Abstract
Multiple drug-resistant bacterial strains are showing new different mechanisms to overcome the antimicrobial action, which reduces the efficacy of conventional antibiotics. Therefore, drug discovery research has focused on developing fast, effective, and safe alternatives to prevent this multi-resistance. Phlorotannins are a diverse class of polyphenols, secondary metabolites described in brown algae, that are mainly constituted of polymers of phloroglucinol and, depending on their linkage and structure, can be classified mainly as fucols, fucophlorethols, eckols, and phloroethols. These polyphenols have been described in both macro- and microalgae, suggesting that they can be recovered from a great variety of sources. Phlorotannins have been extensively described to possess several biological properties, foremost as antioxidant and antimicrobial compounds. Several in vitro reports have described that phlorotannins showed growth inhibition and bactericidal effects against Gram+ (e.g., Bacillus cereus, Streptococcus epidermidis, Staphylococcus aureus) and Gram− bacteria (e.g., Salmonella sp., Campylobacter jejuni, Pseudomonas aeruginosa), also including antibiotic-resistant strains such as MRSA. Although the mechanisms of action of this group of compounds has not been fully elucidated, tannins could interact with membrane proteins and key metabolic enzymes, impeding bacterial growth and resulting in membrane lysis. Moreover, different phlorotannins were able to inhibit bacterial biofilm formation, production of quorum-sensing molecules, and also viral replication (e.g., influenza). Few in vivo studies support their effectiveness as antibiotics, whereas clinical trials studying other properties consistently report high bioavailability and null toxicity of phlorotannins. Considering current evidence, phlorotannins could be considered as interesting candidates for antibiotic therapy clinical trials. The diversity of these natural compounds provides a promising gateway for researchers and the pharmaceutical industry to develop novel nontoxic, cost-effective, and highly efficient antibacterial formulations with a broad scope of applications.
1. Introduction
Antibiotic resistance has become the topmost threat to public health in the 21st century, with more than 40 countries sharing reports on antimicrobial resistance, which signifies criticality [1]. As such, there is a need for alternative compounds and treatments that may function as antibiotics in order to deal with this issue. In the last decade, research on natural compounds from plant and algae sources has rapidly increased, with a great number of natural molecules characterized and described. In this context, marine algae, a traditional food and medicinal East-Asian ingredient, has proven to be an excellent source of natural molecules with numerous potential and effective applications in human health [2,3]. In specific, brown algae possess some unique natural compounds with various bioactive properties, such as polysaccharides (laminaran, fucoidan, alginate), lectins, alkaloids, or polyphenols, such as phlorotannins (PT) [4]. PT are composed of polymeric units of phloroglucinol (1,3,5-trihydroxybenzene), with molecular weights ranging between 126–650 kDa. They are classified into six major groups, according to the type of linkages between phloroglucinol units and the number of hydroxyl groups (Figure 1) [5,6]:
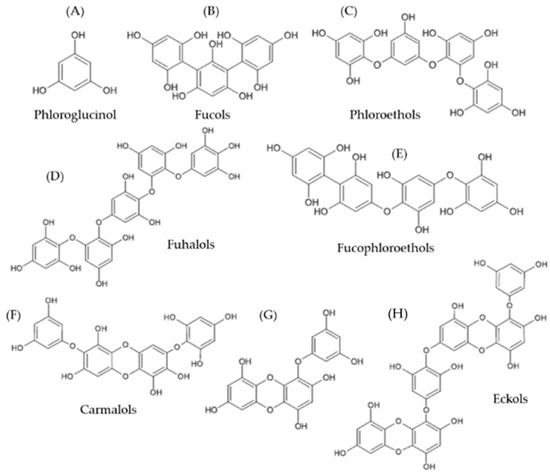
Figure 1.
