Abstract
This study aimed to investigate the antimicrobial effect of cold atmospheric plasma on microbially contaminated liquid films and dried surfaces. Additionally, the contribution of plasma-generated UV radiation to total inactivation was assessed. Experiments were performed using the nearfield module of the relyon piezobrush PZ3 plasma pen on Staphylococcus carnosus, Pseudomonas fluorescens, Streptococcus vestibularis and Candida auris. It was observed that in liquid, there seemed to be no obvious general sensitivity differences between Gram-positive and Gram-negative bacteria. However, all bacteria in liquids were clearly more susceptible to plasma than the yeast. In contrast, there was no significant difference between C. auris and S. carnosus on dried surfaces. The plasma emissions exhibited strong UVA and UVB radiation and only weak emissions in the UVC range. The antimicrobial impacts of the UVA and UVB emissions were negligible. However, an estimation based on two simplifications revealed that the UVC radiation probably contributed considerably to the inactivation properties of the plasma. This might be a special feature of near-field plasma application on thin liquid samples.
1. Introduction
More than 25 years ago, it was first observed that so-called cold or nonthermal plasma can inactivate microorganisms without damaging materials and some biological tissues [1]. This plasma is (partially) ionized gas or air in which free electrons have a high temperature while the heavier gas molecules remain relatively cold. The plasma emits UV radiation and visible light, but the observed antimicrobial effect of cold atmospheric plasma is not attributed to UV radiation but mainly to reactive oxygen and nitrogen species such as O, 1O2, O2−, O3, OH, NO, NO2, … [2,3,4,5]. The reactive species attack cell structures and lead to the death of the cell, and different cells or microorganisms can have different sensitivities. For example, it is assumed that Gram-negative and Gram-positive bacteria differ in their sensitivity and that fungi are generally less sensitive than bacteria [6,7,8,9].
Plasma is applied in the cleaning and disinfection of surfaces and liquids, e.g., in the health and food sector [5,6,7,8,10]. Often, dry surfaces are examined, but liquids are also examined in different volumes.
In the study presented here, the inactivation of Gram-positive and Gram-negative bacteria is investigated in comparison to a yeast. Surrogates of significant pathogens are selected for this purpose. The specimens are microorganisms in small liquid volumes with defined thickness, such as liquid layers on wounds or washed hands or food, which are compared to dried microorganisms. In addition, the importance of UV emissions from plasma is assessed. Therefore, microorganisms whose UV sensitivity is known are selected.
2. Materials and Methods
2.1. Microbiology
For the inactivation experiments, different microorganisms were cultivated. The yeast Candida auris was selected as a representative fungus. C. auris was cultivated in liquid YEPG (yeast extract peptone glucose) and on M129 agar plates. As a Gram-negative bacterium, Pseudomonas fluorescens (DSM 4358) was chosen, and as Gram-positive bacteria, Staphylococcus carnosus (DSM 20501) and Streptococcus vestibularis (DSM 5636) were chosen. P. fluorescens was cultivated in M535, S. carnosus was cultivated in M92 and S. vestibularis was cultivated in a brain heart infusion (BHI) medium at a temperature of 37 °C, with the exception of the 30 °C cultivation of P. fluorescens. Descriptions of all media can be found in [11].
After reaching the mid-exponential phase of the cultivation procedure, samples were taken from each culture and centrifuged at 7000× rpm for 5 min. The resulting pellet was then resuspended with PBS (phosphate-buffered saline), and the centrifugation process was repeated. The washed samples were diluted until a population density of 8 × 106 to 1 × 108 colony-forming units per ml (CFU/mL) was reached.
2.2. Plasma Source and Sample Treatment
For the inactivation experiments, a piezobrush PZ3 pen from relyon plasma GmbH (Regensburg, Germany) was equipped with its nearfield nozzle, and it generated a cold atmospheric air plasma. The plasma emission was measured using a spectrophotometer, a CAS 140D unit from Instruments Systems (Munich, Germany), by placing the plasma pen and a grounded wire directly in front of the aperture of an integrating sphere, as illustrated in Figure 1. The distance between the pen and wire was about 2 mm. Together with published log-reduction doses and an antimicrobial action spectrum, the plasma emission was used to assess the UV contribution to the total antimicrobial impact of the plasma.
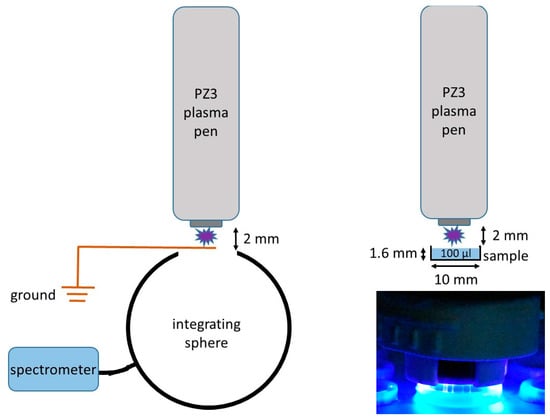
Figure 1.
