Infiltration of CsPbI3:EuI2 Perovskites into TiO2 Spongy Layers Deposited by gig-lox Sputtering Processes
Abstract
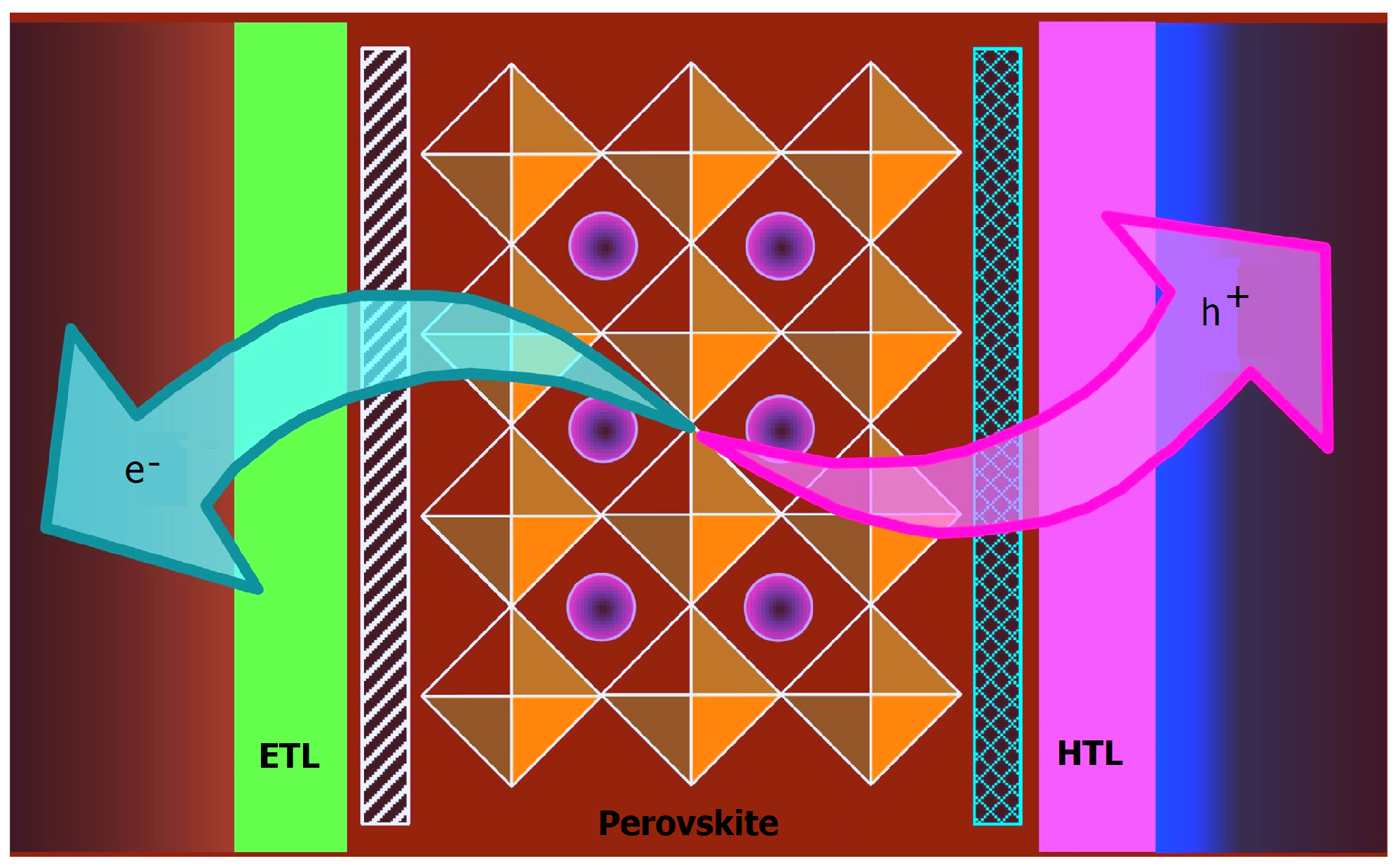
1. Introduction
2. Materials and Methods
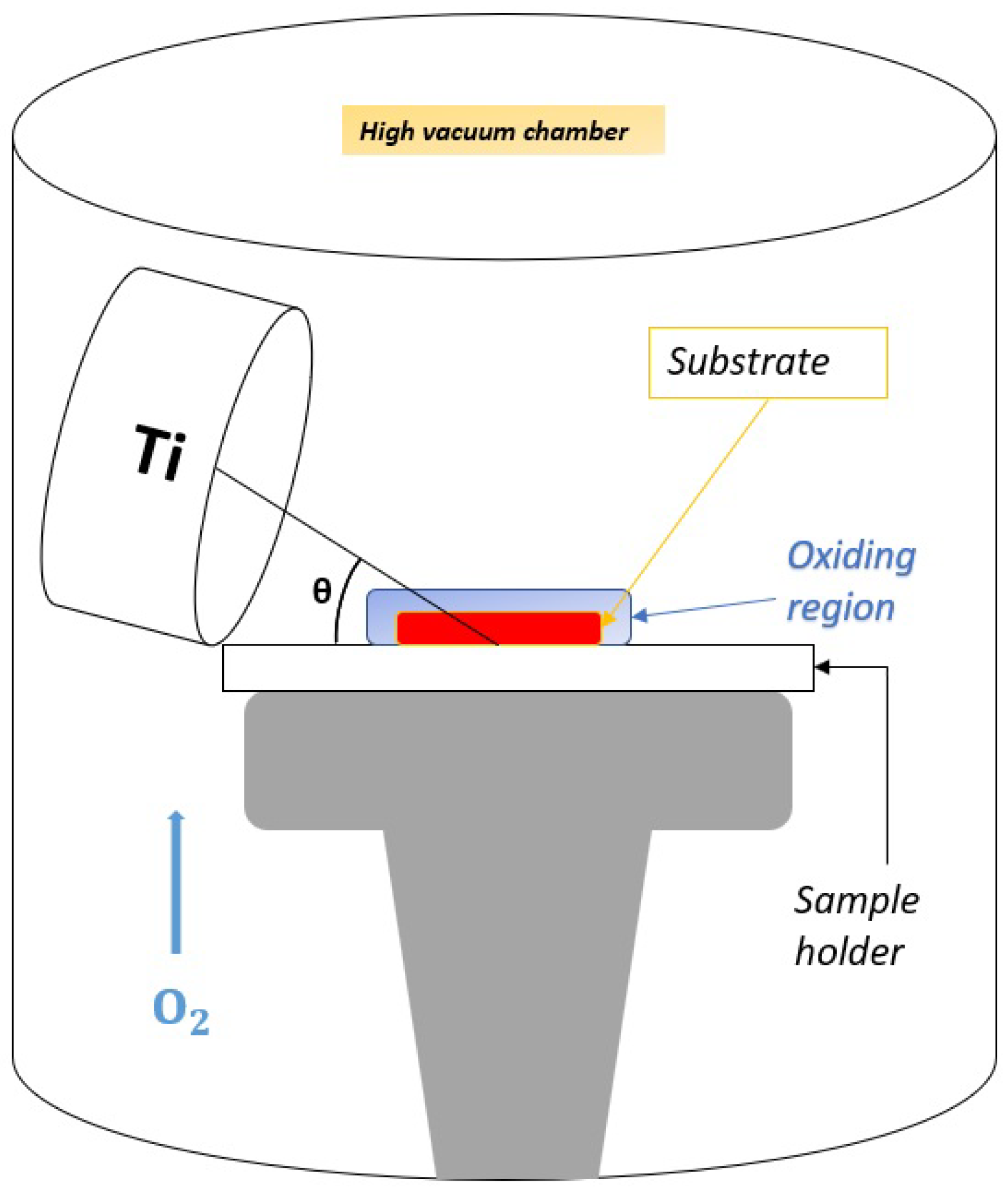
2.1. TiO Deposition
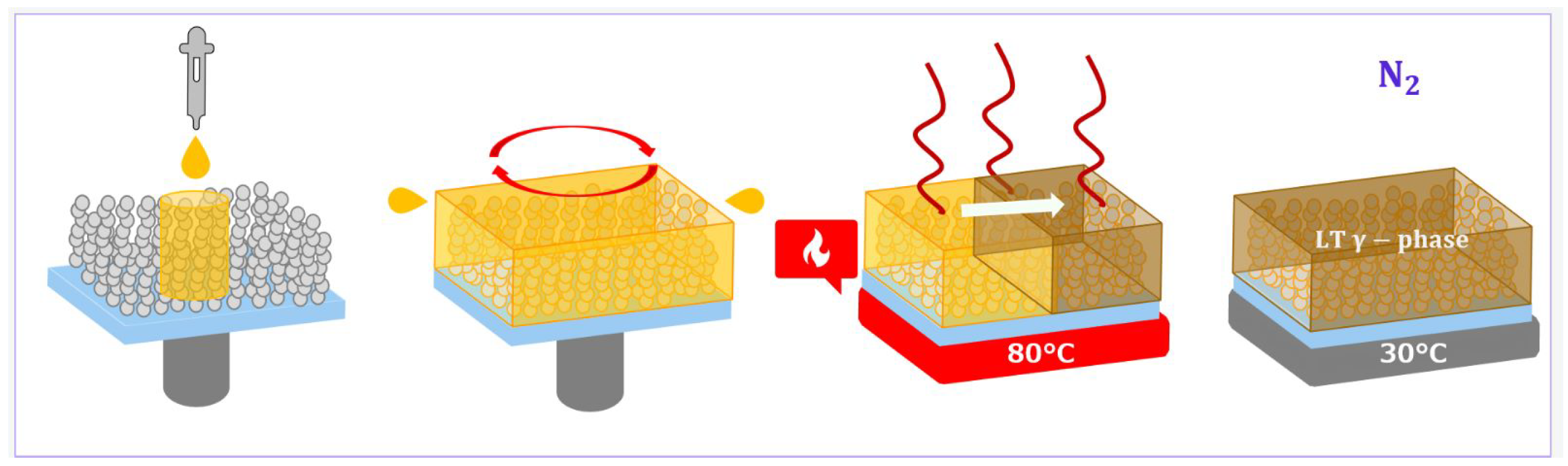
2.2. Perovskite Film Fabrication
2.3. TiO Samples Characterization
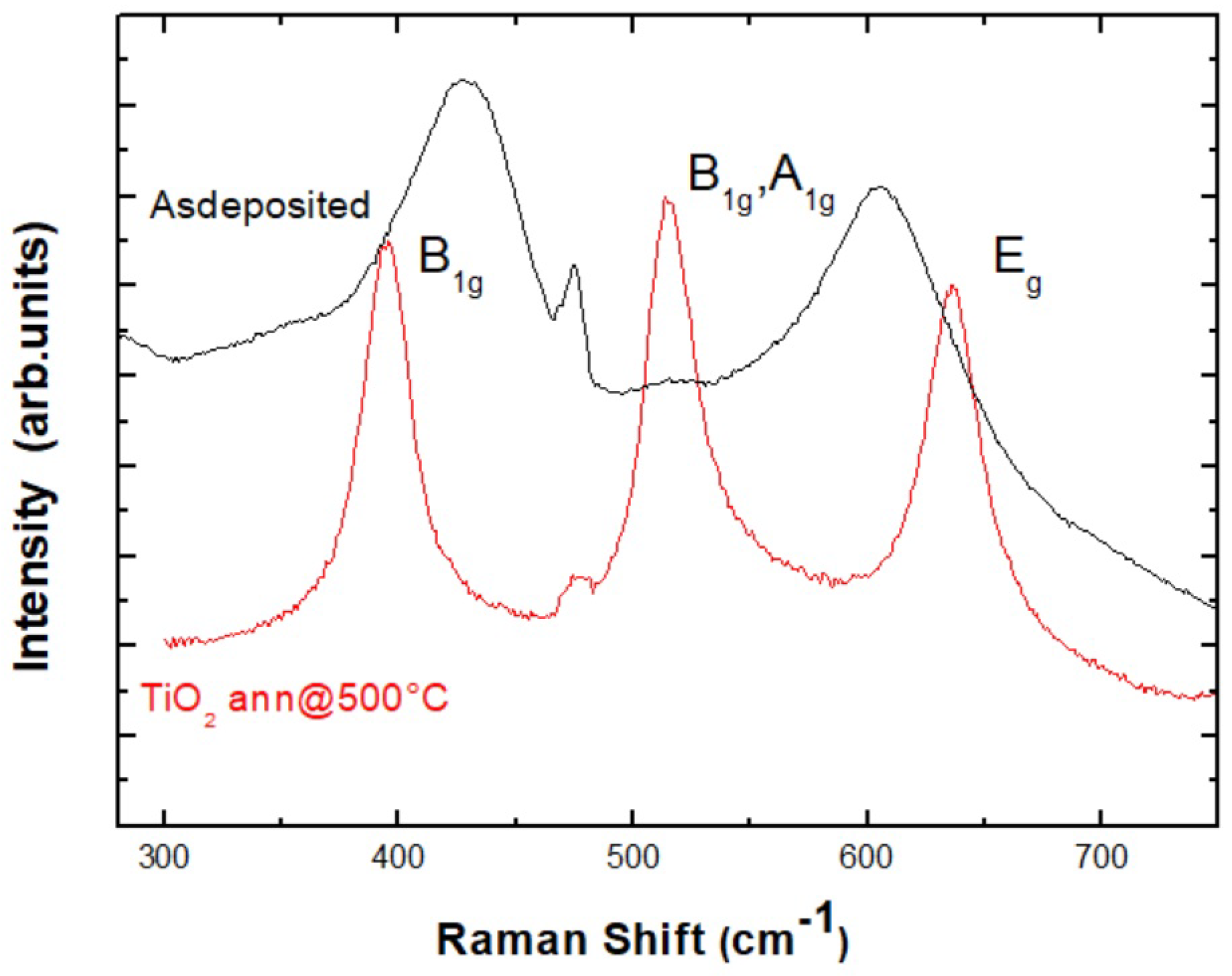
2.3.1. Micro-Raman Analysis
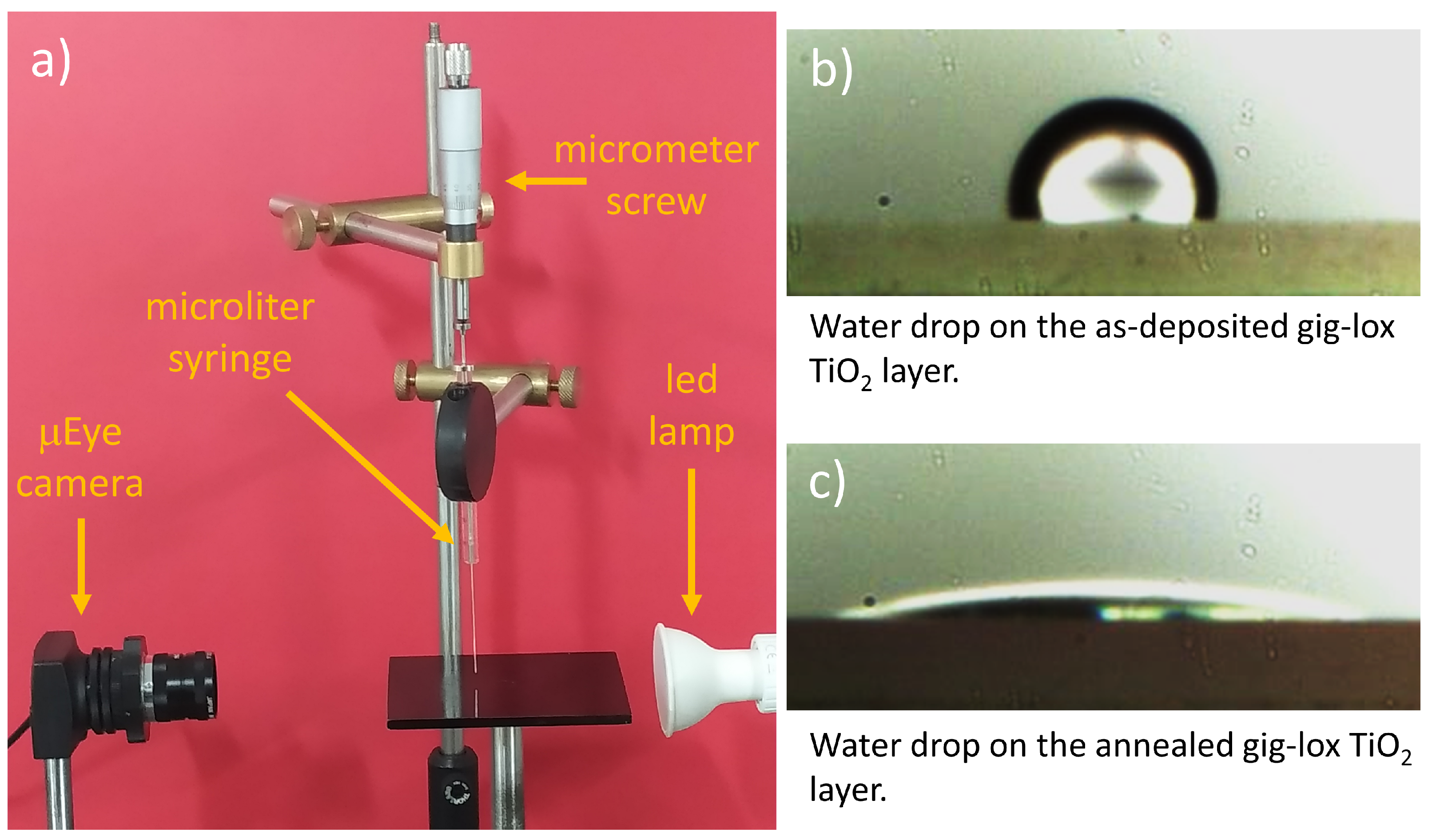
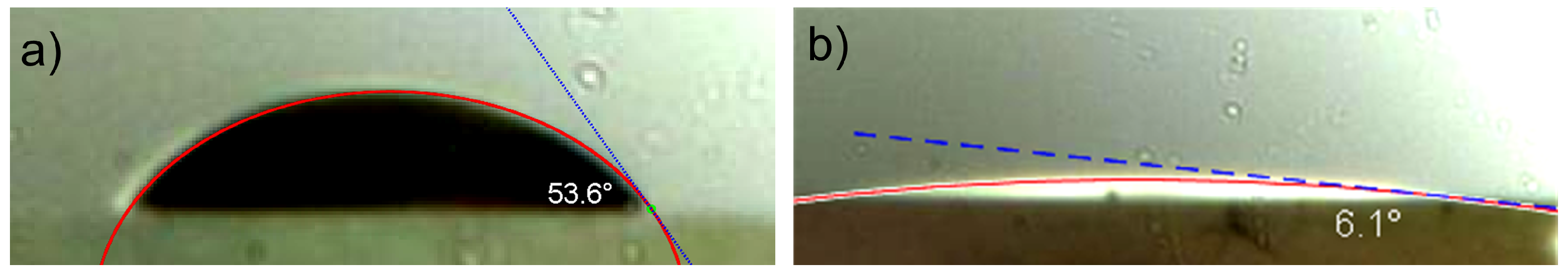
2.3.2. Contact Angle Analysis
2.3.3. Transmission Electron Microscopy Analysis
2.3.4. X-ray Diffraction Analysis
2.3.5. Spectroscopic Ellipsometry Analysis
2.3.6. Photoluminescence Spectroscopy Analysis
3. Results and Discussion
3.1. Micro-Raman Results
3.2. Contact Angle Results
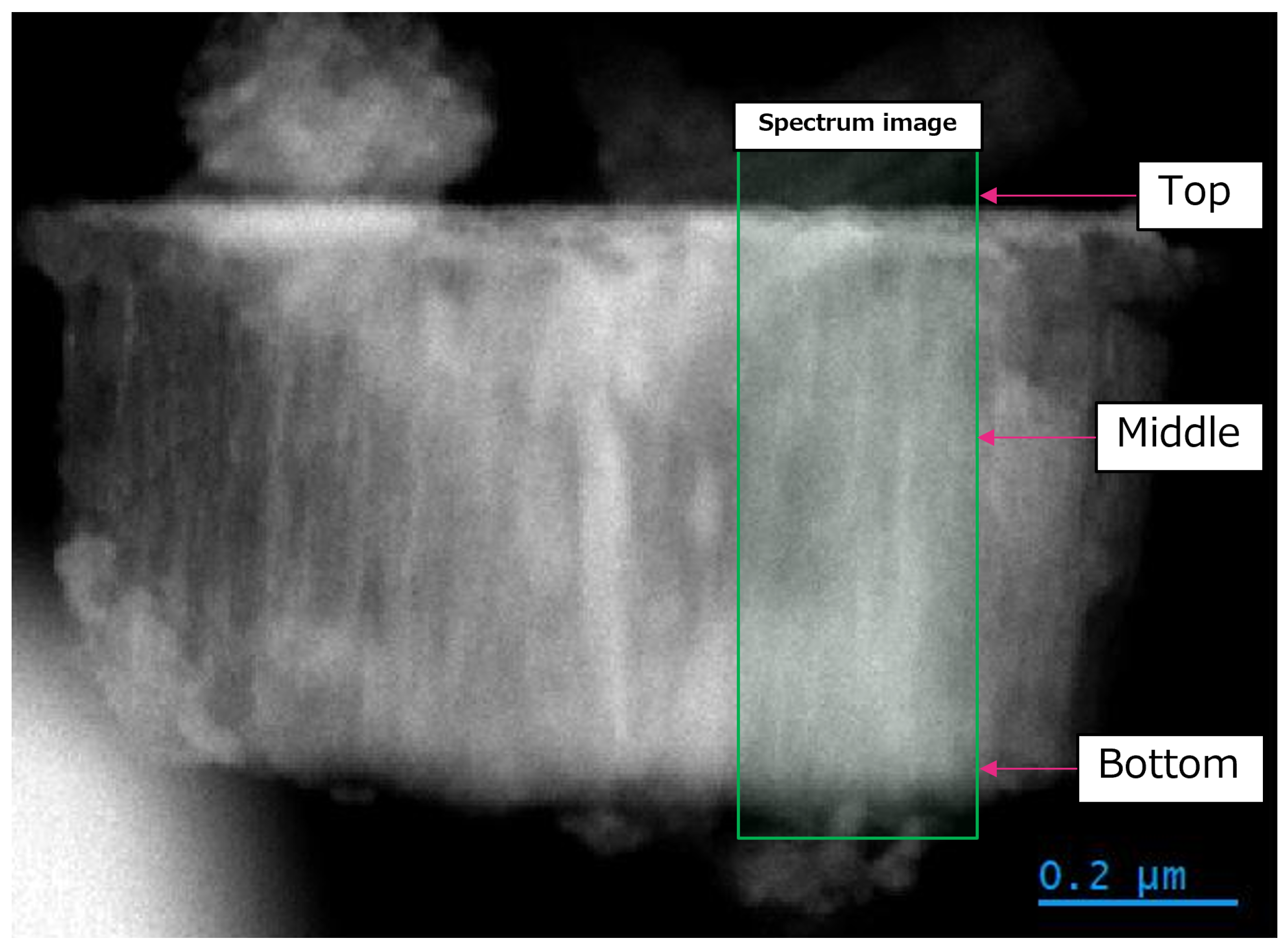
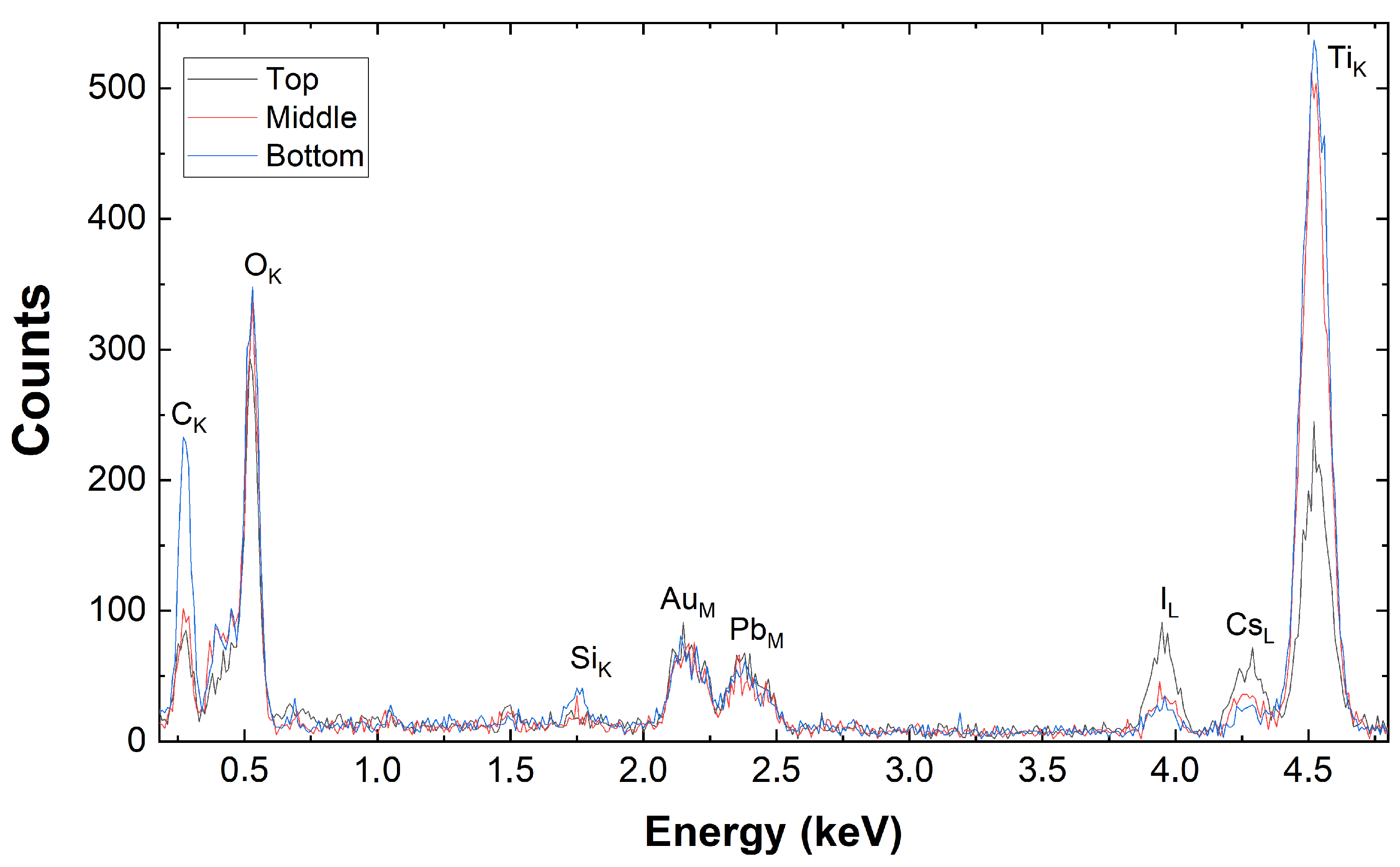
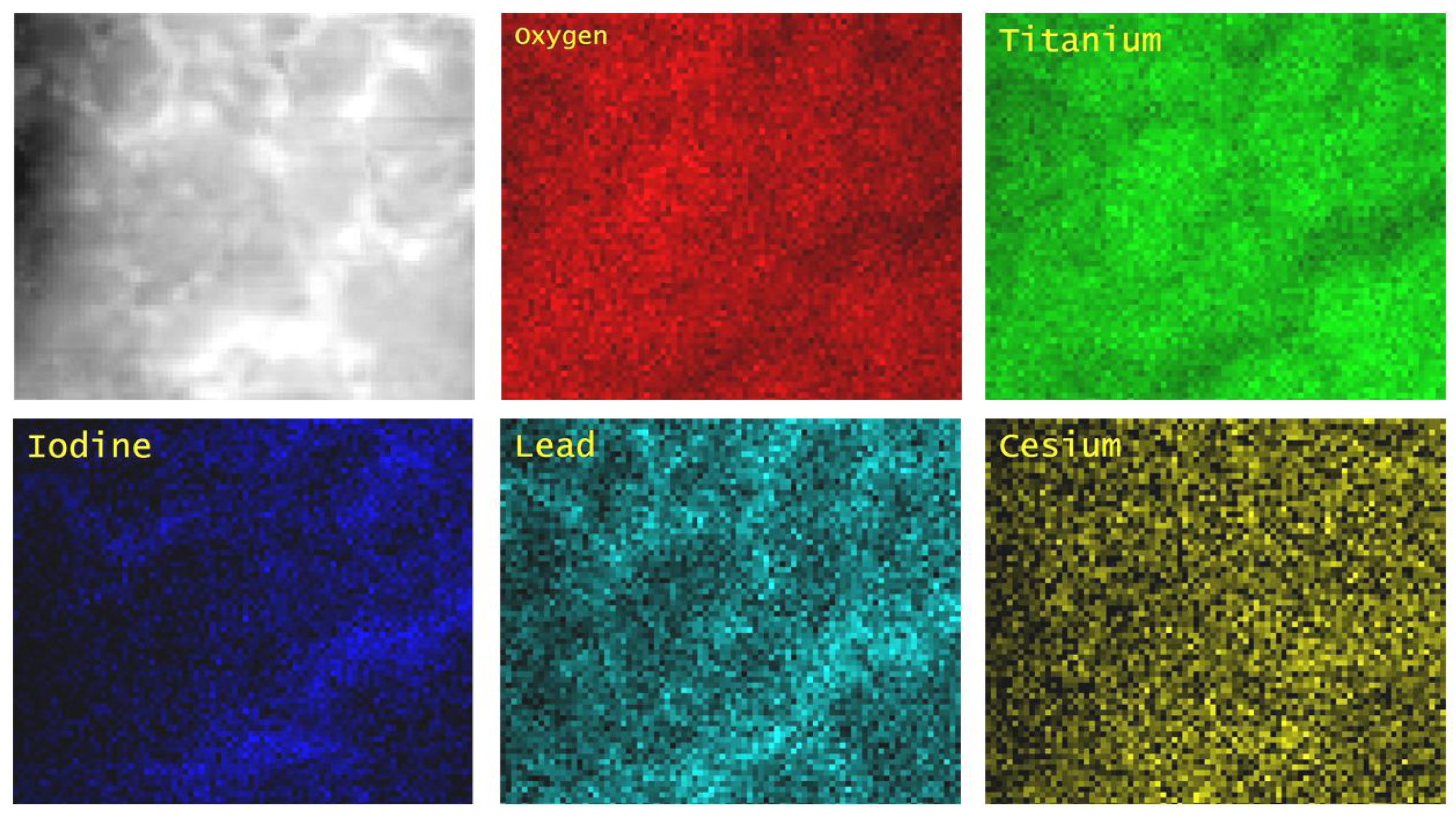
3.3. Transmission Electron Microscopy Results
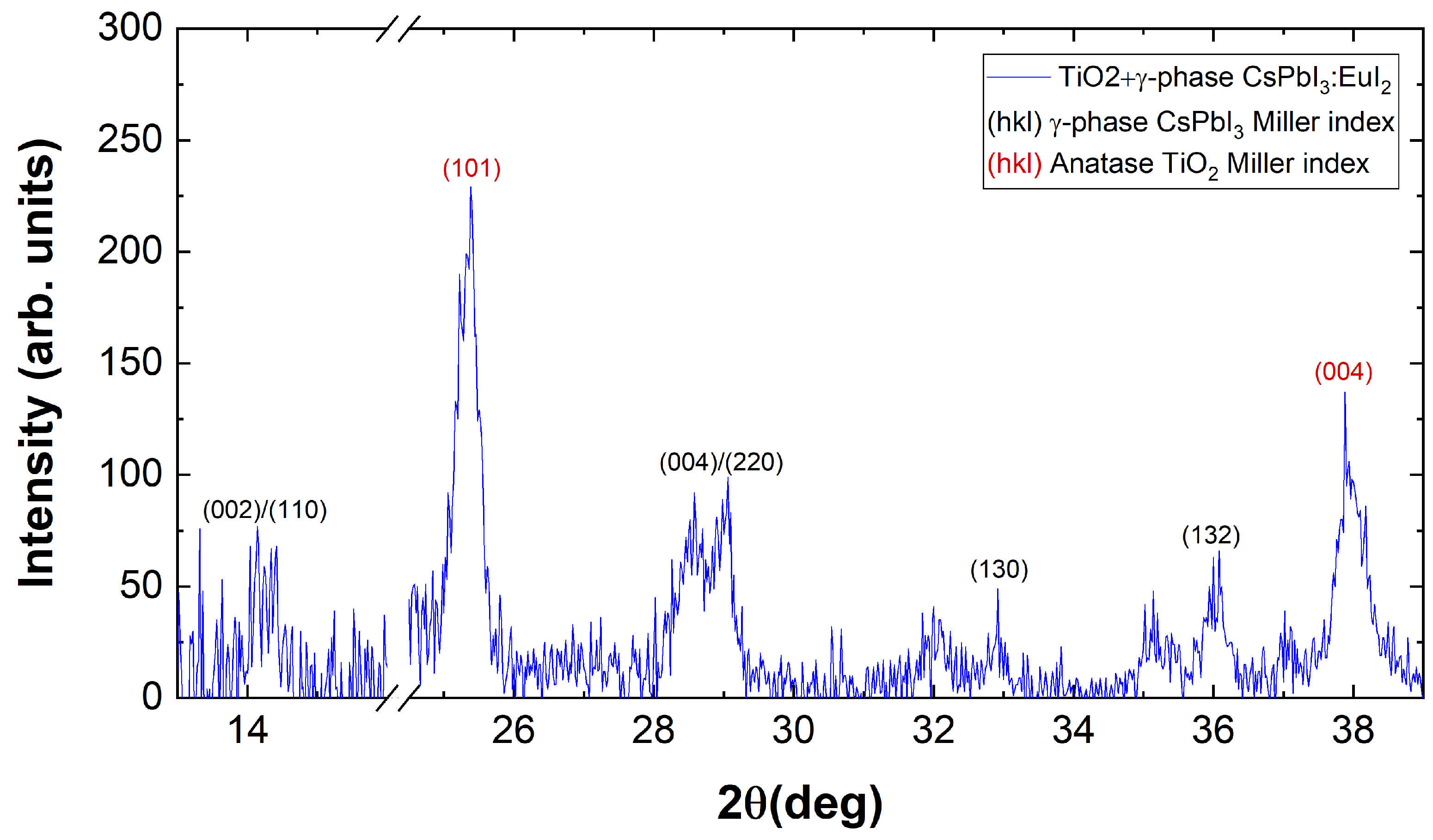
3.4. X-ray Diffraction Results
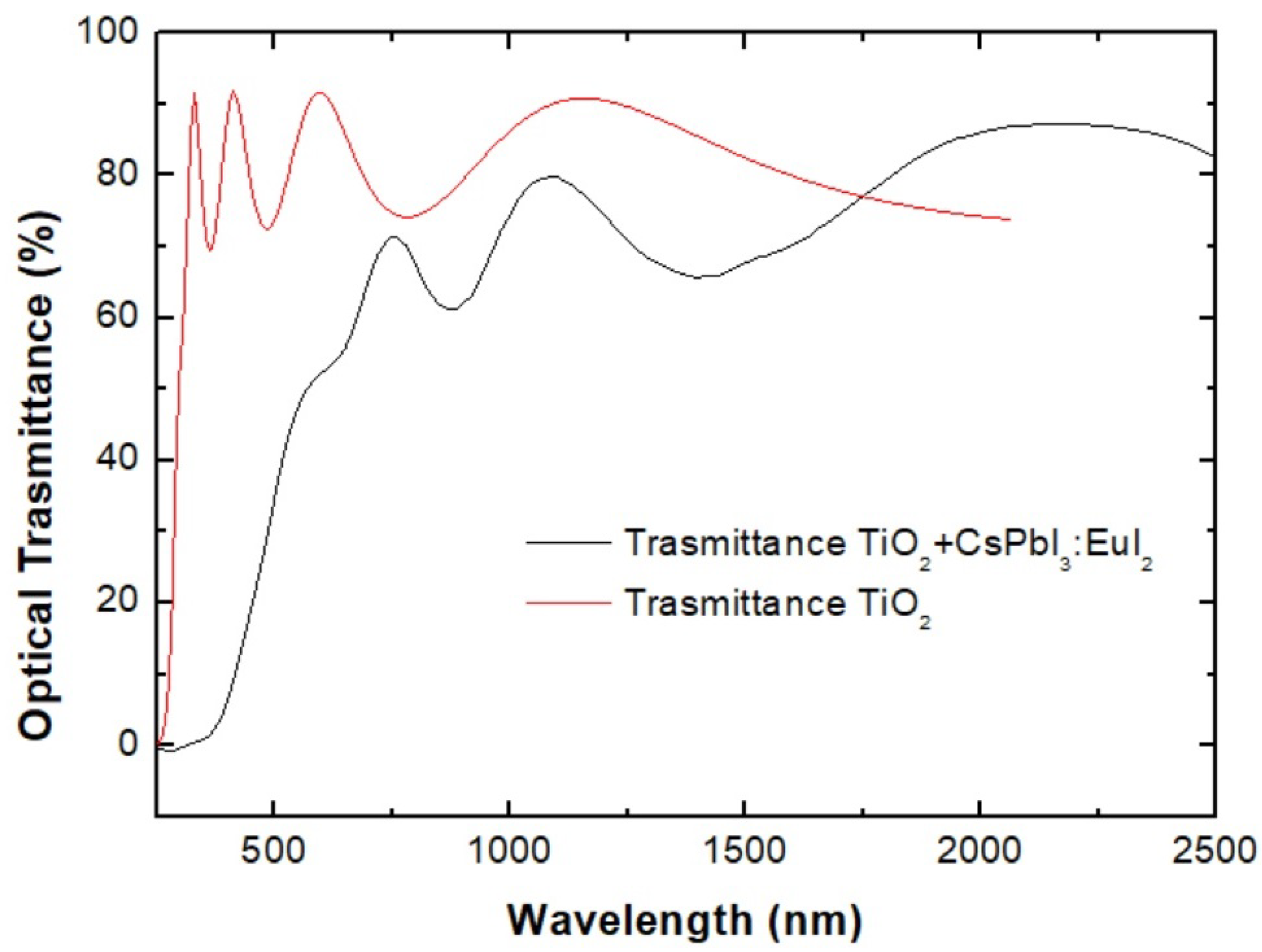
3.5. Spectroscopic Ellipsometry Results
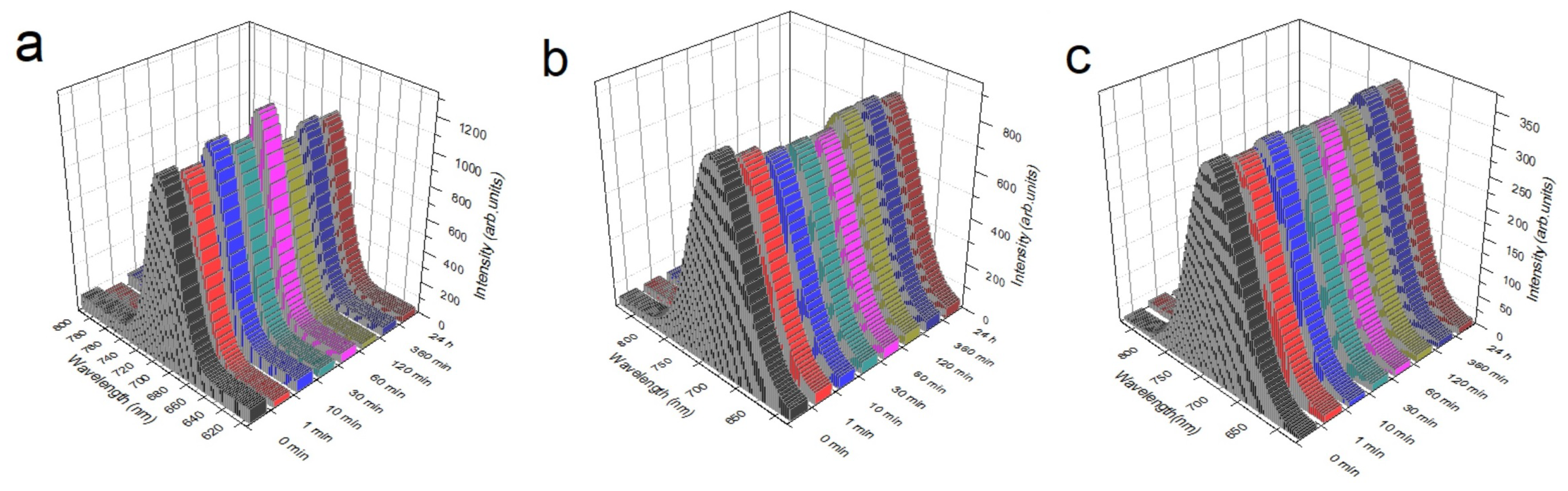
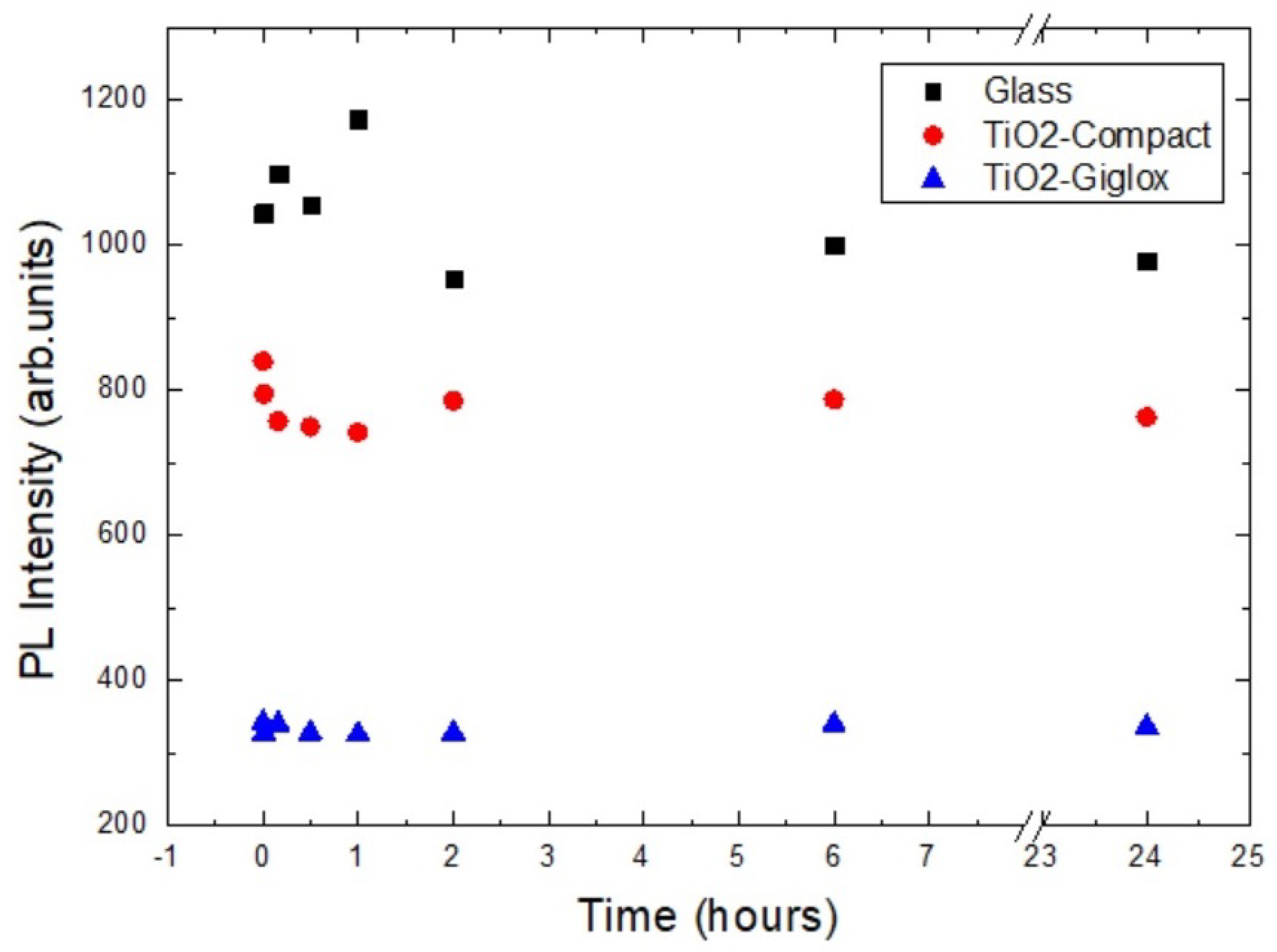
3.6. Photoluminescence Spectroscopy Results
- CsPbI:EuI/glass;
- CsPbI:EuI/TiO-compact/glass;
- CsPbI:EuI/TiO-giglox/glass.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| ETL | Electron-transport layer |
| PSC | Perovskite solar cell |
| TiO | Titanium dioxide |
| TEM | Transmission electron microscopy |
| STEM | Scanning transmission electron microscopy |
| EDX | Energy-dispersive X-ray analysis |
| PL | Photoluminescence spectroscopy |
References
