Protective Effects of New Antioxidants in OTA-Treated Chicken Kidney †
Abstract
:1. Introduction
2. Experiments
2.1. Ethical Statement
2.2. Animal Treatments and Experimental Plan
2.3. RNA Extraction and Complementary DNA Synthesis
2.4. Primer Design for Selected Genes of Interest (GOI) and Reverse Transcription-Quantitative Polymerase Chain Reaction (RT-qPCR)
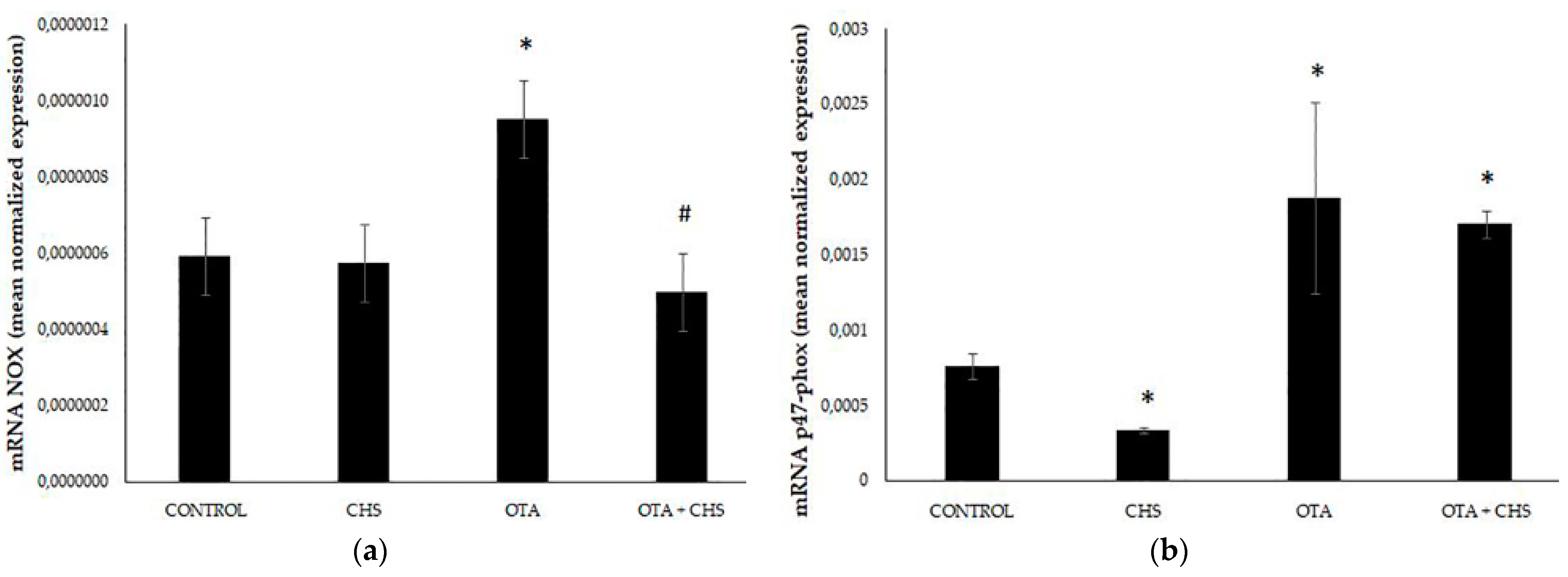
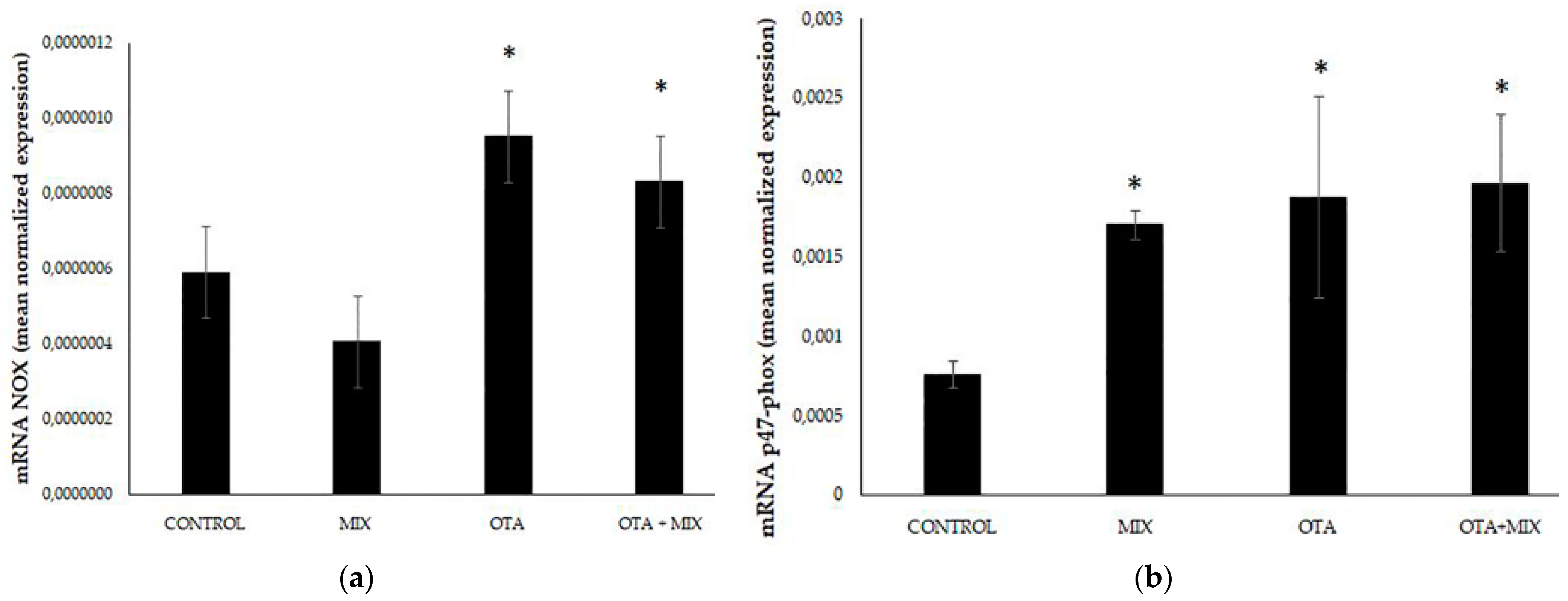
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OTA | Ochratoxin A |
| ROS | Reactive Oxygen Species |
| NOX | NADPH oxidase 4 |
References
- Wang, Y.; Wang, L.; Liu, F.; Wang, Q.; Selvaraj, J.; Xing, F.; Zhao, Y.; Liu, Y. Ochratoxin A Producing Fungi, Biosynthetic Pathway and Regulatory Mechanisms. Toxins 2016, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.; Song, G.; Lim, W. Effects of mycotoxin-contaminated feed on farm animals. J. Hazard. Mater. 2020, 389, 122087. [Google Scholar] [CrossRef] [PubMed]
- Bui-Klimke, T.R.; Wu, F. Ochratoxin A and Human Health Risk: A Review of the Evidence. Crit. Rev. Food Sci. Nutr. 2015, 55, 1860–1869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battacone, G.; Nudda, A.; Pulina, G. Effects of Ochratoxin A on Livestock Production. Toxins 2010, 2, 1796–1824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malir, F.; Ostry, V.; Pfohl-Leszkowicz, A.; Malir, J.; Toman, J. Ochratoxin A: 50 Years of Research. Toxins 2016, 8, 191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biró, K.; Solti, L.; Barna-Vetró, I.; Bagó, G.; Glávits, R.; Szabó, E.; Fink-Gremmels, J. Tissue distribution of ochratoxin A as determined by HPLC and ELISA and histopathological effects in chickens. Avian Pathol. 2002, 31, 141–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Xie, S.; Xu, F.; Liu, A.; Wang, Y.; Chen, D.; Pan, Y.; Huang, L.; Peng, D.; Wang, X.; et al. Ochratoxin A: Toxicity, oxidative stress and metabolism. Food Chem. Toxicol. 2018, 112, 320–331. [Google Scholar] [CrossRef] [PubMed]
- Agriopoulou, S.; Stamatelopoulou, E.; Varzakas, T. Advances in Occurrence, Importance, and Mycotoxin Control Strategies: Prevention and Detoxification in Foods. Foods 2020, 9, 137. [Google Scholar] [CrossRef] [PubMed]