Left: schematic setup of the measurement of the plasma emission. Right: schematic setup of the plasma treatment of samples in 10 mm wells. Both are unscaled representations! Right bottom: photograph of the plasma-treated sample.
For the plasma treatment of small liquid volumes with defined thickness, an array of wells with a diameter of 10 mm and a height of 2 mm was printed in resin using BioMed Clear Resin from Formlabs (Berlin, Germany). Samples with a volume of 100 µL (sample height: 1.6 mm) could be placed in it beforehand. To perform suspension experiments, the liquid samples were treated directly with cold plasma. To perform experiments with a contaminated dry surface, the suspension was dried for three hours prior to the plasma treatment.
The plasma was applied for periods of up to 7 min for liquid samples and up to 2 min for dried ones. For liquid samples, the temperature was measured using an infrared thermometer, a Raynger MX from Raytek (Berlin, Deutschland). Ozone and pH were measured using Water Test test strips from Tytlyworth (unknown origin) and Dosatest pH test strips for a pH of 4.5–10.0 from VWR (Darmstadt, Germany), respectively.
The respective liquid samples were directly removed after the plasma treatment and spread on agar plates in different dilutions. For the recovery of the dried sample, 100 µL of PBS was added to the well containing the dried microorganisms. Then, the sample volume was agitated with a pipette tip for 15 s before the sample was removed and plated out again. Incubation was carried out at the same temperature as during cultivation, and after 48 h, the grown colonies were counted and evaluated. The determined log reduction values referred to a starting value which was an untreated sample that was filled in a well and recovered as all the plasma-treated samples were.
3. Results
The experiments revealed that all microorganisms (liquid and dried samples) were reduced by several orders of magnitude within a few minutes. The plasma application durations required for a log reduction can be found in Table 1. In the semi-logarithmic representation in Figure 2, the measured data lie more or less on a straight line, which is indicative of an approximate exponential reduction in the microorganisms. The measured physical and chemical properties in the liquid samples are listed in Table 2 as functions of time. They are not assumed to have a large influence on the bacteria and yeast reductions.

Table 1.
Plasma log reduction times for different microorganisms and conditions.

Figure 2.
Log change for the microbial concentrations for the different microorganisms and conditions as functions of time and fitted trend lines.

Table 2.
Ozone concentration, pH and temperature in liquid samples as functions of plasma application duration.
Figure 3 gives the short-wavelength plasma emission spectrum. In the UV region, UVA (315–400 nm) is the strongest with 28.4 µW, followed by UVB (280–315 nm) with 7.0 µW and UVC with 5.4 µW. However, if the antimicrobial action spectrum [12] is considered, the photoinactivation impact of the UVC emissions is about 11x times higher than that of the UVB emissions and 111x times higher than that of the plasma’s UVA emissions. Therefore, UVB and UVA did not play a significant role in the antimicrobial impact of the plasma.
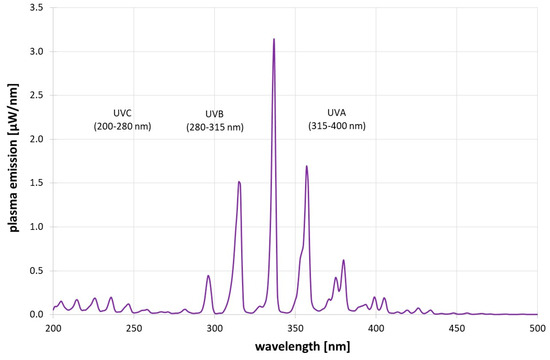
Figure 3.
Measured short-wavelength plasma emissions.
To assess the contribution of the plasma’s UVC emissions, the average UVC irradiation intensity inside the 10 mm well was determined to be 6.9 µW/cm2. The UVC doses applied during the plasma log-reduction time (90% reduction) are given in Table 3 together with known UVC (254 nm) log-reduction doses. Also given are the microbial reductions that would be expected due to UVC application only, with the simplification that UVC photosensitivities can be approximated using published 254 nm values.

Table 3.
Applied UVC doses during plasma log-reduction times (see Table 1) for different microorganisms, published UVC (254 nm) log-reduction doses, and assumed reductions based only on UVC irradiation.
4. Discussion
The cold atmospheric plasma from the piezobrush PZ3 reduced all three bacteria and the yeast C. auris within minutes by orders of magnitude, and the decreases appear to be approximately exponential. A three-phase behavior, as suggested in [3,5], was not observed. In the liquid samples, the yeast was clearly more resistant than the bacteria, but in the dried state, C. auris and S. carnosus appear to be equally susceptible.
The observed differences between the bacteria in liquids were small. The Gram-negative representative lay between the two Gram-positive bacteria in its sensitivity to plasma. Therefore, in this study, there is no evidence of general differences in plasma sensitivity due to bacterial cell wall structure (Gram +/Gram −).
Reactive oxygen and nitrogen species, which attack cell structures, are usually cited as the most important mechanisms in the antimicrobial action of cold plasma. UV radiation is assumed to play a very minor role at atmospheric pressure. Although this study confirms that UVA and UVB radiation do not cause any relevant inactivation, the UVC emissions from the plasma could account for a large contribution to the antimicrobial effect of the plasma in this particular near-field application on small liquid volumes with defined thickness. However, this quantitative statement is not certain because two assumptions were implied that affected the calculated strength of the UVC effect.
