Effects of Tree Leaves, Tannins, and Water Color on Chlorophyll Concentrations in Ponds
Abstract
1. Introduction
2. Materials and Methods
2.1. Effects of Leaf Litter on Chlorophyll Concentrations
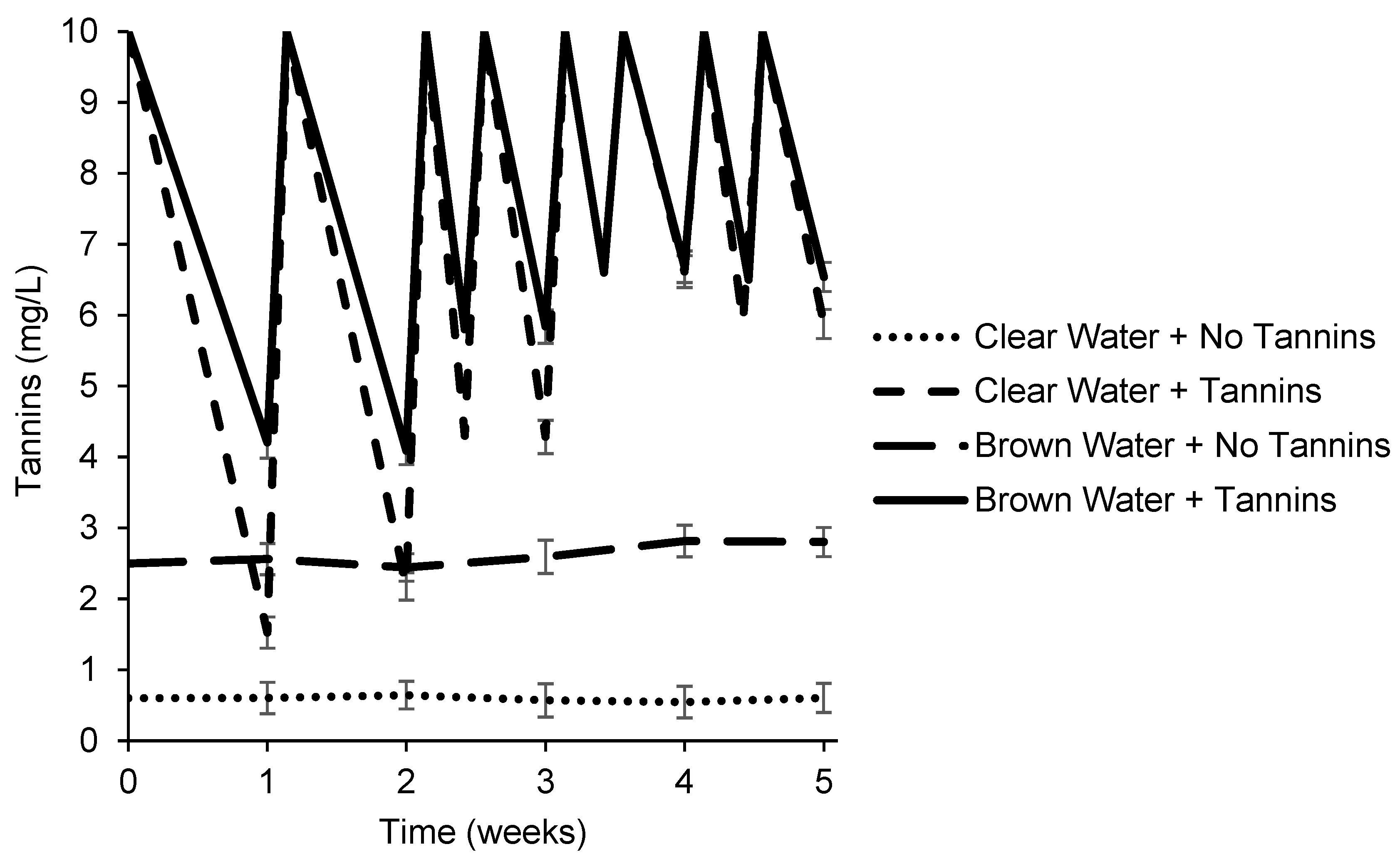
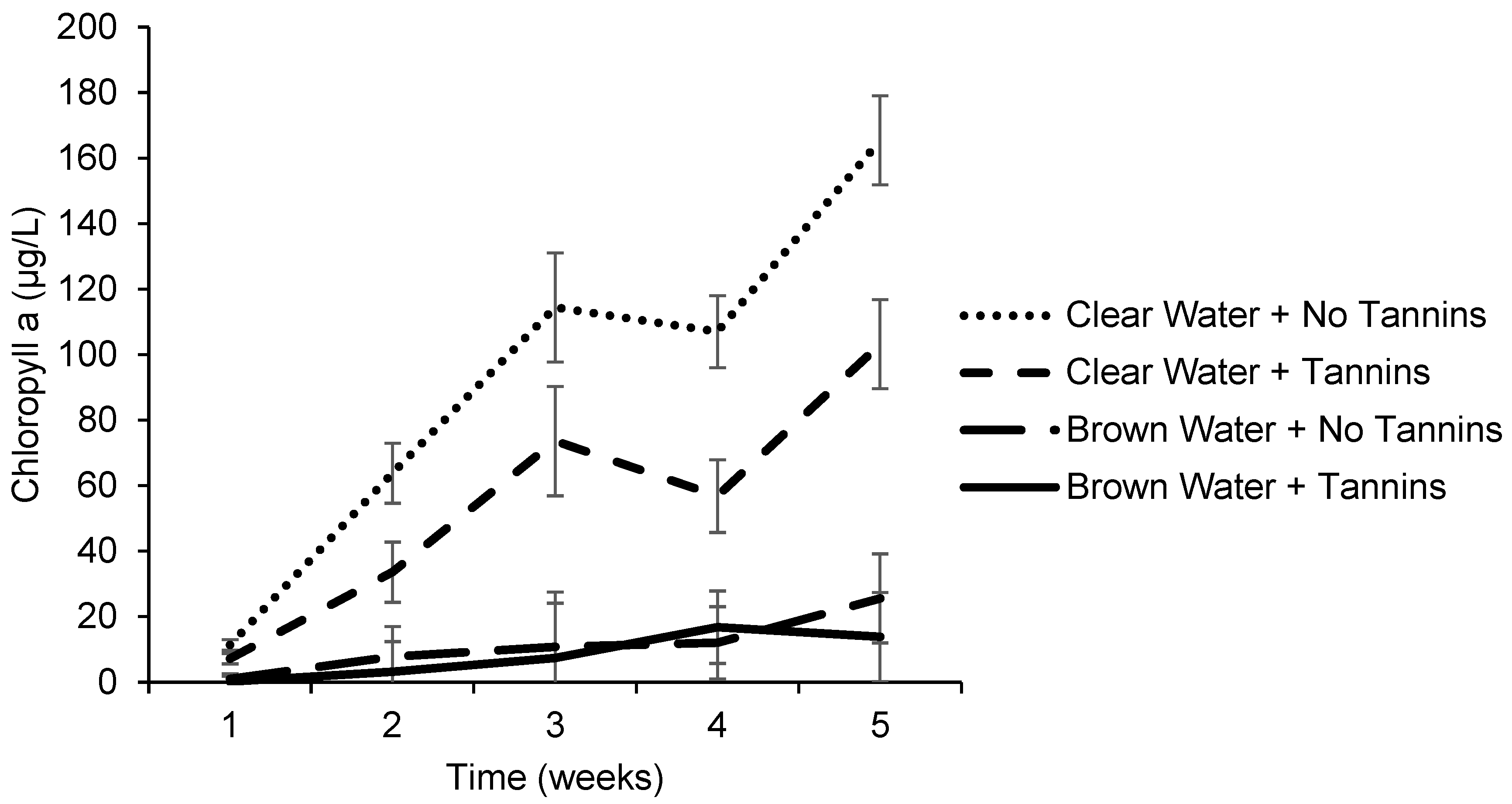
2.2. Effects of Water Color and Tannins on Chlorophyll Concentration
2.3. Statistical Analyses
3. Results
3.1. Effects of Tree Leaves on Chlorophyll Concentration
3.2. Effects of Tannic Acid and Water Color on Chlorophyll Concentrations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vannote, R.L.; Minshall, G.W.; Cummins, K.W.; Sedell, J.R.; Cushing, C.E. The river continuum concept. Can. J. Fish. Aquat. Sci. 1980, 37, 130–137. [Google Scholar] [CrossRef]
- Stoler, A.B.; Relyea, R.A. Reviewing the role of plant litter inputs to forested wetland ecosystems: Leafing through the literature. Ecol. Monogr. 2020, 90, e01400. [Google Scholar] [CrossRef]
- Marks, J.C. Revisiting the fates of dead leaves that fall into streams. Annu. Rev. Ecol. Evol. Syst. 2019, 50, 547–568. [Google Scholar] [CrossRef]
- Earl, J.E.; Semlitsch, R.D. Spatial subsidies, trophic state, and community structure: Examining effects of leaf litter on ponds. Ecosystems 2013, 16, 639–651. [Google Scholar] [CrossRef]
- Earl, J.E.; Semlitsch, R.D. Effect of tannin source and concentration from tree leaves on two species of tadpoles. Environ. Toxicol. Chem. 2015, 34, 120–126. [Google Scholar] [CrossRef]
- Temmink, J.H.M.; Field, J.A.; Van Haastrecht, J.C.; Merkelbach, R.C.M. Acute and sub-acute toxicity of bark tannins in carp (Cyprinus carpio L.). Water Res. 1989, 23, 341–344. [Google Scholar] [CrossRef]
- Xie, Z.; Wang, M.; Deng, Y.; Li, J.; Li, J.; Pang, W.; Xie, L.; Jiang, D.; Huang, Z.; He, T.; et al. Acute toxicity of eucalyptus leachate tannins to zebrafish and the mitigation effect of Fe3+ on tannin toxicity. Ecotoxicol. Environ. Saf. 2022, 229, 113077. [Google Scholar] [CrossRef]
- Battacharya, P.; Swarnakar, S.; Mukhopadhyay, A.; Ghosh, S. Exposure of composite tannery effluent on snail, Pila globosa: A comparative assessment of toxic impacts of the untreated and membrane treated effluents. Ecotoxicol. Environ. Saf. 2016, 126, 45–55. [Google Scholar] [CrossRef]
- Tremolieres, M. Deoxygenating effect and toxicity of ground-up dried coniferous needles and leaves of Canadian trees in water: A preliminary study in comparison with litter of European trees. Water Res. 1988, 22, 21–28. [Google Scholar] [CrossRef]
