Abstract
In previous studies (Marques-da-Silva et al., 2019), we measured the minimum inhibitory concentrations (MICs) of three polyoxovanadates, namely V10, MnV11, and MnV13, against Escherichia coli. MICs were obtained following the standard method, which requires a 16–20 h culture and might neglect the effects of the compounds’ metabolism during incubation. In this work, we studied the action of those compounds against Enterococcus faecalis by monitoring the bacterial growth kinetics, and we observed that the inhibition was evident from the beginning of the exponential phase. Notably, data collected from just a 7 h culture was enough to identify the compounds with stronger antibacterial activity according to standard MICs.
1. Introduction
Polyoxometalates are a well-known group of anionic polynuclear metal oxides (containing VV, TaV, NbV, WVI, and MoVI, usually in their highest oxidation state) with distinct and chemically changeable cluster structures. These compounds present important therapeutic potential as anticancer, antiviral, and antibacterial agents [1].
In previous work [2], we measured the minimum inhibitory concentrations (MICs) of three polyoxovanadates (POVs), namely Na6V10O28 (abbreviated V10), K5MnIVV11O32.10 H2O (MnV11), and K7MnIVV13O38.18 H2O (MnV13) against the Gram-negative bacteria Escherichia coli. The MICs were obtained following the well-accepted serial two-fold dilution method. However, this method requires a 16–20 h culture and might neglect the effects of the compounds’ metabolism/speciation changes during incubation.
There is a clear need for rapid methods to investigate potential antibacterial agents, antibiotic susceptibility, and novel effective combinations against different bacteria [3,4]. Sun et al. (2016) described a method based on OD600 and ATP measurements in a miniaturized set-up to identify new drugs and drug combinations against multidrug-resistant bacteria [3]. Recently, Chakansin et al. (2022) presented a dye-based assay to rapidly screen the antibacterial activity of nanomaterials whose turbidity interferes with conventional methods [4]. Although rapid and simple, it was observed that the dye could be degraded in the presence of nanomaterials with photocatalytic properties at high concentrations.
The application of dye-based assays to test POVs should also be regarded carefully because some of these compounds can catalyze the degradation of dyes [5]. In addition, many POVs are colored, and the color changes with speciation.
Several (bio)chemical modifications of metallodrugs, such as POVs, can affect their bioactivity and putative interactions with proteins [6,7]. Furthermore, the metabolic conversion of POVs by bacteria was reported before [2]. POVs’ speciation and bioactivity can therefore change during incubation with microorganisms [6], and endpoint (single-time) assays, such as the conventional MIC determination method, can fail to detect the influence of compounds’ speciation.
Taking into account these limitations, we further studied the antibacterial action of the POVs against the Gram-positive Enterococcus faecalis in this work by monitoring the growth curve. The method directly following bacterial multiplication is cheap and does not require detection reagents. Moreover, it can be automated and miniaturized for the high-throughput screening of numerous compounds against different bacteria [3]. In addition, the MICs of the POVs against E. faecalis were determined for the first time.
2. Materials and Methods
2.1. Polyoxovanadates, Bacteria and Culture Medium
The synthesis of the POVs and preparation of the stock solutions were performed as described in our previous work [2]. The antibacterial studies were carried out with E. faecalis (ATCC 29212) in LB broth.
2.2. Minimum Inhibitory Concentrations Determination
MICs were measured by the serial two-fold dilution method, using dilutions of each POV in concentrations 1024, 2048, and 4096 μg∙mL−1. After inoculation with the bacteria and incubation for 18 h, the occurrence or absence of bacterial growth was visually examined (turbidity or presence of a cell deposit).
2.3. Bacterial Growth Monitoring
Growth curves of E. faecalis in LB were obtained by monitoring the optical density of the cultures at 600 nm (OD600), for up to 9 h incubation. The POV compounds were tested at 0.5 mM concentration, based on the antibacterial data obtained before with E. coli [2].
3. Results and Discussion
The antibacterial action of V1, V10, MnV11, and MnV13 against E. faecalis was investigated by monitoring the growth of the bacteria at different incubation times and by the conventional MIC determination method.
For the growth curve method, the kinetics of bacterial growth was followed from the OD600 measured in a laboratory spectrophotometer. Figure 1 illustrates the effect of a POV with low or insignificant antibacterial action, V1, and a clearly inhibitory compound, V10. The comparison between the growth curves in the presence of POVs and the corresponding control cultures indicates that the compounds did not affect the lag time of the culture. However, the inhibitory effects of V10, MnV11, and MnV13 on the growth of E. faecalis were evident from the beginning of the exponential phase. The monomeric vanadate species (V1) showed only a modest inhibition of bacterial growth.
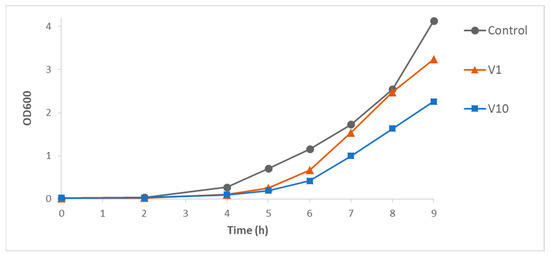
Figure 1.
Time evolution of Enterococcus faecalis growth in the absence and in the presence of V1 or V10 compounds at 0.5 mM concentration.
In addition, the MICs of the POVs against E. faecalis were determined from 18 h cultures in the presence of a series of concentrations up to 4096 μg∙mL−1. The MIC values obtained were 2048 μg∙mL−1 for V10 and MnV11, 4096 μg∙mL−1 for MnV13, and >4096 μg∙mL−1 for V1. These results are in line with the efficacy of the same compounds against E. coli measured previously [2], suggesting that POVs yield broad antibacterial action.
