1. Introduction
Soil pollution is a common risk for plant growth. In many cases human activity leads to heavy metal contamination. One of the most toxic metals for plants is cadmium [
1]. A lot of human activities, such as the metallurgy and electroplating industries, as well as the production of phosphate fertilizers, emit cadmium into environment. Therefore, the search for protective substances that could reduce the negative effects of cadmium on plants is important. Natural raw materials as a source of such protectors, for example, plants and fungi, are preferable compared to synthetic ones. It is well known that fungi have diverse secondary metabolic pathways. It makes them promise for finding new products for plant protection. Xylotrophic fungi have a parasitic or saprotrophic lifestyle, and thus decompose wood. They are widely spread in the forests, and form fruiting bodies with a large biomass. Some of them are rich of organic acids, polysaccharides, proteins, phenols, and triterpenes, which are known as biologically active substances with antioxidant, immunomodulatory, anticancer, and other effects [
2,
3]. Such chemical composition enables their use as bioproducts for plant protection.
Our study deals with the effects of fungi extracts on barley growth under the impact of toxic doze of cadmium ions.
2. Experiments
Barley seeds were germinated on filter paper in Petri dishes at 25 °C, and photoperiod of 16/8 h (day/night). The length of roots and shoots, the content of photosynthetic pigments (80% acetone extracts) in the first leaf were measured on the 5th day of plant growth. The organ size was measured in 30 plants of each variant, chlorophyll content—in 3 average leaf samples and in 3 analytical replications in each of the samples.
For preparation of the extract 10 g of dry mass of Fomes fomentarius fruit bodies were extracted 4 times by 40% ethanol (100 mL) at 50 °C for 40 min with ultrasonic treatment. Extract was evaporated up to 100 mL of final volume and then diluted to concentrations of 2 mg/mL and 1 mg/mL with distilled water, whichis equivalent to 1 and 2 mg of dry fungi biomass in 1 mL of the extract volume.
The content of phenolic compounds in the extract was determined spectrophotometrically by the reaction with Folin–Ciocalteu reagent,; flavonoids concentration—by the aluminum chloride method; and antioxidant activity by the ABTS test [
4,
5]. The number of replicates in each case was 4.
Water solution of cadmium sulfate (250 mM) was used as a toxicant.
Results are presented as mean and standard error. The significance of differences was evaluated by the Mann–Whitney nonparametric U-test.
3. Results
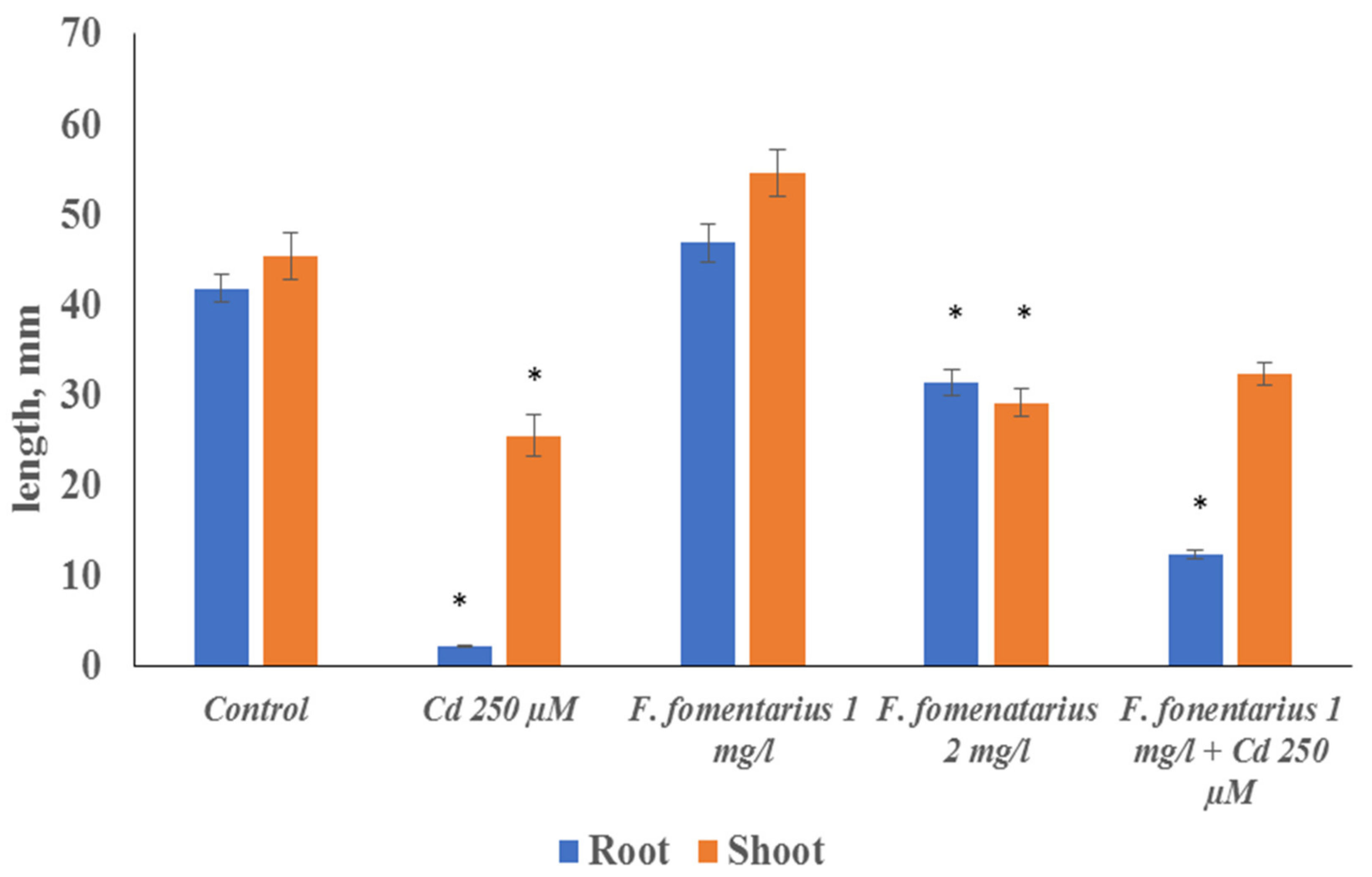
250 μM cadmium ions caused a significant decrease (about 30%) in the shoot length of barley seedlings, in comparison with the control plants. The length of the roots decreased drastically by more than 20 times (
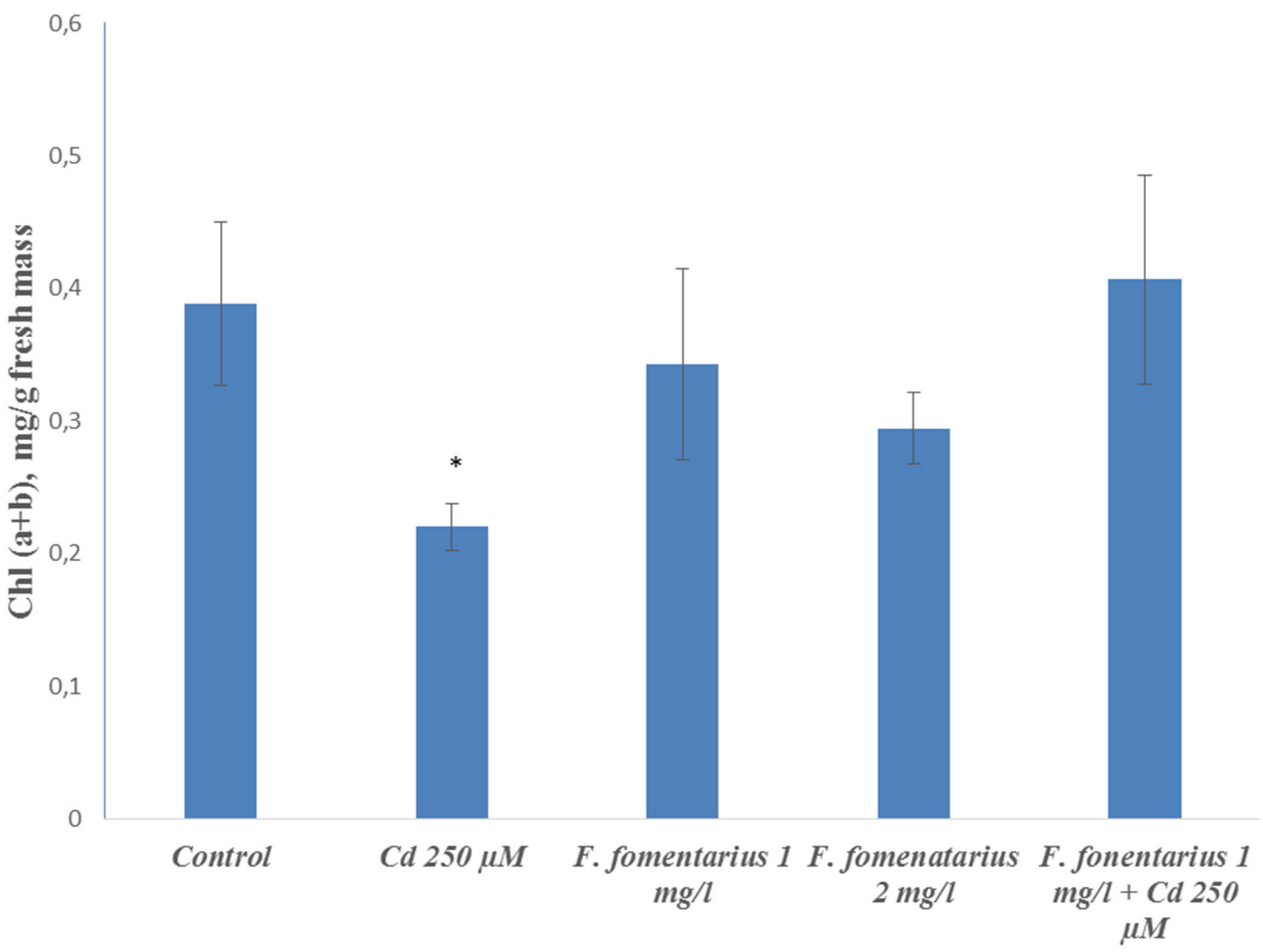
Figure 1). Cadmium ions also caused a significant decline in chlorophyll content (
Figure 2).
