Effects of Long-Term Urban Light Pollution and LED Light Color Temperature on the Behavior of a Holarctic Amphipod Gammarus lacustris Sars, 1863
Abstract
1. Introduction
2. Materials and Methods
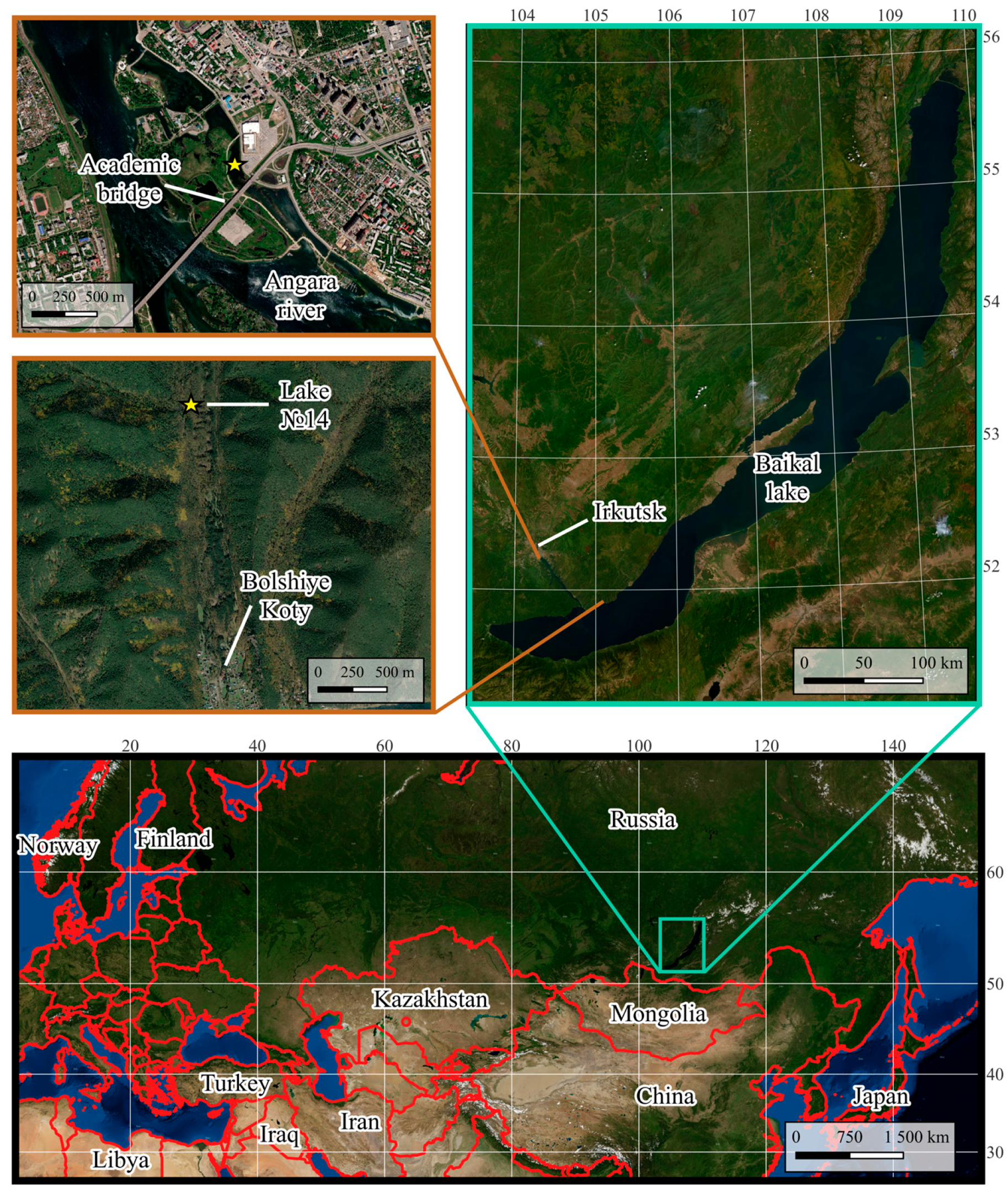
2.1. Capture and Acclimation of Amphipods
2.2. Comparison of the Behavior of Individuals from Different Populations
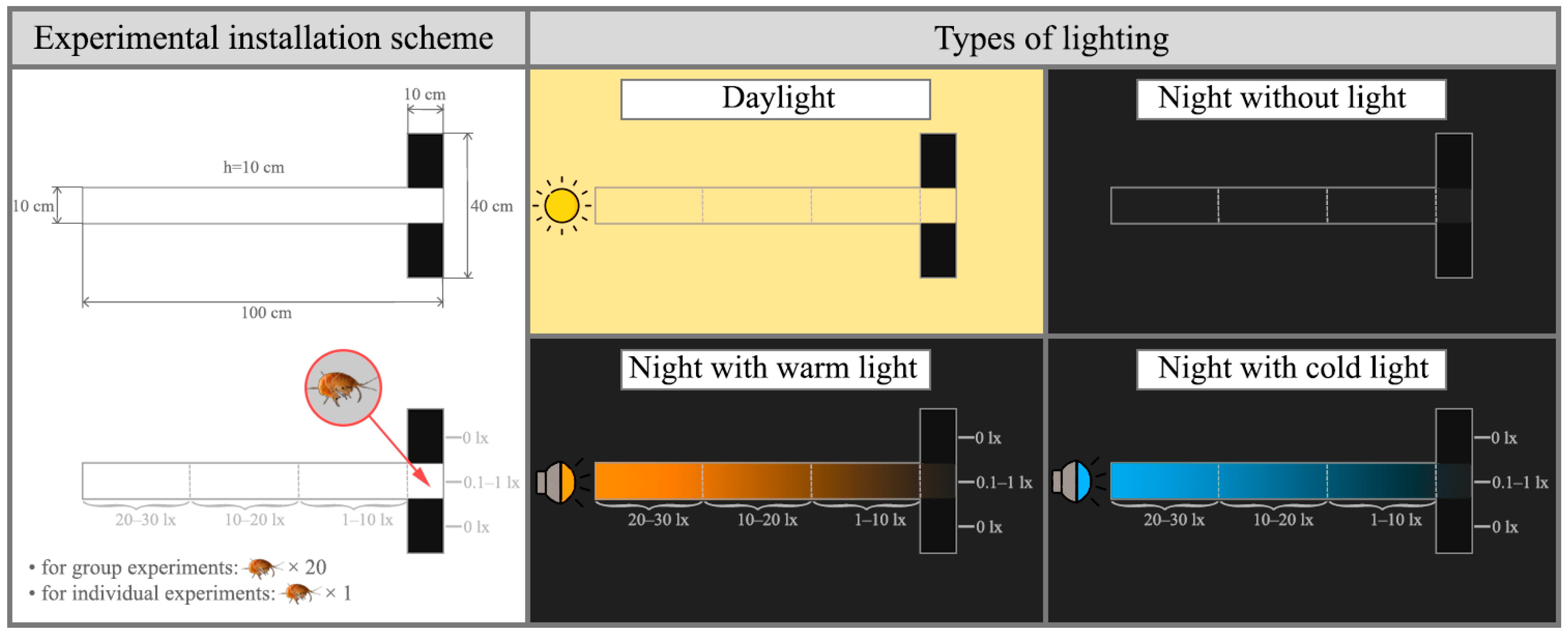
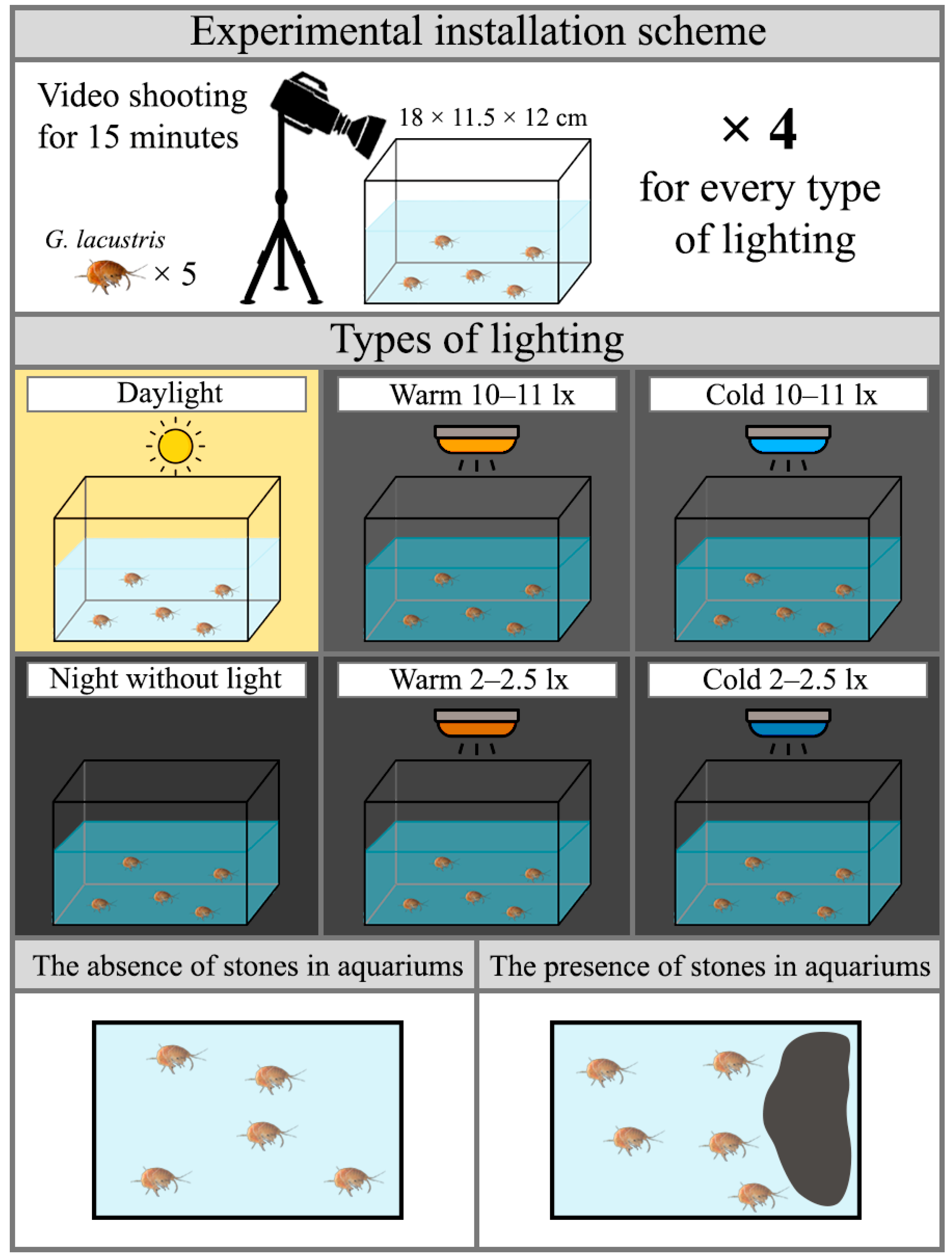
2.3. Evaluation of Amphipod Locomotor Activity
2.4. Data Analysis
3. Results
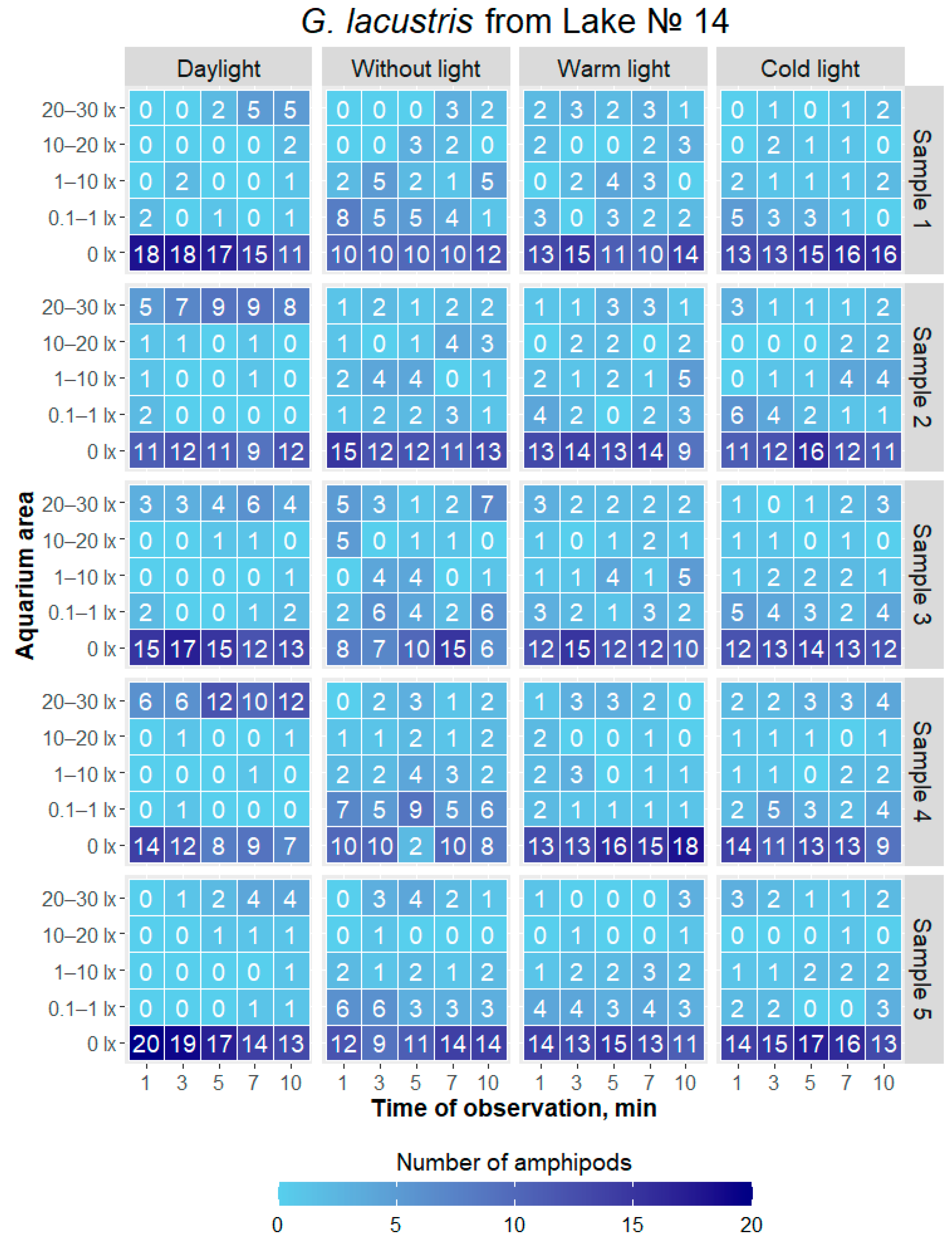
3.1. Response to Different Lighting Conditions of Individuals from Different Populations in Group Experiments
3.2. Response to Different Lighting Conditions of Individuals from Different Populations in Individual Experiments
3.3. Locomotor Activity of Individuals Under Different Lighting Conditions
3.4. Measuring Street Lighting Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LED | Light-emitting diode |
| ALAN | Artificial Light At Night |
References
- Hölker, F.; Jechow, A.; Schroer, S.; Tockner, K.; Gessner, M.O. Light Pollution of Freshwater Ecosystems: Principles, Ecological Impacts and Remedies. Philos. Trans. R. Soc. B 2023, 378, 20220360. [Google Scholar] [CrossRef]
- Falcón, J.; Torriglia, A.; Attia, D.; Viénot, F.; Gronfier, C.; Behar-Cohen, F.; Martinsons, C.; Hicks, D. Exposure to Artificial Light at Night and the Consequences for Flora, Fauna, and Ecosystems. Front. Neurosci. 2020, 14, 602796. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; Lucas, A.J.; Franks, P.J.S.; Schlosser, T.L.; Anderson, C.R.; Send, U.; Davis, K.; Barton, A.D.; Sosik, H.M. Dinoflagellate Vertical Migration Fuels an Intense Red Tide. Proc. Natl. Acad. Sci. USA 2023, 120, e2304590120. [Google Scholar] [CrossRef]
- Moore, M.V.; Pierce, S.M.; Walsh, H.M.; Kvalvik, S.K.; Lim, J.D. Urban Light Pollution Alters the Diel Vertical Migration of Daphnia. SIL Proc. 1922–2010 2000, 27, 779–782. [Google Scholar] [CrossRef]
- Anokhina, L.L. Influence of Moonlight on the Vertical Migrations of Benthopelagic Organisms in the Near-Shore Area of the Black Sea. Oceanology 2006, 46, 385–395. [Google Scholar] [CrossRef]
- Navarro-Barranco, C.; Hughes, L.E. Effects of Light Pollution on the Emergent Fauna of Shallow Marine Ecosystems: Amphipods as a Case Study. Mar. Pollut. Bull. 2015, 94, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Perkin, E.K.; Hölker, F.; Richardson, J.S.; Sadler, J.P.; Wolter, C.; Tockner, K. The Influence of Artificial Light on Stream and Riparian Ecosystems: Questions, Challenges, and Perspectives. Ecosphere 2011, 2, 1–16. [Google Scholar] [CrossRef]
- Ugolini, A.; Borgioli, G.; Galanti, G.; Mercatelli, L.; Hariyama, T. Photoresponses of the Compound Eye of the Sandhopper Talitrus saltator (Crustacea, Amphipoda) in the Ultraviolet-Blue Range. Biol. Bull. 2010, 219, 72–79. [Google Scholar] [CrossRef]
