1. Introduction
Asthma is one of the most common respiratory diseases in the world, with more than 300 million patients diagnosed [
1]. It is described as a chronic disease of the respiratory system, with inflammation of the bronchial airways as the most prominent pathological feature [
2]. The typical symptoms of asthma are wheezing, chest tightness, shortness of breath, evidence of airway obstruction and airway hyperresponsiveness (AHR). Although originally believed to be a single disease, it is now recognized as a complex of multiple phenotypes, each with different severity and treatment response [
3].
Oxidant-antioxidant imbalance plays an important role in the airway inflammation observed in asthmatic patients. Inflammatory cells infiltrating the airways produce several modulators of the inflammatory response, including a range of toxic reactive oxygen species (ROS), which are associated with many of the pathophysiological changes linked with asthma severity, such as increased airway reactivity and secretions, and increased production of chemoattractants [
4]. In fact, when ROS overwhelm antioxidant defenses the severe inflammatory state becomes apparent.
Several studies showed that the interaction between ROS and DNA can produce structural alterations including small-scale insertions, DNA base pair deletions, base modifications, aneuploidy, microsatellite instability, translocation of segments and CI [
5]. In general, ROS is the major source of DNA damage that attacks phosphate, deoxyribose, and base sites, resulting in strand breakage. Among these, double-strand breaks (DSBs) are one of the most cytotoxic forms of DNA damage, and if they are not properly repaired, they can lead to cell death and genomic rearrangements commonly found in cancer cells [
6].
The protective effect of the antioxidant cocktail,
N-Acetyl-
l-cysteine (NAC) and (±)-α-Lipoic acid (ALA), against DSBs measured by CI was previously shown in a study involving FA patients, who are characterized by increased levels of OS and CI [
7]. Since NAC and ALA, independently, were found to suppress airway inflammation and AHR in animal models of asthma, we hypothesize if the same antioxidant cocktail used in FA patients can have also a protective effect in asthmatic patients with severe inflammation caused by OS [
8,
9].
The aim of this study was to evaluate OS-related CI in asthma patients and to study the protective effect of in-vitro administration of NAC and ALA.
2. Experiments
This study included 19 asthma patients. These individuals were divided in two different groups: a “severe asthma” group (n = 15), if they had frequent asthma attacks and needed daily medication, and a “mild asthma” group (n = 4) if they were clinically diagnosed with asthma, but without an attack in the last couple of months and without the need of daily medication. As a control group, healthy blood donors (n = 20) were recruited.
Initially, three sets of experiments were performed. In the first one, lymphocyte cultures were maintained for 72 h without any type of treatment, in order to evaluate the spontaneous CI in cells from all groups of individuals. In the other two experiments, (±)-1,2:3,4-diepoxibutane (DEB), at the concentrations of 0.05 µg/mL and 0.1 µg/mL respectively, was added 24 h after culture initiation, in order to evaluate the OS-related induced CI in cells from all groups of individuals.
Afterwards, three new sets of experiments were performed with cells from individuals presenting increased levels of CI. In the first one, lymphocyte cultures were treated with an antioxidant cocktail containing 20 μM of ALA (Sigma-Aldrich, St. Louis, MO, USA) and 500 μM of NAC (Sigma-Aldrich, St. Louis, MO, USA) 24 h after culture initiation, in order to evaluate its effect on spontaneous CI. In the other two experiments, lymphocyte cultures were pre-treated with the referred antioxidant cocktail 1h30 before DEB exposure (at the same conditions described above), in order to evaluate its effect on OS-related induced CI. The selected concentrations of antioxidants were choosen according to previous in vitro studies [
7,
10].
After 3 days of culture, cells were harvested after 1 h of incubation with colcemid (Gibco, Invitrogen Corporation, Carlsbad, CA, USA) at a concentration of 4 µg/mL, followed by hypotonic treatment with 75 mM KCl (Sigma-Aldrich, St. Louis, MO, USA) and fixed 3 times in solution of acetic acid (Fisher Chemicals, Thermo Fisher Scientific Inc., Waltham, MA, USA) and methanol (Fisher Chemicals, Thermo Fisher Scientific inc., Waltham, MA, USA) in a ratio of 1:3. The resulting suspensions were dropped onto microscope slides and stained for 4 min in a 4% Giemsa solution (Merck, Darmstadt, Germany).
Analysis was performed on 100 metaphases from each experiment, by two independent scorers and in a blinded fashion. Each cell was scored for chromosome number and the number and types of structural abnormalities. The parameters analyzed were percentage of aberrant cells and mean number of breaks per cell.
