A Novel Plant-Based Biostimulant Improves Plant Performances under Drought Stress in Tomato †
Abstract
:1. Introduction
2. Experiments
3. Results
3.1. Leaf Antioxidants and Pigments Content
3.2. Fruit Antioxidants and Pigments Content
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Barnabas, B.; Jager, K.; Feher, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef]
- Costa, J.M.; Ortuño, M.F.; Chaves, M.M. Deficit irrigation as a strategy to save water: Physiology and potential application to horticulture. J. Integr. Plant Biol. 2007, 49, 1421–1434. [Google Scholar] [CrossRef]
- Munns, R.; Pearson, C.J. Effect of water deficit on translocation of carbohydrate in Solanum tuberosum. Funct. Plant Biol. 1974, 1, 529–537. [Google Scholar] [CrossRef]
- Bertamini, M.; Zulini, L.; Muthuchelian, K.; Nedunchezhian, N. Effect of water deficit on photosynthetic and other physiological responses in grapevine (Vitis vinifera L. cv. Riesling) plants. Photosynthetica 2006, 44, 151–154. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Francesca, S.; Arena, C.; Hay Mele, B.; Schettini, C.; Ambrosino, P.; Barone, A.; Rigano, M.M. The use of a plant-based biostimulant improves plant performances and fruit quality in tomato plants grown at elevated temperatures. Agronomy 2020, 10, 363. [Google Scholar] [CrossRef] [Green Version]
- Poorter, H.; Garnier, E. Ecological significance of inherent variation in relative growth rate. In Handbook of Functional Plant Ecology; Pugnaire, F., Valladares, X., Eds.; Marcel Dekker: New York, NY, USA, 1999; pp. 81–120. [Google Scholar]
- Dafni, A. Pollination Ecology: A Practical Approach; Oxford University Press: Oxford, UK, 1992; 250p. [Google Scholar]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Zouari, I.; Salvioli, A.; Chialva, M.; Novero, M.; Miozzi, L.; Tenore, G.C.; Bagnaresi, P.; Bonfante, P. From root to fruit: RNA-Seq analysis shows that arbuscular mycorrhizal symbiosis may affect tomato fruit metabolism. BMC Genom. 2014, 15, 221. [Google Scholar] [CrossRef] [Green Version]
