Toxic Effects of Bisphenol A on L. variegatus and A. punctulata Sea Urchin Embryos
Abstract
1. Introduction
2. Materials and Methods
2.1. Sea Urchin Fertilization Procedure
2.2. Exposure of Embryos to Bisphenol A Solutions
2.3. Light Microscopy Procedure and Data Collection
2.4. Scanning Electron Microscopy Procedure and Data Collection
3. Results
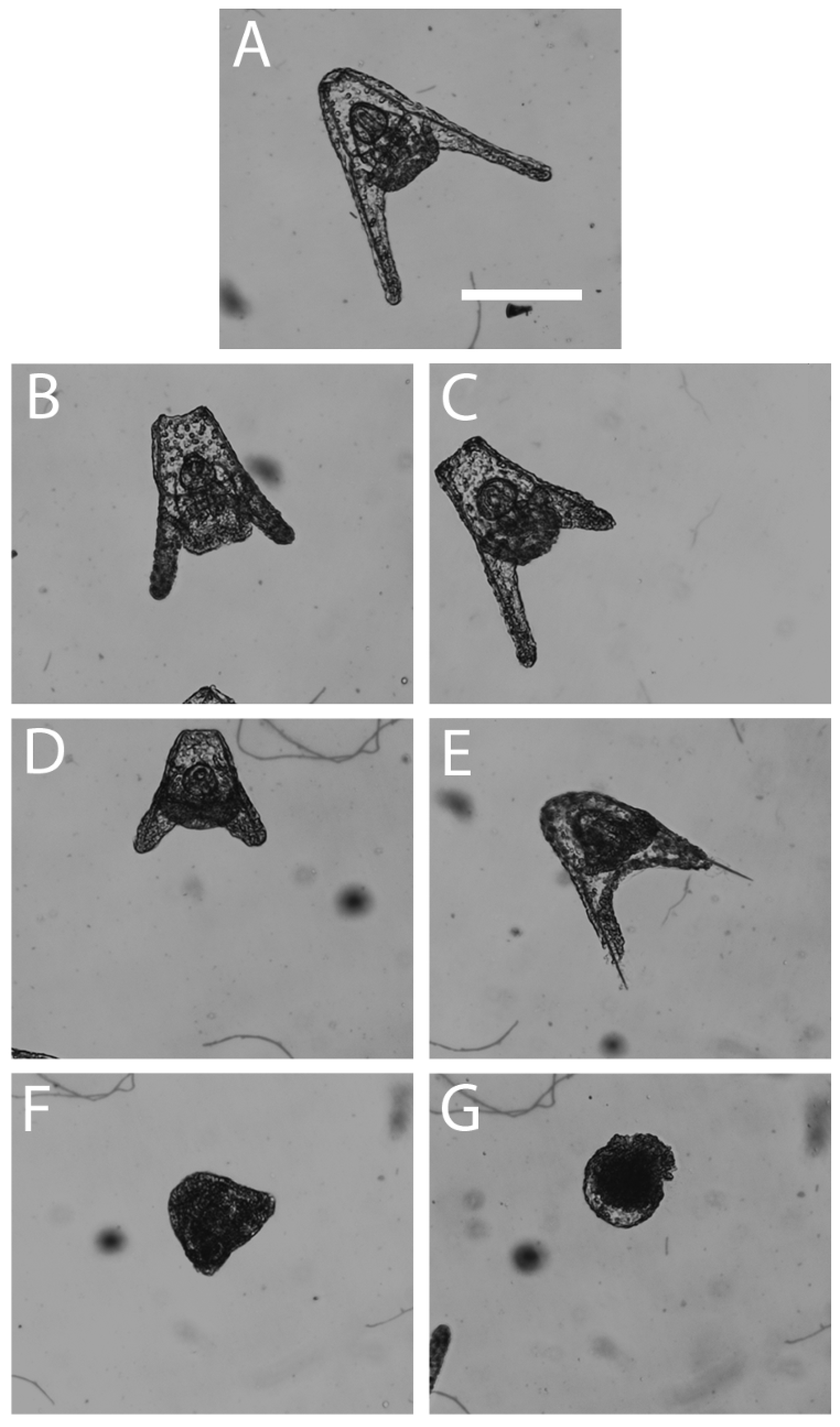
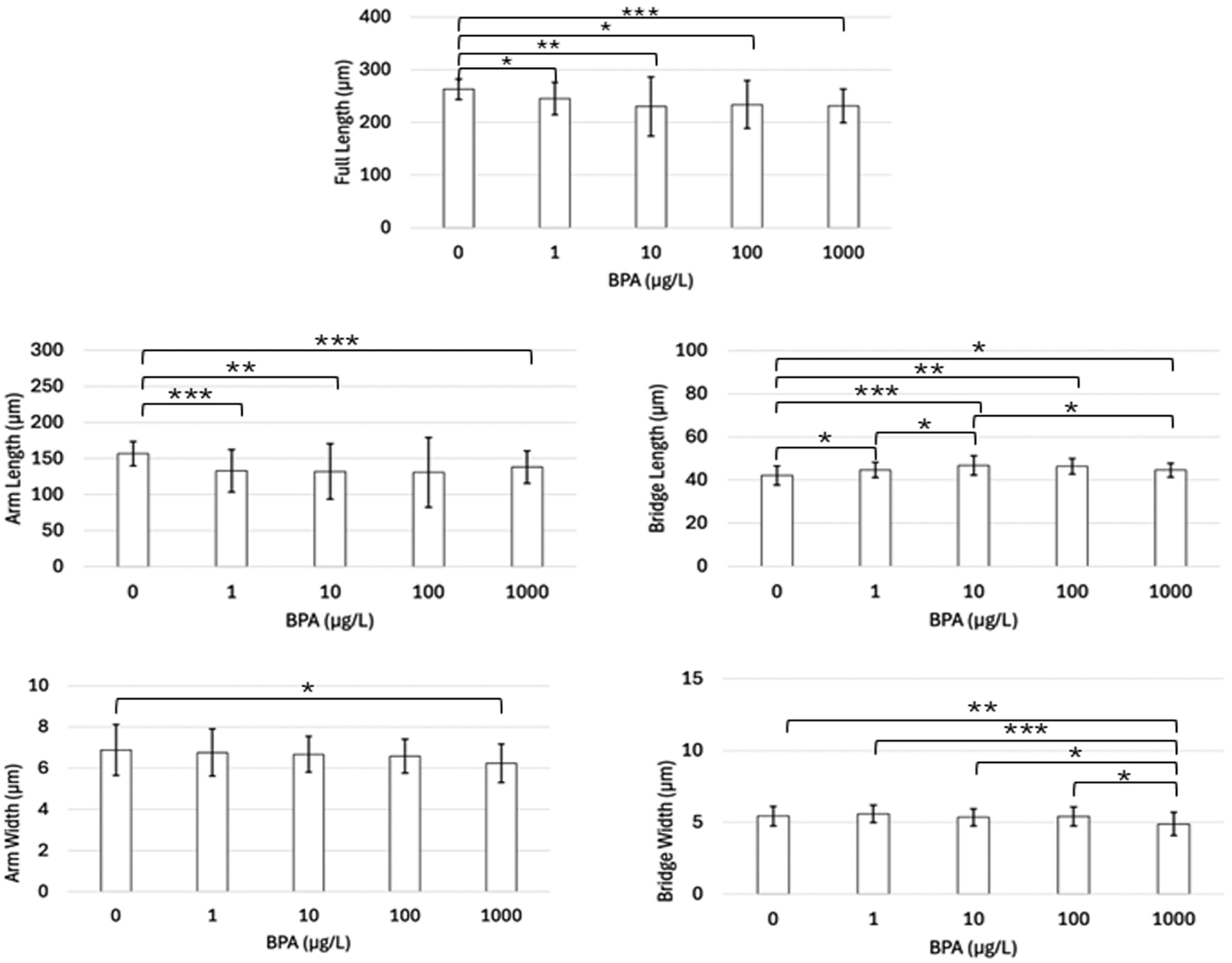
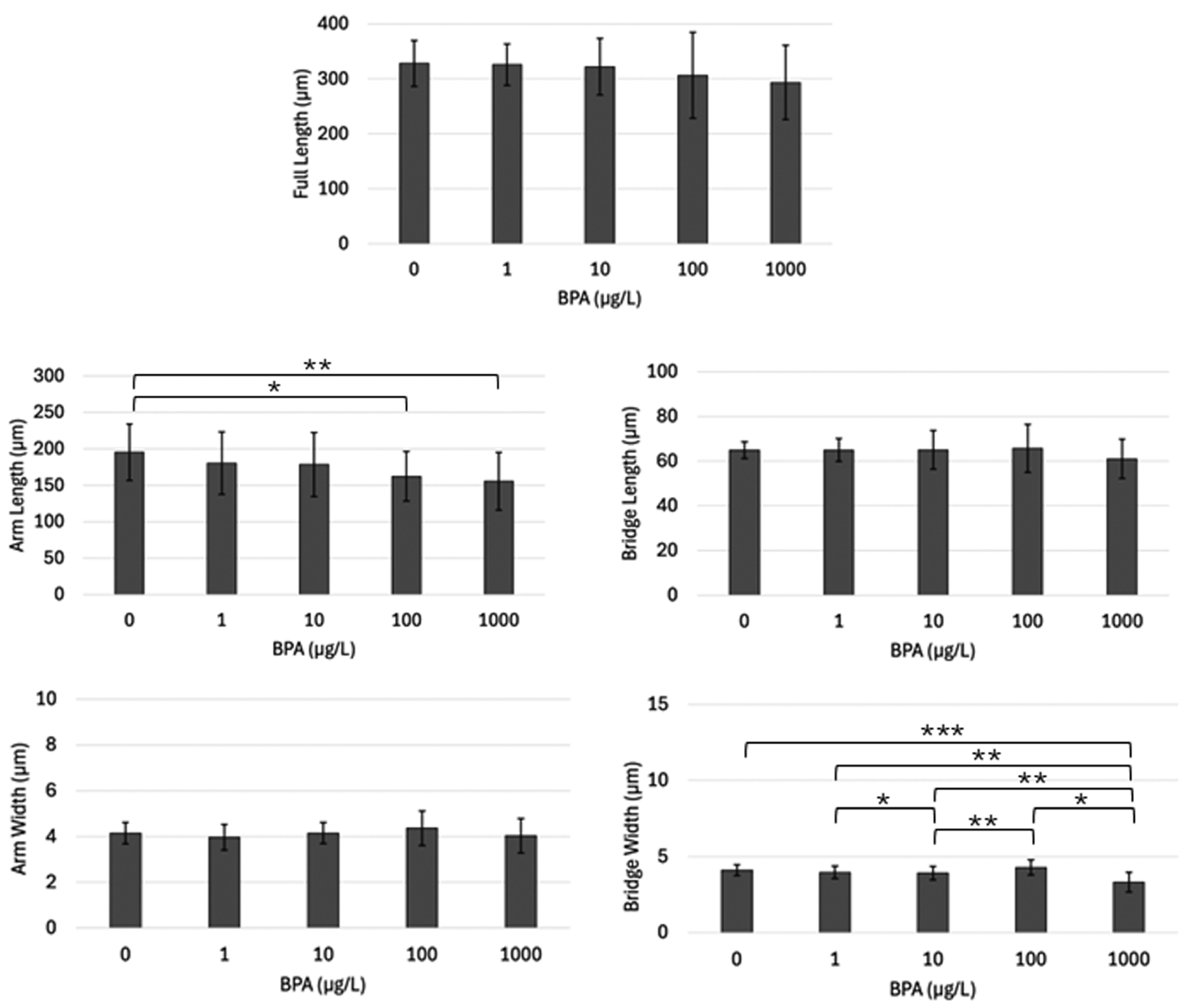
3.1. Light Microscope Analysis
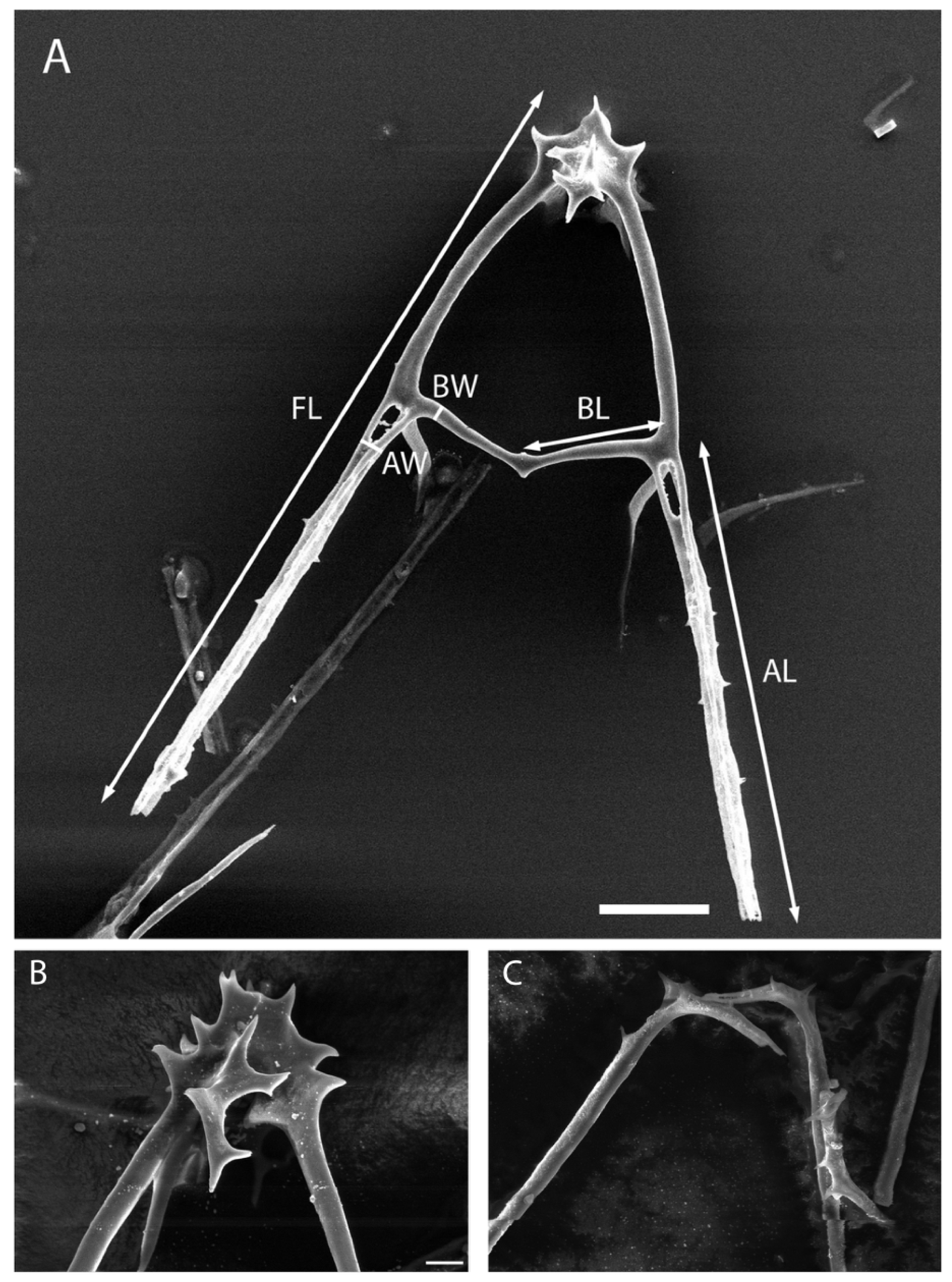
3.2. SEM Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vogel, S.A. The politics of plastics: The making and unmaking of bisphenol A “safety”. Am. J. Public Health 2009, 99, S559–S566. [Google Scholar] [CrossRef] [PubMed]
- Xing, J.; Zhang, S.; Zhang, M.; Hou, J. A critical review of presence, removal and potential impacts of endocrine disruptors bisphenol A. Comp. Biochem. Phys. C 2002, 254, 109275. [Google Scholar] [CrossRef]
- Galloway, T.S.; Lee, B.P.; Buric, I.; Steele, A.M. Plastics additives and human health: A case study of bisphenol A (BPA). In Plastics and the Environment; Hester, R.E., Harrison, R.M., Eds.; Royal Society of Chemistry: London, UK, 2018; pp. 132–153. [Google Scholar] [CrossRef]
- Krishnan, A.V.; Stathis, P.; Permuth, S.F.; Tokes, L.; Feldman, D. Bisphenol-A: An estrogenic substance is released from polycarbonate flasks during autoclaving. Endocrinology 1993, 132, 2279–2286. [Google Scholar] [CrossRef]
- Nagel, S.C.; vom Saal, F.S.; Thayer, K.A.; Dhar, M.G.; Boechler, M.; Welshons, W.V. Relative binding affinity-serum modified access (RBA-SMA) assay predicts the relative in vivo bioactivity of the xenoestrogens bisphenol A and octylphenol. Environ. Health Perspect. 1997, 105, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Colerangle, J.B.; Roy, D. Profound effects of the weak environmental estrogen-like chemical bisphenol A on the growth of the mammary gland of Noble rats. J. Steroid Biochem. 