- Xie, L.; Ma, Z.; Yang, G.; Huang, Y.; Wen, T.; Deng, Y.; Sun, J.; Zheng, S.; Wu, F.; Huang, K.; et al. Study on the inhibition mechanism of eucalyptus tannins against Microcystis aeruginosa. Ecotoxicol. Environ. Saf. 2023, 249, 114452. [Google Scholar] [CrossRef]
- Serrano, L.; Guisande, C. Effects of polyphenolic compounds on phytoplankton. Verhandlungen Int. Ver. Theor. Angew. 1990, 24, 282–288. [Google Scholar] [CrossRef]
- Dodds, W.K.; Whiles, M.R. Freshwater Ecology: Concepts and Environmental Application of Limnology, 3rd ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Wurtsbaugh, W.A.; Paerl, H.W.; Dodds, W.K. Nutrients, eutrophication and harmful algal blooms along the freshwater to marine continuum. WIREs Water 2019, 6, e373. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful algal blooms: A climate change co-stressor in marine and freshwater ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef] [PubMed]
- Patiño, R.; Christensen, V.G.; Graham, J.L.; Rogosch, J.S.; Rosen, B.H. Toxic algae in inland waters of the conterminous United States—A review and synthesis. Water 2023, 15, 2808. [Google Scholar] [CrossRef]
- Backer, L.C.; Manassaram-Baptiste, D.; LePrell, R.; Bolton, B. Cyanobacteria and Algae Blooms: Review of Health and Environmental Data from the Harmful Algal Bloom-Related Illness Surveillance System (HABISS) 2007–2011. Toxins 2015, 7, 1048–1064. [Google Scholar] [CrossRef] [PubMed]
- Leech, D.M.; Pollard, A.I.; Labou, S.G.; Hampton, S.E. Fewer blue lakes and more murky lakes across the continental U.S.: Implications for planktonic food webs. Limnol. Oceanogr. 2018, 63, 2661–2680. [Google Scholar] [CrossRef] [PubMed]
- Matveev, V.; Robson, B.J. Aquatic food web structure and the flow of carbon. Freshw. Rev. 2014, 7, 1–24. [Google Scholar] [CrossRef]
- Tittel, J.; Bissinger, V.; Zippel, B.; Gaedke, U.; Bell, E.; Lorke, A.; Kamjunke, N. Mixotrophs combine resource use to outcompete specialists: Implications for aquatic food webs. Proc. Natl. Acad. Sci. USA 2003, 100, 12776–12781. [Google Scholar] [CrossRef]
- Doi, H. Spatial patterns of autochthonous and allochthonous resources in aquatic food webs. Popul. Ecol. 2009, 51, 57–64. [Google Scholar] [CrossRef]