- Rothsey, S.C.; Andrew, N.R. Orientation of the Beach Hopper Notorchestia Sp. (Amphipoda: Talitridae). J. Crustac. Biol. 2016, 36, 475–484. [Google Scholar] [CrossRef]
- Ciofini, A.; Mercatelli, L.; Hariyama, T.; Ugolini, A. Sky Radiance and Spectral Gradient Are Orienting Cues for the Sandhopper Talitrus saltator (Crustacea, Amphipoda). J. Exp. Biol. 2020, 224, jeb.239574. [Google Scholar] [CrossRef]
- Jechow, A.; Hölker, F. How Dark Is a River? Artificial Light at Night in Aquatic Systems and the Need for Comprehensive Night-time Light Measurements. WIREs Water 2019, 6, e1388. [Google Scholar] [CrossRef]
- Tidau, S.; Whittle, J.; Jenkins, S.R.; Davies, T.W. Artificial Light at Night Reverses Monthly Foraging Pattern under Simulated Moonlight. Biol. Lett. 2022, 18, 20220110. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Sun, H.; Yu, J.; Lin, B. Effects of Correlated Color Temperature of Office Light on Subjective Perception, Mood and Task Performance. Build. Environ. 2022, 224, 109508. [Google Scholar] [CrossRef]
- Sanders, D.; Frago, E.; Kehoe, R.; Patterson, C.; Gaston, K.J. A Meta-Analysis of Biological Impacts of Artificial Light at Night. Nat. Ecol. Evol. 2020, 5, 74–81. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, Y.; Liu, Y.; Liu, S.; Yao, L.; Cao, D. Do Rivers Get Sufficient Sleep—A Global Analysis of Light Pollution in Rivers. Resour. Conserv. Recycl. 2024, 211, 107892. [Google Scholar] [CrossRef]
- Bennie, J.; Davies, T.W.; Cruse, D.; Gaston, K.J. Ecological Effects of Artificial Light at Night on Wild Plants. J. Ecol. 2016, 104, 611–620. [Google Scholar] [CrossRef]
- Jechow, A.; Kyba, C.C.M.; Hölker, F. Mapping the Brightness and Color of Urban to Rural Skyglow with All-Sky Photometry. J. Quant. Spectrosc. Radiat. Transf. 2020, 250, 106988. [Google Scholar] [CrossRef]
- Kyba, C.C.M.; Ruhtz, T.; Fischer, J.; Hölker, F. Cloud Coverage Acts as an Amplifier for Ecological Light Pollution in Urban Ecosystems. PLoS ONE 2011, 6, e17307. [Google Scholar] [CrossRef]
- Manríquez, P.H.; Jara, M.E.; Diaz, M.I.; Quijón, P.A.; Widdicombe, S.; Pulgar, J.; Manríquez, K.; Quintanilla-Ahumada, D.; Duarte, C. Artificial Light Pollution Influences Behavioral and Physiological Traits in a Keystone Predator Species, Concholepas concholepas. Sci. Total Environ. 2019, 661, 543–552. [Google Scholar] [CrossRef]
- Gal, G.; Loew, E.R.; Rudstam, L.G.; Mohammadian, A.M. Light and Diel Vertical Migration: Spectral Sensitivity and Light Avoidance by Mysis relicta. Can. J. Fish. Aquat. Sci. 1999, 56, 311–322. [Google Scholar] [CrossRef]