1997, 60, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Steinmetz, R.; Mitchner, N.A.; Grant, A.; Allen, D.L.; Bigsby, R.M.; Ben-Jonathan, N. The xenoestrogen bisphenol A induces growth, differentiation, and c-fos gene expression in the female reproductive tract. Endocrinology 1998, 139, 2741–2747. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Food Additives, Flavourings, Processing Aids and Materials in Contact with Food; Anton, R.; Barlow, S.; Boskou, D.; Castle, L.; Crebelli, R.; Dekant, W.; Engel, K.-H.; Forsythe, S.; Grunow, W.; et al. Opinion of the Scientific Panel on food additives, flavourings, processing aids and materials in contact with food (AFC) related to 2,2-bis(4-hydroxyphenyl)propane bis(2,3-epoxypropyl)ether (Bisphenol A diglycidyl ether, BADGE). REF. No 13510 and 39700. EFSA J. 2004, 2, 86–126. [Google Scholar] [CrossRef]
- Bloom, M.S.; vom Saal, F.S.; Kim, D.; Taylor, J.A.; Lamb, J.D.; Fujimoto, V.Y. Serum unconjugated bisphenol A concentrations in men may influence embryo quality indicators during in vitro fertilization. Environ. Toxicol. Phar. 2011, 32, 319–323. [Google Scholar] [CrossRef]
- Meeker, J.D.; Ehrlich, S.; Toth, T.L.; Wright, D.L.; Calafat, A.M.; Trisini, A.T.; Ye, X.; Hauser, R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod. Toxicol. 2010, 30, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, Y.; Zhao, J.; Dai, Y.; Luo, X.; Shen, Z.; Chen, X.; Yuan, W.; Shen, Y. Association between serum bisphenol-A and recurrent spontaneous abortion: A 1:2 case-control study, China. Zhonghua Liu Xing Bing Xue Za Zhi 2012, 33, 841–845. [Google Scholar] [PubMed]
- Cantonwine, D.; Meeker, J.D.; Hu, H.; Sánchez, B.N.; Lamadrid-Figueroa, H.; Mercado-García, A.; Fortenberry, G.Z.; Calafat, A.M.; Téllez-Rojo, M.M. Bisphenol A exposure in Mexico City and risk of prematurity: A pilot nested case control study. Environ. Health 2010, 9, 62. [Google Scholar] [CrossRef]
