The Influence of Pine Volatiles on the Growth of an Ophiostomatoid Fungi Associated with Pine Wilt Disease in Pinus pinaster †
Abstract
:1. Introduction
2. Material and Methods
2.1. Chemicals
2.2. Maintance of Leptographium
2.3. Bioassays with the Volatiles
2.4. Data Treatment and Statistical Analysis
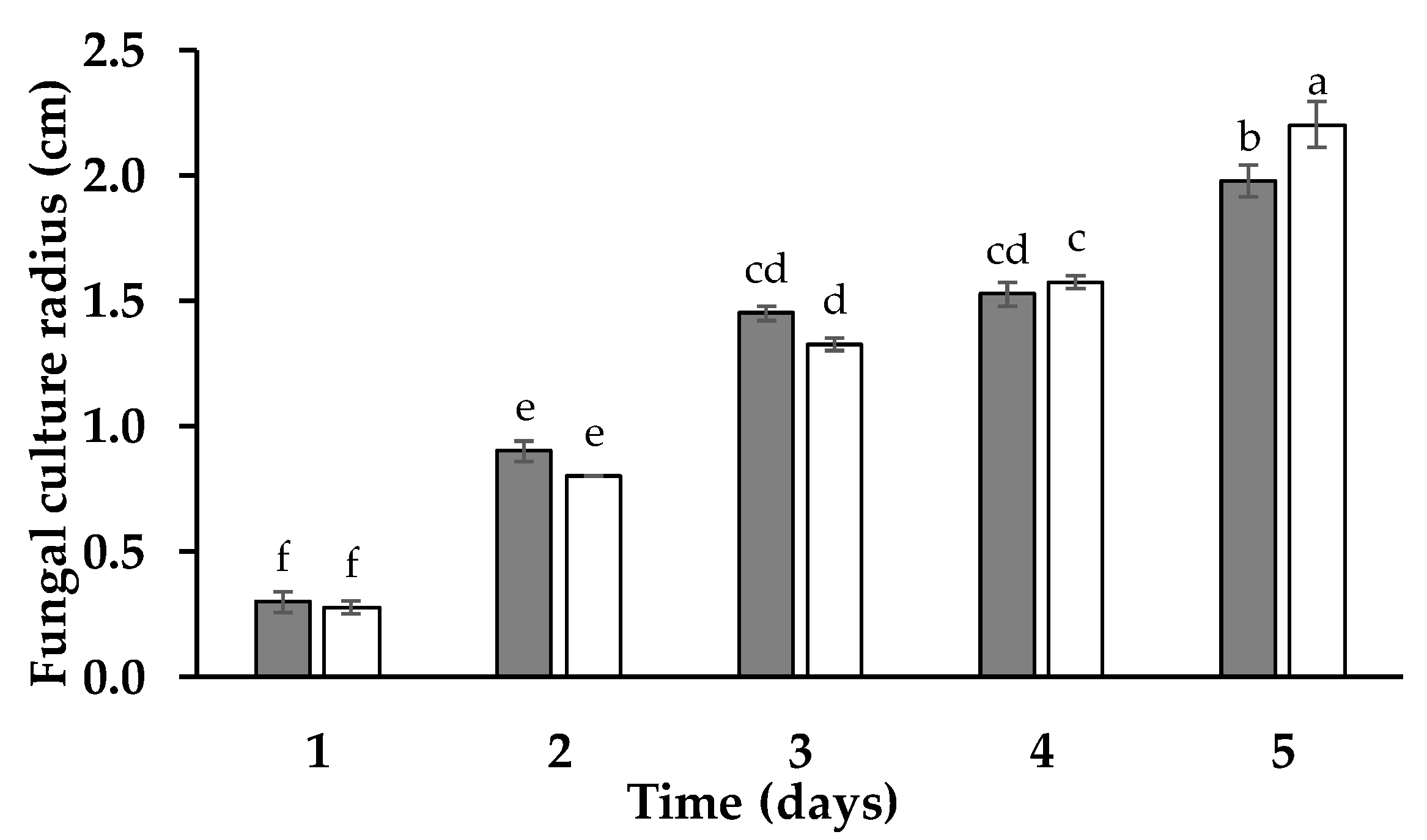
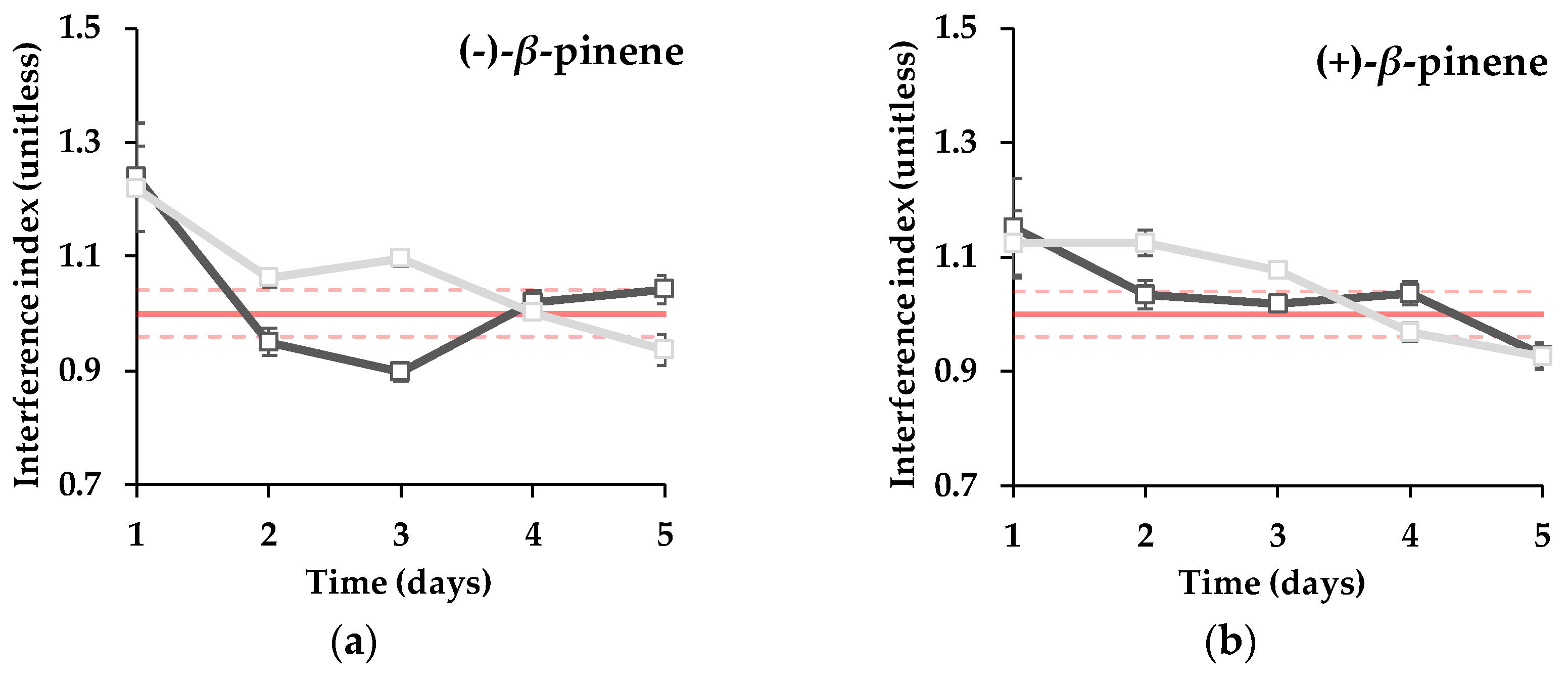
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trollip, C.; Carnegie, A.J.; Dinh, Q.; Kaur, J.; Smith, D.; Mann, R.; Rodoni, B.; Edwards, J. Ophiostomatoid Fungi Associated with Pine Bark Beetles and Infested Pines in South-Eastern Australia, Including Graphilbum ipis-grandicollis sp. nov. IMA Fungus 2021, 12, 24. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.S.L.; Soares, M.; Faria, J.M.S.; Ramos, A.P.; Inácio, M.L. Insights into the Role of Fungi in Pine Wilt Disease. J. Fungi 2021, 7, 780. [Google Scholar] [CrossRef] [PubMed]
- Vicente, C.S.L.; Soares, M.; Faria, J.M.S.; Espada, M.; Mota, M.; Nóbrega, F.; Ramos, A.P.; Inácio, M.L. Fungal Communities of the Pine Wilt Disease Complex: Studying the Interaction of Ophiostomatales with Bursaphelenchus xylophilus. Front. Plant Sci. 2022, 13, 908308. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faria, J.M.S.; Inácio, M.L. The Influence of Pine Volatiles on the Growth of an Ophiostomatoid Fungi Associated with Pine Wilt Disease in Pinus pinaster . Biol. Life Sci. Forum 2024, 31, 9. https://doi.org/10.3390/ECM2023-16454
Faria JMS, Inácio ML. The Influence of Pine Volatiles on the Growth of an Ophiostomatoid Fungi Associated with Pine Wilt Disease in Pinus pinaster . Biology and Life Sciences Forum. 2024; 31(1):9. https://doi.org/10.3390/ECM2023-16454
Chicago/Turabian StyleFaria, Jorge M. S., and Maria L. Inácio. 2024. "The Influence of Pine Volatiles on the Growth of an Ophiostomatoid Fungi Associated with Pine Wilt Disease in Pinus pinaster " Biology and Life Sciences Forum 31, no. 1: 9. https://doi.org/10.3390/ECM2023-16454
APA StyleFaria, J. M. S., & Inácio, M. L. (2024). The Influence of Pine Volatiles on the Growth of an Ophiostomatoid Fungi Associated with Pine Wilt Disease in Pinus pinaster . Biology and Life Sciences Forum, 31(1), 9. https://doi.org/10.3390/ECM2023-16454






