Photoinactivation of Bacterial and Fungal Planktonic/Biofilm Forms Using the Combination of a Porphyrinic Formulation with Potassium Iodide †
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
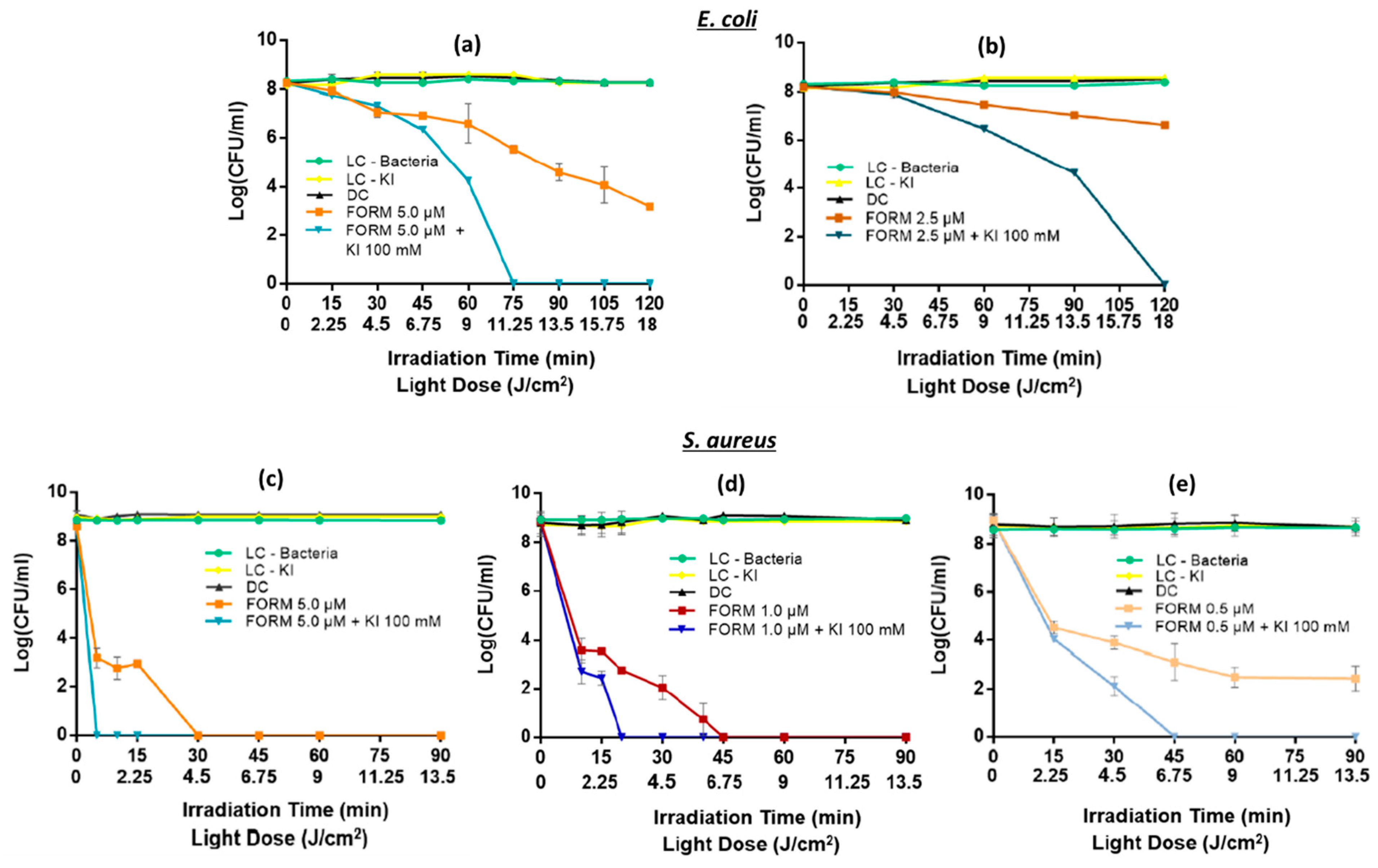
3.1. Photodynamic Inactivation of the Bacterial and Fungal Planktonic Cells
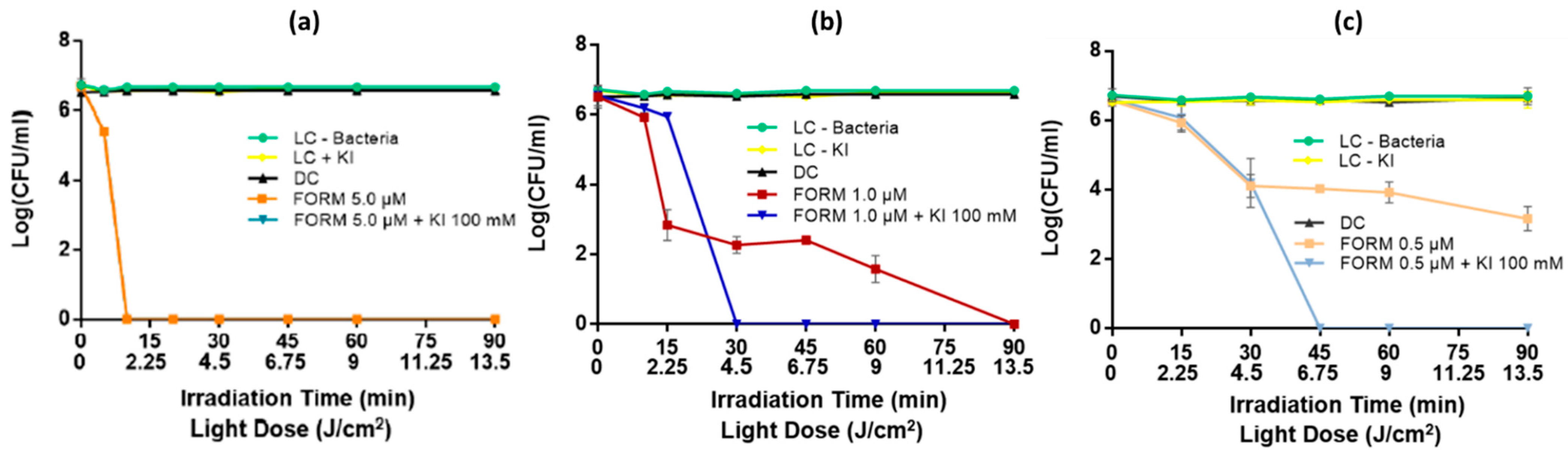
3.2. Photodynamic Inactivation of the Bacterial and Fungal Biofilms
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alves, E.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Cavaleiro, J.A.S.; Cunha, A.; Gomes, N.C.M.; Almeida, A. Photodynamic Antimicrobial Chemotherapy in Aquaculture: Photoinactivation Studies of Vibrio fischeri. PLoS ONE 2011, 6, e20970. [Google Scholar] [CrossRef] [PubMed]
- Alves, E.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Nadais, H.; Almeida, A. Potential Applications of Porphyrins in Photodynamic Inactivation Beyond the Medical Scope. J. Photochem. Photobiol 2015, 22, 34–57. [Google Scholar] [CrossRef]
- Hamblin, M.R. Upconversion in Photodynamic therapy: Plumbing the Depths. Dalton Trans 2018, 47, 8571–8580. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, A.A.; Aoki, A.; Mizutani, K.; Schwarz, F.; Sculean, A.; Wang, C.-Y.; Koshy, G.; Romanos, G.; Shikawa, I.; Zumi, Y. Application of Antimicrobial Photodynamic Therapy in Periodontal and Peri-implant Diseases. Periodontology 2009, 51, 109–140. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, M.Q.; Dias, C.J.; Neves, M.G.P.M.S.; Almeida, A.; Faustino, M.A.F. Revisiting Current Photoactive Materials for Antimicrobial Photodynamic Therapy. Molecules 2018, 23, 2424. [Google Scholar] [CrossRef] [PubMed]