- Werner, J.H. How Much Photovoltaic Efficiency Is Enough? Solar 2022, 2, 215–233. [Google Scholar] [CrossRef]
- Bragagnolo, J.A.; Taretto, K.; Navntoft, C. Solar Energy in Argentina. Solar 2022, 2, 120–140. [Google Scholar] [CrossRef]
- Park, J.; Kim, J.; Yun, H.S.; Paik, M.J.; Noh, E.; Mun, H.J.; Kim, M.G.; Shin, T.J.; Seok, S.I. Controlled growth of perovskite layers with volatile alkylammonium chlorides. Nature 2023, 616, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Tan, Q.; Li, Z.; Luo, G.; Zhang, X.; Che, B.; Chen, G.; Gao, H.; He, D.; Ma, G.; Wang, J.; et al. Inverted perovskite solar cells using dimethylacridine-based dopants. Nature 2023, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Y.; Wang, X.; Lin, R.; Luo, X.; Liu, Z.; Zhou, K.; Xiong, S.; Bao, Q.; Chen, G.; et al. Flexible all-perovskite tandem solar cells approaching 25% efficiency with molecule-bridged hole-selective contact. Nat. Energy 2022, 7, 708–717. [Google Scholar] [CrossRef]
- Min, H.; Lee, D.Y.; Kim, J.; Kim, G.; Lee, K.S.; Kim, J.; Paik, M.J.; Kim, Y.K.; Kim, K.S.; Kim, M.G.; et al. Perovskite solar cells with atomically coherent interlayers on SnO2 electrodes. Nature 2021, 598, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.P.; Cheng, Y.; Yip, H.L.; Zhong, Y.; Fong, P.W.; Li, G.; Ng, A.; Chen, C.; Castriotta, L.A.; Matteocci, F.; et al. Roadmap on Commercialization of Metal Halide Perovskite Photovoltaics. J. Phys. Mater. 2023, 6, 032501. [Google Scholar] [CrossRef]
- Valastro, S.; Smecca, E.; Bongiorno, C.; Spampinato, C.; Mannino, G.; Biagi, S.; Deretzis, I.; Giannazzo, F.; Jena, A.K.; Miyasaka, T.; et al. Out-of-Glovebox Integration of Recyclable Europium-Doped CsPbI3 in Triple-Mesoscopic Carbon-Based Solar Cells Exceeding 9% Efficiency. Solar RRL 2022, 6, 2200267. [Google Scholar] [CrossRef]
