Biomonitoring Air Pollution in Carob Leaves †
Abstract
:1. Introduction
2. Experiments
2.1. Estimating Specific Leaf Area and Chlorophyll Content
2.2. In Situ Measurements of Optical Properties of Fresh Leaves
3. Results
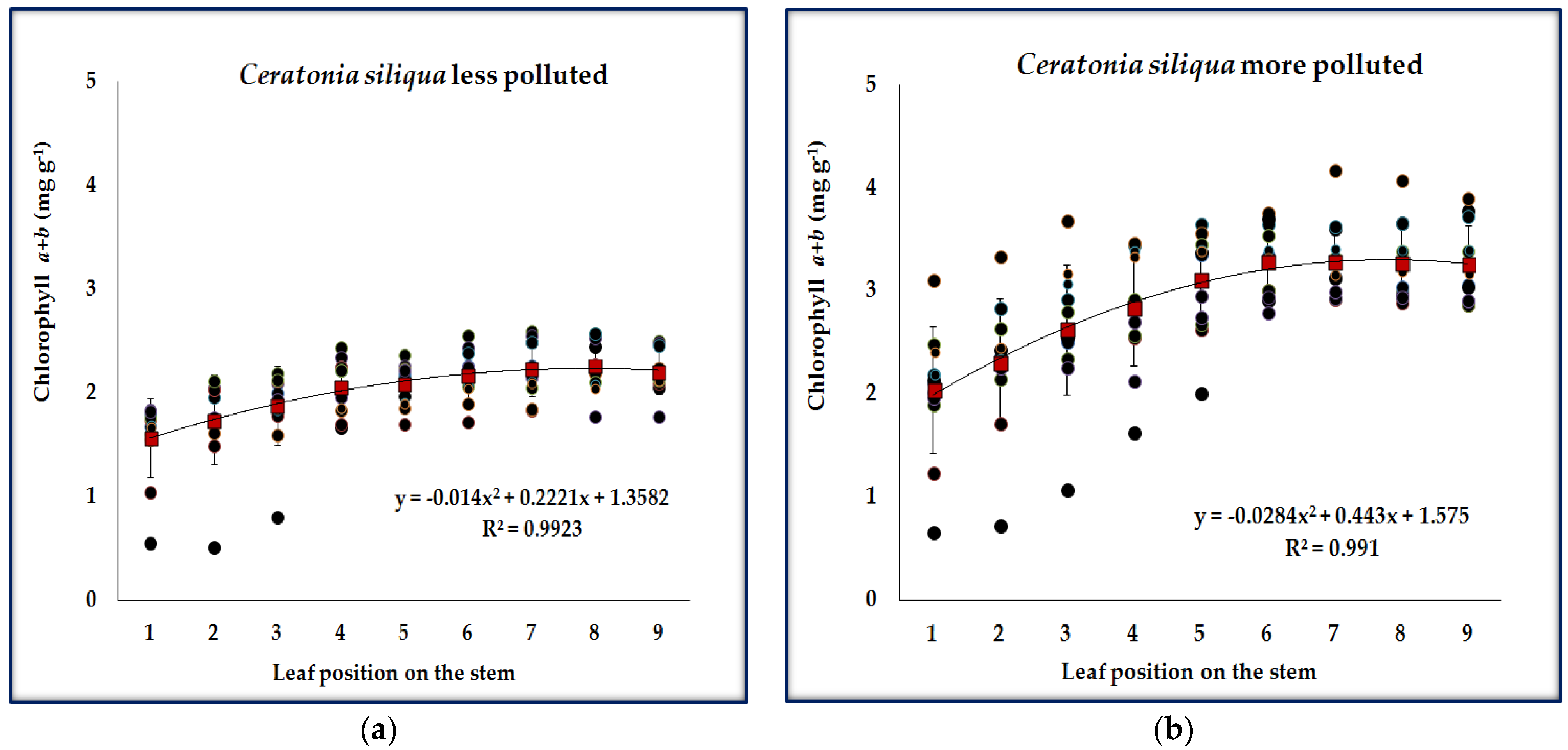
3.1. Chlorophyll Content
3.2. Specific Leaf Area (SLA) (Leaf Area/Dry Weight cm2 g−1)
3.3. Leaf Optical Properties
4. Discussion
Author Contributions
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Falla, J.; Laval-Gilly, P.; Henryon, M.; Morlot, D.; Ferard, J.F. Biological air quality monitoring: A review. Environ. Monit. Assess. 2000, 64, 627–644. [Google Scholar] [CrossRef]
- Meletiou-Christou, M.S.; Nassios, K.; Rhizopoulou, S. A study on the growth rate of Mediterranean plants exposed to the air pollution of the city of Athens. In Mediterranean City Facing Climate Change, 27–28 October 2008; SBOMED: London, UK, 2008; pp. 1–10. [Google Scholar]
- Honour, S.L.; Bell, J.N.B.; Ashenden, T.W.; Cape, J.N.; Power, S.A. Responses of herbaceous plants to urban air pollution: Effects on growth, phenology and leaf surface characteristics. Environ. Pollut. 2009, 157, 1279–1286. [Google Scholar] [CrossRef] [Green Version]
- Molnár, V.É.; Tóthmérész, B.; Szabó, S.; Simon, E. Urban tree leaves’ chlorophyll-a content as a proxy of urbanization. Air Qual. Atmos. Health 2018, 11, 665–671. [Google Scholar] [CrossRef]
- Khalid, N.; Masood, A.; Noman, A.; Aqeel, M.; Qasim, M. Study of the responses of two biomonitor plant species (Datura alba & Ricinus communis) to roadside air pollution. Chemosphere 2019, 235, 832–841. [Google Scholar]
- Meletiou-Christou, M.S.; Rhizopoulou, S. Constraints of photosynthetic performance and water status of four evergreen species co-occurring under field conditions. Bot. Stud. 2012, 53, 325–334. [Google Scholar]
- Leghari, S.K.; Zaidi, M. Effect of air pollution on the leaf morphology of common plant species of Quetta city. Pak. J. Bot. 2013, 5, 447–454. [Google Scholar]
- Meletiou-Christou, M.S.; Rhizopoulou, S. Leaf functional traits of four evergreen species growing in Mediterranean environmental conditions. Acta Physiol. Plant. 2017, 39, 34. [Google Scholar] [CrossRef]
- Bharti, S.K.; Trivedi, A.; Kumar, N. Air pollution tolerance index of plants growing near an industrial site. Urban Clim. 2018, 24, 820–829. [Google Scholar] [CrossRef]
- Balasooriya, B.L.W.K.; Samson, R.; Mbikwa, F.; Boeckx, P.; Van Meirvenne, M. Biomonitoring of urban habitat quality by anatomical and chemical leaf characteristics. Environ. Exp. Bot. 2009, 65, 386–394. [Google Scholar] [CrossRef]