Chemical structure of representative phlorotannin groups. (A) Phloroglucinol monomer; (B) Trifucol; (C) Tetraphlorethol B; (D) Pentafuhalol B; (E), Fucodiphlorethol; (F) Diphlorethohydroxycarmalol; (G) Eckol; (H) Dieckol.
- (i)
- fucols, with aryl-aryl linkages;
- (ii)
- phlorethols, with aryl-ether linkages;
- (iii)
- fucophlorethols, with aryl-aryl and aryl-ether units;
- (iv)
- fuhalols, with aryl-ether linkages and additional hydroxyl groups in every third ring;
- (v)
- carmalols, with a dibenzodioxin moiety and derived from phlorethols;
- (vi)
- eckols, which possess at least one three-ring moiety with a dibenzodioxin element substituted by a phenoxyl group at C-4.
Due to their polymeric structure and number of hydroxyl groups, PT are potent free-radical scavengers and can modulate proteins and chelate metals. These capacities explain the wide range of cellular and ecological roles of phlorotannins in seaweeds. Moreover, PT are herbivore deterrents and protect against desiccation, high UVB radiation, and toxic heavy metals, acting as chelators [7,8]. These compounds have been classified as generally regarded as safe (GRAS) substances by both the Food and Drug Administration (FDA) and the European Food Safety Agency (EFSA) [9,10]. In the EU, phlorotannin-rich extracts from Ecklonia cava are approved as a “novel food” and considered safe to consume, due to an great number of studies supporting their safety [10].
Applications of PT, however, are currently limited as antioxidant cosmetic ingredients, or nutraceuticals for metabolic modulation, as there is a growing body of evidence supported by regulators, suggesting that these marine polyphenols are safe and non-toxic. In addition, several reports suggest their antimicrobial potential. Herein, the potential use of PT as antibiotic compounds based on scientific evidence is briefly reviewed.
3. Discussion and Future Perspectives
Evidence regarding PT as antioxidants and metabolic and inflammatory modulators is vast, with a great number of studies confirming these properties by in vivo studies and clinical trials [6,26]. However, research on their potential applications as antibiotics is still limited, mainly due to the scope of reported studies (number of PT and/or microorganisms tested) and the fact that many of such works have been focused on in vitro studies, which unfortunately may not be translated to effective results in animal models or clinical trials. Nevertheless, the reported inhibitory and bactericidal effects of these molecules, specially purified compounds (e.g., dieckol, phlorofucofuroeckol-A) should be considered effective at low concentrations. Since PT have shown antioxidant, photoprotective, and anti-inflammatory activities, these could be excellent ingredients for topical antibiotic formulations and cosmetics. Moreover, PT are reported to inhibit quorum sensing and limit biofilm formation, which suggests that these compounds could contribute to reducing topical or gut infections [11,17].
Two main factors should be considered regarding potential effectiveness of PT: (1) their generally poor bioavailability and (2) their binding effectiveness to microbial pathogens. Polyphenols and specially highly-polymerized have shown very poor bioavailability (around 5–10% are absorbed), and thus, encapsulation strategies are usually developed [5]. Notably, it has also been described that PT may act as prebiotics but research on this still appears limited, and microbiota alterations based on gut infections and changes due to PT treatment should be further explored [27]. On the other hand, it has been suggested that PTs, similarly to other polyphenols, could interact with cell membrane proteins or metabolic enzymes, precipitating them and disrupting the membrane integrity [3]. This fact was reported as the main factor liable of membrane lysis in MRSA, which also acted in synergy with methicillin [24]. Altogether, despite much needed further research, there is founding evidence suggesting the potential of PT as an effective antimicrobial and antiviral compounds, among other demonstrated properties.