Statistical analysis was performed using the GraphPad Prism 8, version 8.4.3 software. Graphical results are expressed as mean ± SEM and tabular results are expressed as mean ± SEM. Statistical comparison between groups was done using a Two-factor mixed-design ANOVA followed by a multicomparison Turkey’s test.
3. Results
3.1. CI Evaluation in Lymphocyte Cultures from Asthma Patients
3.1.1. Spontaneous CI
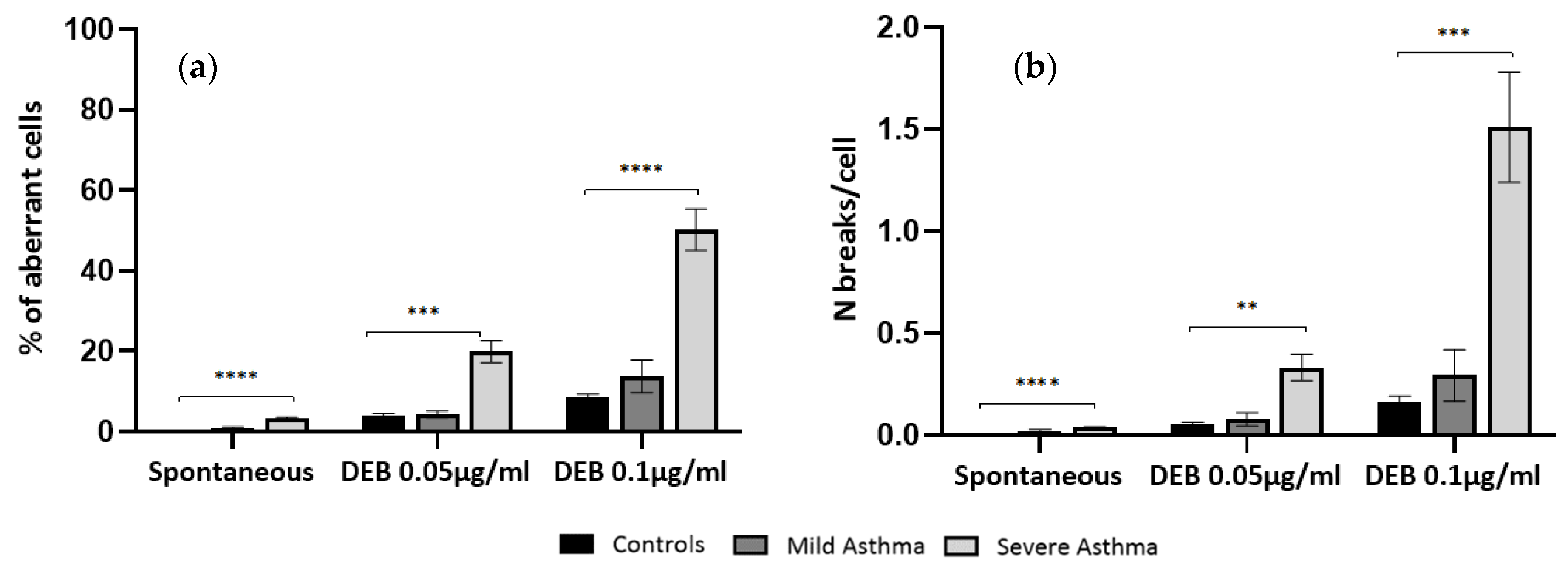
As shown in
Figure 1, lymphocyte cultures from severe asthma patients have a significant increase in the levels of spontaneous CI when compared to controls, either in percentage of aberrant cells (
p < 0.0001) or mean number of breaks per cell (
p < 0.0001). Regarding lymphocyte cultures from mild asthma patients, no significant differences were observed in the levels of spontaneous CI when compared to controls, either in percentage of aberrant cells or mean number of breaks per cell.
3.1.2. OS-Related Induced CI
As shown in
Figure 1, DEB-induced lymphocyte cultures from severe asthma patients have a significant increase in the levels of OS-related CI, when compared to controls, both at 0.05 µg/mL and 0.1 µg/mL DEB concentrations, either in percentage of aberrant cells (
p < 0.001 and
p < 0.0001, respectively) or mean number of breaks per cell (
p < 0.01 and
p < 0.001, respectively).