- Cotrozzi, L.; Townsend, P.A.; Pellegrini, E.; Nali, C.; Couture, J.J. Reflectance spectroscopy: A novel approach to better understand and monitor the impact of air pollution on Mediterranean plants. Environ. Sci. Pollut. Res. 2018, 25, 8249–8267. [Google Scholar] [CrossRef]
- Ramón-Laca, L.; Mabberley, D.J. The ecological status of the carob-tree (Ceratonia siliqua, Leguminosae) in the Mediterranean. Bot. J. Linn. Soc. 2004, 144, 431–436. [Google Scholar] [CrossRef]
- Pratikakis, E.; Rhizopoulou, S.; Psaras, G.K. A phi layer in roots of Ceratonia siliqua L. Bot. Acta 1998, 111, 93–98. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Nunes, M.A. Some adaptative photosynthetic characteristics of a sun plant (Ceratonia siliqua) and a shade plant (Coffea arabica). In Components of Productivity of Mediterranean-Climate Regions. Basic and Applied Aspects; Margaris, N.S., Mooney, H.A., Eds.; Dr W. Junk Publishers: Hague, The Nethelands, 1981; pp. 85–89. [Google Scholar]
- Chimona, C.; Rhizopoulou, S. Water economy through matching plant root elongation to Mediterranean landscapes. World J. Res. Rev. 2017, 5, 22–24. [Google Scholar] [CrossRef]
- Christodoulakis, N.S. Structural diversity and adaptations in some Mediterranean evergreen sclerophyllous species. Environ. Exp. Bot. 1992, 32, 295–305. [Google Scholar] [CrossRef]
- Nunes, M.A.; Linskens, H.F. Some aspects of the structure and regulation of Ceratonia siliqua L. stomata. Port. Acta Biol. 1980, 16, 165–174. [Google Scholar]
- Shahzad, A.; Akhtar, R.; Bukhari, N.A.; Perveen, K. High incidence regeneration system in Ceratonia siliqua L. articulated with SEM and biochemical analysis during developmental stages. Trees 2017, 31, 1149–1163. [Google Scholar] [CrossRef]
- Diamantoglou, S.; Mitrakos, K. Leaf longevity in Mediterranean evergreen sclerophylls. In Components of Productivity of Mediterranean-Climate Regions. Basic and Applied Aspects; Margaris, N.S., Mooney, H.A., Eds.; Dr W. Junk Publishers: Hague, The Nethelands, 1981; pp. 17–19. [Google Scholar]
- Rhizopoulou, S.; Davies, W.J. Influence of soil drying on root development, water relations and leaf growth of Ceratonia siliqua L. Oecologia 1991, 88, 41–47. [Google Scholar] [CrossRef]
- Rhizopoulou, S.; Mitrakos, K. Water relations of evergreen sclerophylls. I. Seasonal changes in the water relations of eleven species from the same environment. Ann. Bot. 1990, 65, 171–178. [Google Scholar] [CrossRef]
- Hellenic Ministry of Environment and Energy 2018. Available online: https://ypen.gov.gr/wp-content/uploads/legacy/Files/Perivallon/Poiotita%20Atmosfairas/Ektheseis/Ekthesi2018.pdf (accessed on 20 April 2019).