- Marciel, L.; Rosalina, F.; Ana, M.; Mariana, M.; Neves, M.G.P.M.S.; Adelaide, A. An Efficient Formulation Based on Cationic Porphyrins to Photoinactivate Staphylococcus aureus and Escherichia coli. Future Med. Chem. 2018, 10, 1821–1833. [Google Scholar] [CrossRef] [PubMed]
- Martins, D.; Mesquita, M.Q.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Reis, L.; Figueira, E.; Almeida, A. Photoinactivation of Pseudomonas syringae pv. actinidiae in Kiwifruit Plants by Cationic Porphyrins. Planta 2018, 248, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Vieira, C.; Gomes, A.T.P.C.; Mesquita, M.Q.; Moura, N.M.M.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. An Insight Into the Potentiation Effect of Potassium Iodide on aPDT Efficacy. Front. Microbiol. 2018, 9, 2665. [Google Scholar] [CrossRef] [PubMed]
- Freire, F.; Ferraresi, C.; Jorge, A.O.C.; Hamblin, M.R. Photodynamic Therapy of Oral Candida Infection in A Mouse Model. Photochem. Photobiol. B 2016, 159, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Dai, T.; Wang, M.; Vecchio, D.; Chiang, L.Y.; Hamblin, M.R. Potentiation of Antimicrobial Photodynamic Inactivation Mediated by a Cationic Fullerene by Added Iodide: In vitro and in vivo Studies. Nanomedicine 2015, 10, 603–614. [Google Scholar] [CrossRef]
- Simões, C.; Gomes, M.C.; Neves, M.G.P.M.S.; Cunha, A.; Tomé, J.P.C.; Tomé, A.C.; Cavaleiro, J.A.S.; Almeida, A.; Faustino, M.A.F. Photodynamic Inactivation of Escherichia coli with Cationic Meso-tetraarylporphyrins—The Charge Number and Charge Distribution Effects. Catal. Today 2016, 266, 197–204. [Google Scholar] [CrossRef]