- Carpenter, S.R.; Cole, J.J.; Pace, M.L.; Van de Bogert, M.C.; Bade, D.L.; Bastviken, D.; Gille, C.M.; Hodgson, J.R.; Kitchell, J.F.; Kritzberg, E.S. Ecosystem subsidies: Terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology 2005, 86, 2737–2750. [Google Scholar] [CrossRef]
- Earl, J.E.; Castello, P.O.; Cohagen, K.E.; Semlitsch, R.D. Effects of subsidy quality on reciprocal subsidies: How leaf litter species changes frog biomass export. Oecologia 2014, 175, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Stoler, A.B.; Relyea, R.A. Living in the litter: The influence of tree leaf litter on wetland communities. Oikos 2011, 120, 862–872. [Google Scholar] [CrossRef]
- Siders, A.C.; Compson, Z.G.; Hungate, B.A.; Dijkstra, P.; Koch, G.W.; Wymore, A.S.; Grandy, A.S.; Marks, J.C. Litter identity affects assimilation of carbon and nitrogen by a shredding caddisfly. Ecosphere 2018, 9, e02340. [Google Scholar] [CrossRef]
- Stephens, J.P.; Berven, K.A.; Tiegs, S.D. Anthropogenic changes to leaf litter input affect the fitness of a larval amphibian. Freshw. Biol. 2013, 58, 1631–1646. [Google Scholar] [CrossRef]
- Stephens, J.P.; Berven, K.A.; Tiegs, S.D.; Raffel, T.R. Ecological stoichiometry quantitatively predicts responses of tadpoles to a food quality gradient. Ecology 2015, 96, 2070–2076. [Google Scholar] [CrossRef]
- Salminen, J.-P.; Karonen, M. Chemical ecology of tannins and other phenolics: We need a change in approach. Funct. Ecol. 2011, 25, 325–338. [Google Scholar] [CrossRef]
- Ostrofsky, M.L. Effect of tannins on leaf processing and conditioning rates in aquatic ecosystems: An empirical approach. Can. J. Fish. Aquat. Sci. 1993, 50, 1176–1180. [Google Scholar] [CrossRef]
- Compson, Z.G.; Hungate, B.A.; Whitham, T.G.; Koch, G.W.; Dijkstra, P.; Siders, A.C.; Wojtowicz, T.; Jacobs, R.; Rakestraw, D.N.; Allred, K.E.; et al. Linking tree genetics and stream consumers: Isotopic tracers elucidate controls on carbon and nitrogen assimiliation. Ecology 2018, 99, 1759–1770. [Google Scholar] [CrossRef]
- Oliveira, R.; Martinez, A.; Gonçalves, A.L.; Canhoto, C. Intra-specific leaf trait variability controls leaf decomposition of Vitis vinifera L. cultivars in streams. Aquat. Ecol. 2022, 56, 47–57. [Google Scholar] [CrossRef]
- Phlips, E.J.; Cichra, M.; Aldridge, F.J.; Jembeck, J. Light availability and variations in phytoplankton standing crops in a nutrient-rich blackwater river. Limnol. Oceanogr. 2000, 45, 916–929. [Google Scholar] [CrossRef]