- Che Halin, D.S.; Azhari, A.W.; Mohd Salleh, M.A.A.; Muhammad Nadzri, N.I.; Vizureanu, P.; Abdullah, M.M.A.B.; Wahab, J.A.; Sandu, A.V. Metal-Doped TiO2 Thin Film as an Electron Transfer Layer for Perovskite Solar Cells: A Review. Coatings 2022, 13, 4. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Chen, X.; Chen, C.; Chen, P.; Wang, Z.; Duan, Y. Functional metal oxides in perovskite solar cells. ChemPhysChem 2019, 20, 2580–2586. [Google Scholar] [CrossRef]
- Ding, Y.; Ding, B.; Kanda, H.; Usiobo, O.J.; Gallet, T.; Yang, Z.; Liu, Y.; Huang, H.; Sheng, J.; Liu, C.; et al. Single-crystalline TiO2 nanoparticles for stable and efficient perovskite modules. Nat. Nanotechnol. 2022, 17, 598–605. [Google Scholar] [CrossRef]
- Valastro, S.; Smecca, E.; Mannino, G.; Bongiorno, C.; Fisicaro, G.; Goedecker, S.; Arena, V.; Spampinato, C.; Deretzis, I.; Dattilo, S.; et al. Preventing lead leakage in perovskite solar cells with a sustainable titanium dioxide sponge. Nat. Sustain. 2023, 1–10. [Google Scholar] [CrossRef]
- Sanzaro, S.; Smecca, E.; Mannino, G.; Bongiorno, C.; Pellegrino, G.; Neri, F.; Malandrino, G.; Catalano, M.R.; Condorelli, G.G.; Iacobellis, R.; et al. Multi-Scale-Porosity TiO2 scaffolds grown by innovative sputtering methods for high throughput hybrid photovoltaics. Sci. Rep. 2016, 6, 39509. [Google Scholar] [CrossRef]
