Molecular Identification of Lactic Acid Producing Bacteria Isolated from Alheira, a Traditional Portuguese Fermented Sausage †
Abstract
1. Introduction
2. Materials and Methods
2.1. Reactivation of Cryopreserved Samples
2.2. DNA Extraction
2.3. 16S rRNA Amplification
2.4. Sanger Sequencing
2.5. Sequence Analysis
2.6. Screening of LAB for Acidifying Capacity, Proteolytic Activity, and Antimicrobial Capacity
2.7. Data Analysis
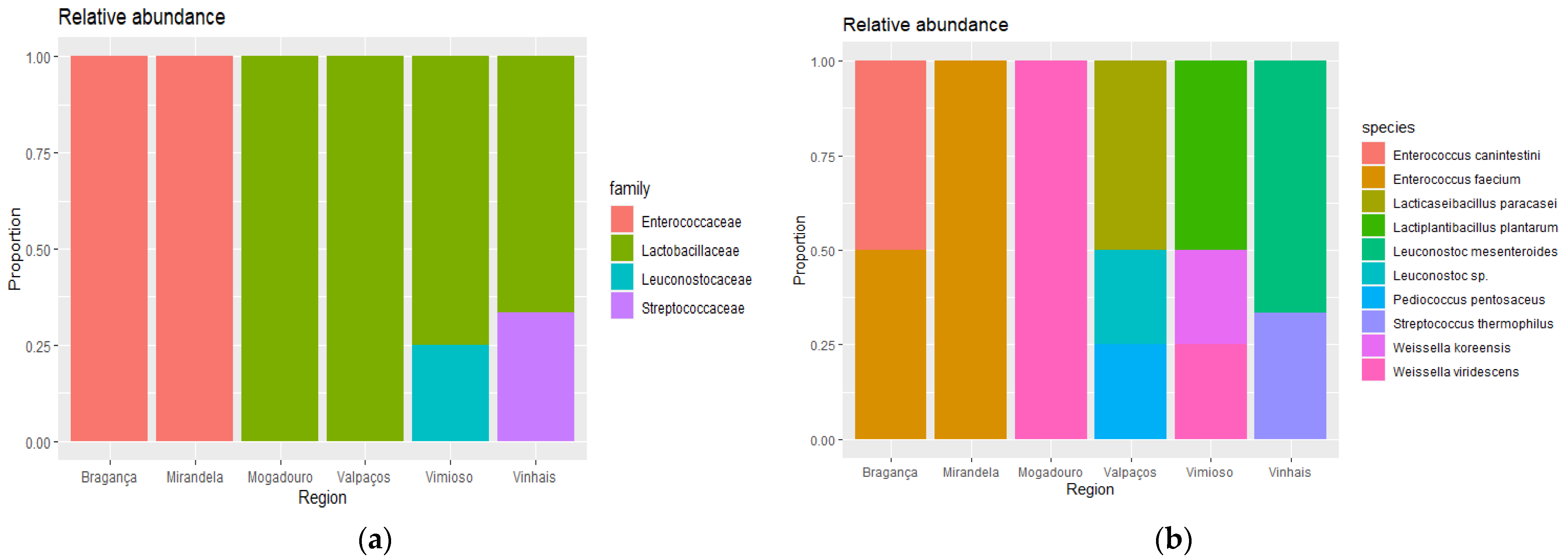
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- CIM-TTM—Comunidade Intermunicipal das Terras de Trás-os-Montes. Guia Prático Capacitação para a Internacionalização Carnes e Enchidos; InovCluster: Bragança, Portugal, 2020; 17p, Available online: https://www.cim-ttm.pt/cimttm/uploads/document/file/233/carnes_e_enchidos.pdf (accessed on 1 July 2022).
- Albano, H.; Henriques, I.; Correia, A.; Hogg, T.; Teixeira, P. Characterization of microbial population of ‘Alheira’ (a traditional Portuguese fermented sausage) by PCR-DGGE and traditional cultural microbiological methods. J. Appl. Microbiol. 2008, 105, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.C.; Lejzerowicz, F.; Poirel, M.; Shaffer, J.P.; Jiang, L.; Aksenov, A.; Litwin, N.; Humphrey, G.; Martino, C.; Miller-Montgomery, S.; et al. Consumption of Fermented Foods Is Associated with Systematic Differences in the Gut Microbiome and Metabolome. mSystems 2020, 5, e00901-19. [Google Scholar] [CrossRef] [PubMed]
- Albano, H.; Todorov, S.D.; van Reenen, C.A.; Hogg, T.; Dicks, L.M.; Teixeira, P. Characterization of two bacteriocins produced by Pediococcus acidilactici isolated from “Alheira”, a fermented sausage traditionally produced in Portugal. Int. J. Food Microbiol. 2007, 116, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Hou, Q.; Bai, X.; Li, W.; Gao, X.; Zhang, F.; Sun, Z.; Zhang, H. Design of Primers for Evaluation of Lactic Acid Bacteria Populations in Complex Biological Samples. Front. Microbiol. 2018, 9, 2045. Available online: https://www.frontiersin.org/articles/10.3389/fmicb.2018.02045 (accessed on 1 July 2022). [PubMed]
- Faria, A.S.; Fernandes, N.; Cadavez, V.; Gonzales-Barron, U. Screening of lactic acid bacteria isolated from artisanally produced “Alheira” fermented sausages as potential starter cultures. In Proceedings of the 2nd International Electronic Conference on Foods—“Future Foods and Food Technologies for a Sustainable World”, 15–30 October 2021; MDPI: Basel, Switzerland, 2021. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 1 July 2022).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Toledano, A.; Jordano, R.; López, C.; Medina, L.M. Proteolytic Activity of Lactic Acid Bacteria Strains and Fungal Biota for Potential Use as Starter Cultures in Dry-Cured Ham. J. Food Prot. 2011, 74, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M. D-Lactic Acid as a Metabolite: Toxicology, Diagnosis, and Detection. BioMed Res. Int. 2020, 2020, 3419034. [Google Scholar] [CrossRef] [PubMed]
- Dicks, L.; Mellett, F.; Hoffman, L. Use of bacteriocin-producing starter cultures of Lactobacillus plantarum and Lactobacillus curvatus in production of ostrich meat salami. Meat Sci. 2004, 66, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Endres, C.M.; Castro, M.S.; Trevisol, L.D.; Severo, J.M.; Mann, M.B.; Varela, A.P.M.; Frazzon, A.P.G.; Mayer, F.Q.; Frazzon, J. Molecular characterization of the bacterial communities present in sheep’s milk and cheese produced in South Brazilian Region via 16S rRNA gene metabarcoding sequencing. LWT 2021, 147, 111579. [Google Scholar] [CrossRef]