- Luimstra, V.M.; Verspagen, J.M.H.; Xu, T.; Schuurmans, J.M.; Huisman, J. Changes in water color shift competition between phytoplankton species with contrasting light-harvesting strategies. Ecology 2020, 101, e02951. [Google Scholar] [CrossRef]
- Blanchet, C.C.; Arzel, C.; Davranche, A.; Kahilainen, K.K.; Secondi, J.; Taipale, S.; Lindberg, H.; Loehr, J.; Manninen-Johansen, S.; Sundell, J.; et al. Ecology and the extent of freshwater browning—What we know and what should be studied next in the context of global change. Sci. Total Environ. 2022, 812, 152420. [Google Scholar] [CrossRef] [PubMed]
- Kritzberg, E.S.; Hasselquist, E.M.; Škerlep, M.; Löfgren, S.; Olsson, O.; Stadmark, J.; Valinia, S.; Hansson, L.-A.; Laudon, H. Browning of freshwaters: Consequences to ecosystem services, underlying drivers, and potential mitigation measures. Ambio 2020, 49, 375–390. [Google Scholar] [CrossRef]
- Pagano, T.; Bida, M.; Kenny, J.E. Trends in levels of allochthonous dissolved organic carbon in natural water: A review of potential mechanisms under a changing climate. Water 2014, 6, 2862–2897. [Google Scholar] [CrossRef]
- Mattsson, T.; Kortelainen, P.; Laubel, A.; Evans, D.; Pujo-Pay, M.; Räike, A.; Conan, P. Export of dissolved organic matter in relation to land use along a European climatic gradient. Sci. Total Environ. 2009, 407, 1967–1976. [Google Scholar] [CrossRef]
- Pile, L.S.; Wang, G.G.; Stovall, J.P.; Siemann, E.; Wheeler, G.S.; Gabler, C.A. Mechanisms of Chinese Tallow (Triadica sebifera) invasion and their management implications—A review. For. Ecol. Manag. 2017, 404, 1–13. [Google Scholar] [CrossRef]
- Cameron, G.N.; LaPoint, T.W. Effects of tannins on the decomposition of Chinese tallow leaves by terrestrial and aquatic invertebrates. Oecologia 1978, 32, 349–366. [Google Scholar] [CrossRef]
- Cameron, G.N.; Spencer, S.R. Rapid leaf decay and nutrient release in a Chinese tallow forest. Oecologia 1989, 80, 222–228. [Google Scholar] [CrossRef]
- Edwards, D.J. The Impact of Leaf Litter Diversity on the Colonization of Aquatic Insects; Louisiana Tech Universtiy: Ruston, LA, USA, 2023. [Google Scholar]
- Petersen, R.C.; Cummins, K.W. Leaf processing in a woodland stream. Freshw. Biol. 1974, 4, 343–368. [Google Scholar] [CrossRef]