- Alberti, A.; Smecca, E.; Sanzaro, S.; Bongiorno, C.; Giannazzo, F.; Mannino, G.; La Magna, A.; Liu, M.; Vivo, P.; Listorti, A.; et al. Nanostructured TiO2 grown by low-temperature reactive sputtering for planar perovskite solar cells. ACS Appl. Energy Mater. 2019, 2, 6218–6229. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhuang, J.; Ma, Z.; Lu, H.; Xia, H.; Zhou, W.; Zhang, T.; Li, H. Mixed-phase Mesoporous TiO2 Film for High Efficiency Perovskite Solar Cells. Chem. Res. Chin. Univ. 2019, 35, 101–108. [Google Scholar] [CrossRef]
- Nosonovsky, M.; Ramachandran, R. Geometric interpretation of surface tension equilibrium in superhydrophobic systems. Entropy 2015, 17, 4684–4700. [Google Scholar] [CrossRef]
- Leger, L.; Joanny, J. Liquid spreading. Rep. Prog. Phys. 1992, 55, 431. [Google Scholar] [CrossRef]
- Tadmor, R. Open problems in wetting phenomena: Pinning retention forces. Langmuir 2021, 37, 6357–6372. [Google Scholar] [CrossRef]
- Tauc, J.; Menth, A.; Wood, D. Optical and magnetic investigations of the localized states in semiconducting glasses. Phys. Rev. Lett. 1970, 25, 749. [Google Scholar] [CrossRef]
- Jellison, G., Jr.; Modine, F. Parameterization of the optical functions of amorphous materials in the interband region. Appl. Phys. Lett 1996, 69, 371–373, Erratum in 1996, 69, 2137. [Google Scholar] [CrossRef]
- Capper, P.; Willoughby, A.; Kasap, S.O. Optical Properties of Materials and Their Applications; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Li, C.; Belkin, D.; Li, Y.; Yan, P.; Hu, M.; Ge, N.; Jiang, H.; Montgomery, E.; Lin, P.; Wang, Z.; et al. Efficient and self-adaptive in-situ learning in multilayer memristor neural networks. Nat. Commun. 2018, 9, 2385. [Google Scholar] [CrossRef] [PubMed]
