Abstract
Germination and seed quality of China aster are crucial features that affect seedling survival and establishment when seeded directly in a field. Moreover, freak weather events in changing climate scenarios and biotic stress have often resulted in poor seedling quality and survival of China aster. Subsequently, the impact of a range of priming techniques on germination, seedling survival and growth of cv. Powderpuff of China aster newly introduced in Kashmir valley was scrutinized at the Plant Tissue Culture Laboratory. Seeds were subjected to two treatment methods (3 hydro-priming and 2 halo-priming), constituting a total of six treatment combinations (T0–T5) in CRD (completely randomized design), with four replications. The analyzed variables were seedling survival percentage, germination percentage, seedling collar diameter, seedling fresh weight, shoot–root ratio and the number of leaves/seedling. The analyzed data on the influence of priming treatments on germination percentage are depicted, showing that different priming agents have a significant influence on pre- and post-germination attributes. Significantly, maximum germination percentage (87.50%), seedling survival percentage (81.95), seedling fresh weight (0.0031 g), seedling collar diameter (0.101 cm), number of leaves seedling−1 (7.01) and shoot–root ratio (1.044) were recorded in treatment T5 (2% KNO3 18 h) and minimum (42.50) in case of control (T0), i.e., un-primed seeds. Halo-conditioning with KNO3 for 12 h significantly improved germination percentage, seedling survival percentage, seedling diameter, leaf number per seedling and shoot–root ratio. In conclusion, KNO3 played a vital role in the establishment and survival of seedlings in the field, under Kashmir conditions.
1. Introduction
Callistephus chinensis (L.) Nees, more commonly known as China aster, and which belongs to the family Asteraceae, is an essential industrial flower crop. The genus Callistephus draws its name from two Greek words: Kalistos, meaning “most beautiful” and Stephos meaning “a crown”. It is estimated to be grown in an area of 3500 ha in India [1]. Amongst annual flowers, it ranks third, only after Chrysanthemum and Marigold [2]. Its farming and cultivation have become trendy around larger cities due to its versatile uses as a loose and cut flower. The crop is used to make enchanting bouquets, buttonholes and garlands. In ornamental farming/gardening, it is used as a cut flower, loose flower, a bedding plant, a pot plant and an herbaceous border. Thus, it has been extensively grown in South Asia and many other regions. Owing to the ever-increasing demand for quality seeds of Aster throughout India, there is a need to increase seed quality that will ensure good returns for growers. In India, China aster seeds available with nurseries are usually of poor quality, which is an impediment to the plant’s wider cultivation.
Seed quality (germination, seedling stand, survival) is a decisive feature that decides the geological distribution and cultivation of any crop. China aster is highly sensitive to edaphic conditions such as distribution of soil microbe inoculums, especially fusarium, resulting in poor germination, seedling survival, seedling stand and, consequently, in poor quality seed and meager returns. Furthermore, freak weather events due to the changing climate have often resulted in poor seed germination, seedling establishment and growth in India and elsewhere. Seed germination is a complex physiological course of action related to absorption of H2O, metabolic breakdown of stored matter, seed respiration intended for energy metabolism, transcript (mRNA) production and mitochondrial refurbishment and multiplication [3]. In recent years, many tactics have been utilized to accelerate the speed of germination and improve seedling uniformity. Pre-sowing treatment of seed priming has been proven as an effectual strategy for achieving rapid uniform emergence, as well as for recuperating seed vigor, viability and seedling stand, even under hostile situations. These methods can be classified as biological, chemical and physical. Numerous studies have shown that priming (hydropriming, halopriming) reinforces the seeds to withstand various abiotic stresses during germination. The probable mechanisms stimulated by priming include membrane repair due to the accumulation of signaling proteins, epigenetic changes and the enhanced antioxidant activity [4] of starch metabolism under various conditions. A range of aspects, such as cultivar, plant species, priming duration and priming substrate, influences seeds’ responses to priming treatment. However, reports regarding possible effects of seed priming on aster seed quality attributes and seedling survival/established are scant. Therefore, the identification of the most effective priming agent is necessary to achieve the desired results, such as improved germination, seedling survival and vigor. Consequently, the intent of the investigation was to probe the effects of seed priming on the seed germination and seedling survival of China aster.