- Clesceri, L.S.; Greenberg, A.E.; Trussell, R.R. (Eds.) Standard Methods for the Examination of Water and Wastewater, 17th ed.; American Public Health Association: Washington, DC, USA, 1989; p. 1624. [Google Scholar]
- Jones, J.B., Jr. Plant tissue analysis in micronutrients. In Micronutrients in Agriculture, 2nd ed.; Mordvedt, J.J., Ed.; Soil Science Society of America: Madison, WI, USA, 1991; Volume 4, pp. 447–522. [Google Scholar]
- Hamdhani, H.; Eppenhimer, D.E.; Walker, D.; Bogan, M.T. Performance of a handheld chlorophyll-a fluorometer: Potential use for rapid algae monitoring. Water 2021, 13, 1409. [Google Scholar] [CrossRef]
- Cremella, B.; Huot, Y.; Bonilla, S. Interpretation of total phytoplankton and cyanobacteria fluorescence from cross-calibrated flourometers, including sensitivity to turbidity and colored dissolved organic matter. Limnol. Oceanogr. Methods 2018, 16, 881–894. [Google Scholar] [CrossRef]
- Kuha, J.; Jarvinen, M.; Salmi, P.; Karjalainen, J. Calibration of in situ chlorophyll flourometers for organic matter. Hydrobiologia 2020, 847, 4377–4387. [Google Scholar] [CrossRef]
- Carlson, R.E.; Shapiro, J. Dissolved humic substances: A major source of error in flourometric analyses involving lake waters. Limnol. Oceanogr. 1981, 26, 785–790. [Google Scholar] [CrossRef]
- Leppa, M.; Karjalainen, J.; Holopainen, A. In vivo-fluorescence and chlorophyll a determination in Finnish humic lakes. Aqua Fenn. 1995, 25, 33–38. [Google Scholar]
- Maerz, J.C.; Brown, C.J.; Chapin, C.T.; Blossey, B. Can secondary compounds of an invasive plant affect larval amphibians? Funct. Ecol. 2005, 19, 970–975. [Google Scholar] [CrossRef]
- Serrano, L. Leaching from vegetation of soluble polyphenolic compounds, and their abuandance in temporary ponds in the Doñana National Park (SW Spain). Hydrobiologia 1992, 229, 43–50. [Google Scholar] [CrossRef]
- Haverkamp, N.; Beauducel, A. Violation of the sphericity assumption and its effect on type-I error rates in repeated measures ANOVA and multi-level linear models (MLM). Front. Psychol. 2017, 8, 1841. [Google Scholar] [CrossRef]
- Abdi, H. The Greenhouse-Geisser Correction. In Encyclopedia of Research Design; Salkind, N., Ed.; Sage: Thousand Oaks, CA, USA, 2010; pp. 1–10. [Google Scholar]
- Fey, S.B.; Mertens, A.N.; Beversdorf, L.J.; McMahon, K.D.; Cottingham, K.L. Recognizing cross-ecosystem responses to changing temperatures: Soil warming impacts pelagic food webs. Oikos 2015, 124, 1473–1481. [Google Scholar] [CrossRef]
