Abstract
The two endemic plant species Silene leucophylla and Silene schimperiana (Caryophyllaceae) are native to the Sinai Peninsula which is considered as one of the floristically richest phytogeographical hot spot regions in of the Mediterranean basin. The biodiversity of the Sinai Peninsula is crucial for conservation and sustainable development in the area. Endemic plant species of the Sinai Peninsula are vulnerable to anthropogenic threats due to their relatively low population sizes. In the current study, we reinvestigated the taxonomic statues of two medicinally important and endangered species. The integrated approach involved macro- and micro-morphological traits using a Scanning Electron Microscope (SEM) as well as phylogenetic analysis were conducted, while phylogenetic analysis were also conducted. Phylogenetic reconstruction using Bayesian Inference based on DNA sequences of nuclear (ITS) and chloroplast (rbcL and matK) markers retrieved the species phylogenies successfully. Silene leucophylla and Silene schimperiana were placed phylogenetically within the whole genus. The sectional classification of the two species was confirmed. Silene leucophylla was placed in section Siphonomorpha while Silene schimperiana allied to section Sclerocalycinae. The current study reaffirmed that the integration of various morphological and molecular approaches is useful for identifying and, determining the taxonomic statues, and revealing the phylogenetic positions of these two endangered plant taxa.
Keywords:
endangered; endemic; Silene; SEM; stomata; molecular systematic; phylogenetic analysis; nrDNA ITS; cpDNA matK 1. Introduction
Anthropogenic risks and environmental transformations are ordinarily believed to be a higher extinction threats for endemic plant species because they are extra vulnerable [1]. Nowadays the botanists is more conscious of the worth of endemic plant species, and their distinct genetic compositions: hence, it is a high-priority to conserve them [1,2]. Egypt is situated in the southeast of the Mediterranean coast which almost contains almost 7% of the plants from around the world [1,2]. Abdelaal et al. [3] recorded 48 endemic taxa in Egypt.
Silene L. (tribe Sileneae) is considered one of the largest genera in the Caryophyllaceae family, with about 850 species distributed in the Northern Hemisphere, temperate regions of the Mediterranean zone, and central and western Asia [4,5]. Jafari et al. [4] divided Silene into of three subgenera (Lychnis (L.) Greuter, Behenantha Schur, and Silene) as well as 34 sections, using morphological and phylogenetic analyses. Twenty-nine Silene taxa were recognized in Egypt three of which them are endemic S. leucophylla Boiss., S. schimperiana Boiss., and S. oreosinaica Chowdhuri [6,7,8,9,10]. S. leucophylla and S. schimperiana have been allied to subg. Silene, below theSiphonomorpha and Sclerocalycinae sections, respectively [10,11,12]. The macro and micromorphological characteristics of leaf epidermal cells have important taxonomical features and play a vital role in the discrimination between taxa within the Caryophyllaceae family and for the discrimination of Silene taxa at the specific level [13,14,15,16].
Phylogenetic analysis is essential for identifyingstructural and functional characteristics related to biodiversity against an evolutionary background [17]; therefore, the utilization of the phylogenetic data is important for ecological, taxonomical, and evolution studies [18,19]. Identifying the phylogenetic relationships of large genera such as Silene is always challenging. A new taxonomic underpinning study of the infrageneric classification of Silene species based on nrDNA ITS and cpDNA rps16 sequences was has been conducted by Jafai et al. [4]. In the current study, the morphological and molecular phylogenetic data for S. leucophylla and S. schimperiana species were merged. The aim of the current study was to contribute to designation and identification of these species by, and revealing the phylogenetic positions of those endemic species within the whole genus.
2. Experiments
2.1. Plant Materials
For morphological and anatomical analyses, herbarium specimens of S. leucophylla and S. schimperiana were obtained from ASTU herbarium. For molecular analysis, fresh leaf materials were collected from different populations across the species ‘geographic distribution at Saint Katherine, South Sinai, Egypt.