2. Material and Methods
2.1. Experimental Setup and Treatment Details
The experiment was conducted at the Plant Tissue Culture Laboratory, Division of FLA, SKUAST-Kashmir, Srinagar; J & K. The seeds of China aster were obtained from Department of Floriculture, Govt. of J&K. The seeds were surface sterilized and rinsed with distilled water. Seeds were divided into 6 seed lots (3 hydro-priming and 2 halo-priming). The seeds were employed to 6 different priming treatments (T0–T5) and one untreated lot of seed (T0). The study was evaluated as four replicates in a completely randomized design. The covered Petri plates were placed in an incubator at 25 ± 1 °C and kept at 16 h photoperiod. Seeds with a noticeable radicle were considered as emerged. Data were recorded for 15 days on a daily basis for emergence with subsequent seedling assessment protocol, as demonstrated in the handbook of the Association of Official Seed Analysts (AOSA1990). Ultimate germination percentage was calculated at the end of 15th day. Subsequently, 4 replications with 20 pre-germinated primed seeds were used as a basic sample, and were sown in propagation trays. Then, 30 days after sowing, various attributes—seedling survival percentage, seedling fresh weight, number of leaves per seedling, seedling collar diameter and seedling shoot–root ratio—were calculated empirically
2.2. Germination Percentage (G%)
Four replications with fifty seeds were kept in Petri plates covered with germination papers and then incubated in a seed germinator at . The Petri plates were timely moisturized with distilled water in order to maintain an optimum moisture level. The number of seeds germinated was counted daily for 15 days. A seed with visible radical protrusion was designated as a germinated one. The number of germinated seeds was recorded daily, during the period of 15 days. A seed was considered germinated when its radicle emerged. Germination percentage (G%) was calculated (ISTA, 1985) using the formulae as follows:
where
- n = number germinated seeds;
- N = total number of seeds taken per lot.
2.3. Seedling Survival%
Seedling survival percentage was calculated by setting the empirical formulae as shown below:
where
- = number of seedlings survived after germination;
- = number of seedlings germinated.
The other apportioning attributes, such as seedling fresh weight (SFW), were calculated after 30 days of seedling growth. Then, 10 randomly selected seedlings were taken to calculate the fresh weights. After that, the mean fresh weight per seedling was deducted. Seedling collar diameter (cm) was analyzed after 30 days of seedling survival. The observation was recorded with the aid of digital vernier caliper; the data were recorded in millimeters (mm) and later on converted to centimeters (cm). A number of leaves per seedling from each treatment, i.e., 5 seedlings, were taken randomly, and the number of leaves was calculated 30 days after seedling growth. Ultimately seedling shoot–root ratio (S/R ratio) was deducted with dry masses (mg) of shoot and root monitored separately from each treatment at random. The final shoot–root ratio was calculated using the equation as below:
where
- = dry mass of shoot (mg);
- = dry mass of root (mg).
2.4. Statistical Analysis
A completely randomized design (CRD) was employed in the experiments with four replications. Data analysis was carried out with the SAS program (SAS Institute, Cary, NC, USA). Means were compared by analysis of variance (ANOVA) at p ≤ 0.05 level of significance, and differences were divorced by Duncan’s multiple range test.
3. Result and Discussion
Seed priming is a complex biochemical and physiological process modulated by cellular/solute osmotic potential, plant growth regulators and enzymatic activities [5,6]. Reports suggest that germination, seedling survival and the seedling stand of crop plants are restricted and limited by many factors that eventually reduce yield and quality. Hence, it is important to draw attention to the cost-effective strategies that improve crop growth and quality, and that result in improved gross returns for the farming community.
Interestingly the results of our investigation (Figure 1 and Figure 2) suggested that KNO3 priming not only sped up seed germination rate but also considerably enhanced seedling survival, Timson germination index and seedling vigour index, as indicated by longer radical lengths, hypocotyls lengths and shoot–root ratio. Timson germination index and germination percentage were enhanced by priming with H2O and KNO3 (Table 1). Germination percentage of primed seeds was higher with increased KNO3 concentration and time of exposure. The extent of priming with H2O and KNO3 manipulated germination percentage differentially, i.e., long-standing priming had an additional constructive outcome on germination. Treatment with 2% KNO3 solution for 18 h resulted in a practically more acceptable germination percentage (85–87.50%). Therefore, priming with 2% KNO3 solution for a minimum of 12 and 18 h was deemed appropriate for increasing germination percentages. Even seeds subjected to H2O priming for different durations showed more rapid germination (65% at 6 h, 70% at 12 h and 77.50% at 18 h) compared to control (42.50%). Similarly, Timson germination index improved with increased priming duration (5.833 in 2 KNO3 18 h, 5.667 in 2%KNO3 12 h, 5.167 in H2O 18 h, 4.667 in H2O 12 h and 4.333 in H2O 6 h) compared to control (2.833). This indicates that expanding the duration of priming treatment might result in a more positive influence on Timson germination index. It has long been known that KNO3 solution is an appropriate chemical approach for promoting germination in several plant species [7]; improved germination percentage and Timson germination index KNO3 treatment might be due to enhanced nitric oxide (NO) production in germinating seeds as a consequence of nitrite and nitrate decomposition [8,9]. This change may possibly be a retort to the interior alteration hastened by exposure to potassium nitrate (KNO3). NO production is known to encourage the accessibility of nitrates in germinating seeds, and it interacts with seed embryo photosynthesis [6,10]. Furthermore, KNO3 is known to be engaged in endosperm putrefaction and it augments the activity of amylase and protease, which may perhaps have contributed to enhanced germination and other physiological indices [11]. Numerous studies have reported improved germination indices with KNO3 treatment [8,12].