- Ludvigsen, M.; Berge, J.; Geoffroy, M.; Cohen, J.H.; De La Torre, P.R.; Nornes, S.M.; Singh, H.; Sørensen, A.J.; Daase, M.; Johnsen, G. Use of an Autonomous Surface Vehicle Reveals Small-Scale Diel Vertical Migrations of Zooplankton and Susceptibility to Light Pollution under Low Solar Irradiance. Sci. Adv. 2018, 4, eaap9887. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, M.; Jermacz, Ł.; Glazińska, P.; Kulasek, M.; Kobak, J. Artificial Light at Night (ALAN) Affects Behaviour, but Does Not Change Oxidative Status in Freshwater Shredders. Environ. Pollut. 2022, 306, 119476. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Ahumada, D.; Quijón, P.A.; Jahnsen-Guzmán, N.; Lynn, K.D.; Pulgar, J.; Palma, J.; Manríquez, P.H.; Duarte, C. Splitting Light Pollution: Wavelength Effects on the Activity of Two Sandy Beach Species. Environ. Pollut. 2024, 356, 124317. [Google Scholar] [CrossRef] [PubMed]
- Brüning, A.; Hölker, F.; Franke, S.; Kleiner, W.; Kloas, W. Impact of Different Colours of Artificial Light at Night on Melatonin Rhythm and Gene Expression of Gonadotropins in European Perch. Sci. Total Environ. 2016, 543, 214–222. [Google Scholar] [CrossRef]
- Garratt, M.J.; Jenkins, S.R.; Davies, T.W. Mapping the Consequences of Artificial Light at Night for Intertidal Ecosystems. Sci. Total Environ. 2019, 691, 760–768. [Google Scholar] [CrossRef]
- Hölker, F.; Bolliger, J.; Davies, T.W.; Giavi, S.; Jechow, A.; Kalinkat, G.; Longcore, T.; Spoelstra, K.; Tidau, S.; Visser, M.E.; et al. 11 Pressing Research Questions on How Light Pollution Affects Biodiversity. Front. Ecol. Evol. 2021, 9, 767177. [Google Scholar] [CrossRef]
- Tüzün, N.; De Meester, L.; Hölker, F. Eco-Evolutionary Feedbacks under Artificial Light at Night. iScience 2025, 28, 112616. [Google Scholar] [CrossRef]
- Meyer, L.A.; Sullivan, S.M.P. Bright Lights, Big City: Influences of Ecological Light Pollution on Reciprocal Stream–Riparian Invertebrate Fluxes. Ecol. Appl. 2013, 23, 1322–1330. [Google Scholar] [CrossRef]
- Burke, L.M.; Davies, T.W.; Wilcockson, D.; Jenkins, S.; Ellison, A. Artificial Light and Cloud Cover Interact to Disrupt Celestial Migrations at Night. Sci. Total Environ. 2024, 943, 173790. [Google Scholar] [CrossRef]
- Schligler, J.; Cortese, D.; Beldade, R.; Swearer, S.E.; Mills, S.C. Long-Term Exposure to Artificial Light at Night in the Wild Decreases Survival and Growth of a Coral Reef Fish. Proc. R. Soc. B 2021, 288, 20210454. [Google Scholar] [CrossRef]
- Sanna, G.; Domenici, P.; Maggi, E. Artificial Light at Night Alters the Locomotor Behavior of the Mediterranean Sea Urchin Paracentrotus lividus. Mar. Pollut. Bull. 2024, 206, 116782. [Google Scholar] [CrossRef]
- Nuñez, J.D.; Sbragaglia, V.; Spivak, E.D.; Chiaradia, N.M.; Luppi, T.A. The Magnitude of Behavioural Responses to Artificial Light at Night Depends on the Ecological Context in a Coastal Marine Ecosystem Engineer. Mar. Environ. Res. 2021, 165, 105238. [Google Scholar] [CrossRef] [PubMed]
- Matafonov, D.V.; Bazova, N.V. Decline of Gammarus lacustris Sars (Crustacea: Amphipoda) Population in the Delta of the Selenga River. Biol. Bull. Russ. Acad. Sci. 2014, 41, 168–175. [Google Scholar] [CrossRef]