- Hao, J.; Wang, J.; Zhao, W.; Ding, L.; Gao, E.; Yuan, W. Effect of bisphenol A exposure on sex hormone level in occupational women. Wei Sheng Yan Jiu 2011, 40, 312–319. [Google Scholar] [PubMed]
- Kandaraki, E.; Chatzigeorgiou, A.; Livadas, S.; Palioura, E.; Economou, F.; Koutsilieris, M.; Palimeri, S.; Panidis, D.; Diamanti-Kandarakis, E. Endocrine disruptors and polycystic ovary syndrome (PCOS): Elevated serum levels of bisphenol A in women with PCOS. J. Clin. Endocr. Metab. 2011, 96, E480–E484. [Google Scholar] [CrossRef]
- Rees Clayton, E.M.; Todd, M.; Dowd, J.B.; Aiello, A.E. The impact of bisphenol A and triclosan on immune parameters in the U.S. population, NHANES 2003–2006. Environ. Health Perspect. 2011, 119, 390–396. [Google Scholar] [CrossRef]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.-T.; Wareham, N.J.; et al. Urinary bisphenol A concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef]
- Yang, Y.J.; Hong, Y.-C.; Oh, S.-Y.; Park, M.-S.; Kim, H.; Leem, J.-H.; Ha, E.-H. Bisphenol A exposure is associated with oxidative stress and inflammation in postmenopausal women. Environ. Res. 2009, 109, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol A concentration with medical disorders and laboratory abnormalities in adults. J. Am. Med. Assoc. 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Melzer, D.; Harries, L.; Cipelli, R.; Henley, W.; Money, C.; McCormack, P.; Young, A.; Guralnik, J.; Ferrucci, L.; Bandinelli, S.; et al. Bisphenol A exposure is associated with in vivo estrogenic gene expression in adults. Environ. Health Perspect. 2011, 119, 1788–1793. [Google Scholar] [CrossRef] [PubMed]
- Rochester, J.R. Bisphenol A and human health: A review of the literature. Reprod. Toxicol. 2013, 42, 132–155. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-H.; Kondo, F. Bisphenol A degradation in seawater is different from that in river water. Chemosphere 2005, 60, 1288–1292. [Google Scholar] [CrossRef]
