Formulation and Evaluation of Sugarcane-Bagasse-Based Biocontrol Agents for Sustainable Phytopathogen Management †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Isolation of PGPR Strains
2.2. Evaluation of Antagonistic Activity Using Antibiosis Method
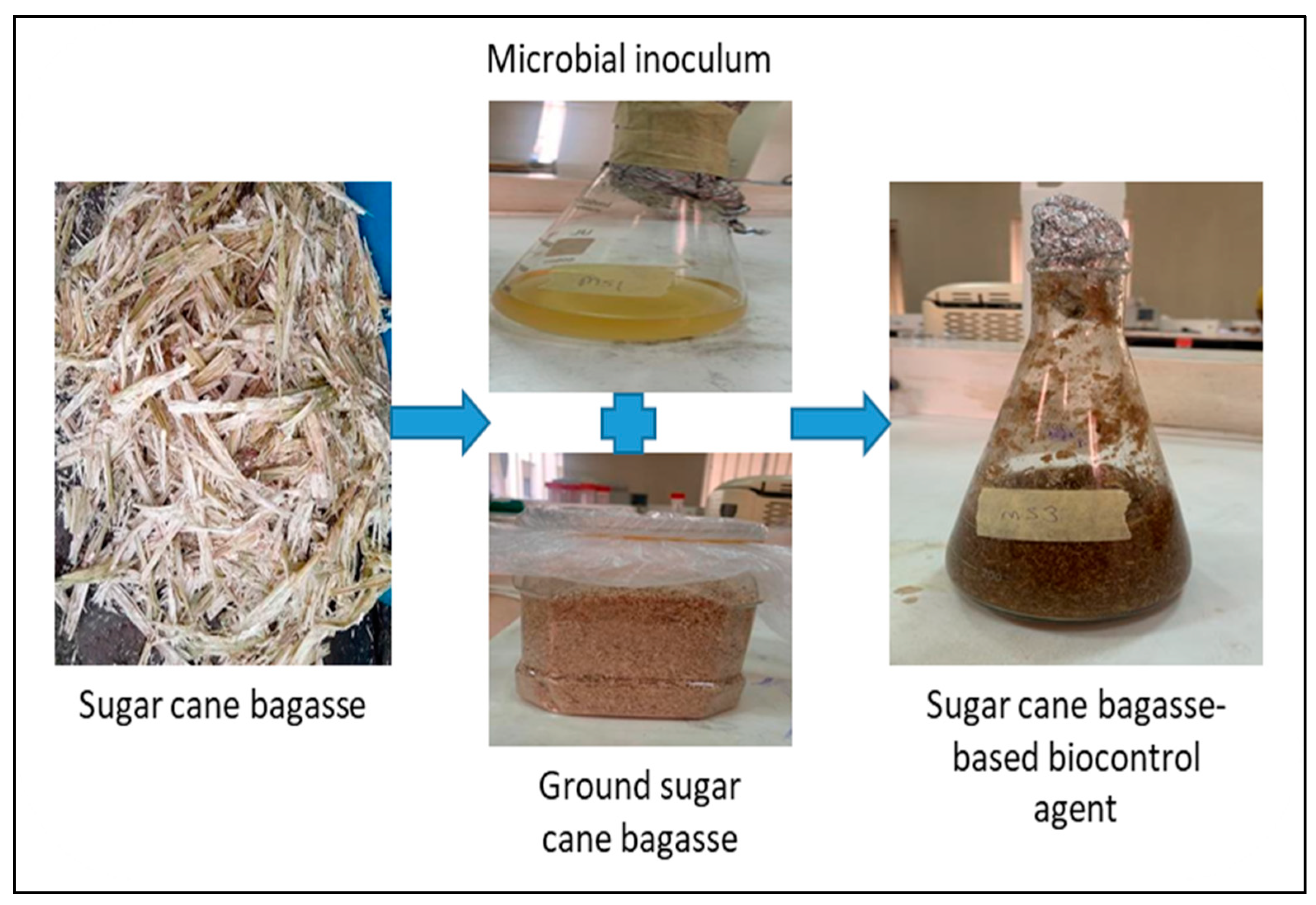
2.3. Inoculum Preparation and Formulation of Sugarcane-Bagasse-Based Biocontrol Agents
2.4. Determination of the Water Absorption and Adhesion Capacity of the Formulant
2.5. Physicochemical Analysis of Soil Sample Prior to Cultivation
2.6. Evaluation of Formulated Biocontrol Agents on Maize Cultivation under Greenhouse Conditions
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ali, S.; Tyagi, A.; Bae, H. Plant Microbiome: An Ocean of Possibilities for Improving Disease Resistance in Plants. Microorganisms 2023, 11, 392. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, V.; Dhanjal, D.S.; Singh, J. Biological Control Agents: Diversity, Ecological Significances, and Biotechnological Applications. In Natural Bioactive Products in Sustainable Agriculture; Singh, J., Yadav, A., Eds.; Springer: Singapore, 2020; pp. 31–44. [Google Scholar] [CrossRef]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and PGPR under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Arseneault, T.; Filion, M. Biocontrol through antibiosis: Exploring the role played by subinhibitory concentrations of antibiotics in soil and their impact on plant pathogens. Can. J. Plant Pathol. 2017, 39, 267–274. [Google Scholar] [CrossRef]
- Dutta, J.; Thakur, D. Evaluation of multifarious plant growth promoting traits, antagonistic potential and phylogenetic affiliation of rhizobacteria associated with commercial tea plants grown in Darjeeling, India. PLoS ONE 2017, 12, e0182302. [Google Scholar] [CrossRef]
- Aloo, B.N.; Mbega, E.R.; Makumba, B.A.; Tumuhairwe, J.B. Effects of carrier materials and storage temperatures on the viability and stability of three biofertilizer inoculants obtained from potato (Solanum tuberosum L.) rhizosphere. Agriculture 2022, 12, 140. [Google Scholar] [CrossRef]
- Raza, Q.U.A.; Bashir, M.A.; Rehim, A.; Sial, M.U.; Ali Raza, H.M.; Atif, H.M.; Geng, Y. Sugarcane industrial byproducts as challenges to environmental safety and their remedies: A review. Water 2021, 13, 3495. [Google Scholar] [CrossRef]
- Verma, P.; Yadav, A.N.; Khannam, K.S.; Kumar, S.; Saxena, A.K.; Suman, A. Molecular diversity and multifarious plant growth promoting attributes of Bacilli associated with wheat (Triticum aestivum L.) rhizosphere from six diverse agro-ecological zones of India. J. Basic Microbiol. 2016, 56, 44–58. [Google Scholar] [CrossRef]