Figure 1.
(a) Evaluation of seedling in propagation trays; note that vivid brighter green colors indicate an improvement in leaf chlorophyll content; (b) visual differences in various conditioning agents on seedling growth. A sturdier seedling growth with KNO3 may be the main reason for enhanced seedling survival.
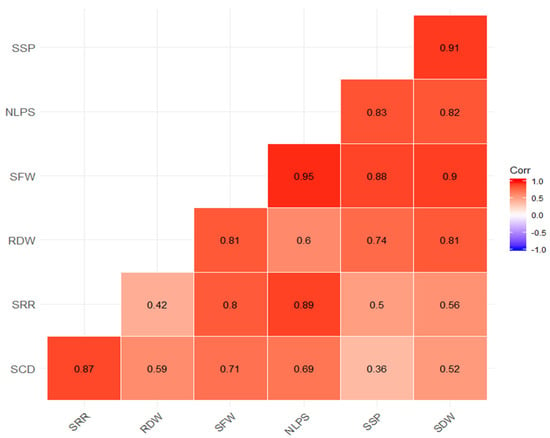
Figure 2.
Visualization of correlation matrix between seedling survival percentage (SSP) vs. number of leaves per seedling (NLPS), seedling fresh weight (SFW), root dry weight (RDW), shoot–root ratio (SRR) and seedling collar diameter (SCD).

Table 1.
Effect of different osmotic agents and priming durations on germination percentage, seedling survival percentage and Timson germination index.
Our study was able to demonstrate an interesting event of significantly enhanced seedling survival percentage in China aster seedlings. Varied priming durations deferentially influenced seedling survival percentages. Improved seedling survival was recorded, with extended priming duration and substrate concentration (81.94% in 2% KNO3 18 h, 80.00% in 2% KNO3 12 h, 64.24 in H2O 18 h), against 52.50% in the case of control, which was deemed as the significantly lowest survival percentage. As noted previously, priming reduces damage due to various abiotic and biotic factors [13] that contribute to better crop performance. Thus, we assumed that KNO3 priming might have improved the defense mechanism against a critical limiting factor, i.e., fusarium wilt of seedling survival. Hence, our findings suggest that KNO3 priming could largely reduce the fungicide treatment on a seedling that contributes to significant input cost. This technique could be undertaken at a larger scale and possibly be user- and environmentally friendly. No or few findings have been reported that suggest KNO3 priming to be an effective strategy to improve seedling survival. As noted earlier [14], KNO3 priming improved seedling survival in Digitalis purpurea. We conducted a correlation analysis between seedling survival percentage and other variables. The visualization of the correlation matrix between seedling survival percentage and other variables is illustrated in Figure 2. The matrixes symbolize positively high correlation between seedling survival percentage, seedling collar diameter, number of leaves per seedling, etc.
From the values of the number of leaves/seedling and seedling collar diameter, we deduced an interesting finding (Table 2). The tendency of priming effect increased with prolonged duration and substrate concentration. The effectiveness of accelerated seedling collar diameter (0.101 cm in 2% KNO3 18 h and 0.086 cm in H2O 18 h) and the number of leaves/seedling (7.014 in 2% KNO3 18 h and 7.006 in 2% KNO3 12 h) was observed in KNO3 and H2O priming compared to 0.068 cm and 3.756, respectively in control. It is noteworthy that seedling raised from KNO3-primed seeds, despite having a maximum number of leaves/seedlings, resulted in lusher, green seedlings, depicting improved chlorophyll content and vigor (Figure 1). These finding could be ascribed to the positive influence of K on the biochemical and physiological process of plant life such as enhanced nitrate reductase (NR) activity [12,15]. NR is important in the creation of the antioxidant mechanism to forge ROS accountable for the volatility of photosynthetic complexes [16,17]. As noted and reported, early KNO3 substantially improves chlorophyll content through cell expansion, osmoregulation and maintenance of cell membrane integrity.