2.2. Morphological and Anatomical Analyses
The stem as well as the leaf abaxial (AB) and adaxial (AD) surfaces were mounted onto stubs with double-sided adhesive tape, coated for 5 min with gold in a polaron JFC-1100E coating unit, then were examined and photographed with a JEOL JSM-IT200 scanning electron microscope unit at Faculty of Science, Alexandria University, Alexandria, Egypt. The quantitative characteristics were measured using image analysis software [20] following and the method used by Barthlott et al. [21].
2.3. Statistical Analysis
All quantitative data were applied using the R-software with the required packages installed [22]. Boxplots were created using the “ggplot2” library [23]. Analysis of variance (ANOVA) was performed using (aov) function. Which it followed bya Post Hoc Tukey Honestly Significant Difference (HSD) test. The “pheatmap” and “ggplot2” packages [23,24], were used to visualize the similarity and dissimilarity within and among two species. The “corrplot2” package was used to visualize the correlation output by drawing the correlogram [25].
2.4. Phylogenetic Analysis
DNA Extraction, PCR Amplification, Sequencing, and Phylogenetic Analysis
Fresh leaf materials used for molecular analyses were collected and preserved in silica gel. DNA was extracted using the cetyltrimethylammonium bromide (CTAB) protocol with some modifications [26]. The PCR amplification was performed at 15 μL volume for ITS with matK, containing 5 U/μL Taq DNA polymerase with 25 μM MgCl2, 10 μM of dNTPs, and 10 μM of each primer. Amplifications were conducted using an Applied Biosystems®-VeritiTM 96-well thermal cycler. PCR products were purified with ExoSAP-IT (USB Corporation, Cleveland, OH, USA). PCR products were sent to Macrogen Spain for direct sequencing in both directions with an ABI 3730XL Genetic Analyzer (Life Technologies Corporation, Carlsbad, CA, USA).
These novel DNA sequences of S. leucophylla and S. schimperiana were deposited in the GenBank under the accession codes ITS: (submitted to GB), and matK: (submitted to GB). The aligned DNA sequences for ITS and matK were used to construct two single markers and a combined dataset. The optimal nucleotide substitution model was estimated using the MrModeltest [27], and executed in MrBayes blocks. A 50% majority role consensus tree was constructed to obtain the posterior probabilities (PP). Posteriori probabilities, values >0.5 at a given branch were considered strong support for the existence of that branch [28].
3. Results
Stem and leaf qualitative and quantitative characteristics are summarized in Supplementary Table S1 and S2, respectively.
3.1. Stem Micromorphology
The stem surface was covered by unicellular non-glandular pustulate trichomes measuring 45–98 × 13–23 μm in S. leucophylla (Figure 1a,b), whereas S. schimperiana had glabrous surface (Figure 2a). The stem epicuticular wax on the stem was film-like in S. leucophylla with irregular flat crystalloid platelets (<1 μm height) with sinuate margin in S. schimperiana. The type of stomatal complex was anomocytic in the investigated species.
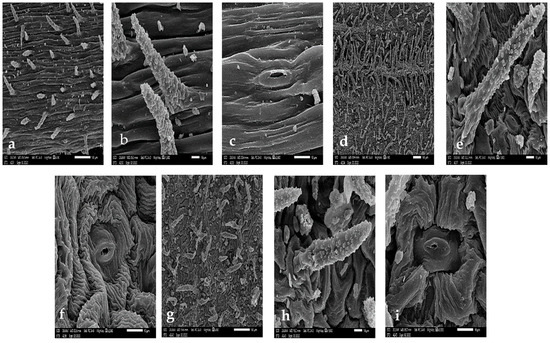
Figure 1.
Scanning Electron Microscope (SEM) photo-micrographs of Silene leucophylla. (a–c) stem. (d–i) leaf. (a) surface. (b) trichome. (c) stomata. (d) abaxial surface. (e) abaxial trichome. (f) abaxial stomata. (g) adaxial surface. (h) adaxial trichome. (i) adaxial stomata.