- Czarnecka, M.; Kobak, J.; Grubisic, M.; Kakareko, T. Disruptive Effect of Artificial Light at Night on Leaf Litter Consumption, Growth and Activity of Freshwater Shredders. Sci. Total Environ. 2021, 786, 147407. [Google Scholar] [CrossRef] [PubMed]
- Hassan, W.; Kobak, J.; Czarnecka, M. Artificial Light at Night Alters Foraging Behavior of Freshwater Amphipods Depending on the Light Spectrum and the Presence of Predation Cues. Curr. Zool. 2024, 71, 432–439. [Google Scholar] [CrossRef]
- Drozdova, P.; Kizenko, A.; Saranchina, A.; Gurkov, A.; Firulyova, M.; Govorukhina, E.; Timofeyev, M. The Diversity of Opsins in Lake Baikal Amphipods (Amphipoda: Gammaridae). BMC Ecol. Evol. 2021, 21, 81. [Google Scholar] [CrossRef]
- Kang, S.Y.; Youn, N.; Yoon, H.C. The Self-Regulatory Power of Environmental Lighting: The Effect of Illuminance and Correlated Color Temperature. J. Environ. Psychol. 2019, 62, 30–41. [Google Scholar] [CrossRef]
- Karnaukhov, D.; Ermolaeva, Y.; Maslennikova, M.; Osadchy, B.; Biritskaya, S.; Lavnikova, A.; Kulbachnaya, N.; Solodkova, A.; Guliguev, A.; Kodatenko, I.; et al. Species-Specific Responses of Baikal Amphipods to Artificial Lighting of Varying Intensity and Spectral Composition. Limnol. Rev. 2025, 25, 11. [Google Scholar] [CrossRef]
- Karnaukhov, D.; Ermolaeva, Y.; Maslennikova, M.; Golubets, D.; Lavnikova, A.; Kodatenko, I.; Guliguev, A.; Rechile, D.; Salovarov, K.; Olimova, A.; et al. Can the Baikal Amphipod Gmelinoides fasciatus (Stebbing, 1899) Have Different Responses to Light Pollution with Different Color Temperatures? J. Mar. Sci. Eng. 2025, 13, 1039. [Google Scholar] [CrossRef]
- Fischer, J.R.; Gangloff, M.M.; Creed, R.P. The Behavioral Responses of 2 Appalachian Crayfish to Cool and Warm Spectrum LED Lights at Night. Freshw. Sci. 2020, 39, 39–46. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, X.; Luo, Q.; Lin, S.; Lyu, M.; Luo, X.; Ke, C.; You, W. Risk Assessment of Persistent Exposure to Artificial Light at Night Revealed Altered Behavior and Metabolic Patterns of Marine Nocturnal Shellfish. Ecol. Indic. 2024, 160, 111807. [Google Scholar] [CrossRef]
- Melnikov Yu, I. Goldeneye Bucephala clangula on "Cold" wintering in the source and upper reaches of the Angara River: Changes in sex ratio as a result of climate warming. Russ. Ornithol. J. 2020, 29, 3789–3799. (In Russian) [Google Scholar]
- Solano Lamphar, H.A.; Kocifaj, M. Light Pollution in Ultraviolet and Visible Spectrum: Effect on Different Visual Perceptions. PLoS ONE 2013, 8, e56563. [Google Scholar] [CrossRef] [PubMed]
- Vinebrooke, R.D.; Leavitt, P.R. Differential Responses of Littoral Communities to Ultraviolet Radiation in An Alpine Lake. Ecology 1999, 80, 223–237. [Google Scholar] [CrossRef]