Author Contributions
Conceptualization, J.E., L.C. and M.A.P.; methodology, J.E. and L.C.; validation, P.G.-P., R.P.-G., M.F.-C. and L.C.; formal analysis, J.E., C.L.-L. and L.C.; investigation, J.E., C.L.-L. and A.C.-C.; resources, F.F.S. and S.B.; data curation, P.O., L.C. and M.F.-C.; writing—original draft preparation, J.E., C.L.-L. and A.C.-C.; writing—review and editing, J.E. and L.C.; visualization, L.C., P.G.-P., R.P.-G. and P.O.; supervision, P.G.-P., R.P.-G., M.F.-C. and L.C.; project administration, M.A.P. and J.S.-G.; funding acquisition, M.A.P. and J.S.-G. All authors have read and agreed to the published version of the manuscript.
Funding
The research leading to these results was supported by MICINN supporting the Ramón y Cajal grant for M.A. Prieto (RYC-2017-22891), María Zambrano grant for R. Perez-Gregorio (CO34991493-20220101ALE481), and the FPU grant for A. Carreira-Casais (FPU2016/06135), by Xunta de Galicia for supporting the program EXCELENCIA-ED431F 2020/12, the post-doctoral grant of M. Fraga-Corral (ED481B-2019/096), and L. Cassani (ED481B-2021/152). The research leading to these results was supported by the European Union through the “NextGenerationEU” program supporting the “Margarita Salas” grant awarded to P. Garcia-Perez, and the EcoChestnut Project (Erasmus+ KA202) that supports the work of J. Echave. Authors are grateful to Ibero-American Program on Science and Technology (CYTED—AQUA-CIBUS, P317RT0003), to the Bio Based Industries Joint Undertaking (JU) under grant agreement No 888003 UP4HEALTH Project (H2020-BBI-JTI-2019) that supports the work of P. Otero and C. Lourenço-Lopes and to AlgaMar company for the collaboration. The JU receives support from the European Union’s Horizon 2020 research and innovation program and the Bio Based Industries Consortium. The project SYSTEMIC Knowledge hub on Nutrition and Food Security has received funding from national research funding parties in Belgium (FWO), France (INRA), Germany (BLE), Italy (MIPAAF), Latvia (IZM), Norway (RCN), Portugal (FCT), and Spain (AEI) in a joint action of JPI HDHL, JPI-OCEANS, and FACCE-JPI launched in 2019 under the ERA-NET ERA-HDHL (n° 696295).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Antimicrobial Resistance Division Global Antimicrobial Resistance and Use Surveillance System (GLASS) Report; WHO: Geneva, Switzerland, 2020; ISBN 9789240005587. [Google Scholar]
- Pérez, M.; Falqué, E.; Domínguez, H. Antimicrobial Action of Compounds from Marine Seaweed. Mar. Drugs 2016, 14, 52. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, S.; Mazumdar, A.; Moulick, A.; Adam, V. Algal Metabolites: An Inevitable Substitute for Antibiotics. Biotechnol. Adv. 2020, 43, 107571. [Google Scholar] [CrossRef]
- Silva, A.; Silva, S.A.; Lourenço-Lopes, C.; Jimenez-Lopez, C.; Carpena, M.; Gullón, P.; Fraga-Corral, M.; Domingues, V.F.; Fátima Barroso, M.; Simal-Gandara, J.; et al. Antibacterial Use of Macroalgae Compounds against Foodborne Pathogens. Antibiotics 2020, 9, 712. [Google Scholar] [CrossRef]
