of abdominal infection in the department of Kouto (COTE D’ IVOIRE). Sch. J. Appl. Med. Sci. 2013, 1, 56–61. [Google Scholar]
- Vijaya, L.; Phatak, A.; Chandra, N. Antioxidant and free radical scavenging activity of Hygrophila schulli (Buch.-Ham.) Almeida and Almeida seeds. Adv. Bio Res. 2010, 1, 7. [Google Scholar]
- Chun-Weng, P.; Sri, N.M.; Halijah, I.; Norhanom, A.W. Antioxidant properties of crude and fractionated extracts of Alpinia mutica rhizomes and their total phenolic content. Afr. J. Pharm. Pharmacol. 2011, 5, 842–852. [Google Scholar]
- Amoussa, A.M.; Lagnika, L.; Tchatchedre, M.; Laleye, A.; Sanni, A. Acute toxicity and antifungal effects of Acacia ataxacantha (Bark). Int. J. Pharmacogn. Phytochem. Res. 2015, 7, 661–668. [Google Scholar]
- Reenu, J.; Azeez, S.; Bhageerathy, C. In vitro antioxidant potential in sequential extracts of curcuma caesia roxb. rhizomes. Indian J. Pharm. Sci. 2015, 77, 41. [Google Scholar] [CrossRef] [PubMed]
- Kanwal, R.; Arshad, M.; Bibi, Y.; Asif, S.; Khalil, S. Evaluation of Ethnopharmacological and Antioxidant Potential of Zanthoxylum armatum DC. J. Chem. 2015, 2015, 925654. [Google Scholar] [CrossRef]
- Fanta, Y.; Taiwe, S.G.; Ngatcha, Z.S.; Khan, M.A.; Agbor, G.A.; Ur-Rahman, N.; Ngo, B.E. Quantification of Bioactive compounds and evaluation of the antioxidant activity of Carissa edulis Valh (Apocynaceae) leaves. Sci. World J. 2019, 2019, 7549620. [Google Scholar]
- Ozioko, S.C.; Madueke, A.C.; Anosike, A.C. Phytochemical composition and free radical scavenging activity of Cucurbita maxima fruit juice. Res. J. Adv. Sci. 2020, 1, 17–26. [Google Scholar]
- Lateef, A.; Ojo, S.A.; Elegbede, J.A.; Azeez, M.A.; Yekeen, T.A.; Akinboro, A. Evaluation of some biosynthesized silver nanoparticles for biomedical applications: Hydrogen peroxide scavenging, anticoagulant and thrombolytic activities. J. Clust. Sci. 2016, 28, 1379–1392. [Google Scholar] [CrossRef]
- Borokini, T.I.; Lawal, I.O. Traditional medicine practices among the Yoruba people of Nigeria: A historical perspective. J. Med. Plants Stud. 2014, 2, 20–33. [Google Scholar]
- Use of Tropical Plant Database, UTPD. Chasmanthera dependens. Available online: http://:tropical.theferns.info (accessed on 28 April 2016).
- Mosango, D.M. Chasmanthera dependens Hochst. Flora 2008, 27, 1844. [Google Scholar]
- Wahab, O.M. Ethnomedicinal Antiepileptic plants used in parts of Oyo and Osun States, Nigeria. BRI 2015, 8, 77–81. [Google Scholar]
- Onabanjo, A.O.; John, T.A.; Sokale, A.A.; Samuel, O.T. Analgesic and Antiinflammatory Effects of Chasmanthera dependens. Int. J. Pharmacogn. 1991, 29, 24–28. [Google Scholar] [CrossRef]
- Kola-Mustapha, A.T.; Yohanna, K.A.; Ghazali, Y.O.; Ayotunde, H.T. Design, formulation and evaluation of Chasmanthera dependens Hochst and Chenopodium ambrosioides Linn based gel for its analgesic and anti- inflammatory activities. Heliyon 2020, 6, e04894. [Google Scholar] [CrossRef]
- Augustine, C.; Anosike, C. Anti-Ulcerogenic Effect of the Chloroform Extract of Chasmanthera dependens on Indomethacin and Aspirin-Induced Ulcer in Rats. J. Nutr. Ecol. Food Res. 2017, 4, 131–137. [Google Scholar] [CrossRef]
- Quadri, A.L.; Yakubu, M.T. Fertility enhancing activity and toxicity profile of aqueous extract of Chasmanthera dependens roots in male rats. Andrologia 2017, 49, e12775. [Google Scholar] [CrossRef]
- Ogbozor, C.S.; Anosike, C.A. The Effect of Chloroform Extract of Chasmenthera dependens on Carbon Tetrachloride (Ccl4) Induced Hepatotoxicity. Glob. Sci. J. 2020, 8. [Google Scholar]
- Abiola, T.S.; Susan, A.O.; Olusegun, B.O. Tannin-Rich Extract of Chasmanthera dependens Stem Potential in Piroxicam-Induced Nephrotoxicity in Adult Male Wistar Rats. Am. J. Mol. Biol. 2020, 10, 29–43. [Google Scholar] [CrossRef][Green Version]
- Lorke, D. A new practical approach to practical acute toxicity testing. Acute Toxicol. 1983, 53, 275–289. [Google Scholar] [CrossRef]
- Wallis, T.E. Textbook of Pharmacognosy, 5th ed.; J and A Churchil Ltd.: London, UK, 1967; pp. 81–82. [Google Scholar]
- Harborne, J.B. Phytochemical Methods, 11th ed.; Chapman and Hall: New York, NY, USA, 1973. [Google Scholar]
- Swain, T. Tannins and Lignins. In Herbivores: Their Interaction with Secondary Plant Metabolites; Rosenthal, G.A., Janzen, D.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 657–682. [Google Scholar]