- Rubbo, M.J.; Kiesecker, J.M. Leaf litter composition and community structure: Translating regional species changes into local dynamics. Ecology 2004, 85, 2519–2525. [Google Scholar] [CrossRef]
- Earl, J.E.; Semlitsch, R.D. Reciprocal subsidies in ponds: Does leaf input increase frog biomass export? Oecologia 2012, 170, 1077–1087. [Google Scholar] [CrossRef]
- Cohen, J.S.; Maerz, J.C.; Blossey, B. Traits, not origin, explain impacts of plants on larval amphibians. Ecol. Appl. 2012, 22, 218–228. [Google Scholar] [CrossRef]
- Karlsson, J.; Byström, P.; Ask, J.; Ask, P.; Persson, L.; Jansson, M. Light limitation of nutrient-poor lake ecosystems. Nature 2009, 460, 506–509. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.I. The influence of humic substances on lacustrine planktonic food chains. Hydrobiologia 1992, 229, 73–91. [Google Scholar] [CrossRef]
- Wilken, S.; Soares, M.; Urritia-Cordero, P.; Ratcovich, J.; Ekvall, M.K.; Van Donk, E.; Hansson, L.-A. Primary producers or consumers? Increasing phytoplankton bacterivory along a gradient of lake warming and browning. Limnol. Oceanogr. 2018, 63, S142–S155. [Google Scholar] [CrossRef]
- Senar, O.E.; Creed, I.F.; Trick, C.G. Lake browning may fuel phytoplankton biomass and trigger shifts in phytoplanktion communitiies in temperate lakes. Aquat. Sci. 2021, 83, 21. [Google Scholar] [CrossRef]
- Finstad, A.G.; Andersen, T.; Larsen, S.; Tominaga, K.; Blumentrath, S.; de Wit, H.A.; Tømmervik, H.; Hessen, D.O. From greening to browning: Catchment vegetation development and reduced S-deposition promote organic carbon load on decadal time scales in Nordic lakes. Sci. Rep. 2016, 6, 31944. [Google Scholar] [CrossRef]
- Peacock, M.; Burden, A.; Cooper, M.; Dunn, C.; Evans, C.D.; Fenner, N.; Freeman, C.; Gough, R.; Hughes, D.; Hughes, S.; et al. Quantifying dissolved organic carbon concentrations in upland catchments using phenolic proxy measurements. J. Hydrol. 2013, 477, 251–260. [Google Scholar] [CrossRef]
- Kang, H.; Freeman, C.; Kim, S.-Y. Variations of DOC and phenolics in pore-water of peatlands. Korean J. Limnol. 2002, 35, 306–311. [Google Scholar]
- Leonard, N.E. The Effects of the Invasive Exotic Chinese Tallow Tree (Triadica sebifera) on Amphibians and Aquatic Invertebrates. Ph.D. Thesis, University of New Orleans, New Oleans, LA, USA, 2008. [Google Scholar]