- Sandak, J.; Sandak, A.; Zitek, A.; Hintestoisser, B.; Picchi, G. Development of low-cost portable spectrometers for detection of wood defects. Sensors 2020, 20, 545. [Google Scholar] [CrossRef] [PubMed]
- Muthee, D.K.; Dejene, B.F. Effect of annealing temperature on structural, optical, and photocatalytic properties of titanium dioxide nanoparticles. Heliyon 2021, 7, e07269. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Seok, S.I. Steps toward efficient inorganic–organic hybrid perovskite solar cells. MRS Bull. 2015, 40, 648–653. [Google Scholar] [CrossRef]
- Frontera, P.; Malara, A.; Stelitano, S.; Leonardi, S.G.; Bonavita, A.; Fazio, E.; Antonucci, P.; Neri, G.; Neri, F.; Santangelo, S. Characterisation and H2O2 sensing properties of TiO2-CNTs/Pt electro-catalysts. Mater. Chem. Phys. 2016, 170, 129–137. [Google Scholar] [CrossRef]
- Son, Y.; Lee, M.K.; Park, Y.C. Contact Angle Relaxation on Amorphous, Mixed-Phase (Anatase+ Rutile), and Anatase TiO2 Films and Its Mechanism. Langmuir 2021, 37, 1850–1860. [Google Scholar] [CrossRef]
- Sanzaro, S.; Zontone, F.; Grosso, D.; Bottein, T.; Neri, F.; Smecca, E.; Mannino, G.; Bongiorno, C.; Spinella, C.; La Magna, A.; et al. Bimodal porosity and stability of a TiO2 gig-lox sponge infiltrated with methyl-ammonium lead iodide perovskite. Nanomaterials 2019, 9, 1300. [Google Scholar] [CrossRef]
- Spampinato, C.; Valastro, S.; Smecca, E.; Arena, V.; Mannino, G.; La Magna, A.; Corsaro, C.; Neri, F.; Fazio, E.; Alberti, A. Spongy TiO2 layers deposited by gig-lox sputtering processes: Contact angle measurements. J. Vac. Sci. Technol. Nanotechnol. Microelectron. Mater. Process. Meas. Phenom. 2023, 41, 012802. [Google Scholar] [CrossRef]