- Fabrello, J.; Matozzo, V. Bisphenol analogs in aquatic environments and their effects on marine species—A review. J. Mar. Sci. Eng. 2022, 10, 1271–1287. [Google Scholar] [CrossRef]
- Yamazaki, E.; Yamashita, N.; Taniyasu, S.; Lam, J.; Lam, P.K.S.; Moon, H.-B.; Jeong, Y.; Kannan, P.; Achyuthan, H.; Munuswamy, N.; et al. Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotox. Environ. Saf. 2015, 122, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wu, L.-H.; Liu, G.-Q.; Shi, L.; Guo, Y. Occurrence and ecological risk assessment of eight endocrine-disrupting chemicals in urban river water and sediments of South China. Arch. Environ. Contam. Toxicol. 2018, 75, 224–235. [Google Scholar] [CrossRef]
- Ozhan, K.; Kocaman, E. Temporal and spatial distributions of bisphenol A in marine and freshwaters in Turkey. Arch. Environ. Contam. Toxicol. 2019, 76, 246–254. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, N.; Zhang, Y.; Hu, H.; Zhao, M.; Jin, H. Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in sea water and sediment from East China Sea. Chemosphere 2022, 287, 132218. [Google Scholar] [CrossRef] [PubMed]
- Sohoni, P.; Tyler, C.R.; Hurd, K.; Caunter, J.; Hetheridge, M.; Williams, T.; Woods, C.; Evans, M.; Toy, R.; Gargas, M.; et al. Reproductive effects of long-term exposure to bisphenol A in the fathead minnow (Pimephales promelas). Environ. Sci. Technol. 2001, 35, 2917–2925. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Shi, W.; Tang, Y.; Zhou, W.; Sun, H.; Zhang, J.; Yan, M.; Hu, L.; Liu, G. Microplastics and bisphenol A hamper gonadal development of whiteleg shrimp (Litopenaeus vannamei) by interfering with metabolism and disrupting hormone regulation. Sci. Total Environ. 2022, 810, 152354. [Google Scholar] [CrossRef] [PubMed]
