1. Introduction
In recent years, the awareness of healthy eating has changed a lot. Although fruits and vegetables are rich in healthy ingredients and have a positive effect on human health, food poisoning can be caused when they are consumed raw. This can be the case if fruits or vegetables have any pathogenic microorganisms on them. A good example is the EHEC epidemic in 2011 in Germany. Nearly 4000 people got ill and over 50 people died after eating lettuce and sprouts, which were contaminated with enterohemorrhagic
Escherichia coli (
E. coli) [
1,
2].
Contamination can occur both during growth in the field and after harvesting [
3,
4,
5]. In order to prevent such outbreaks of food poisoning and diseases due to contaminated fruits and vegetables, there are various approaches for disinfection. These include chemical treatments (e.g., with chlorine dioxide, organic acids) and non-thermal physical treatments (e.g., disinfection with light or radiation) [
6,
7].
In the field of chemical decontamination, e.g., with chlorine dioxide, microbial effectiveness has already been proven. However, these processes can have negative effects on the color of the product [
8]. There are also several physical disinfection options for decontaminating food. Besides the use of cold plasma, high hydrostatic pressure and ionized radiation, UV radiation and visible blue or violet light also exhibit antimicrobial properties. The physical methods that are not based on radiation are usually very costly and have a strong effect on the cells of the irradiated product [
6]. Regarding the legal situation, it is forbidden in Germany to sell food that has been treated with ionized radiation. However, irradiation in the spectral range of UV radiation and visible light is permitted [
9].
The antimicrobial effect of UV-C radiation has been known for over 100 years. It is already being used in different applications including disinfection in healthcare settings such as hospitals [
10]. Unfortunately, the effect of UV-C radiation can be harmful to humans as it can lead to serious illnesses such as cancer through changes in cell DNA. With a wavelength range of 100–280 nm, UV-C radiation is the most energetic range of optical radiation. The physical and biological properties of UV radiation are, in turn, dependent on the respective wavelength [
11]. The range from 200 nm to 230 nm is called Far-UV-C. In contrast to the longer UV-C wavelengths, Far UV-C is less harmful to humans due to a lower penetration depth into the skin caused by absorption of the proteins of dead skin cells in the Stratum corneum [
12]. For this reason, the question arises whether the irradiation of foods such as lamb’s lettuce and chicory with UV-C and Far-UV-C radiation has a significant influence on the spread of pathogens, especially
E. coli [
13,
14,
15].
The aim of this project is therefore to analyze the radiation disinfection among Far-UV-C and UV-C that reduces pathogens, like E. coli, on different kinds of plants. For this purpose, irradiation experiments with 222 and 254 nm radiation are carried out and the irradiated samples are compared with non-irradiated control samples. Not only the differences in bacterial reduction between the different wavelengths, but also between the plants are investigated. Therefore, chicory (Cichorium intybus var. foliosum) seedlings and packed lamb’s lettuce are irradiated for the experiments. The contamination of the plants takes place through selective inoculation with a fixed number of bacteria. This allows quantitative statements to be made about the inactivation of bacteria after irradiation. The reduction in bacteria is investigated with non-pathogenic E. coli transformed with a pGLO plasmid due to the properties of ampicillin resistance and the GFP fluorescence (green fluorescent protein) under black light, which facilitates the evaluation by reducing the interference of other, already present, unwanted microorganisms on the experimental results.
2. Materials and Methods
For the cultivation of
E. coli × pGLO (HB101 K12), obtained from Bio-Rad (Hercules, CA, USA) and described by Deutch [
16], the Luria–Bertani (LB) culture medium is used for all experiments. To ensure the identification of
E. coli × pGLO, 1 mL of the antibiotic ampicillin and 30 mL of a L(+)-arabinose solution (20% arabinose, 80% distilled water) are added to the liquid medium after autoclaving and before pouring out the plates. The antibiotic serves to select bacterial strains so that only the ampicillin-resistant bacteria grow on the agar plates. The arabinose is needed to activate the fluorescent effect (GFP synthesis).