- Cassani, L.; Gomez-Zavaglia, A.; Jimenez-Lopez, C.; Lourenço-Lopes, C.; Prieto, M.A.; Simal-Gandara, J. Seaweed-Based Natural Ingredients: Stability of Phlorotannins during Extraction, Storage, Passage through the Gastrointestinal Tract and Potential Incorporation into Functional Foods. Food Res. Int. 2020, 137, 109676. [Google Scholar] [CrossRef] [PubMed]
- Erpel, F.; Mateos, R.; Pérez-Jiménez, J.; Pérez-Correa, J.R. Phlorotannins: From Isolation and Structural Characterization, to the Evaluation of Their Antidiabetic and Anticancer Potential. Food Res. Int. 2020, 137, 109589. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Mu, T.; Sun, H.; Garcia-Vaquero, M. Phlorotannins: A Review of Extraction Methods, Structural Characteristics, Bioactivities, Bioavailability, and Future Trends. Algal Res. 2021, 60, 102484. [Google Scholar] [CrossRef]
- Tang, J.; Wang, W.; Chu, W. Antimicrobial and Anti-Quorum Sensing Activities of Phlorotannins From Seaweed (Hizikia Fusiforme). Front. Cell Infect. Microbiol. 2020, 10, 1–9. [Google Scholar] [CrossRef]
- Food and Drug Administration. GRAS Notice No. GRN 000661; Centre for Food Safety & Applied Nutrition: College Park, MD, USA, 2017. [Google Scholar]
- EFSA Panel on Dietetic Products Nutrition and Allergies. Safety of Ecklonia Cava Phlorotannins as a Novel Food Pursuant to Regulation (EC) No 258/97. EFSA J. 2017, 15, 5003. [Google Scholar] [CrossRef]
- Nagayama, K.; Iwamura, Y.; Shibata, T.; Hirayama, I.; Nakamura, T. Bactericidal Activity of Phlorotannins from the Brown Alga Ecklonia Kurome. J. Antimicrob. Chemother. 2002, 50, 889–893. [Google Scholar] [CrossRef]
- Choi, J.-G.; Kang, O.; Brice, O.; Lee, Y.; Chae, H.; Oh, Y.-C.; Sohn, D.-H.; Park, H.; Choi, H.-G.; Kim, S.-G.; et al. Antibacterial Activity of Ecklonia Cava Against Methicillin-Resistant Staphylococcus Aureus and Salmonella Spp. Foodborne Pathog. Dis. 2010, 7, 435–441. [Google Scholar] [CrossRef]
- Lee, J.-H.; Eom, S.-H.; Lee, E.-H.; Jung, Y.-J.; Kim, H.-J.; Jo, M.-R.; Son, K.-T.; Lee, H.-J.; Kim, J.H.; Lee, M.-S.; et al. In Vitro Antibacterial and Synergistic Effect of Phlorotannins Isolated from Edible Brown Seaweed Eisenia Bicyclis against Acne-Related Bacteria. ALGAE 2014, 29, 47–55. [Google Scholar] [CrossRef]
- Kim, H.J.; Dasagrandhi, C.; Kim, S.H.; Kim, B.G.; Eom, S.H.; Kim, Y.M. In Vitro Antibacterial Activity of Phlorotannins from Edible Brown Algae, Eisenia Bicyclis Against Streptomycin-Resistant Listeria Monocytogenes. Indian J Microbiol. 2018, 58, 105–108. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.M.; Doan, T.P.; Quy Ha, T.K.; Kim, H.W.; Lee, B.W.; Tung Pham, H.T.; Cho, T.O.; Oh, W.K. Dereplication by High-Performance Liquid Chromatography (HPLC) with Quadrupole-Time-of-Flight Mass Spectroscopy (QTOF-MS) and Antiviral Activities of Phlorotannins from Ecklonia Cava. Mar. Drugs 2019, 17, 149. [Google Scholar] [CrossRef]
- Karadeniz, F.; Kang, K.H.; Park, J.W.; Park, S.J.; Kim, S.K. Anti-HIV-1 Activity of Phlorotannin Derivative 8,4‴-Dieckol from Korean Brown Alga Ecklonia Cava. Biosci. Biotechnol. Biochem. 2014, 78, 1151–1158. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Liu, Q.; Xu, C.; Yu, J.; Zhao, L.; Guo, Q. Damage to the Membrane Permeability and Cell Death of Vibrio Parahaemolyticus Caused by Phlorotannins with Low Molecular Weight from Sargassum Thunbergii. J. Aquat. Food Prod. Technol. 2016, 25, 323–333. [Google Scholar] [CrossRef]