- Janer, G.G.; LeBlanc, A.; Porte, C. A comparative study on androgen metabolism in three invertebrate species. Gen. Comp. Endocr. 2005, 143, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Pagano, G.; Cipollaro, M.; Corsale, G.; Esposito, A.; Ragucci, E.; Giordano, G.G.; Trieff, N.M. The sea urchin: Bioassay for the assessment of damage from environmental contaminants. In Community Toxicity Testing, ASTM STP 920; Cairns, J., Ed.; American Society for Testing and Materials: Philadelphia, PA, USA, 1986; pp. 66–92. [Google Scholar] [CrossRef]
- Kobayashi, N. Marine pollution bioassay by using sea urchin eggs in the Tanabe Bay, Wakayama Prefecture, Japan, 1970–1987. Mar. Pollut. Bull. 1991, 23, 709–713. [Google Scholar] [CrossRef]
- Roepke, T.A.; Snyder, M.J.; Cherr, G.N. Estradiol and endocrine disrupting compounds adversely affect development of sea urchin embryos at environmentally relevant concentrations. Aquat. Toxicol. 2005, 71, 155–173. [Google Scholar] [CrossRef] [PubMed]
- Kiyomoto, M.; Kikuchi, A.; Unuma, T.; Yokota, Y. Effects of ethinyl estradiol and bisphenol A on the development of sea urchin embryos and juveniles. Mar. Biol. 2006, 149, 57–63. [Google Scholar] [CrossRef]
- Arslan, Ö.Ç.; Hatice, P. Effects of bisphenol A on the embryonic development of sea urchin (Paracentrotus lividus). Environ. Toxicol. 2008, 23, 387–392. [Google Scholar] [CrossRef]
- da Silva, A.Q.; de Souza Abessa, D.M. Toxicity of three emerging contaminants to non-target marine organisms. Environ. Sci. Pollut. Res. 2019, 26, 18354–18364. [Google Scholar] [CrossRef] [PubMed]
- Rezg, R.; Oral, R.; Tez, S.; Mornagui, B.; Pagano, G.; Trifu Oggi, T. Cytogenetic and developmental toxicity of bisphenol A and bisphenol S in Arbacia lixula sea urchin embryos. Ecotoxicology 2022, 31, 1087–1095. [Google Scholar] [CrossRef]
- Tato, T.; Salgueiro-González, N.; León, V.M.; González, S.; Beiras, R. Ecotoxicological evaluation of the risk posed by bisphenol A, triclosan, and 4-nonylphenol in coastal waters using early life stages of marine organisms (Isochrysis galbana, Mytilus galloprovincialis, Paracentrotus lividus, and Acartia clausi). Environ. Pollut. 2018, 232, 173–182. [Google Scholar] [CrossRef] [PubMed]
