1. Introduction
Brassica napus L. (oilseed rape, rapeseed) is used as a source of vegetable oil and protein, animal feed and biodiesel. It is one of the most important crops in moderate climate zone, due to its remarkable cold tolerance. However, oilseed rape is highly susceptible to fungal deceases, including powdery mildew
Erysiphe crucifertaum [
1,
2].
Powdery mildew is an obligate biotrophic pathogen, most common in the end of the growing season [
1]. Infection can reduce the yield of rapeseed and its quality, causing chlorosis, necrosis and dehydration. Resistance to this pathogen is very rare in
B. napus and was only achieved by hybridization with distant relatives [
3]. Commercial cultivars resistant to this fungal disease have not been selected yet. The impossibility to grow
E. crucifertaum in culture media apart from the plant complicates associated studies.
Plant physiological reactions to powdery mildew infection are poorly studied. Glutathione plays an important role in plant resistance to oxidative stress.
Reduced Glutathione (
GSH) is involved in detoxification of ROS and toxic molecules, catalyzed by glutathione S-transferases (GSTs). Glutathione disulfide (GSSG) is formed during this process [
4]. Induction of the level of GSH and GST upon exposure to stress provides better protection of the plant cell. Although GSTs are usually associated with tolerance to heavy metals [
5], there are data on the role of several GST genes in resistance to other stress factors such as extreme temperatures [
6] and fungal diseases [
7,
8,
9,
10]. Therefore, it is supposed that described mechanisms can also work during plant–fungus interactions, they have never been studied in oilseed rape infected by
E. crucifertaum and the actual level of GSTs and glutathione in healthy and infected plants have never been evaluated.
2. Experiments
2.1. Biological Material
B. napus of cultivar “Ratnik” were used in the experiments. Seeds were sown in the experimental plot in natural conditions and in the laboratory in vessels filled with commercial soil (Geolia, Russia) under 10,000 lux generated by LED grow light.
E. crucifertaum was isolated from the infected B. napus plants and distinguished on the basis of the sequencing of ITS DNA marker. Leaves of the experimental plants were rubbed with the leaf of the infected plant to spread infection. Control plants remained untreated.
After 30 days, 42 plants with severe signs of infection and 30 control plants, from both the laboratory and field experiments, were used for spectrophotometric GSH, GSSG and GST assay. Each plant with increased resistance to powdery mildew was studied individually.
2.2. Spectrophotometric Assay
Fresh leaves of
B. napus were homogenized and used to measure the level of GSH, GSSG and GST by
spectrophotometric assay simultaneously. Levels of glutathione and glutathione disulfide were determined using o-phthalaldehyde (79,760, Sigma-Aldrich, St. Louis, MO, USA) as a fluorescent reagent; a derivatization of GSH to prevent GSH autooxidation was performed using N-ethylmaleimide (E1271, Sigma-Aldrich) [
11]. GST activity was measured using the model substrate 1-chloro-2,4-dinitrobenzene (138630, Sigma-Aldrich) [
12]. All measurements were performed in 6 replications in a 96-well plate using a Perkin Elmer LS 55 Luminescence Spectrometer (US).
3. Results
Most of the
B. napus plants demonstrated severe signs of infection within 30 days after treatment, however several individuals were less susceptible to it (
Figure 1). In general, 12 plants with increased resistance to powdery mildew were found and examined. Out of them, six plants were cultivated in the field, and six in the laboratory.
There was a difference in GSH and GSSG content and GST activity not only between infected and control plants, but also between plants cultivated in laboratory and in the field. All measured parameters were increased in the latter. Therefore, the results were evaluated separately for laboratory and field experiment.
