1. Introduction
As a result of the global awareness of greenhouse gas emissions, air pollution, and energy security issues, many governments and researchers around the world have been motivated to search for alternative energies that must be environmentally friendly, clean, affordable, and sustainable [
1]. Seeking an ecofriendly, cost-effective, and feasible alternative to overcome the energy crisis is one of the most significant challenges humanity faces today [
2]. Solar energy is one of the attractive solutions to replace fossil fuels, among other diverse alternative energy sources, such as nuclear power, tides, hydro, and wind. Due to the abundance of solar energy throughout the world, its low cost, and environmental friendliness, it has piqued the interest of academia and industry [
3]. Solar energy is harnessed through photovoltaic and solar thermal collectors. The latter is a promising solar energy harvesting technology that could be used for a variety of purposes, including domestic hot water, solar thermal generation, industrial cooling, seawater desalination [
4], and electricity generation, among other things [
5]. The solar selective absorber surface is the vital component of the solar thermal collector because it absorbs high radiant energy in the UV–Vis–NIR range of the solar spectrum and exhibits low emittance in the infrared range [
6,
7]. Photo-thermal conversion efficiency requires high temperature, and hence operating temperatures of the collectors can rise up to 1100 °C. However, as the temperature rises, the loss of thermal radiation energy increases, and the surface’s structure and performance are affected to a large extent [
8]. Zhiyan Yang et al. investigated the solar selectivity and air thermal stability of a spray-fabricated cobalt–nickel–iron oxide coating. The thermal stability of the two-layer Ni
0.9Fe
0.1Co
2O
y coatings was evaluated by continuously heating at 500 °C in the air for 48 h. According to this report, the solar absorptance (α) remained unchanged at 0.93, while the emittance (ε) increased gradually from 0.11 to 0.30 [
9]. Xiang-Hu et al. reported the structure, optical properties, and thermal stability of Al
2O
3-WC nanocomposite ceramic spectrally selective solar absorbers, which exhibit α of 0.94 and ε of 0.08 at 600 °C. Based on their report, the absorber exhibits good thermal stability in a vacuum at 600 °C for 5 h. The absorber seems to exhibit good thermal stability in a vacuum at 600 °C for 5 h [
7]. The operating temperature of the most common solar absorbing coatings is between 200 and 600 °C. As a result, a type of solar coating with exceptional thermal stability is sorely needed in environments with high temperatures (T > 600 °C) [
5].
The most commonly used methods in the world for preparing solar selective coatings include magnetron sputtering, paint coating, and sol-gel [
10,
11]. Yuping Ning et al. used DC magnetron sputtering to fabricate a NiCrAlO/Al
2O
3 solar selective coating, which exhibits high absorptance and low emittance of 0.964 and 0.066 at 25 °C [
12]. Vasiliy Pelenovich et al. demonstrated the possibility of using non-equilibrium reactive RF magnetron sputtering to deposit graded solar selective absorbers. The authors record the highest absorptance and emittance at 0.909 and 0.0670, respectively [
13]. To enhance the thermal stability of the solar selective metal/dielectric multilayer, Ying Wu et al. employed a multi-target magnetron sputtering method to form Cu, SiO
2, and Cr layers, whereas the Al
2O
3 layer was formed by atomic-layer-deposition (ALD). According to the authors, the as-deposited sample showed α of ~0.954 and ε of ~0.196 (773 K), demonstrating its good optical properties. After heat treatment at 500 °C for 72 h, α drops from ~0.033 to 0.951, while ε decreases from ~0.028 to 0.168 [
14]. Adiba et al. investigated the structural and optical properties of sol-gel-synthesized NiO nanoparticles for selective solar absorbers and transparent heat mirror applications. The authors determined the optical band gap of the nanoparticles using UV-Vis absorption spectroscopy and discovered that their absorption edge is in the ultraviolet region of the solar spectrum, confirming their potential for use as selective solar absorbers and transparent heat mirrors [
15]. Qihua et al. demonstrated that a (sol-gel prepared) reduced-graphene-oxide-based, spectrally selective absorber (rGO-SSA) has a low thermal emittance (ε = 0.04) and a high solar absorption of α = 0.92 at 800 °C [
16]. The CoCuMnOx spinel ceramic film was deposited onto stainless steel 304 through the sol-gel dip coating method to form a solar selective coating that exhibits a selectivity of 0.85 [
17]. Tesfamichael et al. synthesized and characterized a FeMnCuOx particle-based solar selective absorbing paint coating. Black carbon pigment was combined with silicone and phenoxy resin to form the coating [
18].
