Control of Carrot Seed-Borne Pathogens by Aromatic Plants Distillates †
Abstract
:1. Introduction
2. Experiments
2.1. Seed Preparation
2.2. Essential Oils Production
2.3. Medium Preparation
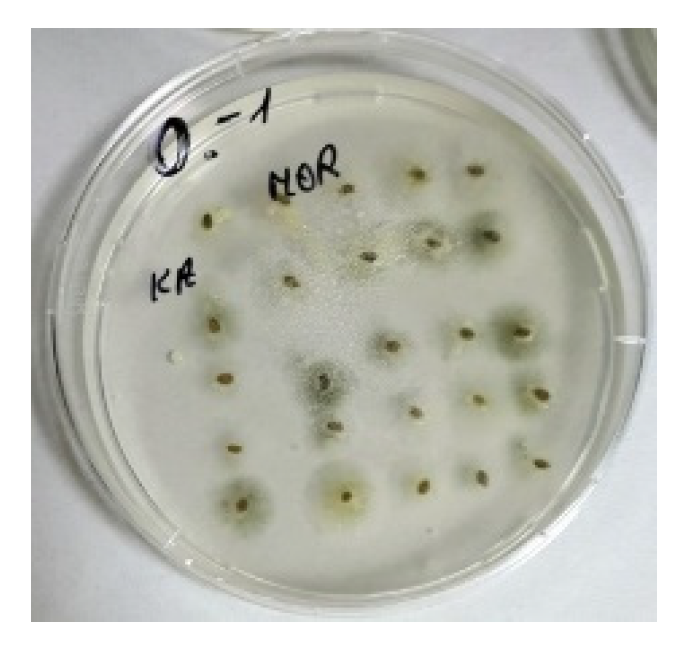
2.4. Essential Oils Effect on Pathogens
2.5. Statistics
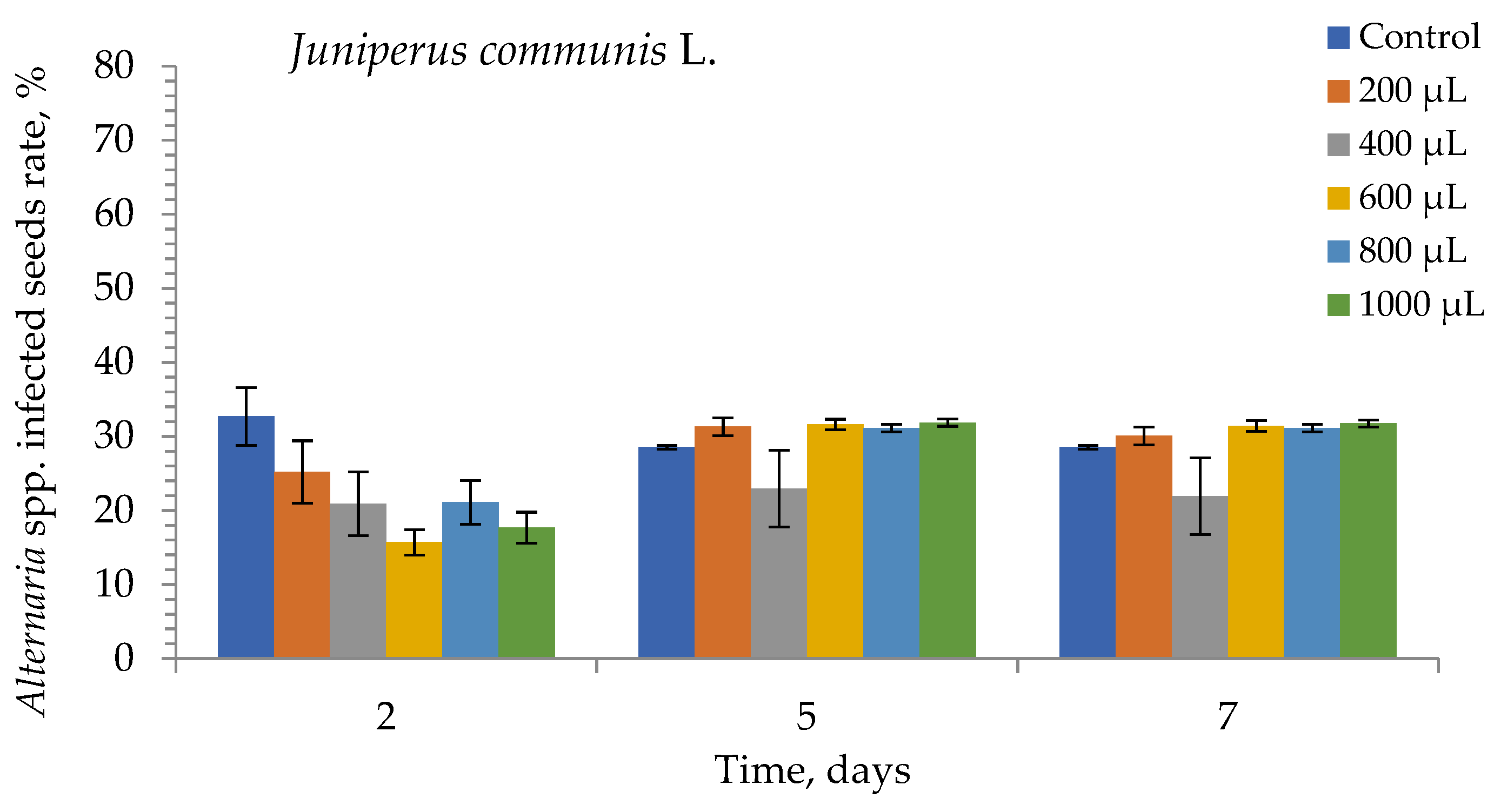
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| LAMMC | Lithuanian Research Centre for Agriculture and Forestry |
| IH | Institute of Horticulture |
| PDA | Potato dextrose agar |
| EOs | Essential oils |
| ANOVA | Analysis of variance |
References
- Que, F.; Hou, X.; Wang, G.; Xu, Z.; Tan, G.; Li, T.; Wang, Y.; Khadr, A.; Xiong, A. Advances in research on the carrot, an important root vegetable in the Apiaceae family. Hortic. Res. Engl. 2019, 6, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Michael, T.P.; VanBuren, R. Progress, challenges and the future of crop genomes. Curr. Opin. Plant Biol. 2015, 24, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Luby, C.H.; Maeda, H.A.; Goldman, I.L. Genetic and phenological variation of tocochromanol (vitamin E) content in wild (Daucus carota L. var. carota) and domesticated carrot (D. carota L. var. sativa). Hortic. Res. Engl. 2014, 1, 14015. [Google Scholar] [CrossRef]
- EPPO Standard. PP 2/1 (1) Guideline on good plant protection practice: Umbelliferous crops. Bull. OEPP/EPPO Bull. 1994, 24, 233–240. [Google Scholar]
- Zhang, X.; Wang, R.; Ning, H.; Li, W.; Bai, Y.; Li, Y. Evaluation and management of fungal-infected carrot seeds. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.M. Carrot diseases and their management. In Diseases of Fruits and Vegetables; Naqvi, S.A.M.H., Ed.; Springer: Dordrecht, The Netherlands, 2004; Volume I, pp. 397–439. [Google Scholar] [CrossRef]