Table 2.
Effect of different osmotic agents and priming durations on seedling fresh weight (g), seedling collar diameter (cm) and number of leaves/seedling.
The results of our study suggested that halopriming (KNO3) not only accelerated germination percentage, Timson germination index and seedling survival, but also significantly enhanced seedling fresh weight, shoot–root ratio as indicated by longer radical lengths and shoot–root dry weight compared to control (Table 3). Seeds subjected to 2% KNO3 priming for 18 h showed a marked build-up in seedling fresh weight (0.036 g), shoot dry weight (0.352 g), root dry weight and shoot–root ratio (1.044) compared to control, i.e., 0.018 g, 0.298 g and 0.572, respectively. It has been demonstrated in many crops that KNO3 priming results in a significantly increased physiological response [18]. Plants raised from primed seeds are known to show structural amendments at all three levels (root, stem and leaf), and the improved performance of plants is accredited to enhanced structural and cortical components, including vascular bundle thickness in leaf and increased pith cell area in stem [12]. Seeds primed in KNO3 produced seedling with a maximum fresh weight per seedling in tomato [19]. It has been observed that the highest shoot fresh weight is produced with KNO3 priming in safflower [19]. Similarly, numerous reports suggest that KNO3 priming improves physiological aspects such as fresh weight, dry weight and root–shoot lengths, in contrast to non-primed seeds [20,21].

Table 3.
Effect of different osmotic agents and priming durations on shoot dry weight (g), root dry weight and shoot–root ratio.
4. Conclusions
In summary, seeds primed with KNO3 not only accelerated germination percentage, Timson germination index, the number of leaves/seedling, seedling collar diameter and dry matter, but also significantly enhanced seedling survival percentages compared to control. In practice, it is recommended that China aster “Powderpuff” seeds can be treated in 2% KNO3 solution for 18 h to obtain optimum seed germination and seedling rate/survival for favorable establishment in the field. Moreover, our findings advocate that KNO3 priming can be used as a cost-effective strategy to alleviate seedling survival at a larger scale. From a farmers/growers perspective, this technique could be promising to replace the obnoxious activity of pesticide application for improving seedling survival, with no tangible ill effects on soil, seeds or human health.
Author Contributions
Conceptualization, methodology, investigation, formal analysis, writing-original draft preparation, writing-review and editing, M.A.W. and F.K.; data compilation, lab supervision, software, drafting technical programme, A.D., I.T.N., S.I. and N.B. All authors have read and agreed to the published version of the manuscript.
Funding
The research received no external funding.
Acknowledgments
We would like to express our sincere gratitude to the in-charge “Plant Tissue Culture Laboratory, Department of FLA” for the kind support. The principal author is also highly obliged to UGC, GoI for providing the monetary support in the form of “Maulana Azad National Fellowship”.
Conflicts of Interest
Authors declare that they have no conflicts of interest.