- Beirão, S.; Fernandes, S.; Coelho, J.; Faustino, M.A.F.; Tomé, J.P.C.; Neves, M.G.P.M.S.; Tomé, A.C.; Almeida, A.; Cunha, A. Photodynamic Inactivation of Bacterial and Yeast Biofilms with A Cationic Porphyrin. Photochem. Photobiol 2014, 90, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.; Faustino, M.A.F.; Neves, M.G.P.M.S.; Cunha, A.; Almeida, A. Photodynamic Inactivation of Mammalian Viruses and Bacteriophages. Viruses 2012, 4, 1034–1074. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, C.; Bartolomeu, M.; Santos, A.R.; Mesquita, M.Q.; Gomes, A.T.P.C.; Neves, M.G.P.M.S.; Faustino, M.A.F.; Almeida, A. Photoinactivation of Bacterial and Fungal Planktonic/Biofilm Forms Using the Combination of a Porphyrinic Formulation with Potassium Iodide. Med. Sci. Forum 2022, 12, 13. https://doi.org/10.3390/eca2022-12711
Vieira C, Bartolomeu M, Santos AR, Mesquita MQ, Gomes ATPC, Neves MGPMS, Faustino MAF, Almeida A. Photoinactivation of Bacterial and Fungal Planktonic/Biofilm Forms Using the Combination of a Porphyrinic Formulation with Potassium Iodide. Medical Sciences Forum. 2022; 12(1):13. https://doi.org/10.3390/eca2022-12711
Chicago/Turabian StyleVieira, Cátia, Maria Bartolomeu, Adriele R. Santos, Mariana Q. Mesquita, Ana T. P. C. Gomes, Maria Graça P. M. S. Neves, Maria Amparo F. Faustino, and Adelaide Almeida. 2022. "Photoinactivation of Bacterial and Fungal Planktonic/Biofilm Forms Using the Combination of a Porphyrinic Formulation with Potassium Iodide" Medical Sciences Forum 12, no. 1: 13. https://doi.org/10.3390/eca2022-12711
APA StyleVieira, C., Bartolomeu, M., Santos, A. R., Mesquita, M. Q., Gomes, A. T. P. C., Neves, M. G. P. M. S., Faustino, M. A. F., & Almeida, A. (2022). Photoinactivation of Bacterial and Fungal Planktonic/Biofilm Forms Using the Combination of a Porphyrinic Formulation with Potassium Iodide. Medical Sciences Forum, 12(1), 13. https://doi.org/10.3390/eca2022-12711