- Rigano, M.M.; Arena, C.; Di Matteo, A.; Sellitto, S.; Frusciante, L.; Barone, A. Eco-physiological response to water stress of drought-tolerant and drought-sensitive tomato genotypes. Plant Biosyst. 2016, 150, 682–691. [Google Scholar] [CrossRef] [Green Version]
- Stevens, R.; Buret, M.; Garchery, C.; Carretero, Y.; Causse, M. Technique for rapid small-scale analysis of vitamin C levels in fruit and application to a tomato mutant collection. J. Agric. Food Chem. 2006, 54, 6159–6165. [Google Scholar] [CrossRef]
- Rigano, M.M.; Raiola, A.; Tenore, G.C.; Monti, D.M.; Del Giudice, R.; Frusciante, L.; Barone, A. Quantitative trait loci pyramiding can improve the nutritional potential of tomato (Solanum lycopersicum) fruits. J. Agric. Food Chem. 2014, 62, 11519–11527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rigano, M.M.; Lionetti, V.; Raiola, A.; Bellincampi, D.; Barone, A. Pectic enzymes as potential enhancers of ascorbic acid production through the d-galacturonate pathway in Solanaceae. Plant Sci. 2018, 266, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 16, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, C.; Werner, C.; Rodrigues, A.; Caldeira, M. A prolonged dry season and nitrogen deposition interactively affect CO2 fluxes in an annual Mediterranean grassland. Sci. Total Environ. 2019, 654, 978–986. [Google Scholar] [CrossRef] [PubMed]