- González, M.; Caetano, P.; Sánchez, M.E. Testing systemic fungicides for control of Phytophthora oak root disease. For. Pathol. 2017, 47, e12343. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; You, M.P.; Laudinot, V.; Barbetti, M.J.; Aubertot, J.N. Revisiting sustainability of fungicide seed treatments for field crops. Plant Dis. 2020, 104, 610–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, U.B.; Chaurasia, R.; Manzar, N.; Kashyap, A.S.; Malviya, D.; Singh, S.; Kannojia, P.; Sharma, P.K.; Imran, M.; Sharma, A.K. Chemical Management of Seed-Borne Diseases: Achievements and Future Challenges. In Seed-Borne Diseases of Agricultural Crops: Detection, Diagnosis & Management; Kumar, R., Gupta, A., Eds.; Springer: Singapore, 2020; pp. 665–682. [Google Scholar] [CrossRef]
- Töfoli, J.G.; Domingues, R.J.; Tortolo, M.P.L. Effect of various fungicides in the control of Alternaria Leaf Blight in carrot crops. Biol. São Paulo 2019, 81, 1–30. [Google Scholar] [CrossRef]
- Silva, V.; Mol, H.G.; Zomer, P.; Tienstra, M.; Ritsema, C.J.; Geissen, V. Pesticide residues in European agricultural soils–A hidden reality unfolded. Sci. Total Environ. 2019, 653, 1532–1545. [Google Scholar] [CrossRef]
- Cozma, P.; Apostol, L.C.; Hlihor, R.M.; Simion, I.M.; Gavrilescu, M. Overview of human health hazards posed by pesticides in plant products. In Proceedings of the 2017 E-Health and Bioengineering Conference (EHB), Sinaia, Romania, 22–24 June 2017; IEEE: Piscataway, NJ, USA, 2017; Volume 17066084, pp. 293–296. [Google Scholar] [CrossRef]