- Puspita, M.; Déniel, M.; Widowati, I.; Radjasa, O.K.; Douzenel, P.; Marty, C.; Vandanjon, L.; Bedoux, G.; Bourgougnon, N. Total Phenolic Content and Biological Activities of Enzymatic Extracts from Sargassum Muticum (Yendo) Fensholt. J. Appl. Phycol. 2017, 29, 2521–2537. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Stratakos, A.C.; Theodoridou, K.; Dick, J.T.A.; Sheldrake, G.N.; Linton, M.; Corcionivoschi, N.; Walsh, P.J. Polyphenols from Brown Seaweeds as a Potential Antimicrobial Agent in Animal Feeds. ACS Omega 2020, 5, 9093–9103. [Google Scholar] [CrossRef]
- Heavisides, E.; Rouger, C.; Reichel, A.F.; Ulrich, C.; Wenzel-Storjohann, A.; Sebens, S.; Tasdemir, D. Seasonal Variations in the Metabolome and Bioactivity Profile of Fucus Vesiculosus Extracted by an Optimised, Pressurised Liquid Extraction Protocol. Mar. Drugs 2018, 16, 503. [Google Scholar] [CrossRef]
- Bogolitsyn, K.; Dobrodeeva, L.; Druzhinina, A.; Ovchinnikov, D.; Parshina, A.; Shulgina, E. Biological Activity of a Polyphenolic Complex of Arctic Brown Algae. J. Appl. Phycol. 2019, 31, 3341–3348. [Google Scholar] [CrossRef]
- Kim, M.S.; Oh, G.W.; Jang, Y.M.; Ko, S.C.; Park, W.S.; Choi, I.W.; Kim, Y.M.; Jung, W.K. Antimicrobial Hydrogels Based on PVA and Diphlorethohydroxycarmalol (DPHC) Derived from Brown Alga Ishige Okamurae: An in Vitro and in Vivo Study for Wound Dressing Application. Mater. Sci. Eng. C 2020, 107, 110352. [Google Scholar] [CrossRef]
- Yang, H.K.; Jung, M.H.; Avunje, S.; Nikapitiya, C.; Kang, S.Y.; Ryu, Y.B.; Lee, W.S.; Jung, S.J. Efficacy of Algal Ecklonia Cava Extract against Viral Hemorrhagic Septicemia Virus (VHSV). Fish Shellfish Immunol. 2018, 72, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Lee, D.S.; Jung, Y.J.; Park, J.H.; Choi, J. Il; Yim, M.J.; Jeon, J.M.; Kim, H.W.; Son, K.T.; Je, J.Y.; et al. The Mechanism of Antibacterial Activity of Phlorofucofuroeckol-A against Methicillin-Resistant Staphylococcus Aureus. Appl. Microbiol. Biotechnol. 2014, 98, 9795–9804. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.H.; Moon, S.Y.; Lee, D.S.; Kim, H.J.; Park, K.; Lee, E.W.; Kim, T.H.; Chung, Y.H.; Lee, M.S.; Kim, Y.M. In Vitro Antiviral Activity of Dieckol and Phlorofucofuroeckol-A Isolated from Edible Brown Alga Eisenia Bicyclis against Murine Norovirus. Algae 2015, 30, 241–246. [Google Scholar] [CrossRef]
- Lee, S.H.; Jeon, Y.J. Anti-Diabetic Effects of Brown Algae Derived Phlorotannins, Marine Polyphenols through Diverse Mechanisms. Fitoterapia 2013, 86, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Charoensiddhi, S.; Conlon, M.A.; Vuaran, M.S.; Franco, C.M.M.; Zhang, W. Polysaccharide and Phlorotannin-Enriched Extracts of the Brown Seaweed Ecklonia Radiata Influence Human Gut Microbiota and Fermentation in Vitro. J. Appl. Phycol. 2017, 29, 2407–2416. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