- Sivasakthi, S.; Usharani, G.; Saranraj, P. Biocontrol potentiality of plant growth promoting bacteria (PGPR)-Pseudomonas fluorescens and Bacillus subtilis: A review. Afr. J. Agric. Res. 2014, 9, 1265–1277. [Google Scholar]
- Sellem, I.; Triki, M.A.; Elleuch, L.; Cheffi, M.; Chakchouk, A.; Smaoui, S.; Mellouli, L. The use of newly isolated Streptomyces strain TN258 as a potential biocontrol agent of potato tubers leak caused by Pythium ultimum. J. Basic Microbiol. 2017, 57, 393–401. [Google Scholar] [CrossRef]
- Ehis-Eriakha, C.B.; Willy-Vidona, C.; Akemu, S.E. Isolation and Molecular Characterization of Diazotrophic Bacteria in Arable Soils. Int. J. Innov. Sci. Res. Technol. 2022, 7, 1436–1443. [Google Scholar]
- Riaz, U.; Murtaza, G.; Anum, W.; Samreen, T.; Sarfraz, M.; Nazir, M.Z. Plant Growth-Promoting Rhizobacteria (PGPR) as biofertilizers and biopesticides. Microbiota Biofertil. Sustain. Contin. Plant Soil Health 2021, 181–196. [Google Scholar]
- Ansari, A.A.; Jaikishun, S. Vermicomposting of sugarcane bagasse and rice straw and its impact on the cultivation of Phaseolus vulgaris L. in Guyana, South America. J. Agric. Technol. 2011, 7, 225–234. [Google Scholar]
- Norhasnan, N.H.A.; Hassan, M.Z.; Nor, A.F.M.; Zaki, S.A.; Dolah, R.; Jamaludin, K.R.; Aziz, S.A. Physicomechanical Properties of Rice Husk/Coco Peat Reinforced Acrylonitrile Butadiene Styrene Blend Composites. Polymers 2021, 13, 1171. [Google Scholar] [CrossRef]
- Baliyan, N.; Qureshi, K.A.; Jaremko, M.; Rajput, M.; Singh, M.; Dhiman, S.; Kumar, A. Bioformulation Containing Cohorts of Ensifer adhaerens MSN12 and Bacillus cereus MEN8 for the Nutrient Enhancement of Cicer arietinum L. Plants 2022, 11, 3123. [Google Scholar] [CrossRef] [PubMed]
- Salihu, S.O.; Iyya, Z. Assessment of physicochemical parameters and organochlorine pesticide residues in selected vegetable farmlands soil in Zamfara state, Nigeria. Sci. Prog. Res. (SPR) 2022, 2, 559–566. [Google Scholar]
- Ju, W.; Jin, X.; Lei, L.; Guoting, S.; Wei, Z.; Chengjiao, D. Rhizobacteria inoculation benefits nutrient availability for phytostabilization in copper contaminated soil: Drivers from bacterial community structures in rhizosphere. Appl. Soil Ecol. 2020, 150, 103450. [Google Scholar] [CrossRef]
- Mehmood, U.; Inam-ul-Haq, M.; Saeed, M.; Altaf, A.; Azam, F.; Hayat, S. A brief review on plant growth promoting rhizobacteria (PGPR): A key role in plant growth promotion. Plant Prot. 2018, 2, 77–82. [Google Scholar]