- Valdivieso-Ugarte, M.; Gomez-Llorente, C.; Plaza-Díaz, J.; Gil, Á. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients 2019, 11, 2786. [Google Scholar] [CrossRef] [Green Version]
- Moulodi, F.; Khezerlou, A.; Zolfaghari, H.; Mohamadzadeh, A.; Alimoradi, F. Chemical Composition and Antioxidant and Antimicrobial Properties of the Essential Oil of Hyssopus officinalis L. J. Kermanshah Univ. Med. Sci. 2018, 22. [Google Scholar] [CrossRef] [Green Version]
- Ložienė, K.; Venskutonis, P.R. Juniper (Juniperus communis L.) oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 495–500. [Google Scholar] [CrossRef]
- Dauqan, E.M.; Abdullah, A. Medicinal and functional values of thyme (Thymus vulgaris L.) herb. J. Appl. Biol. Biotechnol. 2017, 5, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Karaca, G.; Bilginturan, M.; Olgunsoy, P. Effects of some plant essential oils against fungi on wheat seeds. Indian J. Pharm. Educ. Res. 2017, 51, S385–S388. [Google Scholar] [CrossRef] [Green Version]
- Muthukumar, A.; Sangeetha, G.; Naveenkumar, R. Antimicrobial activity of essential oils against seed borne fungi of rice (Oryza sativa L.). J. Environ. Biol. 2016, 37, 1429–1436. [Google Scholar]
- AOAC. Volatile oil in spices. In Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Volume 1. [Google Scholar]
- Acumedia. A Subsidiary of NEOGEN Corporation. Neogen Food Safety. Potato Dextrose Agar (7149)-Protocol. PI7149 Rev 4. 2011. Available online: http://biotrading.com/assets/productinformatie/acumedia/tds/7149.pdf (accessed on 4 November 2020).