For the preculture, a growth medium is first mixed. For preparation, 50 μL of ampicillin and 1.5 mL of the stock solution are filled into a 50 mL tube, and the tube is then filled up to 50 mL with LB. From this growth medium, 5 mL is filled into a 15 mL tube (breathable). One colony is then taken from a pure culture using an inoculation loop and stirred into the 15 mL tube. This tube is placed in a shaker at 170 rpm and 37 °C for 16 to 48 h. For the main culture, 30 mL of the growth medium, which has already been prepared for the preculture, is filled into an Erlenmeyer flask. Furthermore, 200 μL of the preculture is then added to this flask and then placed in the shaker again for approximately 5 h. The duration is derived from the growth rate of E. coli.
Before the bacteria can be used for inoculation, they must be mixed to a fixed bacterial concentration to ensure the same initial conditions for all tests. For this purpose, the growth medium must first be removed from the bacteria. To do this, 1 mL of bacterial suspension from the main culture is filled into each of two 1.5 mL Eppendorf tubes, and these are then centrifuged at 7000 rpm for 5 min. The supernatant is pipetted off so that only the pallet remains in the tube. The vessel is then filled with 1 mL of PBS and homogenized. This wash step is performed three times. It ensures that there is no growth medium left in the bacterial suspension and that the bacteria do not multiply further. In the next step, an OD measurement is performed using a spectrophotometer (SPECORD 250 Plus, Analytik Jena, Jena, Germany) at 222 nm and 254 nm, and the desired bacterial concentration is determined. Here, 1 mL of pure PBS serves as the reference, which corresponds to a transmission of 100%. By adding bacteria, the transmission is lowered until it reaches 50%, which corresponds to a bacteria concentration of 107 KBE/mL. If the concentration is lower, there may not be enough bacteria on the leaves, and if it is higher, the plated-out agar plates may not be countable. Using the determined ratio of PBS and bacteria, the final solution is prepared in a new vessel. To do this, 2 mL of PBS and the determined number of bacteria are added to a new 15 mL tube. This mixture is used to inoculate the leaves with the aid of a pipette.
The experiments are conducted with chicory seedlings (Cichorium intybus var. foliosum), and packed field lettuce (Valerianella locusta).
Different lamps are used for the irradiation. A Far-UV-C lamp from USHIO (UXFL70-222B4-UIA PXZ120I2 A, USHIO) is used as the source for the 222 nm irradiation. Irradiations in the 254 nm range are performed with a UVP 3-UV lamp (Analytik Jena GmbH+Co. KG, Germany) for the chicory. The radiation source (254 nm) for the field lettuce experiment is a low-pressure mercury lamp (TUV 15 W/G15T8, Philips).
The experimental setup consists of a lamp placed on a rack above the plant. To avoid reflection effects or other optical side effects, the set-up is arranged in a darkened room or in a black box. To identify the illuminance of the respective irradiation source, this is measured with the aid of an optometer (X1, Optometer, Gigahertz-Optik GmbH, Türkenfeld, Germany).
For the contamination of the plants, two circles of about 1 cm are drawn on a leaf, one for the sample to be irradiated and one for the control. Within each of these circles, 5 drops a 10 μL bacterial suspension are applied.
After the leaves have been contaminated, they are dried for about 15 min. After this process, the irradiation is carried out. A constant experimental set-up is used in a darkened room. After irradiation, both the samples and the controls are punched out. The bacteria are separated from the leaves using a homogenizer and an ultrasonic bath and then transferred to a dilution series. This is plated in triplicates for statistical evaluation and stored in an incubator for at least 24 h. After this step, the bacterial colonies that glow under UV radiation are counted and the achieved log level reduction is calculated using an Excel table. From this calculation, a graphical representation of the log reduction is created. For each plant and each irradiation wavelength, three independent experimental runs are performed.