Magnetron sputtering technology produces a coating with excellent bonding and optical properties, but it requires a vacuum environment, which in turns requires expensive equipment for mass production [
19]. Solar absorbers prepared using paint and sol-gel methods suffer from a lack of adhesion between the film and the substrate, and they exhibit high emission, which has a significant negative effect on the absorber’s performance [
20,
21].
Therefore, the hydrothermal method may be a better alternative because it has several advantages that overcome some of the above-mentioned challenges. These include low process cost, simplicity, pollution-free operation, and ease of application on a large scale [
3]. As a result, the method was used to treat the surface of the stainless steel (SS) in this study in order to enhance its solar absorption property and to investigate the effect of annealing temperatures on the structural and optical properties of the treated surface. Indeed, due to its superior qualities compared to other metallic systems, SS is widely used in a wide variety of applications [
22]. Stainless steel (SS) is often used in the fabrication of solar absorbers and can be rarely corroded under normal temperature and alkaline conditions. It has been reported that the corrosion process of the SS can be accelerated by hydrothermal conditions, leading to a special nano/microstructure oxide surface. The hydrothermal condition is very sensitive to film preparation parameters, such as time and temperature, which are very useful for obtaining the desired morphology and exploring an improved new optical phase of the material. For this reason, the hydrothermal heat treatment has been demonstrated to be a feasible technique to prepare solar selective absorbers for a photo-thermal conversion application [
3].
Thermal stability of selective solar absorber coatings is critical, as the absorber degrades over time at operating temperatures when exposed to vacuum or air, reducing the life of the absorber and eventually resulting in failure [