- Mathur, S.B.; Kongsdal, O. Common Laboratory Seed Health Testing Methods for Detecting Fungi, 1st ed.; International Seed Testing Association: Bassersdorf, Switzerland, 2003; 425p, ISBN 3906549356. [Google Scholar]
- Mancini, V.; Murolo, S.; Romanazzi, G. Diagnostic methods for detecting fungal pathogens on vegetable seeds. Plant Pathol. 2016, 65, 691–703. [Google Scholar] [CrossRef]
- Mamgain, A.; Roychowdhury, R.; Tah, J. Alternaria pathogenicity and its strategic controls. Res. J. Biol. 2013, 1, 1–9. [Google Scholar]
- Soković, M.D.; Vukojević, J.; Marin, P.D.; Brkić, D.D.; Vajs, V.; van Griensven, L.J.L.D. Chemical composition of essential oils of Thymus and Mentha species and their antifungal activities. Molecules 2009, 14, 238–249. [Google Scholar] [CrossRef]
- Dorna, H.; Qi, Y.; Szopińska, D. The effect of acetic acid, grapefruit extract and selected essential oils on germination, vigour and health of carrot (Daucus carota L.) seeds. Acta Sci. Polonorum Hortorum Cultus 2018, 17, 27–38. [Google Scholar] [CrossRef]
- Vokou, D.; Chalkos, D.; Karamanlidou, G.; Yiangou, M. Activation of soil respiration and shift of the microbial population balance in soil as a response to Lavandula stoechas essential oil. J. Chem. Ecol. 2002, 28, 755–768. [Google Scholar] [CrossRef]