- Trease, G.E.; Evans, W.C. Pharmacognosy, 12th ed.; Bailliere Tridal: London, UK, 1983. [Google Scholar]
- Brunner, J.H. Direct spectrophotometric determination of saponins. Anal. Chem. 1984, 34, 1314–1326. [Google Scholar]
- Sofowora, E.A. Medicinal Plants and Traditional Medicine in Africa, 3rd ed.; Spectrum Books Ltd.: Ibadan, Nigeria, 2008; p. 439. [Google Scholar]
- Gyamfi, M.A.; Yonamine, M.; Aniya, Y. Free radical scavenging activity of medicinal herbs of Ghana: Thonningia sanguinea on experimentally induced liver injuries. Gen. Pharmacol. 1999, 32, 661–667. [Google Scholar] [CrossRef]
- Sahreen, S.; Khan, M.R.; Khan, R.A. Evaluation of antioxidant activities of various solvent extracts of Carissa Opaca fruits. Food Chem. 2010, 122, 1205–1211. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as ameasure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Ruch, R.J.; Cheng, S.J.; Klaunig, J.E. Prevention of cytotoxicity and inhibition of intracellular communication by antioxidant catechins isolated from Chinese green tea. Carcinogenesis 1989, 10, 1003. [Google Scholar] [CrossRef] [PubMed]
- Garrat, D.C. The Quantitative Analysis of Drugs. J. Pharm. Pharmacol. 1964, 16, 772. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the qualitative and quantitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Satoh, K. Serum lipid peroxide in cerebrovascular disorders determined by a new colorimetric method. Clin. Chim. Acta 1978, 90, 37–43. [Google Scholar] [PubMed]
- Bussmann, R.W.; Sharon, D. Traditional medicinal plant use in Northern Peru: Tracking two thousand years of healing culture. J. Ethnobiol. Ethnomed. 2006, 2, 47. [Google Scholar] [CrossRef] [PubMed]
- Sarian, M.N.; Ahmed, Q.U.; So’ad, S.Z.M.; Alhassan, A.M.; Murugesu, S.; Perumal, V.; Mohamad, S.N.A.S.; Khatib, A.; Latip, J. Antioxidant and antidiabetic effects of flavonoids: A structure-activity relationship based study. BioMed Res. Int. 2017, 2017, 8386065. [Google Scholar] [CrossRef] [PubMed]

| Constituents | Relative Abundance |
|---|---|
| Alkaloids | +++ |
| Phenolics | ++ |
| Saponins | ND |
| Tanins | ++ |
| Carbohydrate | ++ |
| Flavonoids | +++ |
| Steroids | +++ |
| Glycosides | ND |
| Terpenoids | ++ |
| Reducing sugar | + |
| Phytochemical Constituents | Bioavailability (mg) |
|---|---|
| Phenolics | 4907.25 ± 75.33 |
| Alkaloids | 1975 ± 68.84 |
| Carbohydrates | 449.64 ± 59.30 |
| Flavonoids | 319.79 ± 98.93 |
| Terpenes | 674.51 ± 46.18 |
| Reducing sugar | 80.8 ± 36.19 |
| Saponins | - |
| Tannins | 22.47 ± 11.44 |
| Steroids | 4.55 ± 0.32 |
| Results from the quantitative analysis are expressed in means ± SD of triplicate | |
| determinations (n = 10) | |
| Concentration (µg/mL) | Ferric Reducing Antioxidant Power (FRAP) (µg GAE) |
|---|---|
| 15.6 | 21.18 ± 0.15 |
| 31.1 | 0.72 ± 0.06 |
| 62.5 | 0.37 ± 0.03 |
| 125 | 0.19 ± 0.01 |
| 250 | 0.11 ± 0.00 |
| 500 | 0.05 ± 0.00 |
| 1000 | 0.03 ± 0.00 |
| Concentration (µg/mL) | Total Antioxidant Capacity (TAC) (µg AAE) |
|---|---|
| 15.6 | 15.22 ± 7.81 |
| 31.1 | 17.71 ± 7.35 |
| 62.5 | 7.83 ± 3.70 |
| 125 | 3.82 ± 0.74 |
| 250 | 3.06 ± 0.33 |
| 500 | 2.40 ± 0.13 |
| 1000 | 1.68 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madueke, A.; Nwanelo, V.; Tabansi, E.; Onoh, P.; Anichebe, R.; Okosisi, A.; Anosike, A. Evaluation of Antioxidant Properties of Choloroform Extract of Chasmanthera dependens Roots. Med. Sci. Forum 2021, 2, 21. https://doi.org/10.3390/CAHD2020-08606
Madueke A, Nwanelo V, Tabansi E, Onoh P, Anichebe R, Okosisi A, Anosike A. Evaluation of Antioxidant Properties of Choloroform Extract of Chasmanthera dependens Roots. Medical Sciences Forum. 2021; 2(1):21. https://doi.org/10.3390/CAHD2020-08606
Chicago/Turabian StyleMadueke, Augustine, Valentine Nwanelo, Emmanuel Tabansi, Pearl Onoh, Remigus Anichebe, Anayo Okosisi, and Assumpta Anosike. 2021. "Evaluation of Antioxidant Properties of Choloroform Extract of Chasmanthera dependens Roots" Medical Sciences Forum 2, no. 1: 21. https://doi.org/10.3390/CAHD2020-08606
APA StyleMadueke, A., Nwanelo, V., Tabansi, E., Onoh, P., Anichebe, R., Okosisi, A., & Anosike, A. (2021). Evaluation of Antioxidant Properties of Choloroform Extract of Chasmanthera dependens Roots. Medical Sciences Forum, 2(1), 21. https://doi.org/10.3390/CAHD2020-08606