Figure 2.
SEM micrographs of Silene schimperiana. (a,b) stem. (c–f) leaf. (a) surface, (b) stomata. (c) abaxial surface. (d) abaxial stomata. (e) adaxial surface. (f) adaxial stomata.
Geom_boxplot and ANOVA tests data were applied to the quantitative for measured stomatal characteristics (length, width, and area) and stomatal pores (length, width and area confirming the stomatal variations of the two species with analysis significant p-values (p = 0.0054 **, R-squared = 0.9487), which showed a the higher median for the stem stomata of S. leucophylla (p = 0.00713 **) than S. schimperiana (p = 0.00563 **) (Figure 3a).
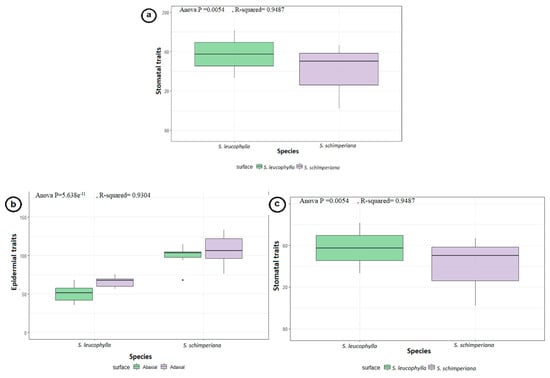
Figure 3.
Boxplots of the quantitative data for (a) stomatal characteristics at the stem micromorphology with the stomatal characteristics (length, width and area) and stomatal pores (length, width and area) in endemic S. leucophylla and S. schimperiana. (b) Epidermal cells sizes shown by micromorphology (length and width) in endemic S. leucophylla and S. schimperiana. (c) stomatal characteristics of the stomatal complex, subsidiary cells and stomatal pores (length, width, and area) respectively in endemic S. leucophylla and S. schimperiana.
3.2. Leaf Epidermal Cells
The epidermal cell characteristics were separately described for abaxial (AB) and adaxial (AD) leaf surfaces. For the primary sculpture, the leaf epidermal cells were parallel or irregularly arranged, and their shapes were oblong to the bone-shape or tetra-, penta-, hexa- to polygonal. Significant variations were distinguished in terms of size of the epidermal cells with ANOVA (p < 2.2 × 10−16 ***, R-squared = 0.9708), with the smallest epidermal cell on both surfaces of S. leucophylla; on the AB surface being in the range of23.51–42.76 × 8.73–19.25 μm (Figure 1d) and the on AD surface in the range of 31.16–52.41 × 12.79–26.97 μm (Figure 1g). The largest cells for S. schimperiana on AB surface were in the range of 30.52–61.40 × 37.85–63.79 μm (Figure 1c) and on the AD surface were in the range of 35.48–91.04 × 32.47–80.59 μm (Figure 1e). These results were confirmed by grouped boxplot for abaxial and adaxial leaf surface (Figure 3b).
The Anticlinal Walls (AW) were usually sunken, irregularly curved in S. leucophylla or straight in S. schimperiana. The relief of the cell boundary was generally channeled, being deeply ribbed on the AB surface and slightly ribbed on AD surface in S. leucophylla and smooth in S. schimperiana. For the secondary sculpture, the fine relief of the cell wall was a regular striate cuticular sculpture in S. leucophylla and was smooth in S. schimperiana. For the tertiary sculpture; the epicuticular secretions were similar to those found on the stem of both S. leucophylla and S. schimperiana.