3. Results
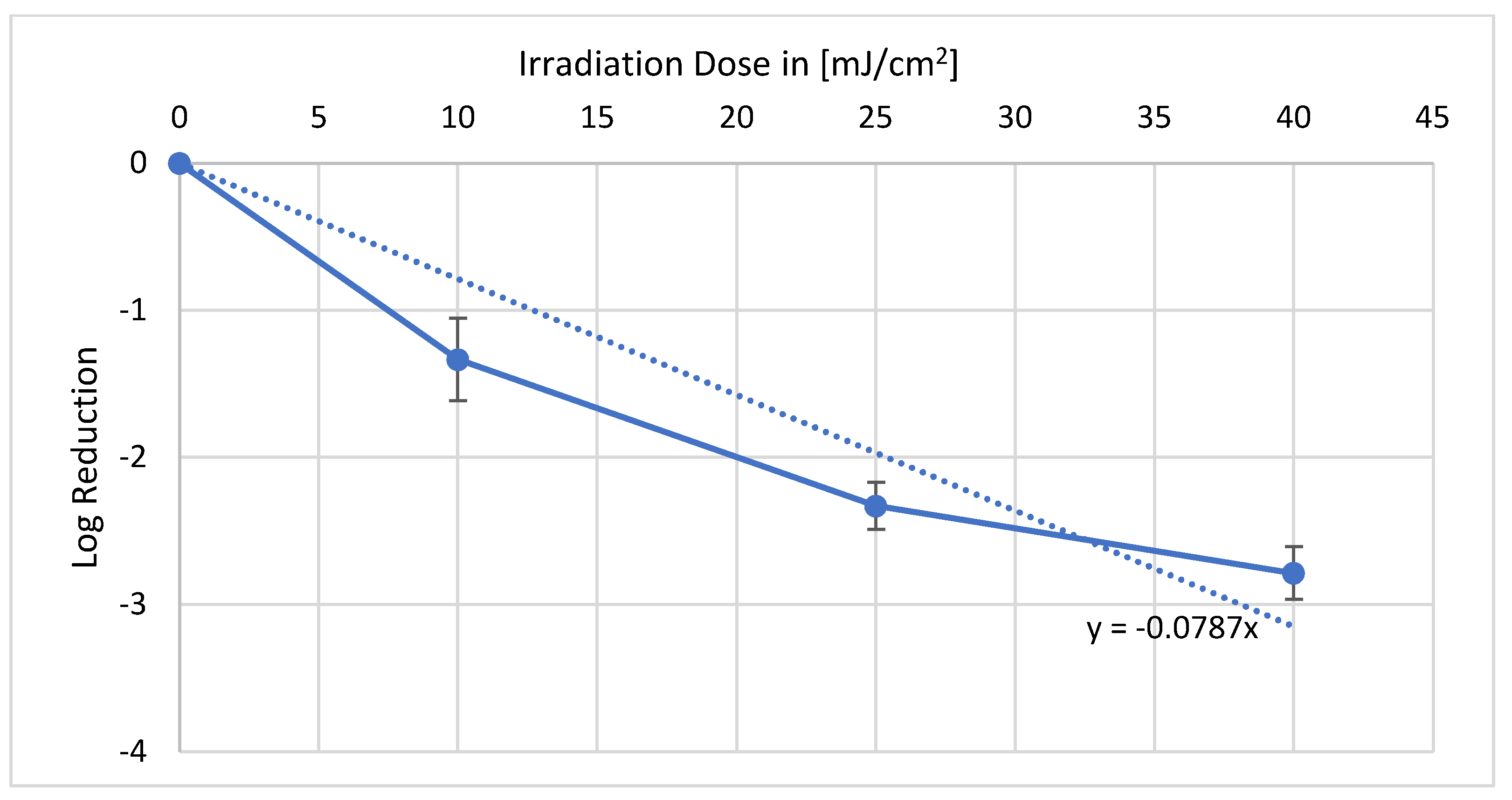
No plant damage was visible for both plants and both wavelengths after irradiation. In the irradiation of
Valerianella locusta with 222 nm, a reproducible reduction could be achieved as illustrated in
Figure 1. With the irradiation dose of 10 mJ/cm
2, a log reduction of 1.33 could be obtained. Furthermore, 25 mJ/cm
2 resulted in a log reduction of 2.33, and 40 mJ/cm
2 resulted in a log reduction of 2.79. The average
E. coli log reduction dose on
Valerianella locusta is 12.7 mJ/cm
2.