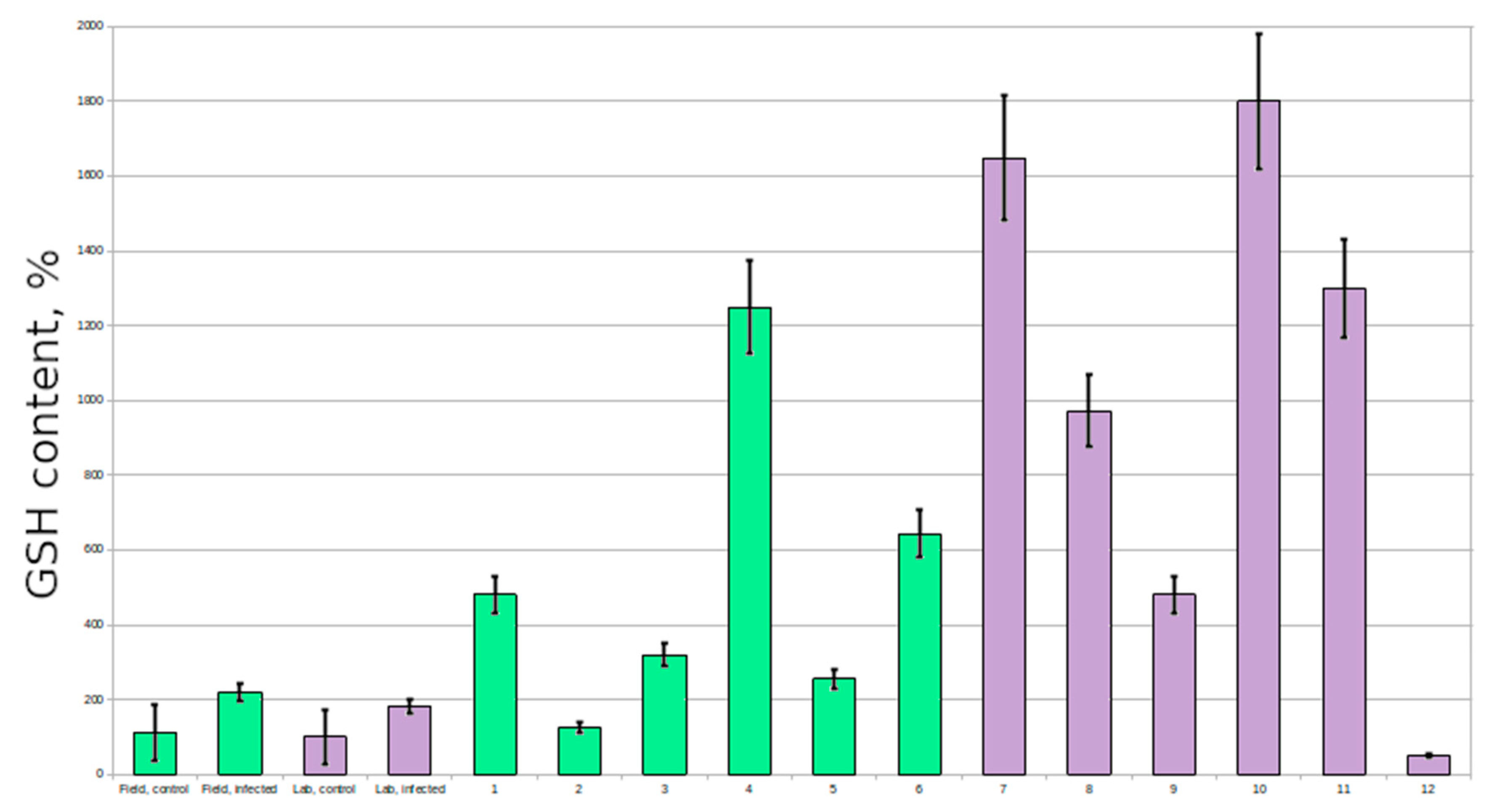
Resistant plants had extremely high glutathione content in comparison with susceptible plants (6–7 times higher in several plants; 2–3 times higher on average) (
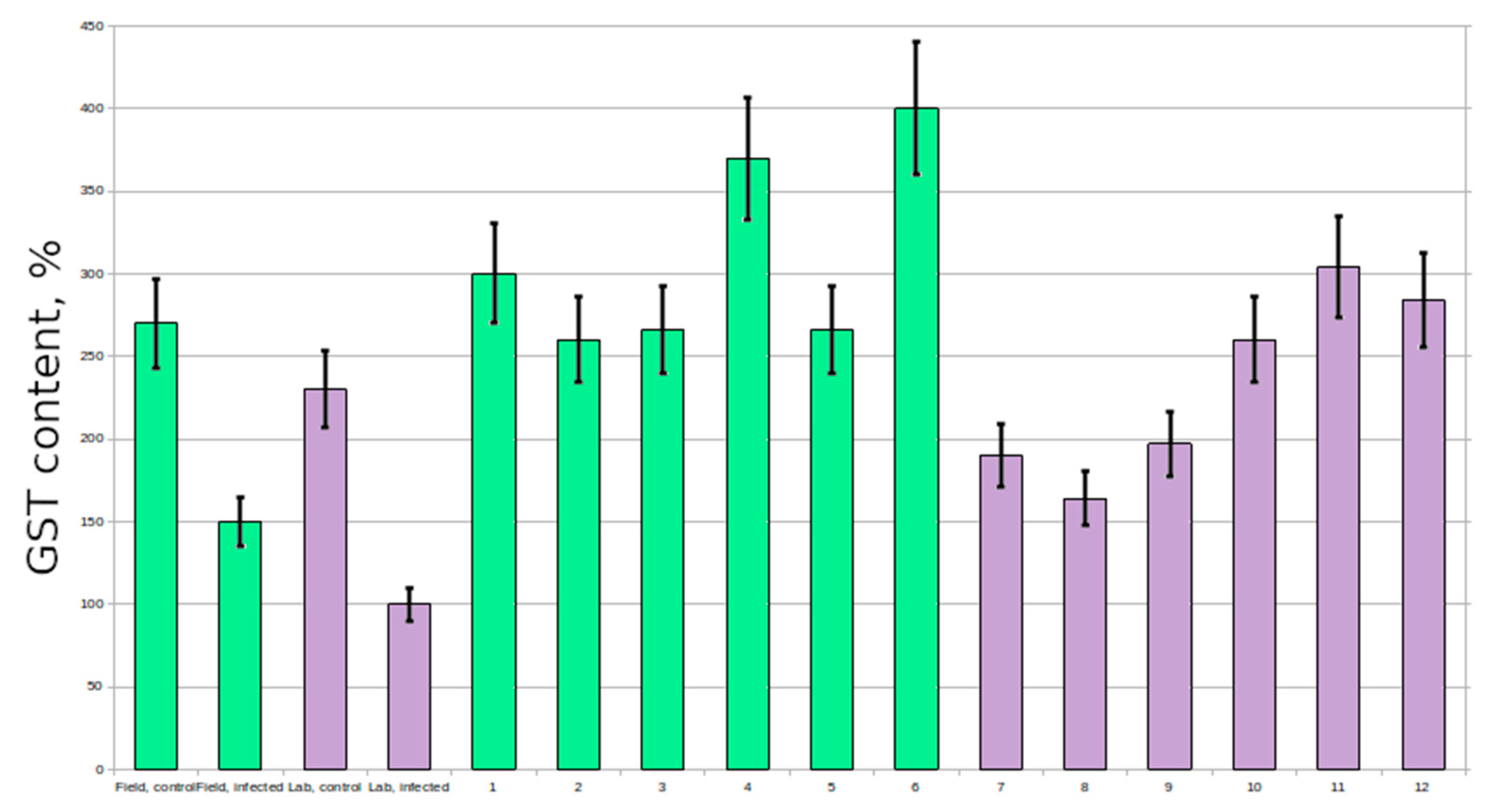
Figure 2); however the activity of GST never varied that much (
Figure 3). In resistant and control plants it was at the same level, however in susceptible plants it was no more than three times lower. In untreated plants GST activity was stable and did not vary significantly, however some plants had increased glutathione levels.
The GSH:GSSG level in most of the susceptible plants was lower than in resistant plants, which is a sign of heavier stress.
4. Discussion
Our results suggest that an increased level of glutathione can give plants an advantage during infection with powdery mildew. The analysis of control plants showed that there are individuals with a physiologically high level of glutathione, and they could be the ones showing resistance to the pathogen. These untreated plants also demonstrated higher a GSH:GSSG level, which is characteristic for the lower oxidative stress.