- Arslan, Ö.Ç.; Karaaslan, M.A.; Nalbantlar, B. Developmental effects of bisphenol A and its analogs bisphenol S, bisphenol F and bisphenol AF on sea urchins Paracentrotus lividus (Lamarck 1816) and Arbacia lixula (Linnaeus 1758). Res. Sq. 2023, 6, 56–70. [Google Scholar] [CrossRef]
- Gambardella, C.; Marcellini, F.; Falugi, C.; Varrella, S.; Corinaldesi, C. Early-stage anomalies in the sea urchin (Paracentrotus lividus) as bioindicators of multiple stressors in the marine environment: Overview and future perspectives. Environ. Pollut. 2021, 287, 117608. [Google Scholar] [CrossRef] [PubMed]
- Ward, T.J.; Kramer, J.R.; Boeri, R.L.; Gorsuch, J.W. Chronic toxicity of silver to the sea urchin (Arbacia punctulata). Environ. Toxicol. Chem. 2009, 25, 1568–1573. [Google Scholar] [CrossRef]
- Perina, F.C.; de Souza Abessa, D.M.; Pinho, G.L.L.; Fillmann, G. Comparative toxicity of antifouling compounds on the development of sea urchin. Ecotoxicology 2011, 20, 1870–1880. [Google Scholar] [CrossRef] [PubMed]
- Böttger, S.A.; McClintock, J.B. The effects of organic and inorganic phosphates on fertilization and early development in the sea urchin Lytechinus variegatus (Echinodermata: Echinoidea). Comp. Biochem. Phys. C 2001, 129, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.; Rittschof, D.; Orihuela, B.; Kwok, K.W.H.; Stafslien, S.; Chisholm, B. The effects of model polysiloxane and fouling-release coatings on embryonic development of a sea urchin (Arbacia punctulata) and a fish (Oryzias latipes). Aquat. Toxicol. 2012, 110–111, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.K.; Lawrence, J.M. Habitats and characteristics of the sea urchins Lytechinus variegatus and Arbacia punctulata (Echinodermata) on the Florida Gulf-Coast Shelf. Mar. Ecol. 2003, 24, 15–30. [Google Scholar] [CrossRef]