- Sherazi, S.T.H.; Shar, Z.H.; Sumbal, G.A.; Tan, E.T.; Bhanger, M.I.; Kara, H.; Nizamani, S.M. Occurrence of ochratoxin A in poultry feeds and feed ingredients from Pakistan. Mycotoxin Res. 2015, 31, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Vila-Donat, P.; Marín, S.; Sanchis, V.; Ramos, A.J. A review of the mycotoxin adsorbing agents, with an emphasis on their multi-binding capacity, for animal feed decontamination. Food Chem. Toxicol. 2018, 114, 246–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Q.; Wu, F.; Wang, J.; Gao, L.; Jiang, L.; Li, H.-D.; Ma, Q.; Liu, X.; Wei, B.; Zhou, L.; et al. Nox4 in renal diseases: An update. Free Radic. Biol. Med. 2018, 124, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Lauritano, C.; Longobardi, C.; Andretta, E.; Elagoz, A.M.; Rapisarda, P.; Di Iorio, M.; Florio, S.; Ciarcia, R. Effects of a Red Orange and Lemon Extract in Obese Diabetic Zucker Rats: Role of Nicotinamide Adenine Dinucleotide Phosphate Oxidase. J. Clin. Med. 2020, 1600. [Google Scholar] [CrossRef] [PubMed]
- Pozzo, L.; Cavallarin, L.; Antoniazzi, S.; Guerre, P.; Biasibetti, E.; Capucchio, M.T.; Schiavone, A. Feeding a diet contaminated with ochratoxin A for broiler chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg). 2. Effects on meat quality, oxidative stress, residues and histological traits. J. Anim. Physiol. Anim. Nutr. 2013, 97, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Damiano, S.; Andretta, E.; Longobardi, C.; Prisco, F.; Paciello, O.; Squillacioti, C.; Mirabella, N.; Florio, S.; Ciarcia, R. Effects of Curcumin on the Renal Toxicity Induced by Ochratoxin A in Rats. Antioxidants 2020, 9, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiano, S.; Iovane, V.; Squillacioti, C.; Mirabella, N.; Prisco, F.; Ariano, A.; Amenta, M.; Giordano, A.; Florio, S.; Ciarcia, R. Red orange and lemon extract prevents the renal toxicity induced by ochratoxin A in rats. J. Cell. Physiol. 2020, 235, 5386–5393. [Google Scholar] [CrossRef] [PubMed]