- Krainer, S.; Hirn, U. Contact angle measurement on porous substrates: Effect of liquid absorption and drop size. Colloids Surf. Physicochem. Eng. Asp. 2021, 619, 126503. [Google Scholar] [CrossRef]
- Hoshian, S.; Jokinen, V.; Hjort, K.; Ras, R.H.; Franssila, S. Amplified and localized photoswitching of TiO2 by micro-and nanostructuring. Acs Appl. Mater. Interfaces 2015, 7, 15593–15599. [Google Scholar] [CrossRef]
- Otitoju, T.; Ahmad, A.; Ooi, B. Superhydrophilic (superwetting) surfaces: A review on fabrication and application. J. Ind. Eng. Chem. 2017, 47, 19–40. [Google Scholar] [CrossRef]
- Shirolkar, M.M.; Phase, D.; Sathe, V.; Rodríguez-Carvajal, J.; Choudhary, R.J.; Kulkarni, S.K. Relation between crystallinity and chemical nature of surface on wettability: A study on pulsed laser deposited TiO2 thin films. J. Appl. Phys. 2011, 109, 123512. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, Z.; Zhou, X. High-efficient hole-transport-material-free carbon-based all-inorganic perovskite solar cells using Cs-doped TiO2 nanorods array as the electron transport layer. J. Alloys Compd. 2022, 922, 166186. [Google Scholar] [CrossRef]
- Arena, V.; Smecca, E.; Valastro, S.; Bongiorno, C.; Fisicaro, G.; Deretzis, I.; Spampinato, C.; Mannino, G.; Dattilo, S.; Scamporrino, A.A.; et al. Lead Detection in a Gig-Lox TiO2 Sponge by X-ray Reflectivity. Nanomaterials 2023, 13, 1397. [Google Scholar] [CrossRef]
- Schmidt-Mende, L.; Dyakonov, V.; Olthof, S.; Ünlü, F.; Lê, K.M.T.; Mathur, S.; Karabanov, A.D.; Lupascu, D.C.; Herz, L.M.; Hinderhofer, A.; et al. Roadmap on organic–inorganic hybrid perovskite semiconductors and devices. Apl Mater. 2021, 9, 109202. [Google Scholar] [CrossRef]