- McEdward, L.R.; Herrera, J.C. Body form and skeletal morphometrics during larval development of the sea urchin Lytechinus variegatus Lamarck. J. Exp. Mar. Biol. Ecol. 1999, 232, 151–176. [Google Scholar] [CrossRef]
- Harvey, E.B. The growth and metamorphosis of the Arbacia punctulata pluteus, and late development of the white halves of centrifuged eggs. Biol. Bull. 1949, 97, 287–299. [Google Scholar] [CrossRef]
- Leahy, P.S. Laboratory culture of Strongylocentrotus purpuratus adults, embryos, and larvae. In Methods in Cell Biology, Volume 27; Schroeder, T.E., Ed.; Academic Press: New York, NY, USA, 1986; pp. 1–14. [Google Scholar]
- Semenova, M.N.; Demchuk, D.V.; Tsyganov, D.V.; Chernysheva, N.B.; Samet, A.V.; Silyanova, E.A.; Kislyi, V.P.; Maksimenko, A.S.; Varakutin, A.E.; Konyushkin, L.D.; et al. Sea urchin embryo model as a reliable in vivo phenotypic screen to characterize selective antimitotic molecules. Comparative evaluation of combretapyrazoles, -isoxazoles, -1,2,3-triazoles, and -pyrroles as tubulin-binding agents. ACS Comb. Sci. 2018, 20, 700–721. [Google Scholar] [CrossRef] [PubMed]
- Semenova, M.N.; Kuptsova, T.S.; Semenova, V.V. Toxicity of organic solvents and surfactants to the sea urchin embryos. Chemosphere 2024, 353, 141589. [Google Scholar] [CrossRef] [PubMed]
- Bellas, J.; Rial, D.; Valdés, J.; Vidal-Liñán, L.; Bertucci, J.I.; Muniategui, S.; León, V.M.; Campillo, J.A. Linking biochemical and individual-level effects of chlorpyrifos, triphenyl phosphate, and bisphenol A on sea urchin (Paracentrotus lividus) larvae. Environ. Sci. Pollut. Res. 2022, 29, 46174–46187. [Google Scholar] [CrossRef] [PubMed]
- Clark, D.M.; Lamare, M.; Barker, M. Response of sea urchin pluteus larvae (Echinodermata: Echinoidea) to reduced seawater pH: A comparison among a tropical, temperate, and a polar species. Mar. Biol. 2009, 156, 1125–1137. [Google Scholar] [CrossRef]