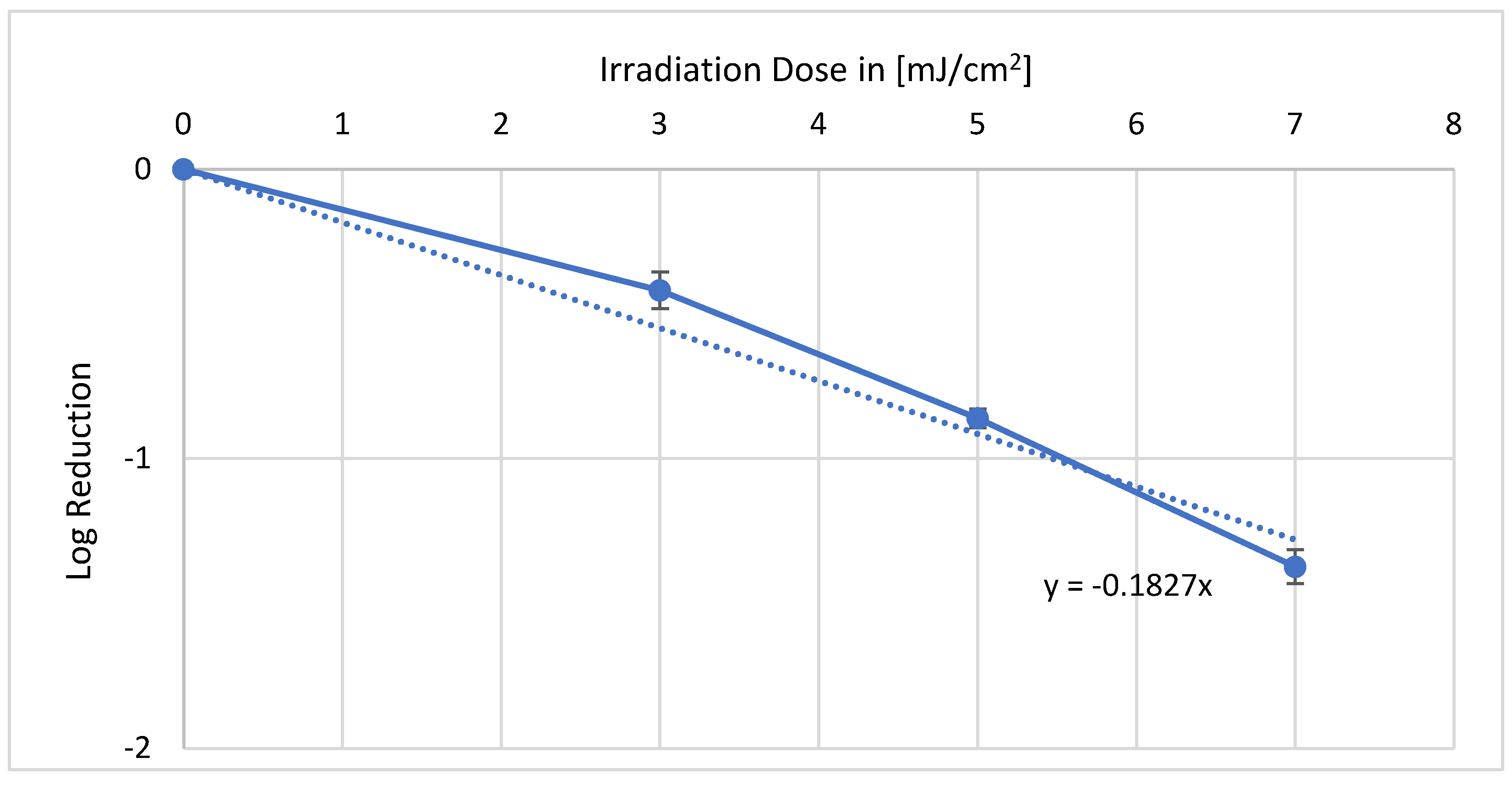
As shown in
Figure 2, the results of the irradiation experiment with 254 nm differ from those of the 222 nm counterpart. Furthermore, 3 mJ/cm
2 caused a log reduction of 0.41, and 5 mJ/cm
2 already led to a log reduction of 0.84. The last irradiation dose of 7 mJ/cm
2 reduced more than 90% of the
E. coli (1.36 log level). From these values, an average log reduction dose of 5.5 mJ/cm
2 can be calculated.