| Species | Agar Media | Inhibition Diameter (mm) | Proteolytic Action (mm) | pH | Lactic Acid Concentration (g/L) | ||
|---|---|---|---|---|---|---|---|
| Salmonella Typhimurium | Listeria monocytogenes | Staphylococcus aureus | |||||
| Lactiplantibacillus plantarum | MRS | 12.65 | 25.86 | 13.88 | 3.34 | 6.466 | 0.092 |
| Lactiplantibacillus plantarum | MRS | 9.31 | 21.64 | 11.23 | 4.47 | 6.324 | 0.493 |
| Lacticaseibacillus paracasei subsp. paracasei | MRS | 9.62 | 19.66 | 8.14 | 4.29 | 6.498 | 0.611 |
| Lacticaseibacillus paracasei | MRS | 9.98 | 15.65 | 10.78 | 0.00 | 6.496 | 0.610 |
| Leuconostoc sp. | MRS | 10.70 | 17.85 | 9.51 | 3.14 | 6.337 | 0.032 |
| Leuconostoc sp. | MRS | 11.37 | 15.09 | 11.08 | 4.37 | 6.345 | 0.032 |
| Pediococcus pentosaceus | MRS | 10.42 | 18.05 | 7.62 | 4.74 | 6.406 | 0.663 |
| Pediococcus pentosaceus | MRS | 10.87 | 20.72 | 10.76 | 5.48 | 6.388 | 0.029 |
| Lacticaseibacillus paracasei | MRS | 10.38 | 17.38 | 10.25 | 4.81 | 6.412 | 0.646 |
| Lacticaseibacillus paracasei | MRS | 9.11 | 18.54 | 10.46 | 0.00 | 5.837 | 0.056 |
| Leuconostoc mesenteroides | MRS | 10.23 | 21.19 | 10.90 | 1.30 | 6.399 | 0.019 |
| Leuconostoc mesenteroides | MRS | 11.53 | 19.42 | 10.21 | 8.26 | 5.916 | 0.031 |
| Enterococcus canintestini | MRS | 10.72 | 17.12 | 9.10 | 2.20 | 5.736 | 0.361 |
| Enterococcus faecium | M17 | 4.35 | 11.66 | 5.24 | 1.11 | 5.564 | 0.270 |
| Enterococcus faecium | M17 | 3.43 | 11.79 | 4.49 | 0.00 | 5.591 | 0.499 |
| Enterococcus faecium | M17 | 5.13 | 11.25 | 5.20 | 0.00 | 5.525 | 0.201 |
| Enterococcus faecium | M17 | 6.13 | 7.55 | 5.56 | 0.00 | 5.618 | 0.282 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, N.; Faria, A.S.; Carvalho, L.; Choupina, A.; Rodrigues, C.; Cadavez, V.; Gonzales-Barron, U. Molecular Identification of Lactic Acid Producing Bacteria Isolated from Alheira, a Traditional Portuguese Fermented Sausage. Biol. Life Sci. Forum 2022, 18, 73. https://doi.org/10.3390/Foods2022-13035
Fernandes N, Faria AS, Carvalho L, Choupina A, Rodrigues C, Cadavez V, Gonzales-Barron U. Molecular Identification of Lactic Acid Producing Bacteria Isolated from Alheira, a Traditional Portuguese Fermented Sausage. Biology and Life Sciences Forum. 2022; 18(1):73. https://doi.org/10.3390/Foods2022-13035
Chicago/Turabian StyleFernandes, Nathália, Ana Sofia Faria, Laís Carvalho, Altino Choupina, Carina Rodrigues, Vasco Cadavez, and Ursula Gonzales-Barron. 2022. "Molecular Identification of Lactic Acid Producing Bacteria Isolated from Alheira, a Traditional Portuguese Fermented Sausage" Biology and Life Sciences Forum 18, no. 1: 73. https://doi.org/10.3390/Foods2022-13035
APA StyleFernandes, N., Faria, A. S., Carvalho, L., Choupina, A., Rodrigues, C., Cadavez, V., & Gonzales-Barron, U. (2022). Molecular Identification of Lactic Acid Producing Bacteria Isolated from Alheira, a Traditional Portuguese Fermented Sausage. Biology and Life Sciences Forum, 18(1), 73. https://doi.org/10.3390/Foods2022-13035