- Canesi, L.; Fabbri, E. Environmental effects of BPA: Focus on aquatic species. Dose Response 2015, 13, 1559325815598304. [Google Scholar] [CrossRef]
- Wu, N.C.; Seebacher, F. Effect of the plastic pollutant bisphenol A on the biology of aquatic organisms: A meta-analysis. Glob. Chang. Biol. 2020, 26, 3821–3833. [Google Scholar] [CrossRef]
- Garrison, M.D.; Storch, P.J.; Eck, W.S.; Adams, V.H.; Fedick, P.W.; Harvey, B.G. BPA-free high-performance sustainable polycarbonates derived from non-estrogenic bio-based phenols. Green Chem. 2021, 20, 8016–8029. [Google Scholar] [CrossRef]
- Guo, Y.; Shi, W.; Liu, Z.; Sun, X.; Wu, J.; Wu, Y. Bisphenol A alternatives continuously contribute to the endocrine disruption in cetaceans. Environ. Int. 2023, 171, 107679. [Google Scholar] [CrossRef]
- Meng, X.; Su, S.; Wei, X.; Wang, S.; Guo, T.; Li, J.; Song, H.; Wang, M.; Wang, Z. Exposure to bisphenol A alternatives bisphenol AF and fluorene-9-bisphenol induces gonadal injuries in male zebrafish. Ecotoxicol. Environ. Saf. 2023, 253, 114634. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunsman, J.D.; Schlesinger, M.C.; McCain, E.R. Toxic Effects of Bisphenol A on L. variegatus and A. punctulata Sea Urchin Embryos. Hydrobiology 2025, 4, 5. https://doi.org/10.3390/hydrobiology4010005
Kunsman JD, Schlesinger MC, McCain ER. Toxic Effects of Bisphenol A on L. variegatus and A. punctulata Sea Urchin Embryos. Hydrobiology. 2025; 4(1):5. https://doi.org/10.3390/hydrobiology4010005
Chicago/Turabian StyleKunsman, Jacob D., Maya C. Schlesinger, and Elizabeth R. McCain. 2025. "Toxic Effects of Bisphenol A on L. variegatus and A. punctulata Sea Urchin Embryos" Hydrobiology 4, no. 1: 5. https://doi.org/10.3390/hydrobiology4010005
APA StyleKunsman, J. D., Schlesinger, M. C., & McCain, E. R. (2025). Toxic Effects of Bisphenol A on L. variegatus and A. punctulata Sea Urchin Embryos. Hydrobiology, 4(1), 5. https://doi.org/10.3390/hydrobiology4010005