- Mäkelä, P.; Munns, R.; Colmer, T.D.; Condon, A.G.; Peltonen-Sainio, P. Effect of foliar applications of glycinebetaine on stomatal conductance, abscisic acid and solute concentrations in leaves of salt-or drought-stressed tomato. Funct. Plant Biol. 1998, 25, 655–663. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar applications of a legume-derived protein hydrolysate elicit dose-dependent increases of growth, leaf mineral composition, yield and fruit quality in two greenhouse tomato cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Sato, S.; Kamiyama, M.; Iwara, T.; Makita, N.; Furukawa, H.; Ikeda, H. Moderate increase of mean daily temperature adversely affects fruit set of Lycopersicum esculentum by distrupting specific physiological processes in male reproductive development. Ann. Bot. 2006, 97, 731–738. [Google Scholar] [CrossRef]
- Guo, Y.Y.; Yu, H.Y.; Kong, D.S.; Yan, F.; Zhang, Y.J. Effects of drought stress on growth and chlorophyll fluorescence of Lycium ruthenicumMurr. Seedling. Photosynthetica 2016, 54, 1–7. [Google Scholar] [CrossRef]
- Ma, P.; Bai, T.; Wang, X.; Ma, F. Effect of light intensity on photosynthesis and photoprotective mechanisms in apple under progressive drought. J. Integr. Agric. 2015, 14, 1755–1766. [Google Scholar] [CrossRef] [Green Version]
- Ertani, A.; Pizzeghello, D.; Francioso, O.; Sambo, P.; Sanchez-Cortes, S.; Nardi, S. Capsicum chinensis L. growth and nutraceutical properties are enhanced by biostimulants in a long-term period: Chemical and metabolomic approaches. Front. Plant Sci. 2014, 5, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erba, D.; Casiraghi, M.C.; Ribas-Agustí, A.; Cáceres, R.; Marfà, O.; Castellari, M. Nutritional value of tomatoes (Solanum lycopersicum L.) grown in greenhouse by different agronomic techniques. J. Food Compos. Anal. 2013, 31, 245–251. [Google Scholar] [CrossRef]
| 100% | 50% | Significance | |||||
|---|---|---|---|---|---|---|---|
| Non-Treated | Treated | Non-Treated | Treated | W | B | W × B | |
| Leaf water potential (Mpa) | 8.67 ± 2.08 a | 7.5 ± 0.87 a | 13.33 ± 2.02 b | 10.33 ± 1.53 ab | ** | ns | ns |