- Altermatt, F.; Ebert, D. Reduced Flight-to-Light Behaviour of Moth Populations Exposed to Long-Term Urban Light Pollution. Biol. Lett. 2016, 12, 20160111. [Google Scholar] [CrossRef]
- Keinath, S.; Hölker, F.; Müller, J.; Rödel, M.-O. Impact of Light Pollution on Moth Morphology–A 137-Year Study in Germany. Basic Appl. Ecol. 2021, 56, 1–10. [Google Scholar] [CrossRef]
- Folt, C.L.; Burns, C.W. Biological drivers of zooplankton patchiness. Trends Ecol. Evol. 1999, 14, 300–305. [Google Scholar] [CrossRef]
- Bartosiewicz, M.; Gliwicz, Z.M. Temporary Intermissions in Capturing Prey (Daphnia) by Planktivorous Fish (Rutilus rutilus): Are They Due to Scramble Competition or the Need for Antipredation Vigilance? Hydrobiologia 2011, 668, 125–136. [Google Scholar] [CrossRef]
- Tałanda, J.; Maszczyk, P.; Babkiewicz, E. The Reaction Distance of a Planktivorous Fish (Scardinius erythrophthalmus) and the Evasiveness of Its Prey (Daphnia pulex × pulicaria) under Different Artificial Light Spectra. Limnology 2018, 19, 311–319. [Google Scholar] [CrossRef]
- Cherry, T.-R.; Kohler, S.A.; Ford, A.T. Sex Specific Differences Recorded in the Behavior of an Amphipod: Implications for Behavioral Toxicology. Front. Mar. Sci. 2020, 7, 370. [Google Scholar] [CrossRef]
- Van Den Berg, S.J.P.; Rodríguez-Sánchez, P.; Zhao, J.; Olusoiji, O.D.; Peeters, E.T.H.M.; Schuijt, L.M. Among-Individual Variation in the Swimming Behaviour of the Amphipod Gammarus pulex under Dark and Light Conditions. Sci. Total Environ. 2023, 872, 162177. [Google Scholar] [CrossRef]
- Kohler, S.A.; Parker, M.O.; Ford, A.T. Species-Specific Behaviours in Amphipods Highlight the Need for Understanding Baseline Behaviours in Ecotoxicology. Aquat. Toxicol. 2018, 202, 173–180. [Google Scholar] [CrossRef]
- Luarte, T.; Bonta, C.C.; Silva-Rodriguez, E.A.; Quijón, P.A.; Miranda, C.; Farias, A.A.; Duarte, C. Light Pollution Reduces Activity, Food Consumption and Growth Rates in a Sandy Beach Invertebrate. Environ. Pollut. 2016, 218, 1147–1153. [Google Scholar] [CrossRef]
- Duarte, C.; Quintanilla-Ahumada, D.; Anguita, C.; Manríquez, P.H.; Widdicombe, S.; Pulgar, J.; Silva-Rodríguez, E.A.; Miranda, C.; Manríquez, K.; Quijón, P.A. Artificial Light Pollution at Night (ALAN) Disrupts the Distribution and Circadian Rhythm of a Sandy Beach Isopod. Environ. Pollut. 2019, 248, 565–573. [Google Scholar] [CrossRef]
- Lynn, K.D.; Quintanilla-Ahumada, D.; Anguita, C.; Widdicombe, S.; Pulgar, J.; Manríquez, P.H.; Quijón, P.A.; Duarte, C. Artificial Light at Night Alters the Activity and Feeding Behaviour of Sandy Beach Amphipods and Pose a Threat to Their Ecological Role in Atlantic Canada. Sci. Total Environ. 2021, 780, 146568. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, C.; Song, Z.; Wang, G. Automatic Monitoring of Relevant Behaviors for Crustacean Production in Aquaculture: A Review. Animals 2021, 11, 2709. [Google Scholar] [CrossRef] [PubMed]