- Abrams, M.D. The red maple paradox: What explains the widespread expansion of red maple in eastern forests? Bioscience 1998, 48, 355–364. [Google Scholar] [CrossRef]
- Fei, S.; Steiner, K.C. Evidence for increasing red maple abundance in the eastern United States. For. Sci. 2007, 53, 473–477. [Google Scholar] [CrossRef]
- Wang, H.-H.; Grant, W.E.; Swannack, T.M.; Gan, J.; Rogers, W.E.; Koralewski, T.E.; Miller, J.H.; Taylor, J.W., Jr. Predicted range expansion of Chinese tallow tree (Triadica sebifera) in forestlands fo the southern United States. Divers. Distrib. 2011, 17, 552–565. [Google Scholar] [CrossRef]
- Libralato, G.; Avezzù, F.; Ghirardini, A.V. Lignin and tannin toxicity to Phaeodactylum tricornutum (Bohlin). J. Hazard. Mater. 2011, 194, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Y.; Wei, Y.; Norgbey, E.; Chen, Y.; Li, R.; Wang, C.; Cheng, Y.; Bofah-Buoh, R. Impact of Eucalyptus residue leaching on iron distribution in reservoir sediments assessed by high-resolution DGT technique. Environ. Sci. Pollut. Res. 2023, 30, 125718–125730. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Hagerman, A.E. Interactive intoxicating and ameliorating effects of tannic acid, aluminum (Al3+), copper (Cu2+), and selenate (SeO42−) in wheat roots: A descriptive and mathematical assessment. Physiol. Plant 2010, 139, 68–79. [Google Scholar] [CrossRef]
- Karrasch, B.; Parra, O.; Cid, H.; Mehrens, M.; Pacheco, P.; Urrutia, R.; Valdovinos, C.; Zaror, C. Effects of pulp and paper mill effluents on the microplankton and microbial self-purification capabilities of the Biobio River, Chile. Sci. Total Environ. 2006, 359, 194–208. [Google Scholar] [CrossRef]
- Motta, C.M.; Simoniello, P.; Arena, C.; Capriello, T.; Panzuto, R.; Vitale, E.; Agnisola, C.; Tizzano, M.; Avallone, B.; Ferrandino, I. Effects of four food dyes on development of three model species, Cucumis sativus, Artemia salina and Danio rerio: Assessment of potential risk for the environment. Environ. Pollut. 2019, 253, 1126–1135. [Google Scholar] [CrossRef] [PubMed]
- Scharnweber, K.; Peura, S.; Attermeyer, K.; Bertilsson, S.; Bolender, L.; Buck, M.; Einarsdóttir, K.; Garcia, S.L.; Gollnisch, R.; Grasset, C.; et al. Comprehensive analysis of chemical and biological problems associated with browning agents used in aquatic studies. Limnol. Oceanogr. Methods 2021, 19, 818–835. [Google Scholar] [CrossRef]