The application of fungal extracts of different concentrations showed different effects. The treatment by the concentration of 1 mg/mL did not significantly affect the length of barley shoot and roots, compared to the control. In the case of the more concentrated extract (2 mg/mL) a significant reduction in roots and shoot was observed. However, both roots and shoots were significantly bigger than in the case with cadmium treatment (
Figure 1).
The chlorophyll content in both the plants treated with cadmium ions and those treated with fungal extracts did not differ from the control (
Figure 2).
For the study of the combined effect of cadmium ions and fungal extract we used afomis extract with 1 mg/mL concentration, as it was not toxic to plants. Under the joint treatment the toxic effect of Cd
2+ was significantly low: the root length was six times higher than under the separate action of cadmium ions. The shoot length increased by 1.5 times compared to the impact of cadmium (
Figure 1). The chlorophyll concentration did not significantly differ from the level of the control plants and was higher than under cadmium ions.
The diluted (1 mg/mL) extract from
F. fomentarius contained 3.5 μg/mL of phenolics (
Table 1).
These compounds are known asantioxidants [
6], and their high content could provide the positive anti-toxicant effect of fungal extract in joint treatment with cadmium ions.
The antioxidant activity of fungal extract (based on ABTS*-test) also showed its quenching potential: the formation of radicals was inhibited by 51%, which was comparable to the standard antioxidant—rutin (
Table 2).
4. Discussion
Cadmium ions are widely spread in contaminated soils and are highly toxic to plants. To a greater extent, they affect the growth of roots rather than shoots, which may be explained by the barrier function of the root. The application of fungal extracts together with toxic concentrations of cadmium ions improved the growth of barley seedlings compared to the separate action of Cd2+. So, the negative effects of cadmium ions on plant growth were reduced by the fungal extract, but the plants did not reach the control level. The pigment content was also higher under the joint treatment compared to the separate action of cadmium ions and reached the control level. The probable reason for these effects is the antioxidant activity of the found biologically active compounds in the extract—phenolics and flavonoids.
To decrease the negative impact of heavy metals ions on plants, different approaches are practiced, for example the use of plant growth promoting bacteria on the contaminated soils the, introduction of metal chelators into the soil and so on. Heavy metal stress in plants is accompanied by oxidative stress, so the use of fungal extracts, which are the natural sources of antioxidants, may be promising for plant protection from heavy metals pollution.
5. Conclusions
Our study has shown that the use of low concentrations of Fomis fomentarius extract (1 mg/mL) did not practically suppress plant growthand also reduced the negative effect of cadmium ions (250 μM) in the case of joint application.
F. fomentarius is a widespread fungus in the forest ecosystems. Its availability as a natural resource, the possibility to cultivate it in vitro, and the low effective concentrations make it possible to recommend this fungus for the production of an ecologically safe plant protective agent, improving plant growth under the heavy metal stress.
Author Contributions
A.E. and I.K. conceived and designed the experiments; I.N., V.N., O.S., A.E. and I.K. performed the experiments; A.E. performed the statistical analyses; A.E. and I.K. analyzed the data; A.E. and I.K. wrote the paper. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available.
Acknowledgments
The research was supported by The Ministry of Education and Science of the Russian federation Agreement No. 02.A03.21.0006.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
ABTS—2,2′-Azino-bis-(3-ethylbenzothiazoline-6-sulfonic acid).
References
- Yang, S.; Gu, S.; He, M.; Tang, X.; Ma, L.Q.; Xu, J.; Liu, X. Policy adjustment impacts Cd, Cu, Ni, Pb and Zn contamination in soils around e-waste area: Concentrations, sources and health risks. Sci. Total Environ. 2020, 471, 140442. [Google Scholar] [CrossRef] [PubMed]
- Lindequist, U.; Niedermeyer, T.; Jülich, W.-D. The Pharmacological Potential of Mushrooms. Evid.-Based Complement. Altern. Med. 2005, 2, 285–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Payamnoor, V.; Kavosi, M.R.; Nazari, J. Polypore fungi of Caucasian alder as a source of antioxidant and antitumor agents. J. For. Res. 2020, 31, 1381–1390. [Google Scholar] [CrossRef]
- Amitava, D.; Kimberly, K. Methods for Measuring Oxidative Stress in the Laboratory. In Antioxidants in Food, Vitamins and Supplements; Amitava, D., Kimberly, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 19–40. [Google Scholar]
- Blainski, A.; Lopes, G.C.; Carlos, J. Application and Analysis of the FolinCiocalteu Method for the Determination of the Total Phenolic Content from Limonium brasiliense L. Molecules 2013, 18, 6852–6865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bojin, L.-A.; Serb, A.-F.; Pascariu, M.-C. Assessment of antioxidant properties of different fomes fomentarius extracts. Farmacia 2020, 68, 322–328. [Google Scholar] [CrossRef]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).