- Weiss, H.M.; Lozano-Álvarez, E.; Briones-Fourzán, P.; Negrete-Soto, F. Using Red Light with Fixed-Site Video Cameras to Study the Behavior of the Spiny Lobster, Panulirus argus, and Associated Animals at Night and Inside Their Shelters. Mar. Technol. Soc. J. 2006, 40, 86–95. [Google Scholar] [CrossRef]
- Karnaukhov, D.; Dolinskaya, E.; Biritskaya, S.; Teplykh, M.; Khomich, A.; Silow, E. Effect of artificial light on the migratory activity of the pelagic amphipod Macrohectopus branickii during daily vertical migration in Lake Baikal. Ecol. Environ. Conserv. 2019, 25, 1693–1695. [Google Scholar]
- Simčič, T.; Brancelj, A. The Effect of Light on Oxygen Consumption in Two Amphipod Crustaceans–the Hypogean Niphargus stygius and the Epigean Gammarus fossarum. Mar. Freshw. Behav. Physiol. 2007, 40, 141–150. [Google Scholar] [CrossRef]
- Davies, T.W.; Duffy, J.P.; Bennie, J.; Gaston, K.J. The Nature, Extent, and Ecological Implications of Marine Light Pollution. Front. Ecol. Environ. 2014, 12, 347–355. [Google Scholar] [CrossRef]
- Sullivan, S.M.P.; Hossler, K.; Meyer, L.A. Artificial Lighting at Night Alters Aquatic-Riparian Invertebrate Food Webs. Ecol. Appl. 2018, 29, e01821. [Google Scholar] [CrossRef]
- Bolton, D.; Mayer-Pinto, M.; Clark, G.F.; Dafforn, K.A.; Brassil, W.A.; Becker, A.; Johnston, E.L. Coastal Urban Lighting Has Ecological Consequences for Multiple Trophic Levels under the Sea. Sci. Total Environ. 2017, 576, 1–9. [Google Scholar] [CrossRef]
- Harrison, S.E.; Gray, S.M. Effects of Light Pollution on Bluegill Foraging Behavior. Trans. Am. Fish. Soc. 2024, 153, 152–162. [Google Scholar] [CrossRef]
- Longcore, T.; Rich, C. Ecological Light Pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
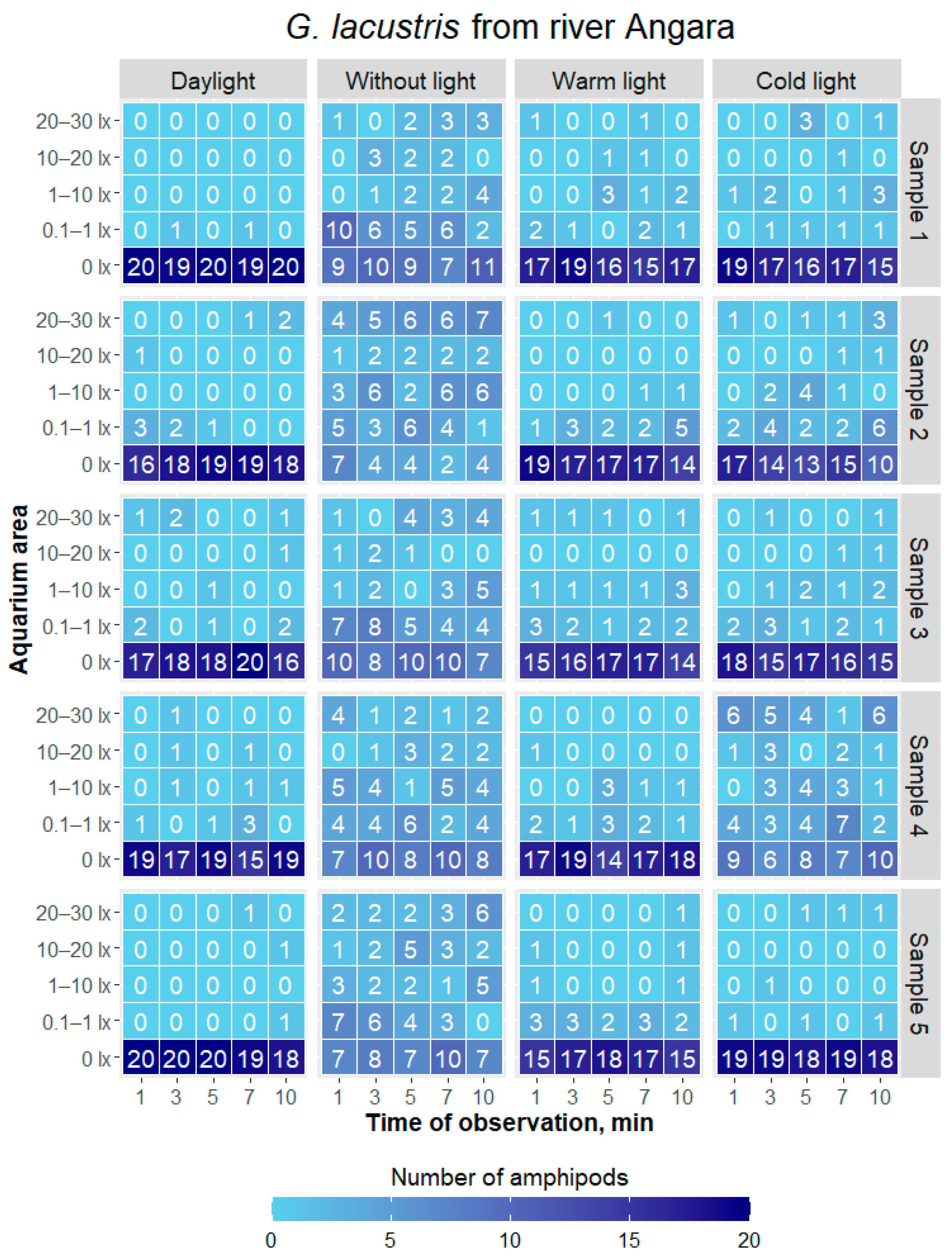
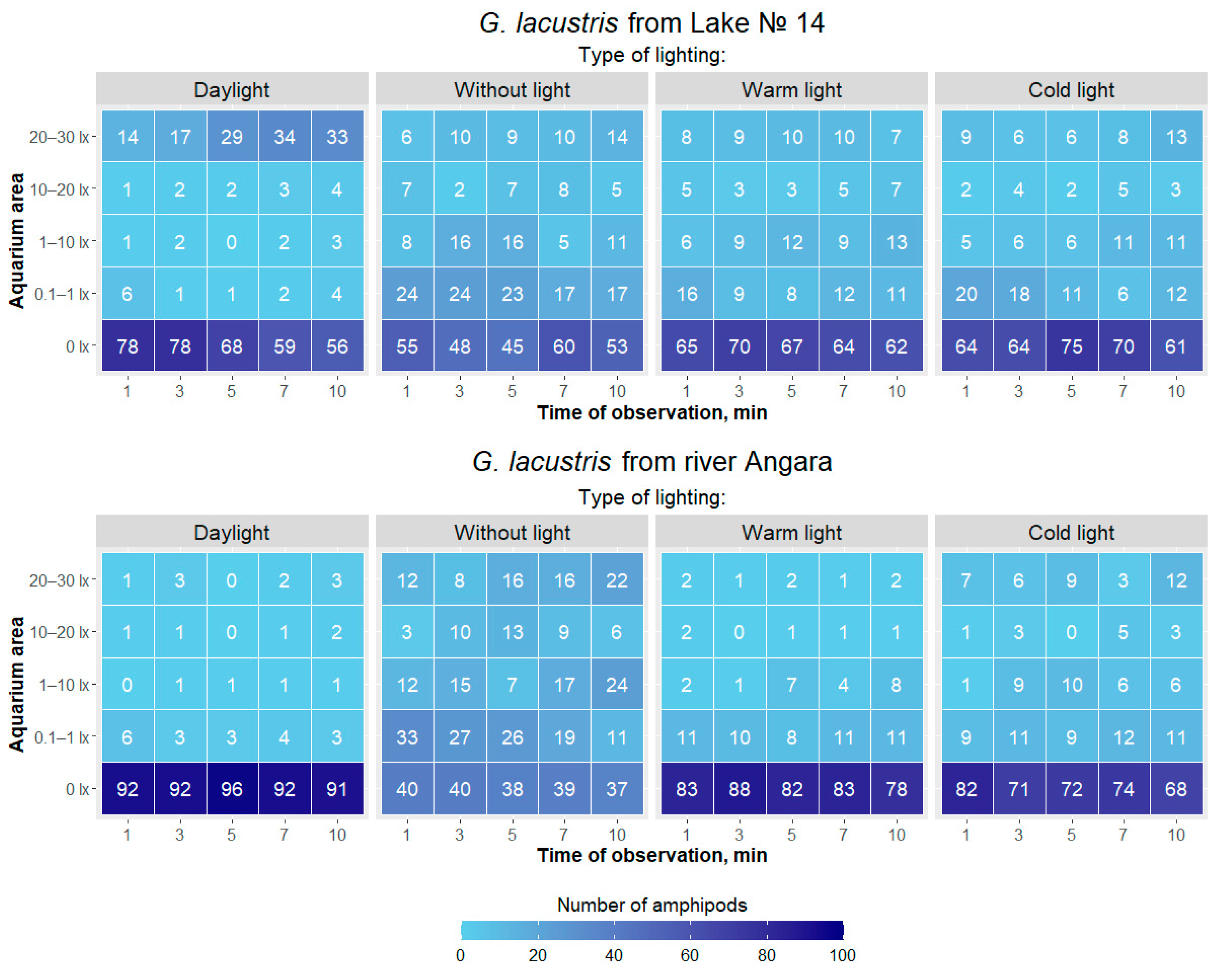

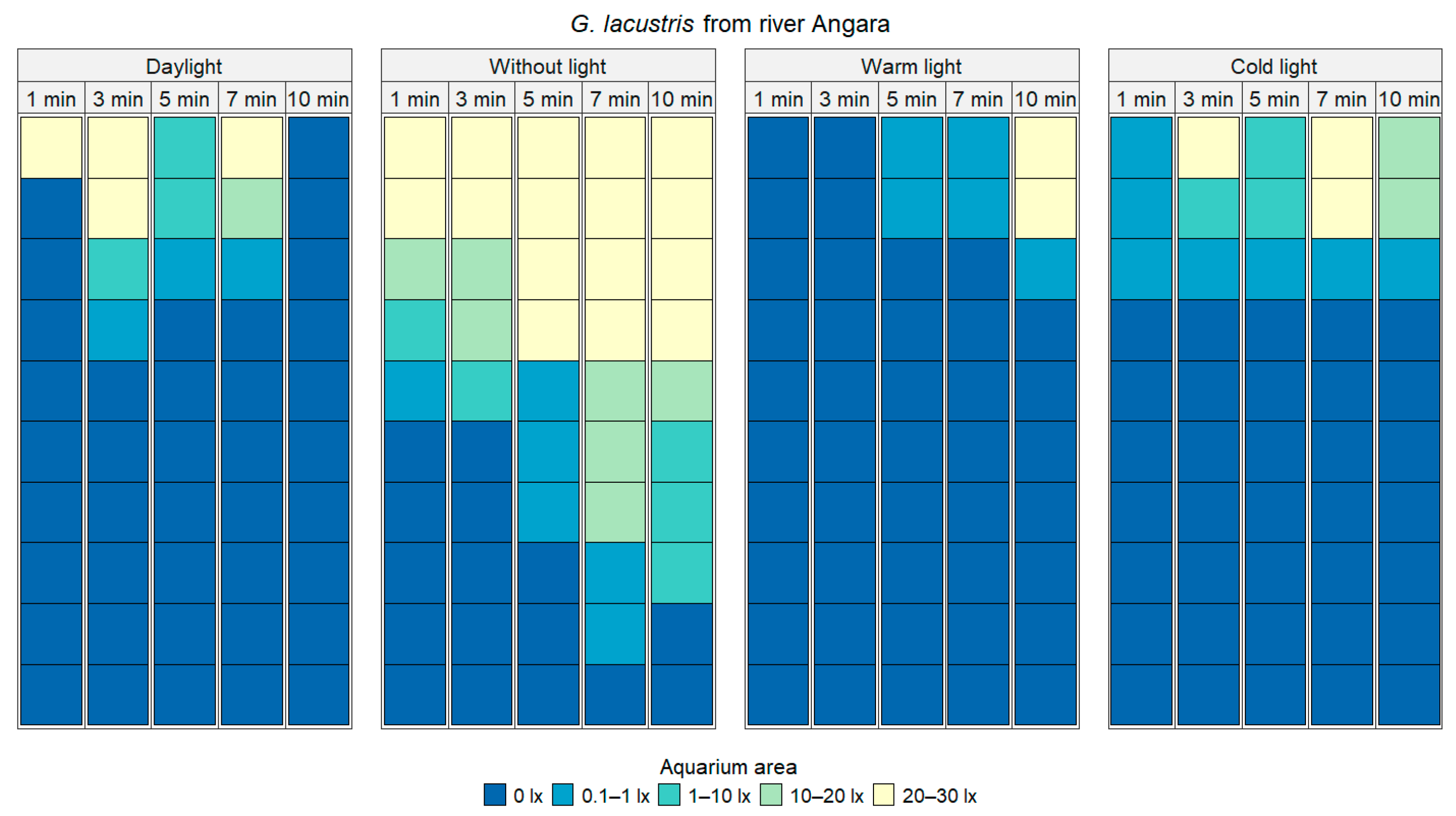
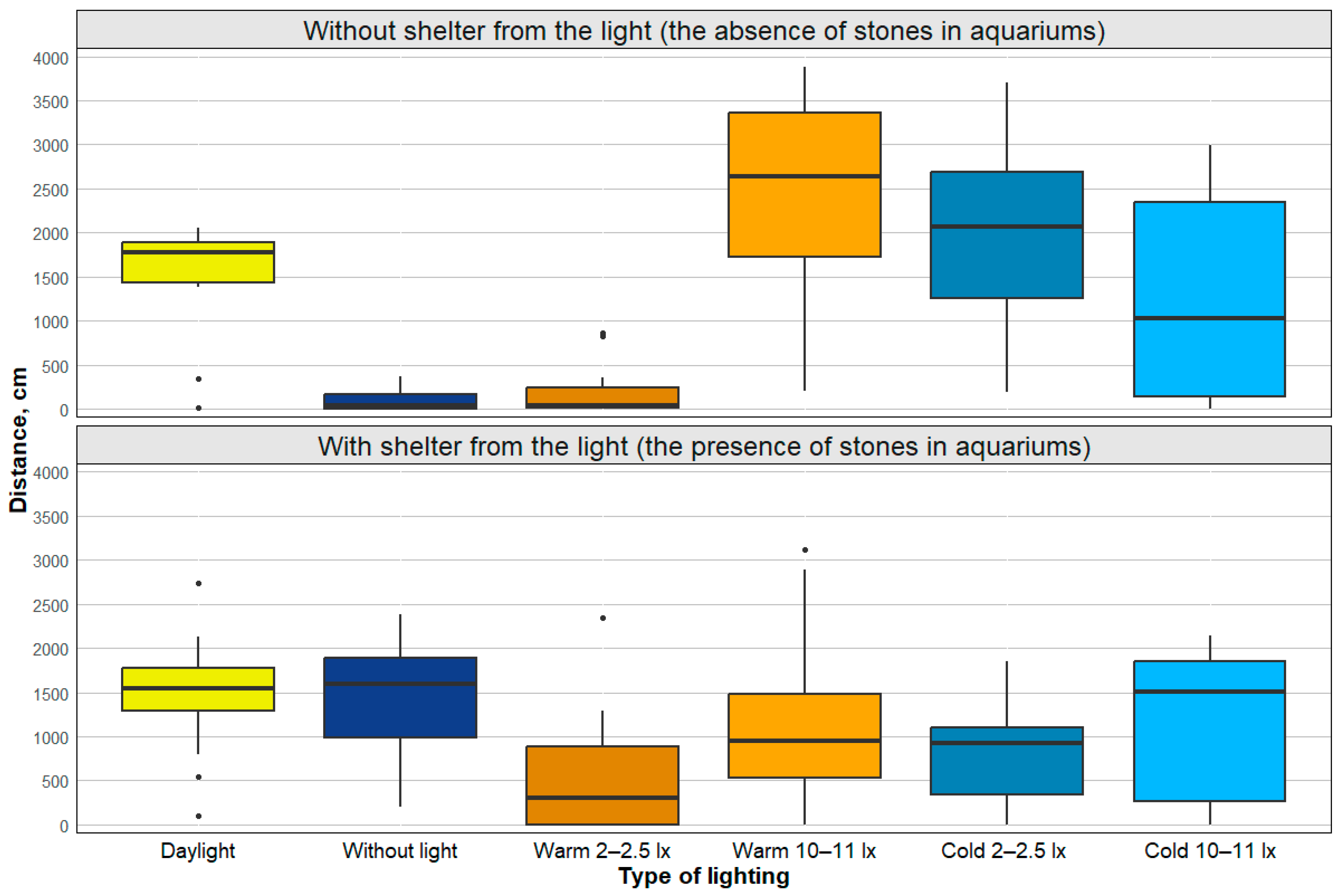

| Type of Lighting | Cold Light | Daylight | Without Light |
|---|---|---|---|
| Daylight | 0.1 | - | - |
| Without light | 0.7 | 0.01 * | - |
| Warm light | 0.9 | 0.08 | 0.9 |
| Daylight (Kruskal–Wallis Chi-Squared = 88.289, df = 4, p-Value < 2.2 × 10−16) | ||||
|---|---|---|---|---|
| Aquarium area | 0 lx | 0.1–1 lx | 1–10 lx | 10–20 lx |
| 0.1–1 lx | 1.9 × 10−11 * | - | - | - |
| 1–10 lx | 2.2 × 10−13 * | 1 | - | - |
| 10–20 lx | 1.6 × 10−11 * | 1 | 1 | - |
| 20–30 lx | 0.01 * | 0.0003 * | 2.5 × 10−5 * | 0.0003 * |
| Without light (Kruskal–Wallis chi-squared = 73.573, df = 4, p-value = 3.992 × 10−15) | ||||
| Aquarium area | 0 lx | 0.1–1 lx | 1–10 lx | 10–20 lx |
| 0.1–1 lx | 0.002 * | - | - | - |
| 1–10 lx | 5.1 × 10−8 * | 0.08 | - | - |
| 10–20 lx | 8.2 × 10−14 * | 0.0001 * | 0.15 | - |
| 20–30 lx | 2.3 × 10−9 * | 0.02 * | 0.6 | 0.3 |
| Warm light (Kruskal–Wallis chi-squared = 70.742, df = 4, p-value = 1.582 × 10−14) | ||||
| Aquarium area | 0 lx | 0.1–1 lx | 1–10 lx | 10–20 lx |