There was also a good correlation between the effect of each compound in the E. faecalis growth curve and the corresponding MIC values (Figure 2). Notably, the inhibition data obtained after 7 h culture was sufficient to identify the POVs with stronger antibacterial activity, V10, MnV11, and MnV13, in agreement with the results of the MIC determination at 18 h. The inhibition of bacterial growth was calculated from the increase in OD600 of cultures in the presence of the tested compound, which was normalized to the increase observed in the control culture that started with the same bacterial inoculum, but without the compounds. At 7 h, V10, MnV11, and MnV13 inhibited bacterial growth by 51%, 84%, and 73%, respectively. At this incubation time, V1 only inhibited 11% of E. faecalis growth, an indication of its low antibacterial activity.
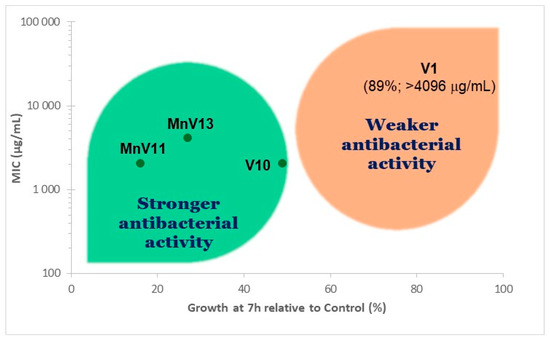
Figure 2.
Relationship between the effect of the tested polyoxovanadates on Enterococcus faecalis growth measured at 7 h incubation and the corresponding minimum inhibitory concentrations (MIC) determined by the serial two-fold dilution method at 18 h.
4. Conclusions
The present study expands our understanding of the antibacterial action of vanadates and manganese polyoxovanadates. The POVs were tested against the Gram-positive bacteria E. faecalis by monitoring the growth of the microorganism at different incubation times and by the conventional MIC determination method.
The MICs herein obtained for the POVs against E. faecalis, and previously against E. coli [2], point to MnV11 as the compound with a higher potential for antibacterial applications, with activity towards both Gram-negative and Gram-positive bacteria.
The growth curve method implemented in this work to assess the antibacterial activity of POVs returned coherent results that validate the method for the fast screening of compounds. The method can provide preliminary indications of high- and low-activity compounds within a few hours.
As a future perspective, the study points out that the automated monitoring of the growth curve can meet the need for rapid methods to screen antibacterial POVs against different bacteria.
Author Contributions
Investigation, D.M.-d.-S. and S.S.M.; writing—original draft preparation, D.M.-d.-S. and R.L.; writing—review and editing, M.A. and R.L. All authors have read and agreed to the published version of the manuscript.
Funding
The authors’ research received Portuguese national funds from FCT—Foundation for Science and Technology through unit projects UIDB/50020/2020, UIDP/50020/2020 and LA/P/0045/2020 (D.M.S.); UIDB/04326/2020 and LA/P/0101/2020 (M.A.); and UIDP/04378/2020, UIDB/04378/2020 and LA/P/0140/2020 (R.L.). S.S.M. thanks the Indian Council of Scientific & Industrial Research (CSIR) for providing financial support (01(2906)/17/EMR-II).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Aureliano, M.; Marques-da-Silva, D.; Serrano, A.; Martins, J.; Faleiro, L.; Fonseca, C.; Fraqueza, G.; Lagoa, R. Polyoxometalates with anticancer, antibacterial and antiviral activities. In Polyoxometalates: Advances, Properties, and Applications, 1st ed.; Jenny Stanford Publishing: United Square, Singapore, 2022; in press. [Google Scholar]
- Marques-Da-Silva, D.; Fraqueza, G.; Lagoa, R.; Vannathan, A.A.; Mal, S.S.; Aureliano, M. Polyoxovanadate inhibition of: Escherichia coli growth shows a reverse correlation with Ca2+-ATPase inhibition. New J. Chem. 2019, 43, 17577–17587. [Google Scholar] [CrossRef]
- Sun, W.; Weingarten, R.A.; Xu, M.; Southall, N.; Dai, S.; Shinn, P.; Sanderson, P.E.; Williamson, P.R.; Frank, K.M.; Zheng, W. Rapid antimicrobial susceptibility test for identification of new therapeutics and drug combinations against multidrug-resistant bacteria. Emerg. Microbes Infect. 2016, 5, e116. [Google Scholar] [CrossRef] [PubMed]
- Chakansin, C.; Yostaworakul, J.; Warin, C.; Kulthong, K.; Boonrungsiman, S. Resazurin rapid screening for antibacterial activities of organic and inorganic nanoparticles: Potential, limitations and precautions. Anal. Biochem. 2022, 637, 114449. [Google Scholar] [CrossRef] [PubMed]
- Missina, J.M.; Leme, L.B.; Postal, K.; Santana, F.S.; Hughes, D.L.; De Sá, E.L.; Ribeiro, R.R.; Nunes, G.G. Accessing decavanadate chemistry with tris(hydroxymethyl)aminomethane, and evaluation of methylene blue bleaching. Polyhedron 2020, 180, 114414. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; Rompel, A.; Crans, D.C. Polyoxovanadates with emerging biomedical activities. Coord. Chem. Rev. 2021, 447, 214143. [Google Scholar] [CrossRef]
- Aureliano, M.; Gumerova, N.I.; Sciortino, G.; Garribba, E.; McLauchlan, C.C.; Rompel, A.; Crans, D.C. Polyoxidovanadates’ interactions with proteins: An overview. Coord. Chem. Rev. 2022, 454, 214344. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).