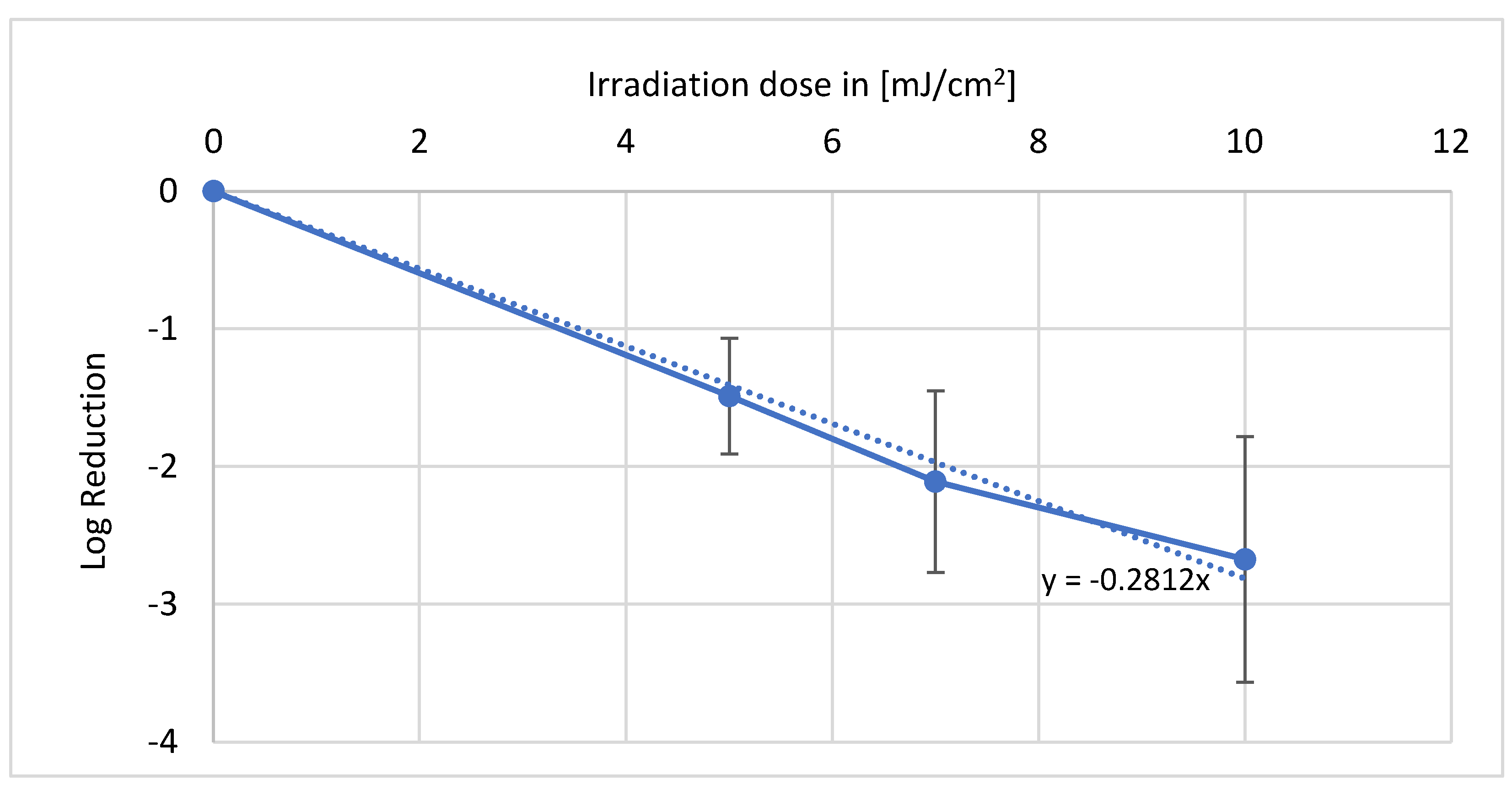
With irradiation in the wavelength range of 222 nm of the living plants, reproducible data could be collected and evaluated. In
Figure 3, it can be observed that a dose of 8 mJ/cm
2 led to a log reduction of 1.35. A dose of 15 mJ/cm
2 resulted in a log reduction of 2.01. At the highest dose of 25 mJ/cm
2, the irradiation resulted in a log reduction of 3.12, which corresponds to a disinfection of more than 99.9% of the bacteria. In summary, a single log reduction was achieved with 7.6 mJ/cm
2.