3.3. Stomatal Complex
The leaves are amphistomatic in the two studied species. The raised diacytic type of stomata was observed in S. schimperiana, while S. leucophylla showed both sunken diacytic and tetracytic stomata. The surface of the guard cells were either smooth in S. leucophylla or epicuticular crustose platelets in S. schimperiana. The smallest stomatal area was recorded in S. leucophylla on the AB surface (46.44–74.64 = 61.42 ± 8.56 μm2; (Figure 1f), while the largest area was recorded in S. schimperiana on the AD surface 102.20–253.50 = 152.30 ± 37.92 μm2 (Figure 1i). Grouped boxplot of abaxial and adaxial leaveas showed variations in at stomatal pore, stomatal complex and subsidiary cells in terms of length, width and area respectively, as measured with ANOVA (p = 5.638 × 10−11 ***, R-squared = 0.9304), proving the previous reading (Figure 3c). Moreover, the lowest Stomatal Index (SI%) values were recorded for S. leucophylla (11.76–12.12 = 11.94 ± 0.25), while the highest SI (12.90–20.69 = 15.10 ± 3.76) values were recorded on the AD surface for S. schimperiana.
Finally, the pheatmap exhibited the variation when understudying the two taxa, where in the two S. leucophylla replicates the measured readings grouped together in separate clusters, showing divergence from the three S. schimperiana replicates, which revealed little divergence, meaning they collected from two places with little difference in altitude range Figure 4a. The correlogram correlation analysis exposed significant relationships among numerous traits (Figure 4b).
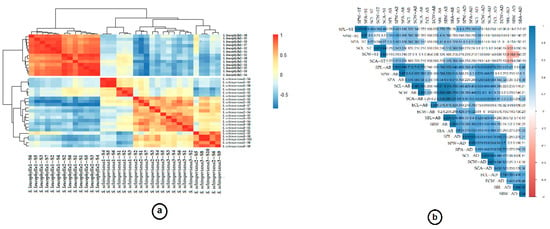
Figure 4.
(a) pheatmap for based on the quantitative data of Stem and leaf micromorphology traits, to visualize the similarity and dissimilarity within and among S. leucophylla and S. schimperiana. (b) Correlogram for quantitative traits. Positive and negative correlations are displayed in blue and in red color, respectively. Correlation coefficients are proportional to color intensity, and the size of the circle is proportional to the correlation coefficients.
3.4. Phylogeny
In the combined circularized phylogenetic tree for nrDNA ITS and cpDNA matK (Figure 5), Atocion lerchenfeldianum, Atocion rupestre, Viscaria alpina, Arenaria densissima, Arenaria polytrichoides, Arenaria smithiana and Bufonia multiceps composed the outgroup taxa. The in-group consisted of 33 Silene taxa. The three strongly supported sections “S. sect. Auctifolia (Posterior Propability PP = 100), S. sect. Auriculate (PP = 100), and S. sect. Silene (PP = 100)” were represented by S. cordifolia, S. schafta, and S. ciliata. Silene leucophylla was placed in Silene sect. Siphonomorpha s.l. (PP = 100), while S. schimperiana was placed in S. sect. Sclerocalycinae s.l. (PP = 98).
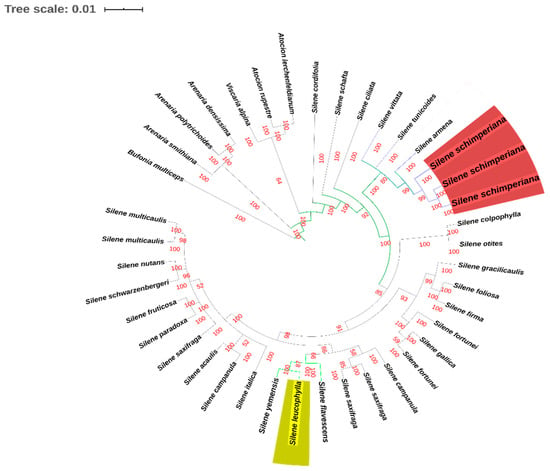
Figure 5.
Bayesian phylogenetic tree based a combined DNA sequences of nrDNA ITS and cpDNA matK.