References
- Chaitra, R.; Patil, V.S. Integrated nutrient management studies in China aster (Callistephus chinensis (L.) Nees). Karnataka J. Agric. Sci. 2007, 20, 689–690. [Google Scholar]
- Sheela, V.L. China aster. Flowers for trade. In Horticultural Science Series 10; New India Pub Agency: New Delhi, India, 2008; pp. 113–127. [Google Scholar]
- Finch-Savage, B. Seeds: Physiology of development, germination and dormancy. Seed Sci. Res. 2013, 23, 289. [Google Scholar] [CrossRef]
- Khan, H.A.; Ayub, C.M.; Pervez, M.A.; Bilal, R.M.; Shahid, M.A.; Ziaf, K. Effect ofseed priming with NaCl on salinity tolerance of hot pepper (Capsicum annuum L.) at seedling stage. Soil Environ. 2009, 28, 81–87. [Google Scholar]
- Huang, Y.; Lin, C.; He, F.; Li, Z.; Guan, Y.; Hu, Q.; Hu, J. Exogenous spermidine improves seed germination of sweet corn via involvement in phytohormone interactions, H2O2 and relevant gene expression. BMC Plant Biol. 2017, 17, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanto, U.; Jutamanee, K.; Osotsapar, Y.; Chaiarree, W.; Jattupornpong, S. Promotive effect of priming with 5-aminolevulinic acid on seed germination capacity, seedling growth and antioxidant enzyme activity in rice subjected to accelerated ageing treatment. Plant Prod. Sci. 2015, 18, 443–454. [Google Scholar] [CrossRef] [Green Version]
- McDonald, M.B. Seed priming. In Seed Technology and Its Biological Basis; Black, M., Bewley, J.D., Eds.; Sheffield Academic Press Ltd.: Sheffield, UK, 2000; pp. 287–325. [Google Scholar]
- Bian, L.; Yang, L.; Wang, J.A.; Shen, H.L. Effects of KNO3 pretreatment and temperature on seed germination of Sorbuspohuashanensis. J. For. Res. 2013, 24, 309–316. [Google Scholar] [CrossRef]
- Renata, B.; Agnieszka, G. Nitric oxide and HCN reduce deep dormancy of apple seeds. Acta Physiol. Plantar. 2006, 28, 281–287. [Google Scholar] [CrossRef]
- Giba, Z.; Grubišić, D.; Konjevĭ, R. Seeking the role of NO in breaking seed dormancy. Plant Cell Monogr. 2006, 5, 91–111. [Google Scholar]
- Gupta, S.M.; Pandey, P.; Grover, A.; Ahmed, Z. Breaking seed dormancy in Hippophaesalicifolia, a high value medicinal plant. Physiol. Mol. Biol. Plants 2011, 17, 403–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafiq, F.; Batool, H.; Raza, S.H.; Hameed, M. Effect of potassium nitrate seed priming on allometry of drought-stressed cotton (Gossypium hirsutum L.). J. Crop SciBiotechnol. 2015, 18, 195–204. [Google Scholar] [CrossRef]
- Rashid, A.; Harris, D.; Hollington, P.A.; Rafiq, M. Improving the yield of mungbean (Vigna radiata) in the North West Frontier Province of Pakistan using on-farm seed priming. Exp. Agric. 2004, 40, 233–244. [Google Scholar] [CrossRef]
- Butler, L.H.; Hay, F.R.; Ellis, R.H.; Smith, R.D.; Murray, T.B. Priming and re-drying improve the survival of mature seeds of Digitalis purpurea during storage. Ann. Bot. 2009, 103, 1261–1270. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Tripathi, R.D.; Dwivedi, S.; Kumar, A.; Trivedi, P.K.; Chakrabarty, D. Lead bioaccumulation potential of an aquatic macrophyte Najasindica are related to antioxidant system. Bioresour. Technol. 2010, 101, 3025–3032. [Google Scholar] [CrossRef]
- Pető, A.; Lehotai, N.; Feigl, G.; Tugyi, N.; Ördög, A.; Gémes, K.; Tari, I.; Erdei, L.; Kolbert, Z. Nitric oxide contributes to copper tolerance by influencing ROS metabolism in Arabidopsis. Plant Cell Rep. 2013, 32, 1913–1923. [Google Scholar] [CrossRef] [PubMed]
- Simaei, M.; Khavari-Nejad, R.A.; Bernard, F. Exogenous application of salicylic acid and nitric oxide on the ionic contents and enzymatic activities in NaCl-stressed soybean plants. Am. J. Plant Sci. 2012, 3, 1495–1503. [Google Scholar] [CrossRef] [Green Version]
- Ahmadvand, G.; Soleimani, F.; Saadatian, B.; Pouya, M. Effect of seed priming with potassium nitrate on germination and emergence traits of two soybean cultivars under salinity stress conditions. Am. Eurasian J. Agric. Environ. Sci. 2012, 12, 769–774. [Google Scholar]
- Mirabi, E.; Hasanabadi, M. Effect of Seed Priming on Some Characteristic of Seedling and Seed Vigor of Tomato (Lycopersiconesculentum). J. Adv. Lab. Res. Biol. 2012, 3, 237–240. [Google Scholar]
- Ghassemi, G.K.; Jabbarpour, S.; Zehtab-Salmasi, S.; Mohammadi, A. Response of winter rapeseed (Brassica napus L.) cultivars to salt priming of seeds. Afr. J. Agric. Res. 2010, 5, 1089–1094. [Google Scholar]
- Yogananda, D.K.; Vyakarnahal, B.S.; Shekhargouda, M. Effect of seed invigoration with growth regulations and micronutrients on germination and seedling vigour of bell pepper cv. California Wonder. Karnataka J. Agric. Sci. 2004, 17, 811–813. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).