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Jacquemoud, S.; Ustin, S. Spectroscopy of Leaf Molecules. In Leaf Optical Properties; Cambridge University Press: Cambridge, UK, 2019; pp. 48–73. [Google Scholar] [CrossRef]
- Stratakis, E.; Zorba, V.; Barberoglou, M.; Fotakis, C.; Shafeev, G.A. Laser writing of nanostructures on bulk Al via its ablation in liquids. Nanotechnology 2009, 20, 105303. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef] [PubMed]




| Months | April | May | June | July | August | September | October | November | December | January | February | March |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S-PM10 | 27 | 3 | 20 | 18 | 19 | 17 | 23 | 15 | 12 | 13 | 13 | 37 |
| U-PM10 | 38 | 32 | 29 | 28 | 26 | 5 | 31 | 25 | 33 | 33 | 27 | 44 |
| S-CO | - | - | - | - | - | - | - | - | - | - | - | - |
| U-CO | 0.5 | 0.4 | 0.3 | 0.4 | 0.3 | 0.2 | 0.4 | 0.5 | 0.9 | 0.8 | 1.5 | 0.7 |
| S-NO | 2 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 1 | 2 | 2 | 1 |
| U-NO | 10 | 5 | 3 | 4 | 1 | 4 | 9 | 11 | 23 | 24 | 11 | 6 |
| S-NO2 | 18 | 14 | 13 | 11 | 6 | 14 | 13 | 14 | 12 | 14 | 13 | 15 |
| U-NO2 | 41 | 28 | 25 | 28 | 16 | 7 | 3 | 29 | 30 | 35 | 24 | 28 |
| S-O3 | 106 | 96 | 101 | 101 | 103 | 96 | 73 | 53 | 52 | 59 | 66 | 79 |
| U-O3 | 60 | 76 | 81 | 80 | 85 | 79 | 63 | 42 | 42 | 44 | 59 | 59 |
| Compound | Absorption Peaks, Wavelengths (nm) |
|---|---|
| Phenolic compounds | 260–370 |
| Chlorophyll a | 430 and 662 |
| Chlorophyll b | 453 and 642 |
| Carotenoids | 460–550 |
| Anthocyanins | 450–550 (maximum at 520) |
| Water | 970, 1200, 1470 and 1900 (maximum) |
| Cellulose–Lignin | 1400–2000 and 2000–2500 (maximum) |
| Protein–Starch–Sugar | 1400 and 2000–2500 (maximum) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulou, S.; Rhizopoulou, S.; Meletiou-Christou, M.-S.; Stratakis, E. Biomonitoring Air Pollution in Carob Leaves. Biol. Life Sci. Forum 2021, 4, 50. https://doi.org/10.3390/IECPS2020-08896
Papadopoulou S, Rhizopoulou S, Meletiou-Christou M-S, Stratakis E. Biomonitoring Air Pollution in Carob Leaves. Biology and Life Sciences Forum. 2021; 4(1):50. https://doi.org/10.3390/IECPS2020-08896
Chicago/Turabian StylePapadopoulou, Sophia, Sophia Rhizopoulou, Maria-Sonia Meletiou-Christou, and Emmanuel Stratakis. 2021. "Biomonitoring Air Pollution in Carob Leaves" Biology and Life Sciences Forum 4, no. 1: 50. https://doi.org/10.3390/IECPS2020-08896
APA StylePapadopoulou, S., Rhizopoulou, S., Meletiou-Christou, M.-S., & Stratakis, E. (2021). Biomonitoring Air Pollution in Carob Leaves. Biology and Life Sciences Forum, 4(1), 50. https://doi.org/10.3390/IECPS2020-08896