4. Discussion
The current study represents the first detailed leaf surface morphology analysis for the Egyptian endemic and near endemic taxa; S. leucophylla and S. schimperiana respectively. Stem and leaf samples of S. schimperiana are characterized by the presence of epicuticular secretions, in the form of irregular flat crystalloid crustose platelets. These waxes have great systematic significance and ecological importance due to the interactions between plants and their environments [21]. Furthermore, both stem and leaf surfaces were covered by unicellular non-glandular pustulate trichomes in S. leucophylla, whereas S. schimperiana had a glabrous surface. Our results are compatible with Stace [29], who suggested that anticlinal wall patterns are corresponding to the habitat and environment, whereby species growing in drier habitats have straight to curved anticlinal walls.
The studied species are amphistomatic, which is a characteristic feature for species occupying xerophytic habitats [30]. In the present study, the stem stomatal complex area for S. leucophylla was 1.2 times larger than for S. schimperiana. On the other hands, leaf measurements of stomatal complex and epidermal cells characteristics were always greater on the AD surface than on the AB surface for both species, while the size of S. schimperiana cells was larger than for S. leucophylla. It was notable that for the former species, the cell size for the stomatal complex area wass 2.6–2.9 times, the stomatal pore area was 24.4–33.5 times and the subsidiary cell area wass 2.69–3.13 times larger than values obtained for the latter species. As mentioned by Rossatto and Kolb [31], species located in shady areas similar to the top of the Saint Katherine Protectorate mountains have a low average Stomatal Index (SI%). This observation is harmonious with our study, as SI values were 12.42% and 14.44% for S. leucophylla and S. schimperiana respectively. In contrast, a high average of SI (up to 95.58%) is noticed for species found in sunny areas [32].
Rohrbach [33] classified S. leucophylla and S. schimperiana at the same Section III. Botryosilene, but in differing at Series 8. Nutantes, and 1. Sclerocalycinae, respectively. While Chowdhuri [10] and Hosny, et al. [12] placed the species with sect. Siphonomorpha and sect. Sclerocalycinae (Subsection Chlorifoliae), respectively. The latest studies Oxelman et al. [11] deal with S. leucophylla as S. subsect. Brachypodae, allied to S. sect. Siphonomorpha while S. schimperiana was allied to S. subsect. Sclerocalycinae.
Our results from the phylogenetic studies showed a combined phylogenetic tree for nrDNA ITS and cpDNA matK confirming that S. leucophylla is related to sect. Siphonomorpha, due to its noticeable S. leucophylla shared with S. yemensis in the same clade and in-group with S. flavescens, which are related to S. sect. Siphonomorpha. While S. schimperiana allied to S. subsect. Sclerocalycinae, which was exhibited at a clade in a group with S. armena, while S. tunicoides was related to S. sect. Sclerocalycinae. This is in line with the phylogenetic studied by Jafari et al. [4], although they didn’t examine the two endemic Egyptianspecies under study.
5. Conclusions
In conclusion, the stem and leaf micromorphology (SEM) results revealed the complete distinctions between the two taxa. We discussed the variation between them that permitted the endemism of S. leucophylla and S. schimperiana. While the phylogenetic studies confirmed the classification of these species to the relevant sections. the classification depended only on the morphological descriptions.
Supplementary Materials
The poster presentation is available online at https://www.mdpi.com/article/10.3390/IECPS2020-08619/s1.