- Kranthiraja, K.; Aryal, S.; Temsal, M.; Sharma, M.; Kaul, A.B. Optical Property and Stability Study of CH3(CH2)3NH3)2 (CH3NH3)3Pb4I13 Ruddlesden Popper 2D Perovskites for Photoabsorbers and Solar Cells and Comparison with 3D MAPbI3. Solar 2022, 2, 385–400. [Google Scholar] [CrossRef]
- Patil, P.; Sangale, S.S.; Kwon, S.N.; Na, S.I. Innovative Approaches to Semi-Transparent Perovskite Solar Cells. Nanomaterials 2023, 13, 1084. [Google Scholar] [CrossRef]
- Ghosh, A.; Mesloub, A.; Touahmia, M.; Ajmi, M. Visual comfort analysis of semi-transparent Perovskite based building integrated photovoltaic window for hot desert climate (Riyadh, Saudi Arabia). Energies 2021, 14, 1043. [Google Scholar] [CrossRef]
- Ito, S.; Kitamura, T.; Wada, Y.; Yanagida, S. Facile fabrication of mesoporous TiO2 electrodes for dye solar cells: Chemical modification and repetitive coating. Sol. Energy Mater. Sol. Cells 2003, 76, 3–13. [Google Scholar] [CrossRef]
- Han, Q.; Ding, J.; Bai, Y.; Li, T.; Ma, J.Y.; Chen, Y.X.; Zhou, Y.; Liu, J.; Ge, Q.Q.; Chen, J.; et al. Carrier dynamics engineering for high-performance electron-transport-layer-free perovskite photovoltaics. Chem 2018, 4, 2405–2417. [Google Scholar] [CrossRef]
- Bahadur, J.; Ghahremani, A.H.; Martin, B.; Druffel, T.; Sunkara, M.K.; Pal, K. Solution processed Mo doped SnO2 as an effective ETL in the fabrication of low temperature planer perovskite solar cell under ambient conditions. Org. Electron. 2019, 67, 159–167. [Google Scholar] [CrossRef]
- Hassanabadi, E.; Latifi, M.; Gualdrón-Reyes, A.F.; Masi, S.; Yoon, S.J.; Poyatos, M.; Julián-López, B.; Mora-Seró, I. Ligand & band gap engineering: Tailoring the protocol synthesis for achieving high-quality CsPbI3 quantum dots. Nanoscale 2020, 12, 14194–14203. [Google Scholar] [PubMed]