| 0.1–1 lx | 4.3 × 10−6 * | - | - | - |
| 1–10 lx | 4.3 × 10−8 * | 0.9 | - | - |
| 10–20 lx | 1.7 × 10−14 * | 0.017 * | 0.16 | - |
| 20–30 lx | 2.3 × 10−8 * | 0.9 | 0.9 | 0.17 |
| Cold light (Kruskal–Wallis chi-squared = 78.753, df = 4, p-value = 3.2 × 10−16) | ||||
| Aquarium area | 0 lx | 0.1–1 lx | 1–10 lx | 10–20 lx |
| 0.1–1 lx | 9.7 × 10−5 * | - | - | - |
| 1–10 lx | 7.7 × 10−9 * | 0.22 | - | - |
| 10–20 lx | 2.9 × 10−16 * | 0.0002 * | 0.08 | - |
| 20–30 lx | 3.5 × 10−8 * | 0.25 | 0.7 | 0.049 * |
| Type of Lighting | Cold Light | Daylight | Without Light |
|---|---|---|---|
| Daylight | 0.007 * | - | - |
| Without light | 0.0004 * | 2.6 × 10−11 * | - |
| Warm light | 0.2 | 0.1 | 2 × 10−6 * |
| Daylight (Kruskal–Wallis Chi-Squared = 78.586, df = 4, p-Value = 3.472 × 10−16) | ||||
|---|---|---|---|---|
| Aquarium area | 0 lx | 0.1–1 lx | 1–10 lx | 10–20 lx |
| 0.1–1 lx | 1.6 × 10−7 * | - | - | - |
| 1–10 lx | 5.6 × 10−13 * | 0.3 | - | - |
| 10–20 lx | 2.3 × 10−12 * | 0.4 | 1 | - |
| 20–30 lx | 8.5 × 10−11 * | 0.9 | 1 | 1 |
| Without light (Kruskal–Wallis chi-squared = 61.508, df = 4, p-value = 1.398 × 10−12) | ||||
| Aquarium area | 0 lx | 0.1–1 lx | 1–10 lx | 10–20 lx |
| 0.1–1 lx | 0.017 * | - | - | - |
| 1–10 lx | 1.5 × 10−6 * | 0.1 | - | - |
| 10–20 lx | 3.5 × 10−12 * | 0.0001 * | 0.11 | - |
| 20–30 lx | 1.1 × 10−6 * | 0.1 | 0.9 | 0.11 |
| Warm light (Kruskal–Wallis chi-squared = 92.921, df = 4, p-value < 2.2 × 10−16) | ||||
| Aquarium area | 0 lx | 0.1–1 lx | 1–10 lx | 10–20 lx |
| 0.1–1 lx | 0.004 * | - | - | - |
| 1–10 lx | 7.1 × 10−9 * | 0.02 * | - | - |
| 10–20 lx | 2.9 × 10−15 * | 8.5 × 10−6 * | 0.1 | - |
| 20–30 lx | 1.2 × 10−13 * | 7.1 × 10−5 * | 0.2 | 0.6 |
| Cold light (Kruskal–Wallis chi-squared = 72.433, df = 4, p-value = 6.952 × 10−15) | ||||
| Aquarium area | 0 lx | 0.1–1 lx | 1–10 lx | 10–20 lx |
| 0.1–1 lx | 1.9 × 10−5 * | - | - | - |
| 1–10 lx | 8.3 × 10−9 * | 0.38 | - | - |
| 10–20 lx | 2.7 × 10−14 * | 0.007 * | 0.35 | - |
| 20–30 lx | 4.7 × 10−9 * | 0.38 | 0.9 | 0.36 |
| Daylight (W = 6701.5) | Without Light (W = 8563) | Warm Light (W = 6074.5) | Cold Light (W = 6946.5) | ||||
|---|---|---|---|---|---|---|---|
| River Angara | River Angara | River Angara | River Angara | ||||
| Lake № 14 | 0.03 * | Lake № 14 | 0.18 | Lake № 14 | 0.001 * | Lake № 14 | 0.12 |
| Type of Lighting | Cold Light | Daylight | Without Light |
|---|---|---|---|
| Daylight | 0.0005 * | - | - |
| Without light | 0.01 * | 0.1 | - |
| Warm light | 0.5 | 0.0005 * | 0.0005 * |
| Type of Lighting | Cold Light | Daylight | Without Light |
|---|---|---|---|
| Daylight | 0.6 | - | - |
| Without light | 0.0007 * | 0.0001 * | - |
| Warm light | 0.2 | 0.2 | 10−7 * |
| Daylight | Without Light | Warm Light | Cold Light | ||||
|---|---|---|---|---|---|---|---|
| River Angara | River Angara | River Angara | River Angara | ||||