Author Contributions
Conceptualization A.E.-B., and A.E.; methodology, A.E.-B., F.Y.E., I.H.N. and A.E.; software, A.E.-B. and F.Y.E.; formal analysis, A.E.-B., I.H.N. and F.Y.E.; data curation, A.E.-B., F.Y.E., and I.H.N.; investigation, A.E.-B., F.Y.E., I.H.N. and A.F.; resources, A.E.-B., A.F., A.E., I.H.N. and A.O.O.; writing—original draft preparation, A.E.-B., F.Y.E., I.H.N., A.F., and A.O.O.; writing—review and editing, A.E.-B., F.Y.E., I.H.N. and A.F.; funding acquisition, A.E.-B., F.Y.E., I.H.N., A.F., A.O.O. and A.E. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Coelho, N.; Gonçalves, S.; Romano, A. Endemic Plant Species Conservation: Biotechnological Approaches. Plants 2020, 9, 345. [Google Scholar] [CrossRef] [Green Version]
- Foggi, B.; Viciani, D.; Baldini, R.M.; Carta, A.; Guidi, T. Conservation assessment of the endemic plants of the Tuscan Archipelago, Italy. Oryx 2015, 49, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Abdelaal, M.; Fois, M.; Fenu, G.; Bacchetta, G. Critical checklist of the endemic vascular plants of Egypt. Phytotaxa 2018, 360, 19–34. [Google Scholar] [CrossRef]
- Jafari, F.; Zarre, S.; Gholipour, A.; Eggens, F.; Rabeler, R.; Oxelman, B. A new taxonomic backbone for the infrageneric classification of the species-rich genus Silene (Caryophyllaceae). Taxon 2020, 69, 337–368. [Google Scholar] [CrossRef]
- Hernández-Ledesma, P.; Berendsohn, W.G.; Borsch, T.; von Mering, S.; Akhani, H.; Arias, S.; Castañeda-Noa, I.; Eggli, U.; Eriksson, R.; Flores-Olvera, H. A taxonomic backbone for the global synthesis of species diversity in the angiosperm order Caryophyllales. Willdenowia 2015, 45, 281–383. [Google Scholar] [CrossRef] [Green Version]
- Radford, E.A.; Catullo, G.; Montmollin, B.D. Important Plant Areas of the South and East Mediterranean Region: Priority Sites for Conservation; IUCN/WWF: Gland, Switzerland, 2011. [Google Scholar]
- Fakhry, A.M.; El-Keblawy, A.; Shabana, H.A.; Gamal, I.E.; Shalouf, A. Microhabitats Affect Population Size and Plant Vigor of Three Critically Endangered Endemic Plants in Southern Sinai Mountains, Egypt. Land 2019, 8, 86. [Google Scholar] [CrossRef] [Green Version]
- Hashim, A.M.; Alharbi, B.M.; Abdulmajeed, A.M.; Elkelish, A.; Hozzein, W.N.; Hassan, H.M. Oxidative stress responses of some endemic plants to high altitudes by intensifying antioxidants and secondary metabolites content. Plants 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Zahran, M.; Wafaa, A.; Samy, A.; Omran, G. Endemic species in Sinai Peninsula, Egypt, with particular reference to Saint Katherine protectorate: I-ecological features. J. Environ. Sci. 2015, 44, 589–609. [Google Scholar]
- Chowdhuri, P. Studies in the genus Silene. Notes from the RGB. Edinburgh 1957, 22, 221–278. [Google Scholar]
- Oxelman, B.; Rautenberg, A.; Thollesson, M.; Larsson, A.; Frajman, B.; Eggens, F.; Petri, A.; Aydin, Z.; Töpel, M.; Brandtberg-Falkman, A. Sileneae taxonomy and systematics. Cited 2013, 4, 2014. [Google Scholar]
- Hosny, A.; el Hadidi, M.; Shamso, E. Taxonomic Studies of Silenoideae (Caryophyllaceae) in Egypt. 1. Systematic revision of the genus Silene L. Taeckholmia 1992, 14, 1–36. [Google Scholar]
- Zarrinkamar, F. Foliar anatomy of the Caryophyllaceae family in Arasbaran, NW. J. Iran. Iran. J. Bot. 2001, 9, 93–102. [Google Scholar]