- Rodríguez, H.; de las Rivas, B.; Gómez-Cordovés, C.; Muñoz, R. Degradation of tannic acid by cell-free extracts of Lactobacillus plantarum. Food Chem. 2008, 107, 664–670. [Google Scholar] [CrossRef]
- Pallardy, S.G.; Nigh, T.A.; Garrett, H.E. Changes in forest composition in central Missouri: 1968–1982. Am. Midl. Nat. 1988, 120, 380–390. [Google Scholar] [CrossRef]
- Abrams, M.D. Fire and the development of oak forests. Bioscience 1992, 42, 346–353. [Google Scholar] [CrossRef]
- Zhu, K.; Woodall, C.W.; Clark, J.S. Failure to migrate: Lack of tree range expansion in response to climate change. Glob. Chang. Biol. 2012, 18, 1042–1052. [Google Scholar] [CrossRef]

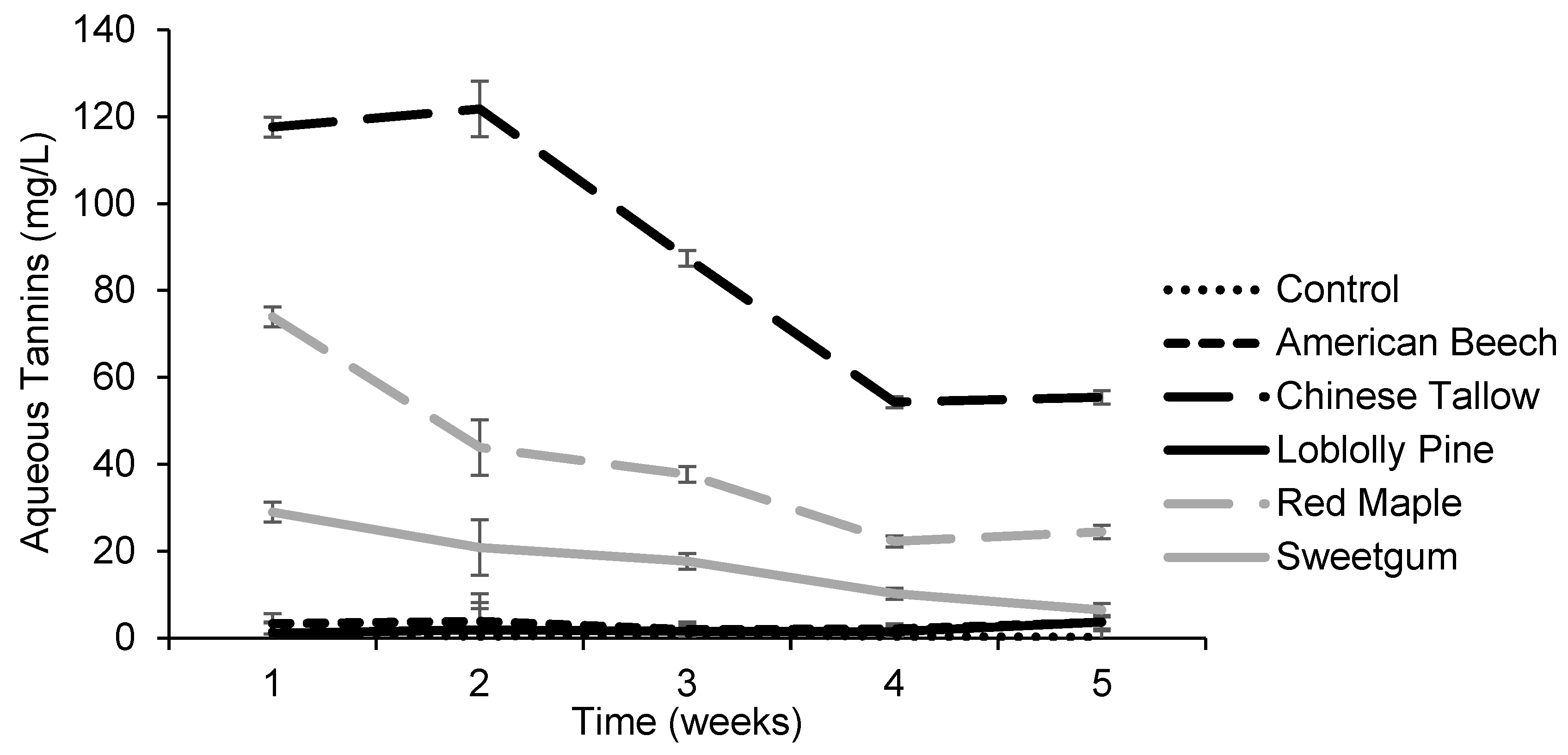



| Common Name | Scientific Name | C (%) | N (%) | P (%) | K (%) | Tannins (mg/g) |
|---|---|---|---|---|---|---|
| Chinese tallow American beech Loblolly pine | Triadica sebiferum | 47.9 a | 0.78 a | 0.10 ab | 0.49 a | 166.6 a |
| Fagus grandifolia | 47.2 a | 0.79 a | 0.11 b | 0.54 a | 27.6 c | |
| Pinus taeda | 51.1 b | 0.49 b | 0.05 a | 0.18 b | 32.4 c | |
| Red maple Sweetgum | Acer rubrum | 46.9 a | 0.95 a | 0.18 c | 0.73 a | 71.8 b |
| Liquidambar styraciflua | 45.5 a | 0.89 a | 0.10 ab | 0.52 a | 35.3 c |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliva, A.; Doolittle, C.M.; Medlock, S.A.; Aubert, J.F.; Earl, J.E. Effects of Tree Leaves, Tannins, and Water Color on Chlorophyll Concentrations in Ponds. Hydrobiology 2024, 3, 263-278. https://doi.org/10.3390/hydrobiology3030017
Oliva A, Doolittle CM, Medlock SA, Aubert JF, Earl JE. Effects of Tree Leaves, Tannins, and Water Color on Chlorophyll Concentrations in Ponds. Hydrobiology. 2024; 3(3):263-278. https://doi.org/10.3390/hydrobiology3030017
Chicago/Turabian StyleOliva, Anna, Christina M. Doolittle, Shelby A. Medlock, Joseph F. Aubert, and Julia E. Earl. 2024. "Effects of Tree Leaves, Tannins, and Water Color on Chlorophyll Concentrations in Ponds" Hydrobiology 3, no. 3: 263-278. https://doi.org/10.3390/hydrobiology3030017
APA StyleOliva, A., Doolittle, C. M., Medlock, S. A., Aubert, J. F., & Earl, J. E. (2024). Effects of Tree Leaves, Tannins, and Water Color on Chlorophyll Concentrations in Ponds. Hydrobiology, 3(3), 263-278. https://doi.org/10.3390/hydrobiology3030017