- Koch, E.; Schmitt, A.; Stephan, D.; Kromphardt, C.; Jahn, M.; Krauthausen, H.J.; Forsberg, G.; Werner, S.; Amein, T.; Wright, S.A.I.; et al. Evaluation of non-chemical seed treatment methods for the control of Alternaria dauci and A. radicina on carrot seeds. Eur. J. Plant Pathol. 2010, 127, 99–112. [Google Scholar] [CrossRef]
- Riccioni, L.; Orzali, L. Activity of tea tree (Melaleuca alternifolia, Cheel) and thyme (Thymus vulgaris, Linnaeus.) essential oils against some pathogenic seed borne fungi. J. Essent. Oil Res. 2011, 23, 43–47. [Google Scholar] [CrossRef]
- Zabka, M.; Pavela, R.; Slezakova, L. Antifungal effect of Pimenta dioica essential oil against dangerous pathogenic and toxinogenic fungi. Ind. Crops Prod. 2009, 30, 250–253. [Google Scholar] [CrossRef]
- Menghani, E.; Sharma, S.K. Antimicrobial activity of Juniperus communis and Solanum xanthocarpum. Int. J. Pharm. Sci. Res. 2012, 3, 2815. [Google Scholar] [CrossRef]
- Fraternale, D.; Ricci, D.; Epifano, F.; Curini, M. Composition and antifungal activity of two essential oils of hyssop (Hyssopus officinalis L.). J. Essent. Oil Res. 2004, 16, 617–622. [Google Scholar] [CrossRef]
