Surveillance and Stewardship Approaches for COVID-19 Novel Therapeutics in England from 2021 to 2022 (ESPAUR Report) †
Abstract
1. Introduction
2. Methods
3. Results
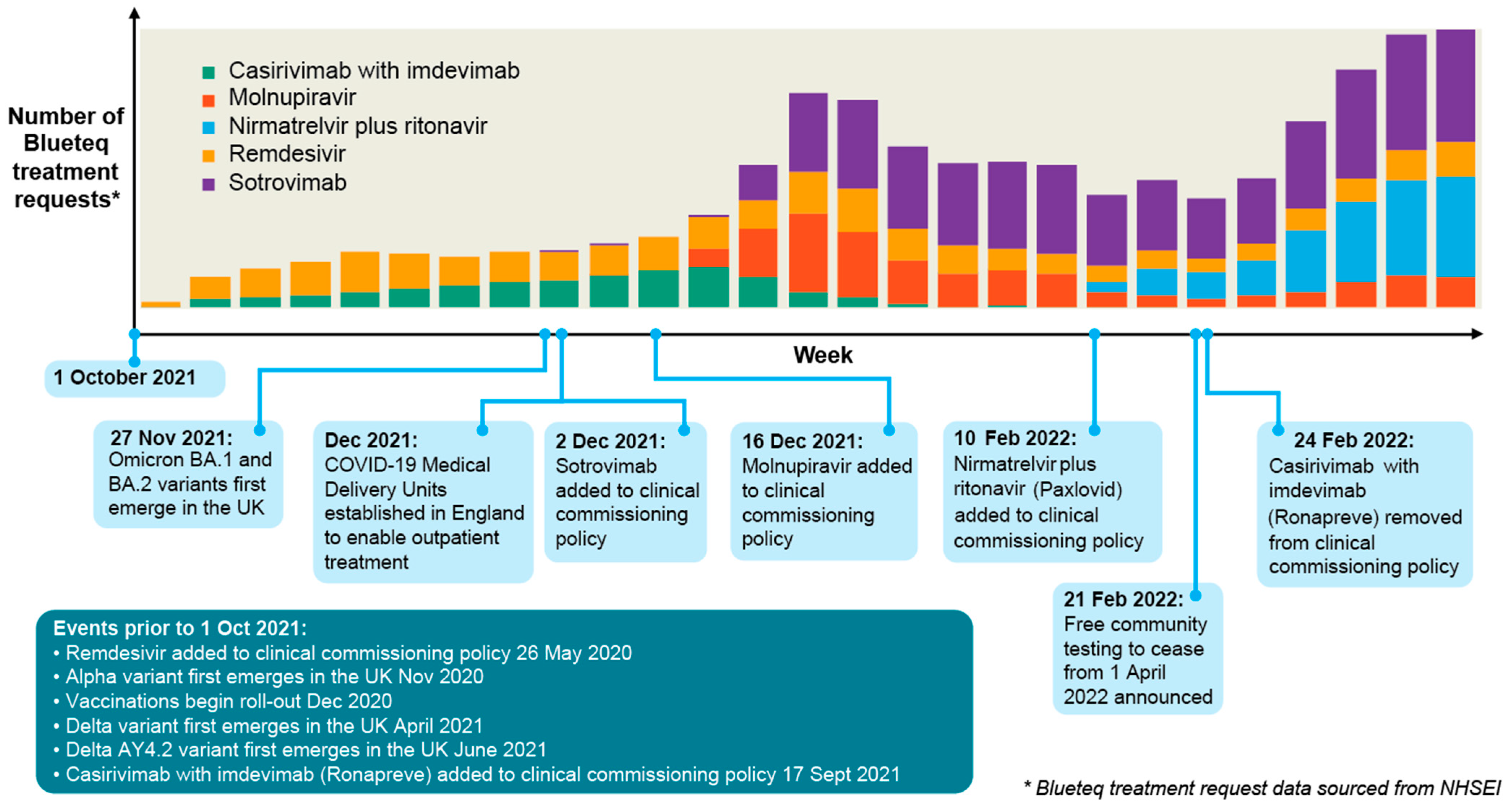
3.1. Treatment Requests (BlueTeq data)
3.2. Comparison of Treatment Requests with Rx-Info Medicines Supply Data
3.3. Genomic Surveillance
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashiru-Oredope, D.; Hopkins, S.; on behalf of the English Surveillance Programme for Antimicrobial Utilization and Resistance Oversight Group; Kessel, A.; Hopkins, S.; Ashiru-Oredope, D.; Brown, B.; Brown, N.; Carter, S.; Charlett, A.; et al. Antimicrobial stewardship: English surveillance programme for antimicrobial utilization and resistance (ESPAUR). J. Antimicrob. Chemother. 2013, 68, 2421–2423. [Google Scholar] [CrossRef] [PubMed]
- Squire, H.; Lochen, A.; Ashiru-Oredope, D.; Hand, K.; Hartman, H.; Triggs-Hodge, C.; Fountain, H.; Bou-Antoun, S.; Gerver, S.; Demirjian, A. Chapter 7 COVID-19 therapeutics. In English Surveillance Programme for Antimicrobial Utilisation and Resistance (ESPAUR) Report 2021 to 2022; UK Health Security Agency: London, UK, 2022. [Google Scholar]
- Twohig, K.A.; Nyberg, T.; Zaidi, A.; Thelwall, S.; Sinnathamby, M.A.; Aliabadi, M.; Seaman, S.R.; Harris, R.J.; Hope, R.; Lopez-Bernal, J.; et al. Hospital admission and emergency care attendance risk for SARS-CoV-2 delta (B.1.617.2) compared with alpha (B.1.1.7) variants of concern: A cohort study. Lancet Infect. Dis. 2022, 22, 35–42. [Google Scholar] [CrossRef] [PubMed]
| NHS Region | No. Requests | Percent | Rates per 100,000 Population | Rates per 100,000 COVID-19 Cases |
|---|---|---|---|---|
| East of England | 7967 | 15% | 121.4 | 589.1 |