- Liu, K.; Garrett, C.; Fadamiro, H.; Kloepper, J.W. Antagonism of black rot in cabbage by mixtures of plant growth-promoting rhizobacteria (PGPR). BioControl 2016, 61, 605–613. [Google Scholar] [CrossRef]
- Ghnaya, T.; Mnassri, M.; Ghabriche, R.; Wali, M.; Poschenrieder, C.; Lutts, S.; Abdelly, C. Nodulation by Sinorhizobium meliloti originated from a mining soil alleviates Cd toxicity and increases Cd-phytoextraction in Medicago sativa L. Front. Plant Sci. 2015, 6, 863. [Google Scholar] [CrossRef]
- Rana, A.; Saharan, B.; Nain, L.; Prasanna, R.; Shivay, Y.S. Enhancing micronutrient uptake and yield of wheat through bacterial PGPR consortia. Soil Sci. Plant Nutr. 2012, 58, 573–582. [Google Scholar] [CrossRef]
- El-Baky, N.A.; Amara, A.A.A.F. Recent approaches towards control of fungal diseases in plants: An updated review. J. Fungi 2021, 7, 900. [Google Scholar] [CrossRef] [PubMed]
- Hassan, T.U.; Bano, A.; Naz, I. Alleviation of heavy metals toxicity by the application of plant growth promoting rhizobacteria and effects on wheat grown in saline sodic field. Int. J. Phytoremed. 2017, 19, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Detraksa, J. Evaluation of plant growth-promoting streptomyces sp. SR13-2 immobilized with sugarcane bagasse and filter cake for promoting rice growth. Food Appl. Biosci. J. 2020, 8, 1–13. [Google Scholar]

| Samples | Experiment |
|---|---|
| POT 1 | 2 kg soil + 30 g ZEEMYC + phytopathogen + healthy seed |
| POT 2 | 2 kg soil + 30 g ZEEPAS + phytopathogen + healthy seed |
| POT 3 | 2 kg soil + 30 g ZEEBAC + phytopathogen + healthy seed |
| POT 4 (Control A) | 2 kg soil + healthy seed |
| POT 5 (Control B) | 2 kg soil + diseased seed (healthy seed impregnated with phytopathogen) |
| Isolates | Zone of Inhibition | Antibiosis Activity |
|---|---|---|
| MS1 | 3.5 mm | + |
| MS3 | 5 mm | + |
| CS2 | 3 mm | + |
| Isolate | Cultural Properties | Gram Stain | Shape | Oxidase | Catalase | H2S | Citrate | Urease | Indole | Glucose | Sucrose | Lactose | Maltose | Fructose | Tentative Identity of Isolates |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS 1 | Round, Cream, Raised, Smooth. Entire, Opaque Dry, Small. | - | Rod | + | + | - | + | + | - | + | - | - | + | - | Mycobacterium spp. |
| MS 3 | Round, Yellowish-green, Flat, Smooth, Entire, opaque, Dry, Small | - | Rod | + | + | + | - | - | + | + | - | - | + | + | Pseudomonas spp. |
| CS 2 | Round, Cream, Raised. Smooth, Entire, Opaque Dry, Large. | + | Rod | - | + | + | - | + | - | + | + | - | + | + | Bacillus spp. |
| Inoculant | Water Capacity |