- Judžentienė, A. Hyssop (Hyssopus officinalis L.) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Academic Press: Cambridge, MA, USA, 2016; pp. 471–479. [Google Scholar] [CrossRef]
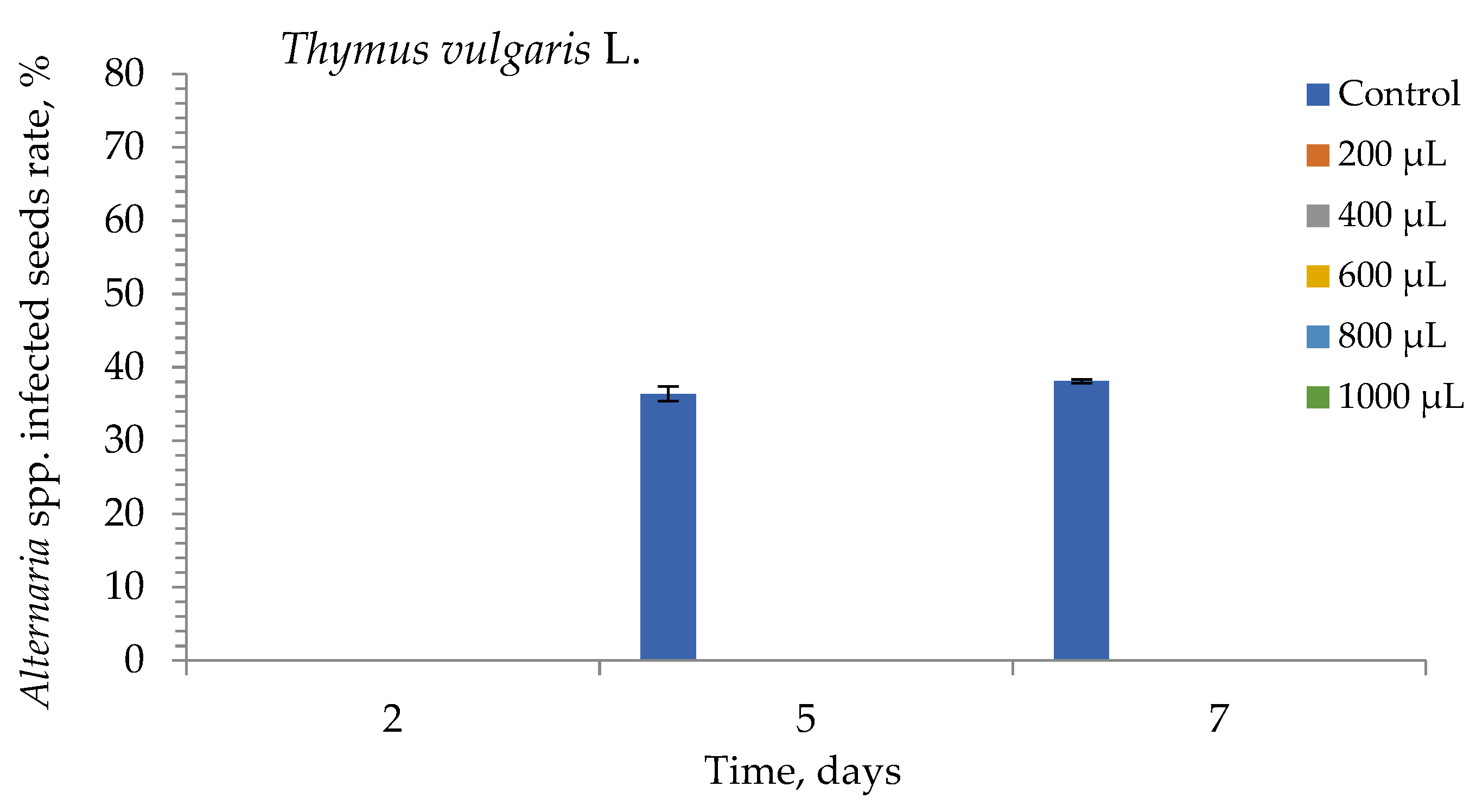
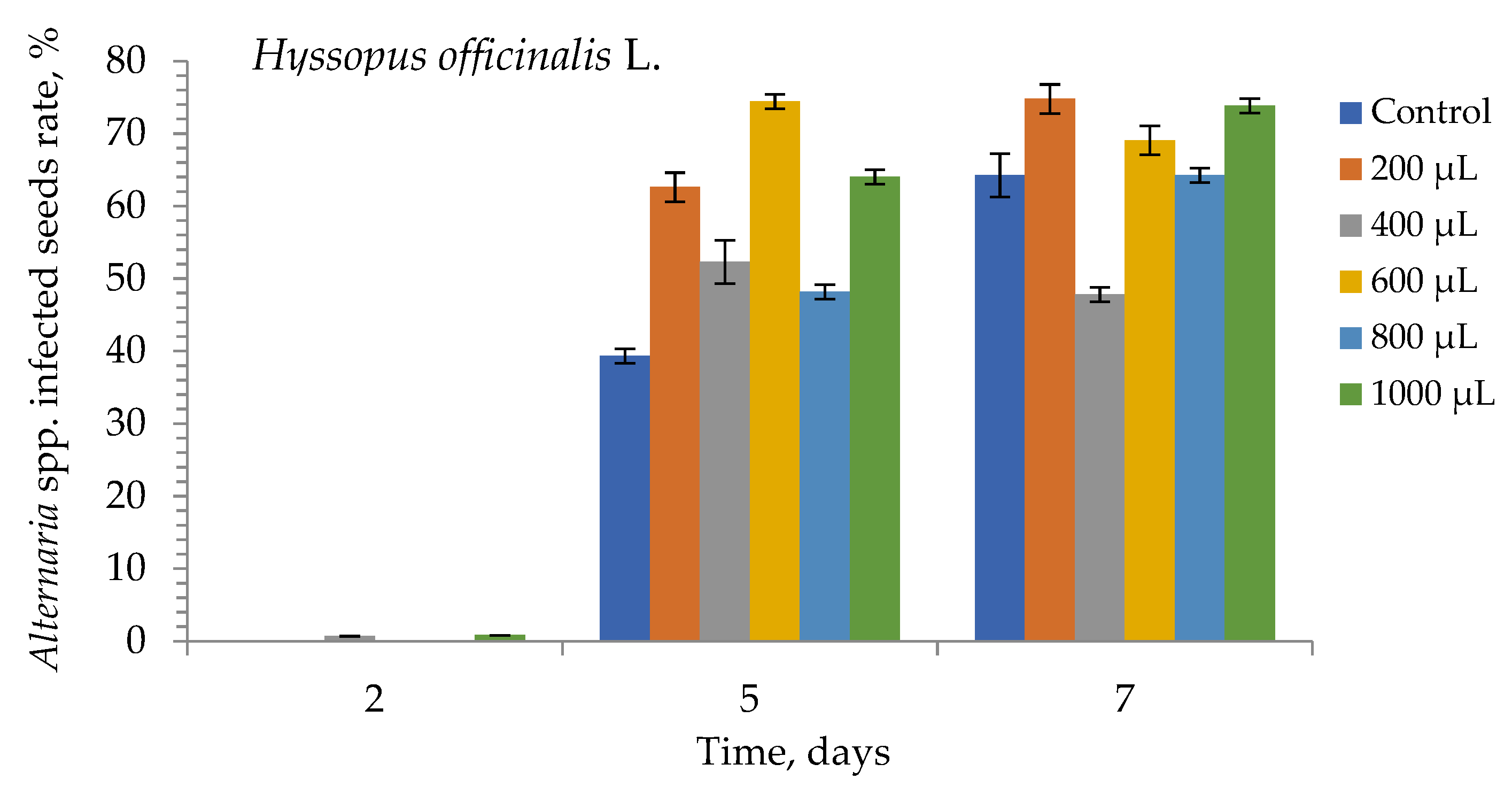
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lukošiūtė, S.; Rasiukevičiūtė, N.; Valiuškaitė, A. Control of Carrot Seed-Borne Pathogens by Aromatic Plants Distillates. Biol. Life Sci. Forum 2021, 4, 29. https://doi.org/10.3390/IECPS2020-08628
Lukošiūtė S, Rasiukevičiūtė N, Valiuškaitė A. Control of Carrot Seed-Borne Pathogens by Aromatic Plants Distillates. Biology and Life Sciences Forum. 2021; 4(1):29. https://doi.org/10.3390/IECPS2020-08628
Chicago/Turabian StyleLukošiūtė, Simona, Neringa Rasiukevičiūtė, and Alma Valiuškaitė. 2021. "Control of Carrot Seed-Borne Pathogens by Aromatic Plants Distillates" Biology and Life Sciences Forum 4, no. 1: 29. https://doi.org/10.3390/IECPS2020-08628
APA StyleLukošiūtė, S., Rasiukevičiūtė, N., & Valiuškaitė, A. (2021). Control of Carrot Seed-Borne Pathogens by Aromatic Plants Distillates. Biology and Life Sciences Forum, 4(1), 29. https://doi.org/10.3390/IECPS2020-08628