| London | 9910 | 19% | 110.1 | 587.5 |
| Southwest | 6018 | 12% | 106.2 | 522.8 |
| Southeast | 8161 | 16% | 91.3 | 436.2 |
| Northeast and Yorkshire | 7115 | 14% | 82.4 | 447.3 |
| Midlands | 8552 | 16% | 80.2 | 433.7 |
| Northwest | 4178 | 8% | 58.9 | 311.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Løchen, A.; Squire, H.; Ashiru-Oredope, D.; Hand, K.S.; Hartman, H.; Triggs-Hodge, C.; Fountain, H.; Bou-Antoun, S.; Demirjian, A.; Gerver, S.M. Surveillance and Stewardship Approaches for COVID-19 Novel Therapeutics in England from 2021 to 2022 (ESPAUR Report). Med. Sci. Forum 2022, 15, 2. https://doi.org/10.3390/msf2022015002
Løchen A, Squire H, Ashiru-Oredope D, Hand KS, Hartman H, Triggs-Hodge C, Fountain H, Bou-Antoun S, Demirjian A, Gerver SM. Surveillance and Stewardship Approaches for COVID-19 Novel Therapeutics in England from 2021 to 2022 (ESPAUR Report). Medical Sciences Forum. 2022; 15(1):2. https://doi.org/10.3390/msf2022015002
Chicago/Turabian StyleLøchen, Alessandra, Hanna Squire, Diane Ashiru-Oredope, Kieran S. Hand, Hassan Hartman, Carry Triggs-Hodge, Holly Fountain, Sabine Bou-Antoun, Alicia Demirjian, and Sarah M. Gerver. 2022. "Surveillance and Stewardship Approaches for COVID-19 Novel Therapeutics in England from 2021 to 2022 (ESPAUR Report)" Medical Sciences Forum 15, no. 1: 2. https://doi.org/10.3390/msf2022015002
APA StyleLøchen, A., Squire, H., Ashiru-Oredope, D., Hand, K. S., Hartman, H., Triggs-Hodge, C., Fountain, H., Bou-Antoun, S., Demirjian, A., & Gerver, S. M. (2022). Surveillance and Stewardship Approaches for COVID-19 Novel Therapeutics in England from 2021 to 2022 (ESPAUR Report). Medical Sciences Forum, 15(1), 2. https://doi.org/10.3390/msf2022015002