| Lake № 14 | 0.0008 * | Lake № 14 | 0.7 | Lake № 14 | 0.6 | Lake № 14 | 0.9 |
| Without Shelter from the Light (the Absence of Stones in Aquariums) | |||||
|---|---|---|---|---|---|
| Type of lighting | Daylight | Cold 10–11 lx | Cold 2–2.5 lx | Without light | Warm 10–11 lx |
| Cold 10–11 lx | 1 | - | - | - | - |
| Cold 2–2.5 lx | 1 | 0.4 | - | - | - |
| Without light | 0.0001 * | 0.002 * | 1.4× 10−6 * | - | - |
| Warm 10–11 lx | 0.2 | 0.03 * | 1 | 2.5 × 10−9 * | - |
| Warm 2–2.5 lx | 0.0004 * | 0.006 * | 5.8 × 10−6 * | 1 | 1.4 × 10−8 * |
| With shelter from the light (the presence of stones in aquariums) | |||||
| Type of lighting | Daylight | Cold 10–11 lx | Cold 2–2.5 lx | Without light | Warm 10–11 lx |
| Cold 10–11 lx | 1 | - | - | - | - |
| Cold 2–2.5 lx | 0.01 * | 0.2 | - | - | - |
| Without light | 1 | 1 | 0.02 * | - | - |
| Warm 10–11 lx | 0.5 | 1 | 1 | 0.6 | - |
| Warm 2–2.5 lx | 0.0003 * | 0.01 * | 1 | 0.0005 * | 0.1 |
| Type of Lighting | p-Value |
|---|---|
| Daylight | 0.2 |
| Cold 10–11 lx | 0.7 |
| Cold 2–2.5 lx | 0.0002 * |
| Without light | 2.757 × 10−10 * |
| Warm 10–11 lx | 0.0008 * |
| Warm 2–2.5 lx | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ermolaeva, Y.; Maslennikova, M.; Golubets, D.; Lavnikova, A.; Kulbachnaya, N.; Biritskaya, S.; Solodkova, A.; Kodatenko, I.; Guliguev, A.; Rechile, D.; et al. Effects of Long-Term Urban Light Pollution and LED Light Color Temperature on the Behavior of a Holarctic Amphipod Gammarus lacustris Sars, 1863. Hydrobiology 2025, 4, 23. https://doi.org/10.3390/hydrobiology4030023
Ermolaeva Y, Maslennikova M, Golubets D, Lavnikova A, Kulbachnaya N, Biritskaya S, Solodkova A, Kodatenko I, Guliguev A, Rechile D, et al. Effects of Long-Term Urban Light Pollution and LED Light Color Temperature on the Behavior of a Holarctic Amphipod Gammarus lacustris Sars, 1863. Hydrobiology. 2025; 4(3):23. https://doi.org/10.3390/hydrobiology4030023
Chicago/Turabian StyleErmolaeva, Yana, Maria Maslennikova, Dmitry Golubets, Arina Lavnikova, Natalia Kulbachnaya, Sofya Biritskaya, Anastasia Solodkova, Ivan Kodatenko, Artem Guliguev, Diana Rechile, and et al. 2025. "Effects of Long-Term Urban Light Pollution and LED Light Color Temperature on the Behavior of a Holarctic Amphipod Gammarus lacustris Sars, 1863" Hydrobiology 4, no. 3: 23. https://doi.org/10.3390/hydrobiology4030023
APA StyleErmolaeva, Y., Maslennikova, M., Golubets, D., Lavnikova, A., Kulbachnaya, N., Biritskaya, S., Solodkova, A., Kodatenko, I., Guliguev, A., Rechile, D., Salovarov, K., Olimova, A., Kondratieva, D., Solomka, A., Slepchenko, A., Bashkirtsev, A., Karnaukhov, D., & Silow, E. (2025). Effects of Long-Term Urban Light Pollution and LED Light Color Temperature on the Behavior of a Holarctic Amphipod Gammarus lacustris Sars, 1863. Hydrobiology, 4(3), 23. https://doi.org/10.3390/hydrobiology4030023