However, there are some data on the increased expression of
GST genes in plants exposed to fungal infection [
7,
8,
9,
10] in wheat, tomato and barley; in our experiments there was no significant difference between control and resistant plants in GST activity. Many of the susceptible plants had reduced GST activity; therefore, this protein is also of research interest.
5. Conclusions
Oilseed rape with naturally increased glutathione content can be used in the selection of resistant cultivars. GST and GSH genes should be also considered as targets for genetic engineering and genome editing.
Author Contributions
E.M. conceived and designed the experiments, analyzed the data and wrote the paper; M.S. and V.A. performed the experiments. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Council on grants of the President of the Russian Federation MK-1146.2020.11.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uloth, M.B.; You, M.P.; Barbetti, M.J. Plant age and ambient temperature: Significant drivers for powdery mildew (Erysiphe cruciferarum) epidemics on oilseed rape (Brassica napus). Plant Pathol. 2018, 67, 445–456. [Google Scholar] [CrossRef]
- Alkooranee, J.T.; Liu, S.; Aledan, T.R.; Yin, Y.; Li, M. First report of powdery mildew caused by Erysiphe cruciferarum on Brassica napus in China. Plant Dis. 2015, 99, 1651. [Google Scholar] [CrossRef]
- Gong, Q.; Dai, C.Y.; Zhang, X.H.; Wang, X.L.; Huang, Z.; Xu, A.X.; Dong, J.G.; Yu, C.Y. Towards breeding of rapeseed (Brassica napus) with alien cytoplasm and powdery mildew resistance from Ethiopian mustard (Brassica carinata). Breed. Sci. 2020, 20017. [Google Scholar] [CrossRef] [PubMed]
- Gullner, G.; Komives, T. Defense reactions of infected plants: Roles of glutathione and glutathione S-transferase enzymes. Acta Phytopathol. Entomol. Hung. 2006, 41, 3–10. [Google Scholar] [CrossRef]
- Kuluev, B.R.; Berezhneva, Z.A.; Mikhaylova, E.V.; Postrigan, B.N.; Knyazev, A.V. Productivity and stress-tolerance of transgenic tobacco plants with constitutive expression of rapeseed glutathione synthetase gene BnGSH. Ecol. Genet. 2017, 15, 12–19. [Google Scholar] [CrossRef][Green Version]
- Kouno, T.; Ezaki, B. Multiple regulation of Arabidopsis AtGST11 gene expression by four transcription factors under abiotic stresses. Physiol. Plant. 2013, 148, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Mauch, F.; Dudler, R. Differential induction of distinct glutathione-S-transferases of wheat by xenobiotics and by pathogen attack. Plant Physiol. 1993, 102, 1193–1201. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.M.; Liu, H.Y.; Xu, H.M.; Li, M.; Kang, Z.S. Analysis of differential transcriptional profiling in wheat infected by Blumeria graminis f. sp. tritici using GeneChip. Mol. Biol. Rep. 2012, 39, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Ma, H.; Zhang, Y.; Ma, Y.; Wang, W.; Geng, H.; Wu, J.; Li, C. Virus-induced gene silencing of a putative glutathione S-transferase gene compromised Ol-1-mediated resistance against powdery mildew in tomato. Plant Mol. Biol. Rep. 2011, 29, 972–978. [Google Scholar] [CrossRef]
- Hossain, M.A.; Mostofa, M.G.; Diaz-Vivancos, P.; Burritt, D.J.; Fujita, M.; Tran, L.S.P. (Eds.) GlutaThione in Plant Growth, Development, and Stress Tolerance; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Hissin, P.J.; Hilf, R. Fluorometric determination of glutathione using o-phthaldialdehyde. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Sappl, P.G.; Carroll, A.J.; Clifton, R.; Lister, R.; Whelan, J.; Harvey Millar, A.; Singh, K.B. The Arabidopsis glutathione transferase gene family displays complex stress regulation and co-silencing multiple genes results in altered metabolic sensitivity to oxidative stress. Plant J. 2009, 58, 53–68. [Google Scholar] [CrossRef] [PubMed]
| Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).