|---|---|
| ZEEMYC | 9.9 g |
| ZEEPAS | 6.9 g |
| ZEEBAC | 8.9 g |
| S/N | Parameter | Mean Value |
|---|---|---|
| 1 | pH | 7.785 |
| 2 | Temperature (°C) | 25.80 |
| 3 | Conductivity (uscm) | 85.15 |
| 4 | Moisture Content (%) | 12.23 |
| 5 | Color | Brownish/Ditto |
| 6 | Phosphorus (mg kg−1) | 29.23 |
| 7 | Nitrate (mg kg−1) | 16.52 |
| 8 | Organic Carbon (%) | 0.32 |
| 9 | Organic Matter (%) | 0.56 |
| 10 | Nickel (mg kg−1) | 0.18 |
| 11 | Zinc (mg kg−1) | 0.44 |
| 12 | Lead (mg kg−1) | 0.098 |
| Days | Growth Parameters | ZEEPAS (cm) | ZEEBAC (cm) | ZEEMYC (cm) | Control A (cm) | Control B |
|---|---|---|---|---|---|---|
| Day 7 | Plant height | 11.65 ± 0.31 b | 11.97 ± 3.41 c | 10.47 ± 0.81 b | 9.03 ± 1.68 b | No growth |
| Shoot length | 9.80 ± 0.35 b | 9.89 ± 0.19 b | 8.37 ± 2.82 c | 7.93 ± 3.59 b | ||
| Root length | 2.40 ± ± 0.69 b | 1.93 ± 0.12 b | 2.56 ± 0.97 b | 1.54 ± 0.94 b | ||
| Day 14 | Plant height | 16.71 ± 5.70 b | 18.57 ± 2.48 b | 16.77 ± 5.87 b | 14.77 ± 5.02 b | No growth |
| Shoot length | 13.61 ± 5.55 b | 15.40 ± 5.05 b | 15.21 ± 5.01 b | 13.73 ± 5.46 b | ||
| Root length | 3.77 ± 2.13 ab | 3.60 ± 2.43 b | 2.67 ± 1.15 b | 2.07 ± 0.12 b | ||
| Day 28 | Plant height | 43.70 ± 1.60 a | 39.97 ± 0.74 a | 36.77 ± 0.31 a | 38.13 ± 0.35 a | No growth |
| Shoot length | 34.37 ± 1.80 a | 35.40 ± 0.95 a | 31.80 ± 0.30 a | 34.80 ± 1.87 a | ||
| Root length | 6.47 ± 0.60 a | 7.73 ± 0.32 a | 5.27 ± 0.40 a | 5.00 ± 0.10 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehis-Eriakha, C.B.; Akemu, S.E.; Tiamiyu, A. Formulation and Evaluation of Sugarcane-Bagasse-Based Biocontrol Agents for Sustainable Phytopathogen Management. Biol. Life Sci. Forum 2023, 27, 52. https://doi.org/10.3390/IECAG2023-15992
Ehis-Eriakha CB, Akemu SE, Tiamiyu A. Formulation and Evaluation of Sugarcane-Bagasse-Based Biocontrol Agents for Sustainable Phytopathogen Management. Biology and Life Sciences Forum. 2023; 27(1):52. https://doi.org/10.3390/IECAG2023-15992
Chicago/Turabian StyleEhis-Eriakha, Chioma Bertha, Stephen Eromosele Akemu, and Azeeza Tiamiyu. 2023. "Formulation and Evaluation of Sugarcane-Bagasse-Based Biocontrol Agents for Sustainable Phytopathogen Management" Biology and Life Sciences Forum 27, no. 1: 52. https://doi.org/10.3390/IECAG2023-15992
APA StyleEhis-Eriakha, C. B., Akemu, S. E., & Tiamiyu, A. (2023). Formulation and Evaluation of Sugarcane-Bagasse-Based Biocontrol Agents for Sustainable Phytopathogen Management. Biology and Life Sciences Forum, 27(1), 52. https://doi.org/10.3390/IECAG2023-15992