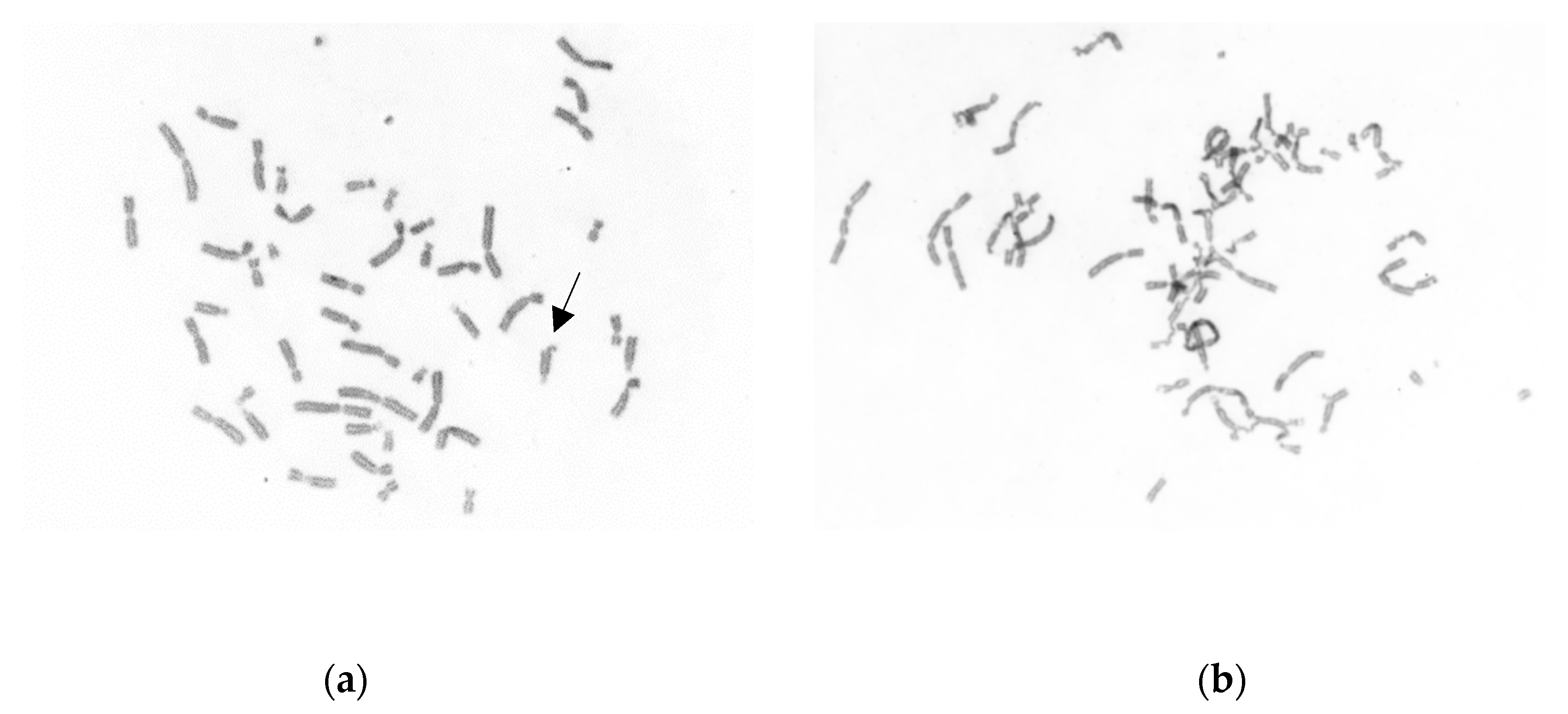
Figure 2 is an example of the mild DEB-induced CI in a cell from a normal individual, where only a chromatid break is seen, and the increased DEB-induced CI in a cell from a severe asthma patient, with multiple chromosome breaks and rearrangements.
Regarding DEB-induced lymphocyte cultures from mild asthma patients, no significant differences were observed in the levels of CI when compared to controls, either in percentage of aberrant cells or mean number of breaks per cell.