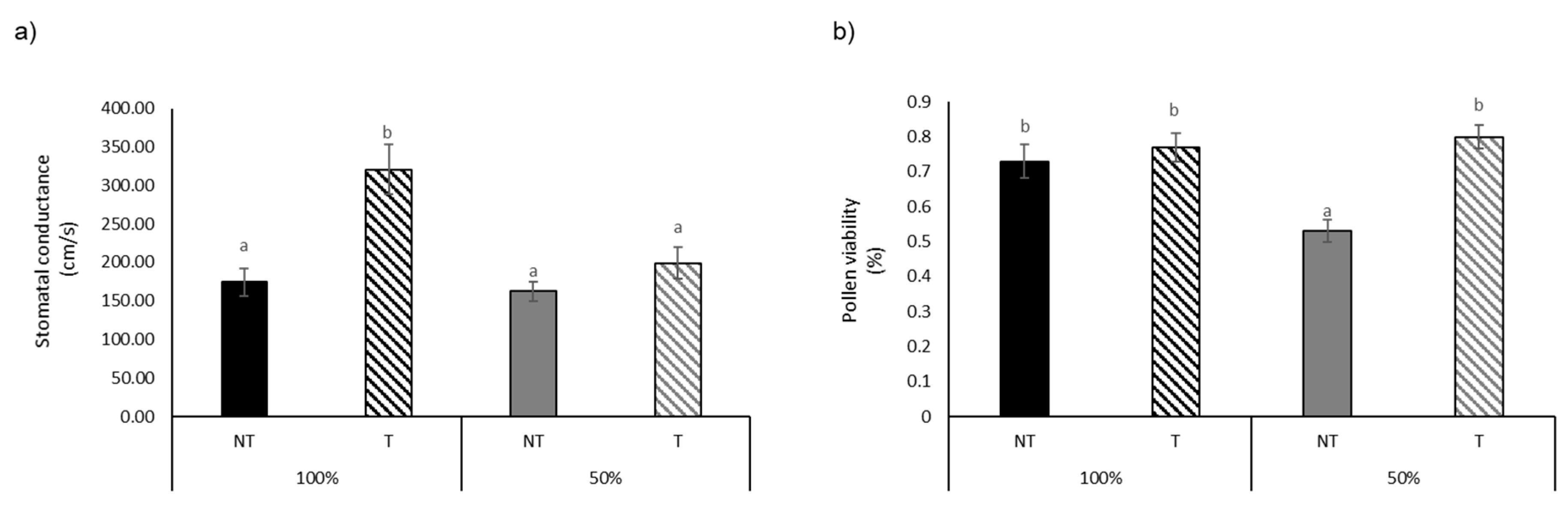
| Stomatal conductance (cm/s) | 174.17 ± 42.79 a | 320.5 ± 79.35 b | 162.17 ± 30.67 a | 199 ± 51.27 a | *** | ** | * |
| Leaf dry matter content (g/g) | 0.072 ± 0.008 bc | 0.103 ± 0.015 c | 0.019 ± 0.012 a | 0.055 ± 0.008 b | *** | ** | ns |
| Shoot FW (kg) | 2.55 ± 0.79 a | 5.07 ±1.85 b | 0.50 ± 0.11 a | 2 ± 0.48 a | ** | ns | ns |
| Pollen viability (%) | 0.73 ± 0.12 b | 0.77 ± 0.1 b | 0.53 ± 0.08 a | 0.8 ± 0.08 b | *** | ** | *** |
| Fruit weight (g) | 7.13 ± 2.16 ab | 8.30 ± 1.16 b | 5.38 ± 1.38 a | 8.40 ± 1.57 b | ns | ** | ns |
| Number of fruit | 123.17 ± 67.14 b | 177 ± 59.58 b | 36.33 ± 38.66 a | 35.17 ± 22.18 a | *** | ns | ns |
| Yield (kg/pt) | 1.25 ± 0.27 b | 1.76 ± 0.60 b | 0.07 ± 0.02 a | 0.44 ± 0.19 a | *** | * | ns |
| 100% | 50% | Significance | |||||
|---|---|---|---|---|---|---|---|
| Non-Treated | Treated | Non-Treated | Treated | W | B | W × B | |
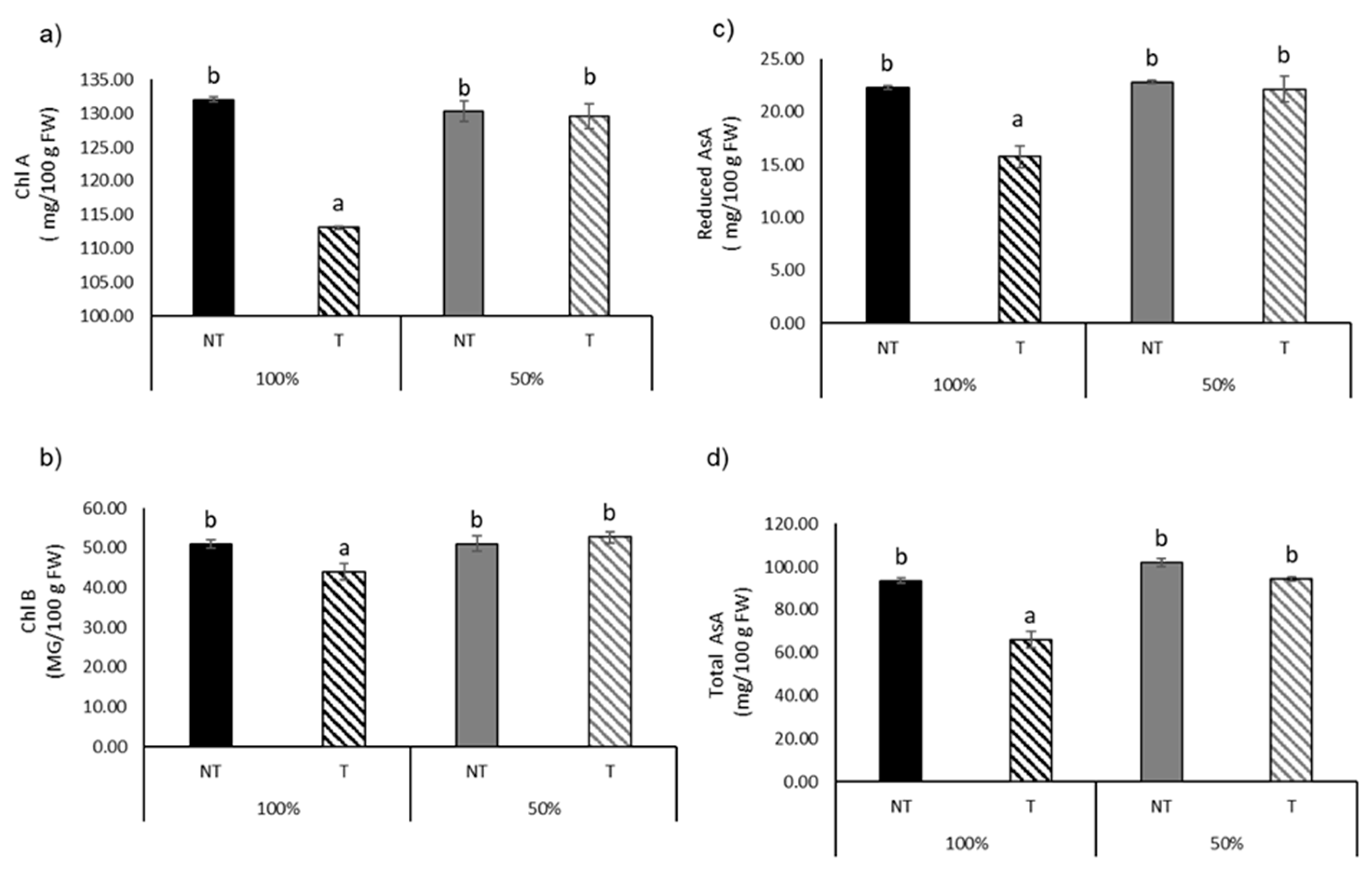
| Total Asa (mg/100 g FW) | 93.51 ± 2.53 b | 65.96 ± 9.58 a | 101.82 ± 4.80 b | 94.35 ± 2.24 b | *** | *** | *** |
| Reduced AsA (mg/100 g FW) | 22.26 ± 0.47 b | 15.73 ± 2.47 a | 22.81 ± 0.42 b | 22.14 ± 2.90 b | *** | *** | ** |
| Carotenoids (mg/100 g FW) | 25.16 ± 3.59 ab | 24.11 ± 2.32 b | 26.22 ± 0.33 ab | 27.43 ± 0.45 b | ** | ns | ns |
| Chl A (mg/100 g FW) | 132.04 ± 0.92 b | 113.097 ± 0.60 a | 130.27 ± 3.76 b | 129.54 ± 4.45 b | *** | *** | *** |
| Chl B (mg/100 g FW) | 51.02 ± 2.50 b | 43.95 ± 4.86 a | 51.05 ± 4.67 b | 52.67 ± 3.53 b | ** | ns | ** |
| Frap (mmol TE/100 g FW) | 179.48 ± 18.14 a | 202.48 ± 65.77 a | 174.38 ± 18.50 a | 345.44 ± 66.35 b | ** | *** | ** |
| 100% | 50% | Significance | |||||
|---|---|---|---|---|---|---|---|
| Non-Treated | Treated | Non-Treated | Treated | W | B | W × B | |