With 254 nm, an applied dose of 5 mJ/cm
2 resulted in a log reduction of 1.49 as depicted in
Figure 4. Increasing the dose from 2 mJ/cm
2 to 7 mJ/cm
2 led to the inactivation of 99% (log level 2.11) of the bacteria. With a longer irradiation duration, and thus increasing dose to a maximum of 10 mJ/cm
2, a further decrease in bacteria to a log reduction of 2.67 could be achieved. This results in an average log reduction of 3.6 mJ/cm
2.
4. Discussion
For the 222 nm and 254 nm irradiation experiments, bacterial reduction was successfully performed on living plants (Cichorium intybus var. foliosum). Finally, a mean log reduction of around 7.6 mJ/cm2 was obtained for these experiments at 222 nm. In comparison, the 254 nm log reduction dose was 3.6 mJ/cm2. Thus, although it can be observed that slightly higher doses are required overall with 222 nm to achieve the same log level reduction, the values are limited to a few millijoules and thus to a few seconds difference in irradiation, even with suitable irradiation intensity and a constant experimental setup. These results are similar to the irradiation results already obtained with packed, harvested lamb’s lettuce leaves. Here, minimally higher mean doses of 12.7 mJ/cm2 (222 nm) and 5.5 mJ/cm2 (254 nm) were required for a 90% reduction. When comparing the results for the different plants, it becomes apparent that lower doses are sufficient to inactivate E. coli on live chicory plants, more so than on the packaged field salad plants, in order to achieve a comparable effect. At 222 nm, an average log reduction dose of 7.6 mJ/cm2 is required for chicory and 12.7 mJ/cm2 for lamb’s lettuce. This illustrates that the selected irradiation method with the appropriate wavelengths also has an effect even before harvesting and can counteract contamination by polluted irrigation water. The experiments with packaged lettuce have also shown a positive reduction in bacteria. This demonstrates that applications both before and after harvest have a beneficial effect.
Compared to other research, the here presented decontaminating properties of UV-C radiation are in concordance with the results of UV-C irradiation of other microbial contaminated food. For example, the irradiation of strawberries with 222 nm resulted in a reduction in fungal spores such as
Botrytis cinerea [
14]. Irradiation experiments have also been carried out on fruits such as apricots for pathogens such as salmonella. This shows that the decontamination method used has also been successfully applied to other bacteria and foodstuffs, especially fruits [
15]. If one compares the fact that higher doses were required for 222 nm than for 254 nm to achieve the same reduction with other research projects, the results are confirmed. For example, a comparative project dealt with the inactivation sensitivity of 222 nm and 254 nm on escape pathogens. Here, it was also shown that at 222 nm, higher irradiation doses in PBS were necessary to achieve a comparable effect as that of 254 nm [
16]. In general, slightly higher doses were needed on the salads—this is probably due to the uneven surfaces and the associated shading.