- Palabiyik, S.S.; Erkekoglu, P.; Zeybek, N.D.; Kizilgun, M.; Baydar, D.E.; Sahin, G.; Giray, B.K. Protective effect of lycopene against ochratoxin A induced renal oxidative stress and apoptosis in rats. Exp. Toxicol. Pathol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Sheu, M.-L.; Shen, C.-C.; Chen, Y.-S.; Chiang, C.-K. Ochratoxin A induces ER stress and apoptosis in mesangial cells via a NADPH oxidase-derived reactive oxygen species-mediated calpain activation pathway. Oncotarget 2017, 8, 19376–19388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Costa, K.-A.; Niculescu, M.D.; Craciunescu, C.N.; Fischer, L.M.; Zeisel, S.H. Choline deficiency increases lymphocyte apoptosis and DNA damage in humans. Am. J. Clin. Nutr. 2006, 84, 88–94. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Primer F Primer R | Amplicon Size | E | R2 | Acc.Number |
|---|---|---|---|---|---|
| NOX | TCGGGTGGCTTGTTGAAGTA- GTCTGTGGGAAATGAGCTTGG | 224 | 90 | 0.99 | NM_053524 |
| p47-phox | TACGCTGCTGTTGAAGAGGA- GATGTCCCCTTTCCTGACCA | 105 | 100 | 0.99 | AY029167.1 |
| 18S | AGAAACGGCTACCACATCCA- CCCTCCAATGGATCCTCGTT | 158 | 93 | 0.99 | NR_046237.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andretta, E.; Longobardi, C.; Laselva, M.; Lauritano, C.; Avantaggiato, G.; Schiavone, A.; Jarriyawattanachaikul, W.; Florio, S.; Damiano, S.; Ciarcia, R. Protective Effects of New Antioxidants in OTA-Treated Chicken Kidney. Med. Sci. Forum 2021, 2, 18. https://doi.org/10.3390/CAHD2020-08617
Andretta E, Longobardi C, Laselva M, Lauritano C, Avantaggiato G, Schiavone A, Jarriyawattanachaikul W, Florio S, Damiano S, Ciarcia R. Protective Effects of New Antioxidants in OTA-Treated Chicken Kidney. Medical Sciences Forum. 2021; 2(1):18. https://doi.org/10.3390/CAHD2020-08617
Chicago/Turabian StyleAndretta, Emanuela, Consiglia Longobardi, Martina Laselva, Chiara Lauritano, Giuseppina Avantaggiato, Achille Schiavone, Watanya Jarriyawattanachaikul, Salvatore Florio, Sara Damiano, and Roberto Ciarcia. 2021. "Protective Effects of New Antioxidants in OTA-Treated Chicken Kidney" Medical Sciences Forum 2, no. 1: 18. https://doi.org/10.3390/CAHD2020-08617
APA StyleAndretta, E., Longobardi, C., Laselva, M., Lauritano, C., Avantaggiato, G., Schiavone, A., Jarriyawattanachaikul, W., Florio, S., Damiano, S., & Ciarcia, R. (2021). Protective Effects of New Antioxidants in OTA-Treated Chicken Kidney. Medical Sciences Forum, 2(1), 18. https://doi.org/10.3390/CAHD2020-08617