First, for simplicity, it was assumed that the photosensitivity of each microorganism in the spectral range 200–280 nm does not vary substantially from its photosensitivity at 254 nm. Second, the plasma emissions were not measured over the sample wells as in the real application but in a special arrangement in front of the integrating sphere of the spectrometer in combination with a grounded wire. Therefore, the UV emissions in this setup might have been higher than in inactivation experiments. On the other hand, the UV intensities of the piezobrush PZ3 with a near-field nozzle measured herein seem to be in the range of the results of Timmermann et al. [9]. This statement is based on the UVA and UVB emissions only as Timmermann et al. published no data below 280 nm.
Both assumptions may lead to an overestimation of UVC’s influence, but the error will probably not be of an order of magnitude and it can thus be assumed that UVC emissions from plasma contribute clearly to the antimicrobial effect of plasma, at least under these particular conditions.
Author Contributions
Conceptualization, A.-M.G., C.L. and M.H.; methodology, A.-M.G., C.L. and M.H.; software, A.-M.G.; validation, A.-M.G. and M.H.; formal analysis, A.-M.G.; investigation, A.-M.G. and M.H.; resources, C.L. and M.H.; writing—original draft preparation, A.-M.G. and M.H.; writing—review and editing, A.-M.G., C.L. and M.H.; visualization, A.-M.G.; supervision, C.L. and M.H.; project administration, M.H.; funding acquisition, C.L. and M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are available upon reasonable request.
Acknowledgments
We are grateful for instrumental support from J. Moisel and C. Dietrich (THU).
Conflicts of Interest
The authors declare that they have no known competing financial interests.
References
- Laroussi, M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans. Plasma Sci. 1996, 24, 1188–1191. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Machala, Z.; Chládeková, L.; Pelach, M. Plasma agents in bio-decontamination by dc discharges in atmospheric air. J. Phys. D Appl. Phys. 2010, 43, 222001. [Google Scholar] [CrossRef]
- de Geyter, N.; Morent, R. Nonthermal plasma sterilization of living and nonliving surfaces. Annu. Rev. Biomed. Eng. 2012, 14, 255–274. [Google Scholar] [CrossRef] [PubMed]
- Laroque, D.A.; Seó, S.T.; Valencia, G.A.; Laurindo, J.B.; Carciofi, B.A.M. Cold plasma in food processing: Design, mechanisms, and application. J. Food Eng. 2022, 312, 110748. [Google Scholar] [CrossRef]
- Niveditha, A.; Pandiselvam, R.; Prasath, V.A.; Singh, S.K.; Gul, K.; Kothakota, A. Application of cold plasma and ozone technology for decontamination of Escherichia coli in foods—A review. Food Control 2021, 130, 108338. [Google Scholar] [CrossRef]
- Katsigiannis, A.S.; Bayliss, D.L.; Walsh, J.L. Cold plasma for the disinfection of industrial food-contact surfaces: An overview of current status and opportunities. Compr. Rev. Food Sci. Food Saf. 2022, 21, 1086–1124. [Google Scholar] [CrossRef] [PubMed]
- Timmermann, E.; Bansemer, R.; Gerling, T.; Hahn, V.; Weltmann, K.-D.; Nettesheim, S.; Puff, M. Piezoelectric-driven plasma pen with multiple nozzles used as a medical device: Risk estimation and antimicrobial efficacy. J. Phys. D Appl. Phys. 2021, 54, 25201. [Google Scholar] [CrossRef]
- Laroussi, M. Plasma Medicine: A Brief Introduction. Plasma 2018, 1, 47–60. [Google Scholar] [CrossRef]
- DSMZ-Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH (DSMZ). MediaDive. October. 2020. Available online: https://mediadive.dsmz.de/ (accessed on 17 July 2023).
- DIN 5031-10:2018-03; Strahlungsphysik im Optischen Bereich und Lichttechnik_-Teil_10: Photobiologisch Wirksame Strahlung, Größen, Kurzzeichen und Wirkungsspektren. Beuth Verlag GmbH: Berlin, Germany, 2018. [CrossRef]
- Gierke, A.-M.; Hessling, M. Investigation on Potential ESKAPE Surrogates for 222 and 254 nm Irradiation Experiments. Front. Microbiol. 2022, 13, 942708. [Google Scholar] [CrossRef] [PubMed]
- Bekawi, D.; Gierke, A.-M.; Hasan, S.; Alzyoud, W.; Hessling, M. Investigation on the sensitivity of Streptococcus cristatus and Streptococcus vestibularis towards 222 and 254 nm UVC irradiation. In Proceedings of the 2nd International Electronic Conference on Microbiology, Online, 1–15 December 2023. [Google Scholar]
- Lemons, A.R.; McClelland, T.L.; Martin, S.B.; Lindsley, W.G.; Green, B.J. Inactivation of the multi-drug resistant pathogen Candida auris using ultraviolet germicidal irradiation (UVGI). J. Hosp. Infect. 2020, 105, 495–501. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).