3.2. Antioxidant Effect against CI in Lymphocyte Cultures from Severe Asthma Patients
3.2.1. Effect of NAC + ALA on Spontaneous CI
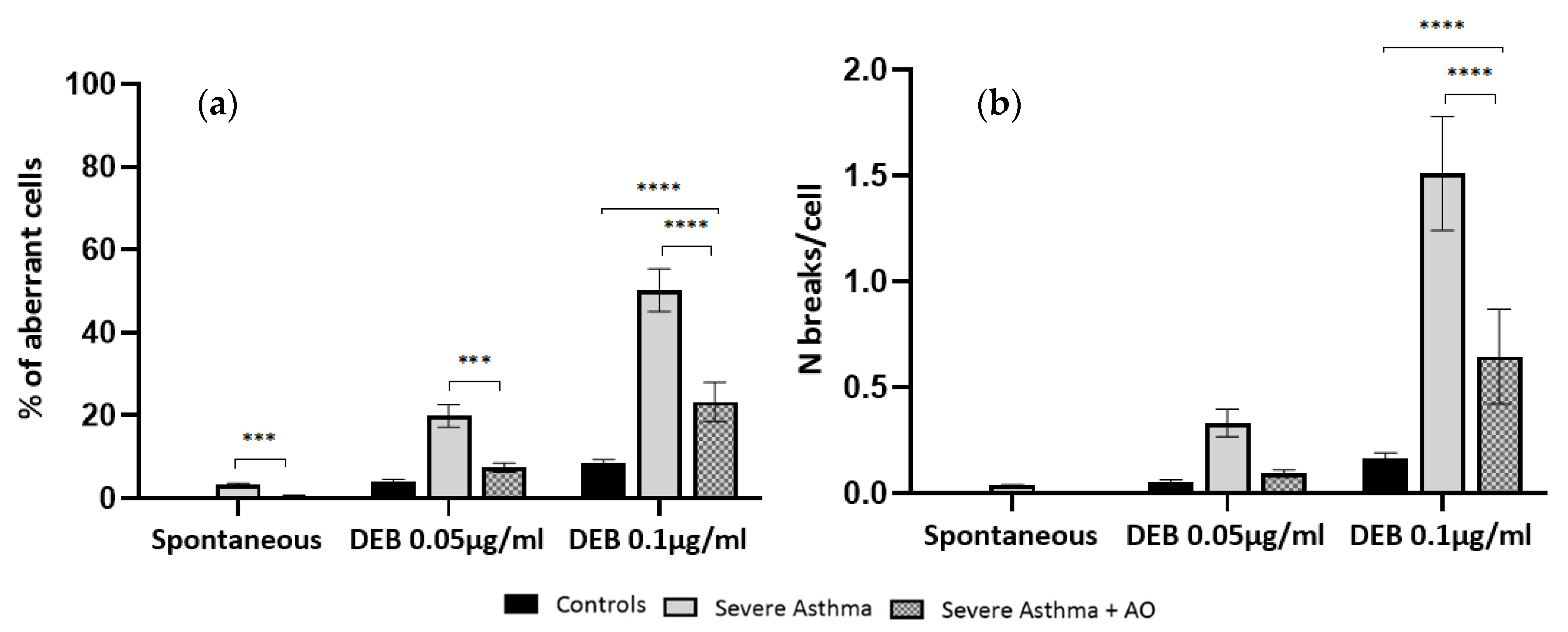
As shown in
Figure 3, lymphocyte cultures from severe asthma patients treated with NAC+ALA have a significant reduction in the percentage of aberrant cells, when compared to cultures of the same group without antioxidant treatment (
p < 0.001). When comparing the NAC + ALA treated cultures with spontaneous cultures from controls, no differences were observed either in percentage of aberrant cells or number of breaks per cell.
3.2.2. Effect of NAC + ALA on OS-Related Induced CI
As shown in
Figure 3, lymphocyte cultures from severe asthma patients exposed to DEB and pre-treated with NAC + ALA have a significant reduction in the percentage of aberrant cells when compared to those only exposed to DEB, both at 0.05 µg/mL and 0.1 µg/mL DEB concentrations (
p < 0.001 and
p < 0.0001, respectively). Regarding the number of breaks per cell, there was only a significant difference at the DEB concentration of 0.1 µg/mL (
p < 0.0001).
When comparing the NAC + ALA pre-treated cultures from severe asthma patients with DEB-induced cultures from controls, only found a significant difference in the number of breaks per cell at the DEB concentration of 0.1 µg/mL (p < 0.0001) was found.
4. Discussion
The first aim of this study was to evaluate cellular levels of CI in cells from asthma patients. Our results clearly show that cultured lymphocytes from severe asthma patients have increased levels of CI, when compared to controls. Interestingly, no significant differences were found in cells from mild asthma patients. The difference between the two groups may be due to the severity of the disease, and consequently to the inflammatory state present at the time. It is well known that when inflammatory cells infiltrate the airways, several modulators of the inflammatory response are produced, including a range of toxic ROS. Knowing the role of OS in the increase of CI, this pathological feature is in agreement with our results, once in the severe asthma patients’ cells a significant increase in the levels of both spontaneous and OS-induced CI were observed. This last result may also indicate that these patients might have a higher sensitivity to the cumulative genotoxic effect of OS, comparatively to normal individuals.