- Zhang, X.; Guo, L.; Zhang, Y.; Cheng, C.; Cheng, Y.; Li, X.; Zhang, J.; Xu, S.; Cao, Y.; Chen, B. Excellent exciton luminescence of CsPbI3 red quantum dots in borate glass. J. Non-Cryst. Solids 2020, 541, 120066. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spampinato, C.; La Magna, P.; Valastro, S.; Smecca, E.; Arena, V.; Bongiorno, C.; Mannino, G.; Fazio, E.; Corsaro, C.; Neri, F.; et al. Infiltration of CsPbI3:EuI2 Perovskites into TiO2 Spongy Layers Deposited by gig-lox Sputtering Processes. Solar 2023, 3, 347-361. https://doi.org/10.3390/solar3030020
Spampinato C, La Magna P, Valastro S, Smecca E, Arena V, Bongiorno C, Mannino G, Fazio E, Corsaro C, Neri F, et al. Infiltration of CsPbI3:EuI2 Perovskites into TiO2 Spongy Layers Deposited by gig-lox Sputtering Processes. Solar. 2023; 3(3):347-361. https://doi.org/10.3390/solar3030020
Chicago/Turabian StyleSpampinato, Carlo, Paola La Magna, Salvatore Valastro, Emanuele Smecca, Valentina Arena, Corrado Bongiorno, Giovanni Mannino, Enza Fazio, Carmelo Corsaro, Fortunato Neri, and et al. 2023. "Infiltration of CsPbI3:EuI2 Perovskites into TiO2 Spongy Layers Deposited by gig-lox Sputtering Processes" Solar 3, no. 3: 347-361. https://doi.org/10.3390/solar3030020
APA StyleSpampinato, C., La Magna, P., Valastro, S., Smecca, E., Arena, V., Bongiorno, C., Mannino, G., Fazio, E., Corsaro, C., Neri, F., & Alberti, A. (2023). Infiltration of CsPbI3:EuI2 Perovskites into TiO2 Spongy Layers Deposited by gig-lox Sputtering Processes. Solar, 3(3), 347-361. https://doi.org/10.3390/solar3030020