23,
24,
25]. Thermal stability testing in the presence of air is critical if the vacuum is breached [
26]. Herein, we report the thermal stability of stainless steel (SS) solar absorbers that are hydrothermally treated and annealed in the temperature range between 300 and 700 °C.
4. Conclusions
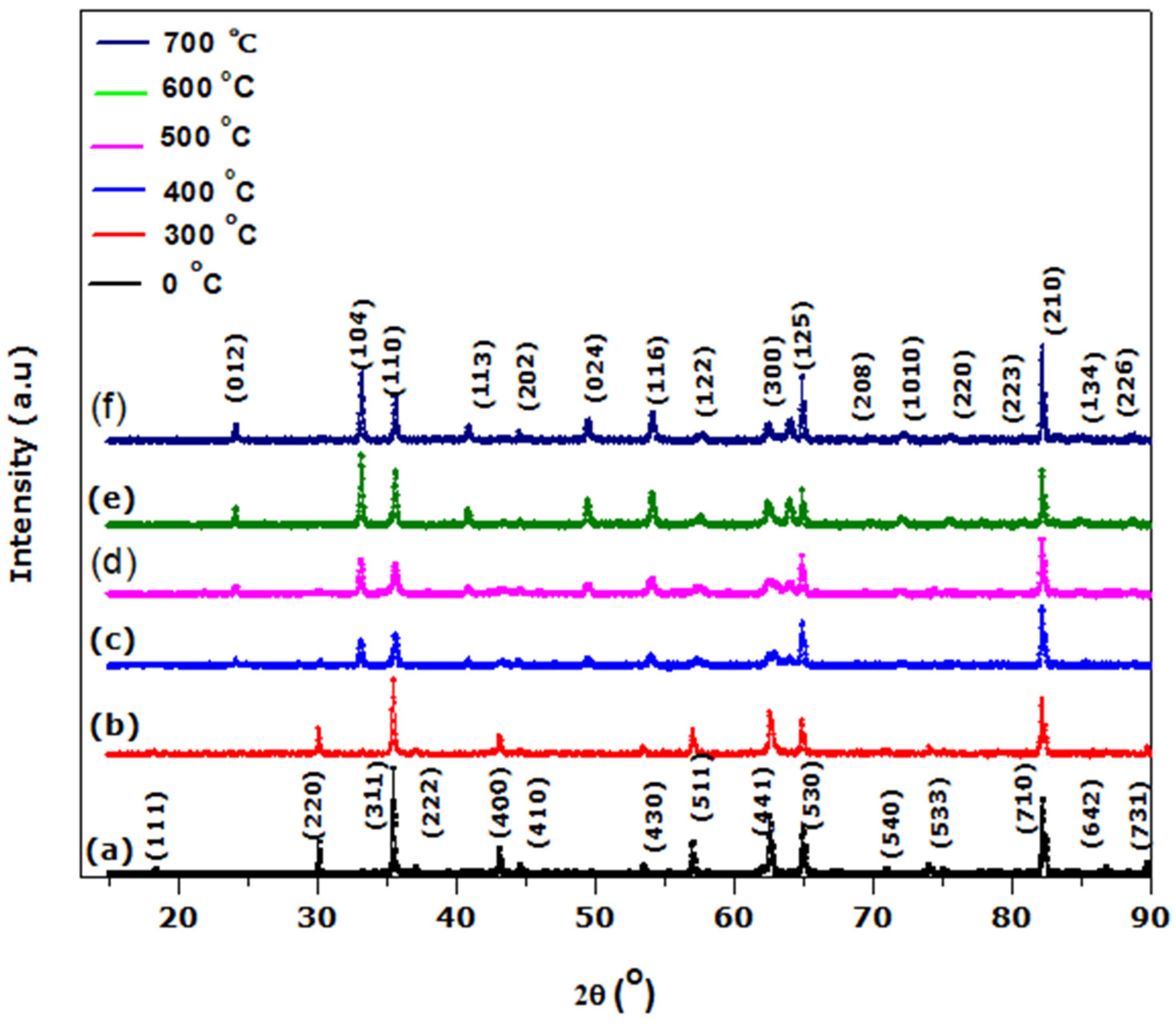
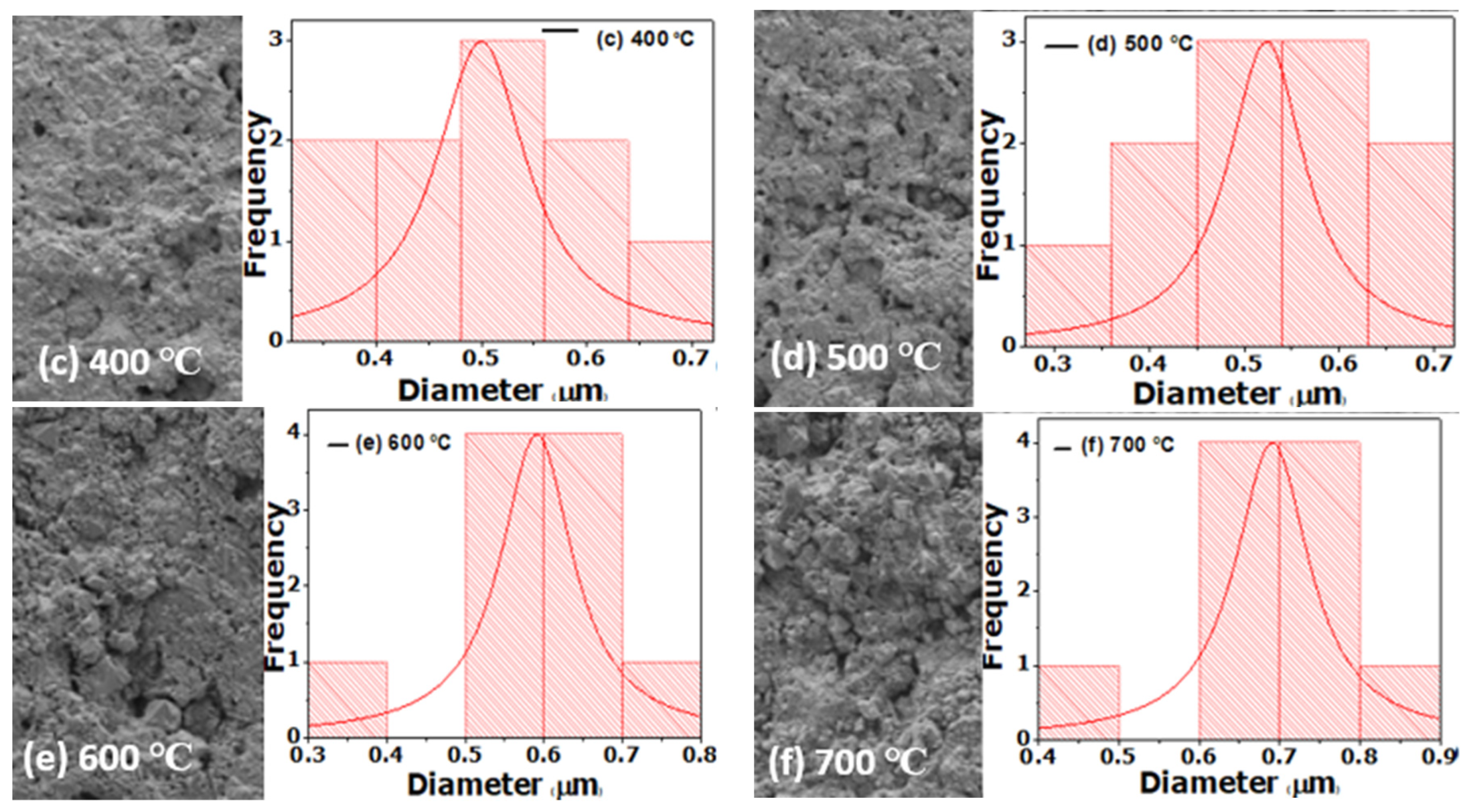
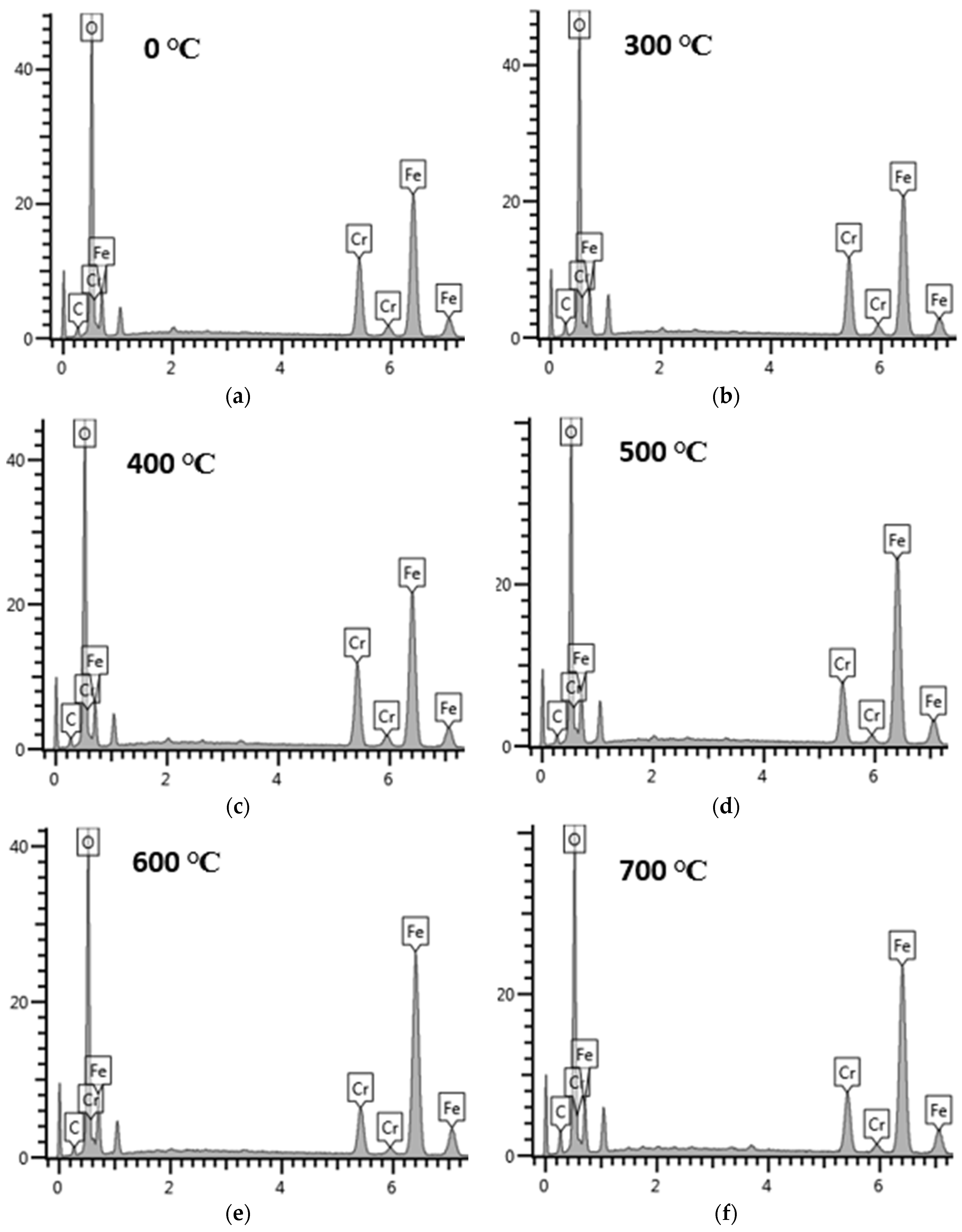
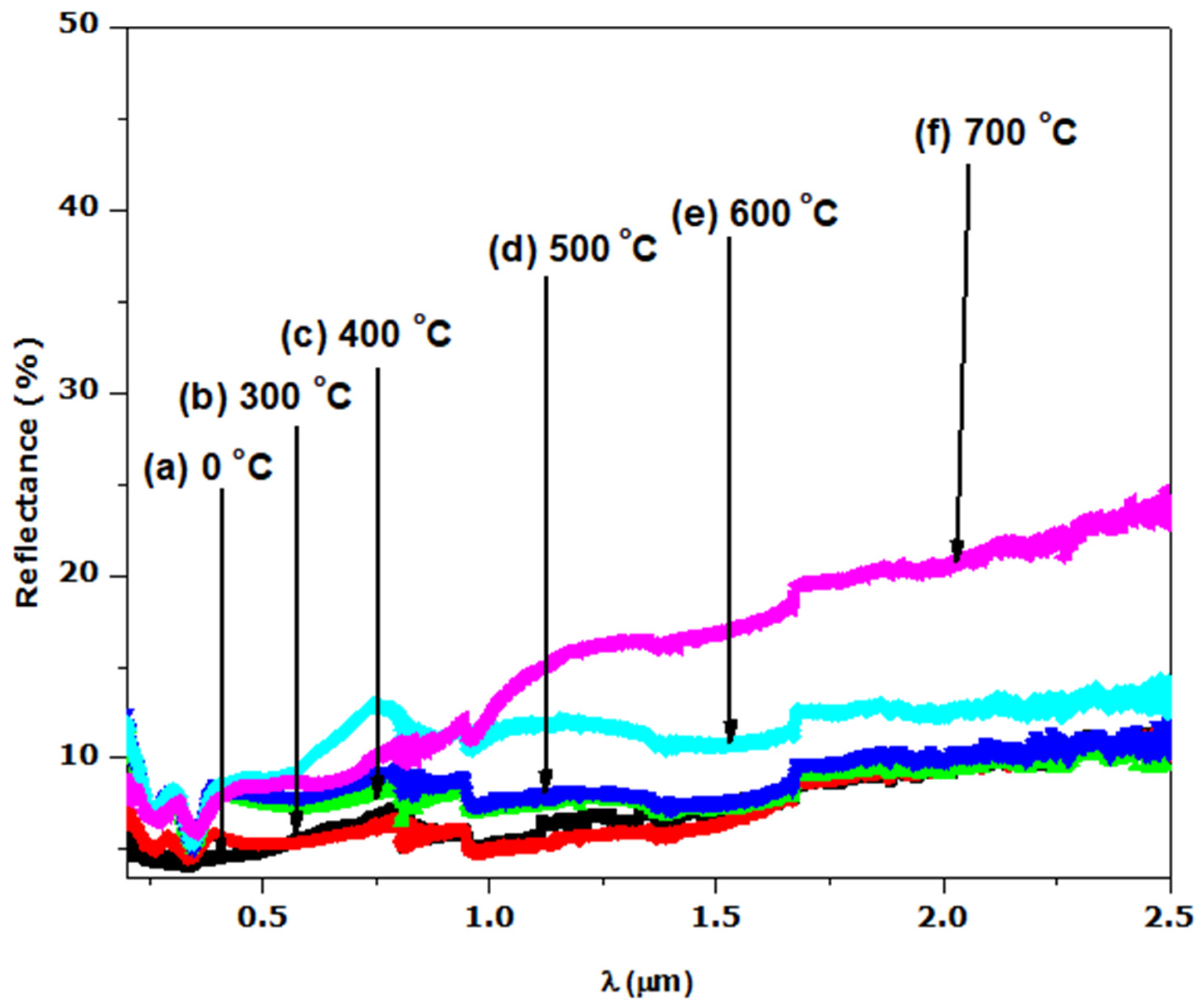
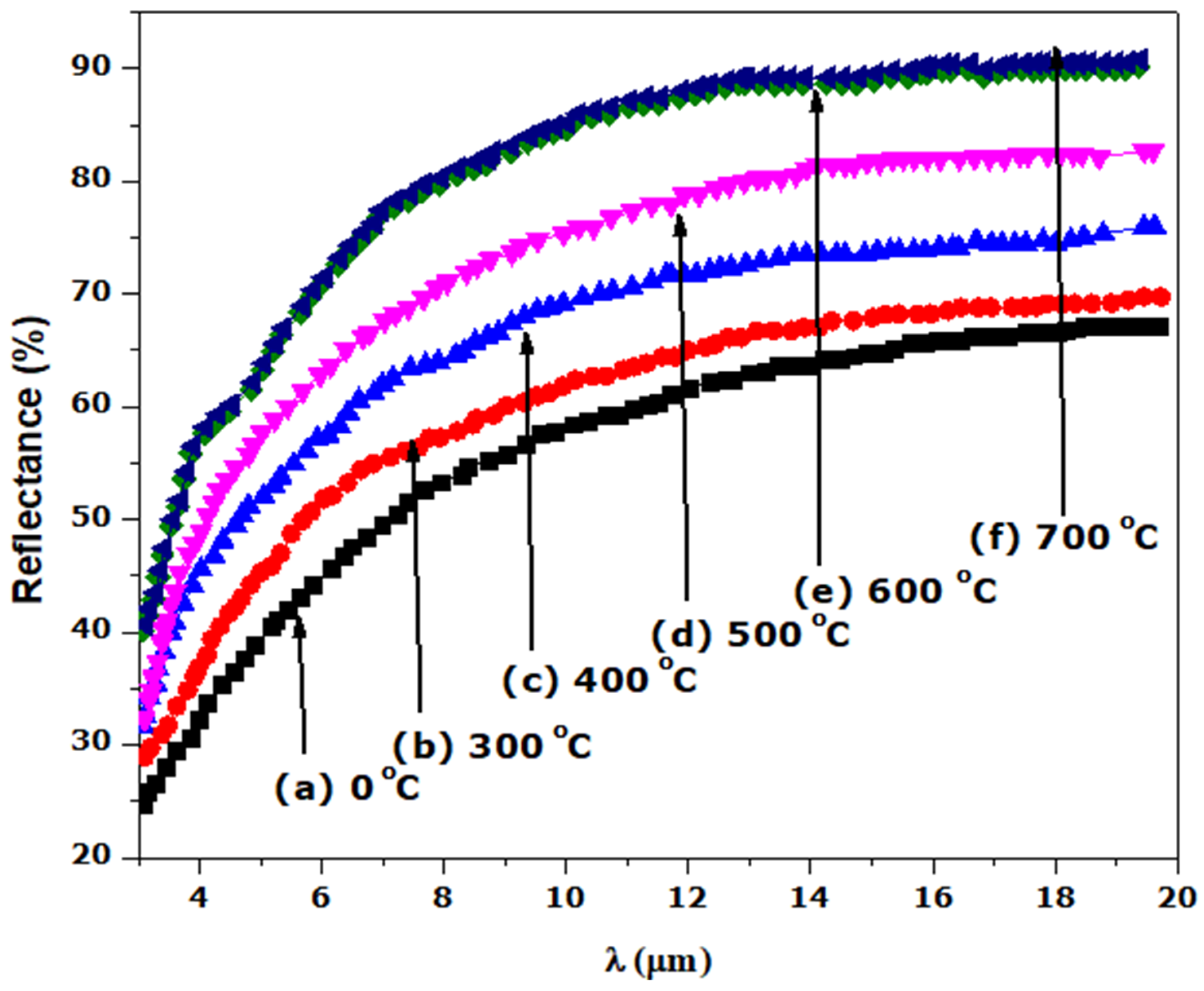
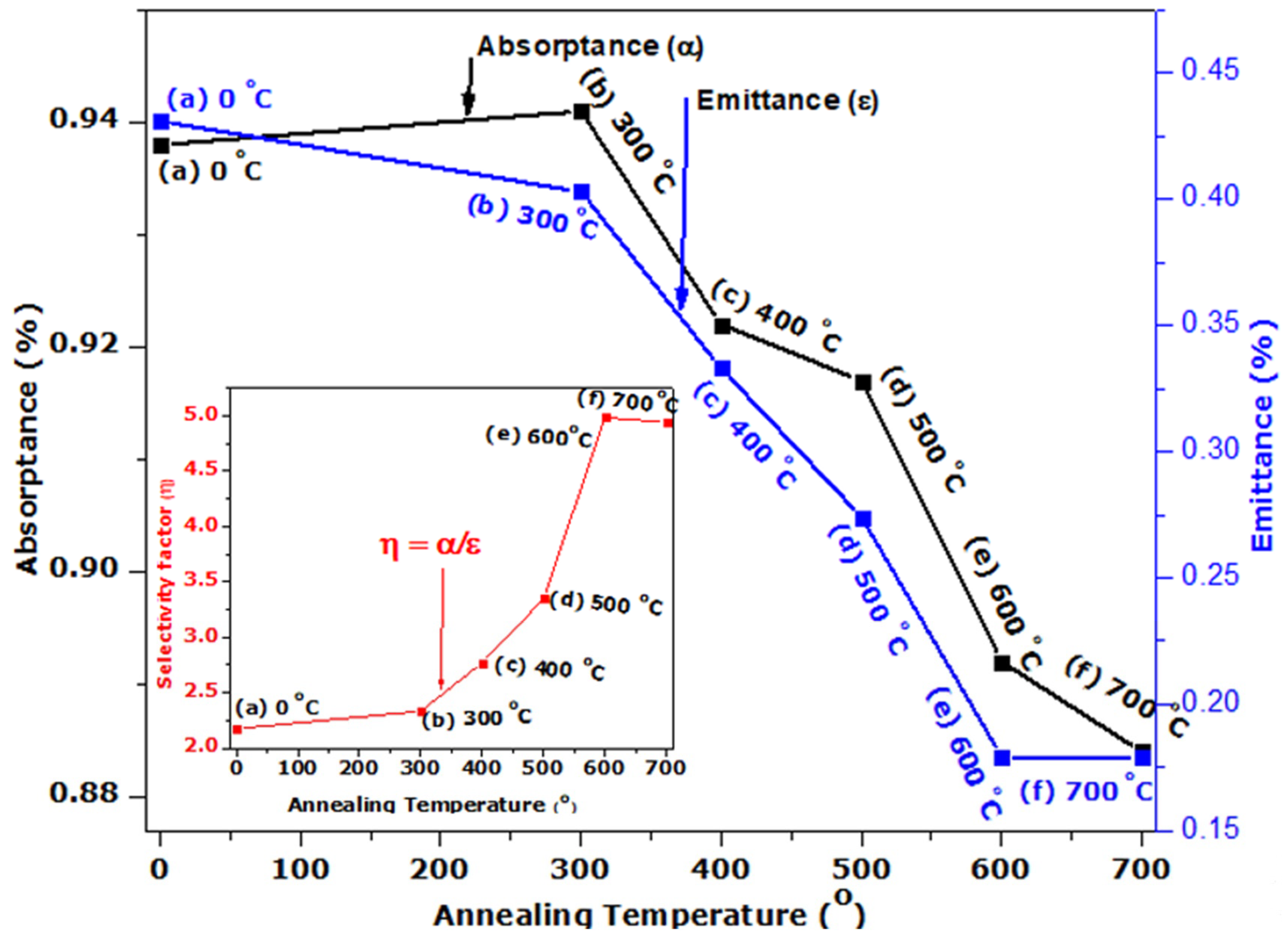