| Total Asa (mg/100 g FW) | 115.40 ± 11.41 b | 100.99 ± 6.68 a | 111.50 ± 7.69 ab | 102.70 ± 8.38 ab | ns | ** | ns |
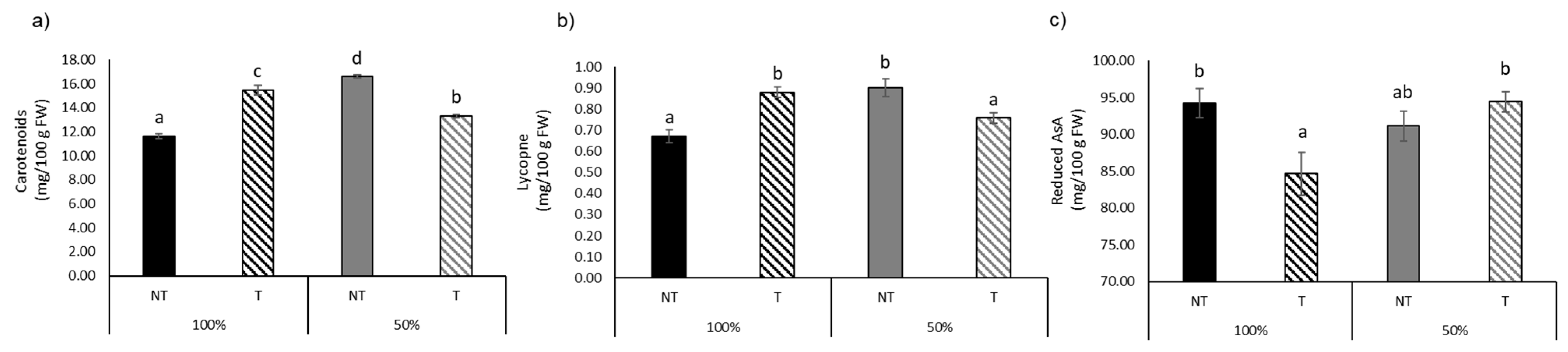
| Reduced AsA (mg/100 g FW) | 94.20 ± 4.90 b | 84.65 ± 7.15 a | 91.11 ± 5.03 ab | 94.43 ± 3.37 b | ns | ns | ** |
| Carotenoids (mg/100 g FW) | 11.61 ± 0.51 a | 15.47 ± 0.95 c | 16.58 ± 0.32 d | 13.31 ± 0.41 b | *** | ns | *** |
| β-Carotene (mg/100 g FW) | 0.34 ± 0.05 a | 0.33 ± 0.03 a | 0.40 ± 0.02 b | 0.37 ± 0.07 ab | ** | ns | ns |
| Lycopene (mg/100 g FW) | 0.67 ± 0.08 a | 0.88 ± 0.06 b | 0.90 ± 0.10 b | 0.76 ± 0.06 a | ns | ns | *** |
| Frap (mmol TE/100 g FW) | 413.55 ± 48.20 a | 426.52 ± 58.38 a | 845.10 ± 79.03 b | 882.24 ± 73.71 b | *** | ns | ns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Francesca, S.; Raimondi, G.; Cirillo, V.; Maggio, A.; Barone, A.; Rigano, M.M. A Novel Plant-Based Biostimulant Improves Plant Performances under Drought Stress in Tomato. Biol. Life Sci. Forum 2021, 4, 52. https://doi.org/10.3390/IECPS2020-08883
Francesca S, Raimondi G, Cirillo V, Maggio A, Barone A, Rigano MM. A Novel Plant-Based Biostimulant Improves Plant Performances under Drought Stress in Tomato. Biology and Life Sciences Forum. 2021; 4(1):52. https://doi.org/10.3390/IECPS2020-08883
Chicago/Turabian StyleFrancesca, Silvana, Giampaolo Raimondi, Valerio Cirillo, Albino Maggio, Amalia Barone, and Maria Manuela Rigano. 2021. "A Novel Plant-Based Biostimulant Improves Plant Performances under Drought Stress in Tomato" Biology and Life Sciences Forum 4, no. 1: 52. https://doi.org/10.3390/IECPS2020-08883
APA StyleFrancesca, S., Raimondi, G., Cirillo, V., Maggio, A., Barone, A., & Rigano, M. M. (2021). A Novel Plant-Based Biostimulant Improves Plant Performances under Drought Stress in Tomato. Biology and Life Sciences Forum, 4(1), 52. https://doi.org/10.3390/IECPS2020-08883