- Ahmad, K.; Khan, M.A.; Ahmad, M.; Zafar, M.; Arshad, M.; Ahmad, F. Taxonomic diversity of stomata in dicot flora of a district tank (NWFP) in Pakistan. Afr. J. Biotechnol. 2009, 8, 1052–1055. [Google Scholar]
- Keshavarzi, M.; Bozchaloyi, S.E. Leaf and stem comparative anatomical analysis of three genera of Alsinoideae (Caryophyllaceae). Iran. J. Bot. 2014, 20, 71–79. [Google Scholar]
- Nejati, E.M.; Ghahremaninejad, F.; Attar, F. Foliar anatomy of the genus Silene L. (Caryophyllaceae) at sectional level in Iran. Iran. J. Bot 2016, 22, 2. [Google Scholar]
- Rolland, J.; Cadotte, M.W.; Davies, J.; Devictor, V.; Lavergne, S.; Mouquet, N.; Pavoine, S.; Rodrigues, A.; Thuiller, W.; Turcati, L. Using Phylogenies in Conservation: New Perspectives; The Royal Society: London, UK, 2012. [Google Scholar]
- Rodrigues, A.S.; Gaston, K.J. Maximising phylogenetic diversity in the selection of networks of conservation areas. Biol. Conserv. 2002, 105, 103–111. [Google Scholar] [CrossRef]
- Hoveka, L.N.; van der Bank, M.; Bezeng, B.S.; Davies, T.J. Identifying biodiversity knowledge gaps for conserving South Africa’s endemic flora. Biodivers. Conserv. 2020, 29, 2803–2819. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Barthlott, W.; Neinhuis, C.; Cutler, D.; Ditsch, F.; Meusel, I.; Theisen, I.; Wilhelmi, H. Classification and terminology of plant epicuticular waxes. Bot. J. Linn. Soc. 1998, 126, 237–260. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2011. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kassambara, A. ggpubr: “ggplot2” Based Publication Ready Plots (Version R Package Version 0.3.0) [Computer Software]; R Development Core Team: Vienna, Austria, 2020. [Google Scholar]
- Soetewey, A. Correlation Coefficient and Correlation Test in R. 2020. Available online: https://www.statsandr.com/blog/correlation-coefficient-and-correlation-test-in-r/ (accessed on 20 February 2021).
- Doyle, J. DNA Protocols for Plants. In Molecular Techniques in Taxonomy; Springer: Berlin/Heidelberg, Germany, 1991; pp. 283–293. [Google Scholar]
- Posada, D.; Crandall, K.A. Modeltest: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [Green Version]
- Mao, Y.R.; Zhang, Y.H.; Nakamura, K.; Guan, B.C.; Qiu, Y.X. Developing DNA barcodes for species identification in Podophylloideae (Berberidaceae). J. Syst. Evol. 2014, 52, 487–499. [Google Scholar] [CrossRef]
- Stace, C.A. Cuticular Studies as an Aid to Plant Taxonomy; Bulletin of the British Museum (Natural History); British Museum (Natural History): London, UK, 1965. [Google Scholar]
- Parkhurst, D.F. The adaptive significance of stomatal occurrence on one or both surfaces of leaves. J. Ecol. 1978, 66, 367–383. [Google Scholar] [CrossRef]
- Rossatto, D.R.; Kolb, R.M. Gochnatia polymorpha (Less.) Cabrera (Asteraceae) changes in leaf structure due to differences in light and edaphic conditions. Acta Bot. Bras. 2010, 24, 605–612. [Google Scholar] [CrossRef]
- Ullah, F.; Zafar, M.; Ahmad, M.; Shah, S.N.; Razzaq, A.; Sohail, A.; Zaman, W.; Çelik, A.; Ayaz, A.; Sultana, S. A systematic approach to the investigation of foliar epidermal anatomy of subfamily Caryophylloideae (Caryophyllaceae). Flora 2018, 246, 61–70. [Google Scholar] [CrossRef]
- Rohrbach, P. Conspectus systematicus specierum generis Silene Ann. Sci. Nat. Bot. 1868, 5, 369–382. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).