In an attempt to study the possible role of antioxidants in the reduction of levels of CI observed in cells from severe asthma patients, an antioxidant cocktail (NAC + ALA) was added to the lymphocytes cultures. This cocktail was previously proven to be effective in reduction of CI in in vitro studies in patients with increased OS-related CI. Our results clearly show that when exposed to the antioxidant cocktail, cells from severe asthma patients had a significant decrease in the levels of CI, both spontaneous and OS-induced. Further studies are still required in order to understand why these particular antioxidants were effective in the reduction of CI in these cells, namely studies of ROS characterization at cellular level and mitochondrial function.
5. Conclusions
In conclusion, the present study provides an important and novel finding that may have a clinical applicability. Understanding how CI due to exposure to OS correlates to asthma patients’ clinical phenotype may be pivotal not only to the design of preventive measures, in order to avoid the cumulative OS genotoxic effect, but also to design patient-specific treatments, like the prophylactic use of antioxidants.
Author Contributions
C.O. designed and performed the experiments, performed research, analyzed data and drafted the manuscript. M.N. performed some of the experiments. T.B., C.C. and J.N. provided blood samples and clinical data from patients, analyzed the experimental data. S.C. coordinated the study and critically revised the manuscript. B.P. coordinated the study, designed experiments, analyzed data, critically revised the manuscript and gave final approval of the version to be published. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of ICBAS-UP (2019-323 (260-DEFI/279-CE).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data are available on request from the corresponding author.
Acknowledgments
Authors wish to thank to all volunteers who provided blood samples, through the Service of Hematology from the Hospital Center of Oporto and Institute of Biomedical Science Abel Salazar. They also thank all the asthma patients for participating in this study.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| OS | oxidative stress |
| CI | chromosome instability |
| AHR | airway hyperresponsiveness |
| ROS | reactive oxygen species |
| DSBs | double-strand breaks |
| NAC | N-acetyl-l-cysteine |
| ALA | (±)-α-Lipoic acid |
| DEB | (±)-1,2:3,4-diepoxibutane |
References
- Masoli, M.; Fabian, D.; Holt, S.; Beasley, R. The global burden of asthma: Executive summary of the GINA Dissemination Committee report. Allergy 2004, 59, 469–478. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Hammad, H. The immunology of asthma. Nat. Immunol. 2015, 16, 45–56. [Google Scholar] [CrossRef]
- White, J.; Paton, J.Y.; Niven, R.; Pinnock, H. Guidelines for the diagnosis and management of asthma: A look at the key differences between BTS/SIGN and NICE. Thorax 2018, 73, 293–297. [Google Scholar] [CrossRef] [Green Version]
- Rosanna, D.P.; Salvatore, C. Reactive oxygen species, inflammation, and lung diseases. Curr. Pharm. Des. 2012, 18, 3889–3900. [Google Scholar] [CrossRef] [PubMed]
- Wiseman, H.; Halliwell, B. Damage to DNA by reactive oxygen and nitrogen species: Role in inflammatory disease and progression to cancer. Biochem. J. 1996, 313, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krejci, L.; Altmannova, V.; Spirek, M.; Zhao, X. Homologous recombination and its regulation. Nucleic Acids Res. 2012, 40, 5795–5818. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Sousa, R.; Fernandes, A.P.; Gonçalves, C.; Barbot, J.; Carvalho, F.; Porto, B. Improvement of genetic stability in lymphocytes from Fanconi anemia patients through the combined effect of α-lipoic acid and N-acetylcysteine. Orphanet J. Rare Dis. 2012, 7, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blesa, S.; Cortijo, J.; Mata, M.; Serrano, A.; Closa, D.; Santangelo, F.; Estrela, J.M.; Suchankova, J.; Morcillo, E.J. Oral N-acetylcysteine attenuates the rat pulmonary inflammatory response to antigen. Eur. Respir. J. 2003, 21, 394–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cho, Y.S.; Lee, J.; Lee, T.H.; Lee, E.Y.; Lee, K.U.; Park, J.Y.; Moon, H.B. alpha-Lipoic acid inhibits airway inflammation and hyperresponsiveness in a mouse model of asthma. J. Allergy Clin. Immunol. 2004, 114, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Ponte, F.; Carvalho, F.; Porto, B. Protective effect of acetyl-l-carnitine and α-lipoic acid against the acute toxicity of diepoxybutane to human lymphocytes. Toxicology 2011, 289, 52–58. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).