We have treated the surface of stainless steel to enhance its solar absorption property using a hydrothermal technique. Following the thermal treatment at different annealing temperatures, the SS absorber was characterized. The SEM images reveal micropores with decreasing grain sizes and increasing full width at half maximum (FWHM) when annealing temperature is increased, as confirmed by XRD analysis. The EDS spectrum indicates the presence of the transitional elements Cr and Fe, which were evident in the optical response. The SS absorber exhibited a lower reflectance at the UV-VIS-NIR wavelength zone, and this can be attributed to the presence of micropores, which were evident in the SEM images. The optical reflectance of the annealed films at the far-IR wavelength zone were high (60 to 90%), resulting in the obtained minimum thermal emission of the annealed SS absorbers. The major components of the as-treated and annealed film that play a significant role in enhancing the selectivity of the SS absorber are Cr, Fe, and O, as evidenced by the EDS result. Cr and Fe are transitional metals that help to enhance the optical infrared reflectance property of a metal/film, whereas element O (deposited on SS absorber through NaOH used during the hydrothermal treatment) enhances the absorption property of the SS absorber. Thus, the oxidized surface (Fe2O3) of the SS exhibits a high absorption characteristic, whereas Fe and Cr exhibit a high infrared reflectance property, which aids in minimizing thermal emission loss from the absorber surface; thus, the existence of these elements (Fe and Cr) in film results in a good selective absorber with the required optical properties for photo-thermal conversion applications.
The as-obtained SS absorbers exhibit spectra selectivity of 2.176 (0.938/0.431), whereas SS annealed at 700 °C exhibits 4.939 (0.884/0.179). These results indicate a significant improvement in the selectivity (absorptivity/emissivity) of the SS annealed at 700 °C. Hence, the annealed SS absorber could be a promising candidate for a photo-thermal conversion application.