Short-Term Responses of Aquatic Ecosystem and Macroinvertebrate Assemblages to Rehabilitation Actions in Martil River (North-Western Morocco)
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Approach
2.2. Methods
2.2.1. Physicochemical Parameters
2.2.2. Hydromorphological Measures
2.2.3. Sampling and Identification of Macroinvertebrates
2.2.4. DPSIR Model
- Driving forces (D): refers to natural and anthropogenic factors that impact the environment
- Pressures (P): refers to how these drivers are produced and expressed at the local
- State (S): refers to the current state of the environment under the pressures exerted
- Impact (I): refers to the changes resulting from the combined impact of natural and anthropogenic pressures on environmental state, human health, and socio-economic aspects.
- Response (R): refers to the measures and practices undertaken to improve the current environmental status.
2.3. Data Analysis
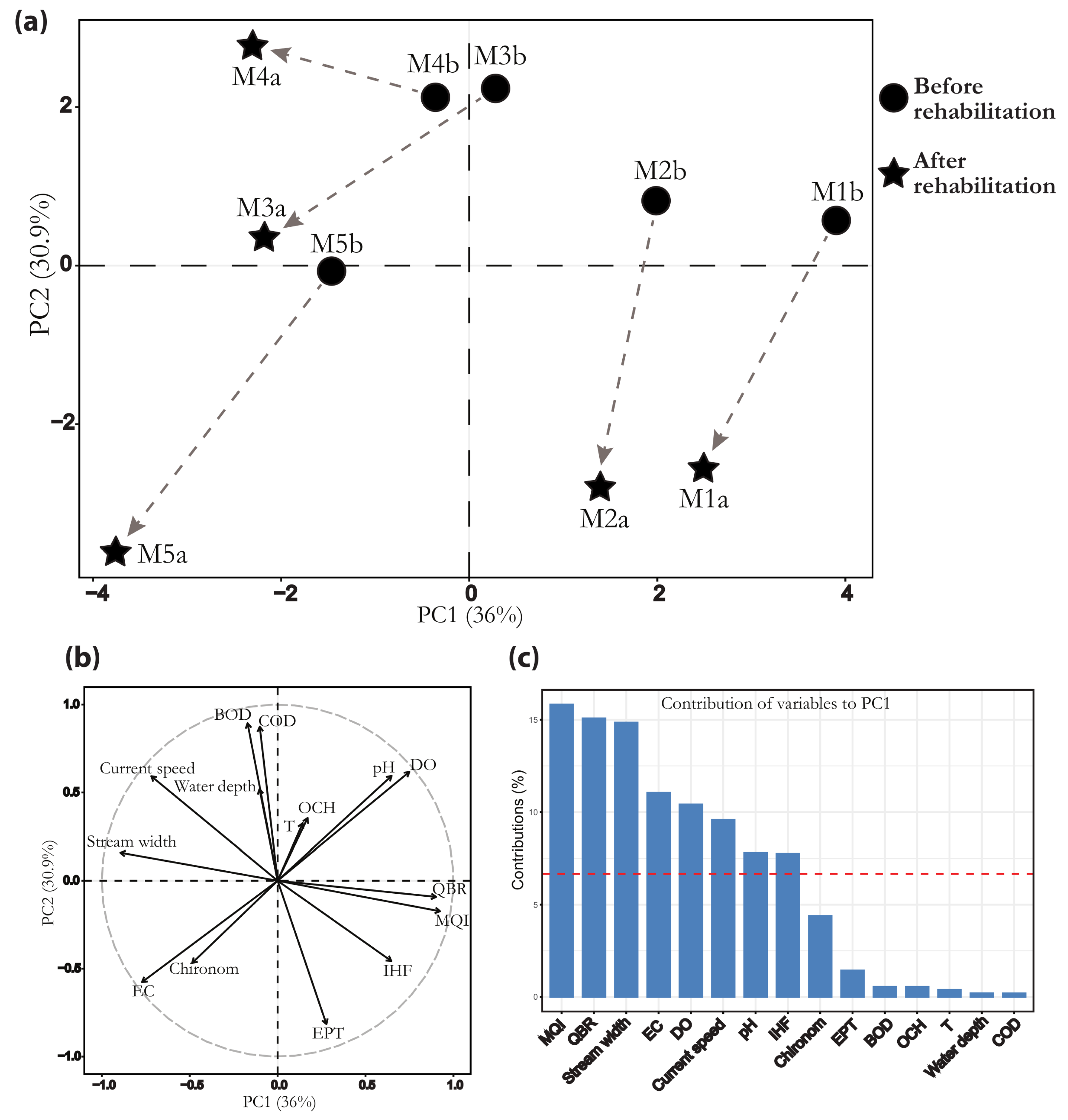
3. Results
3.1. Environmental and Biotic Variables
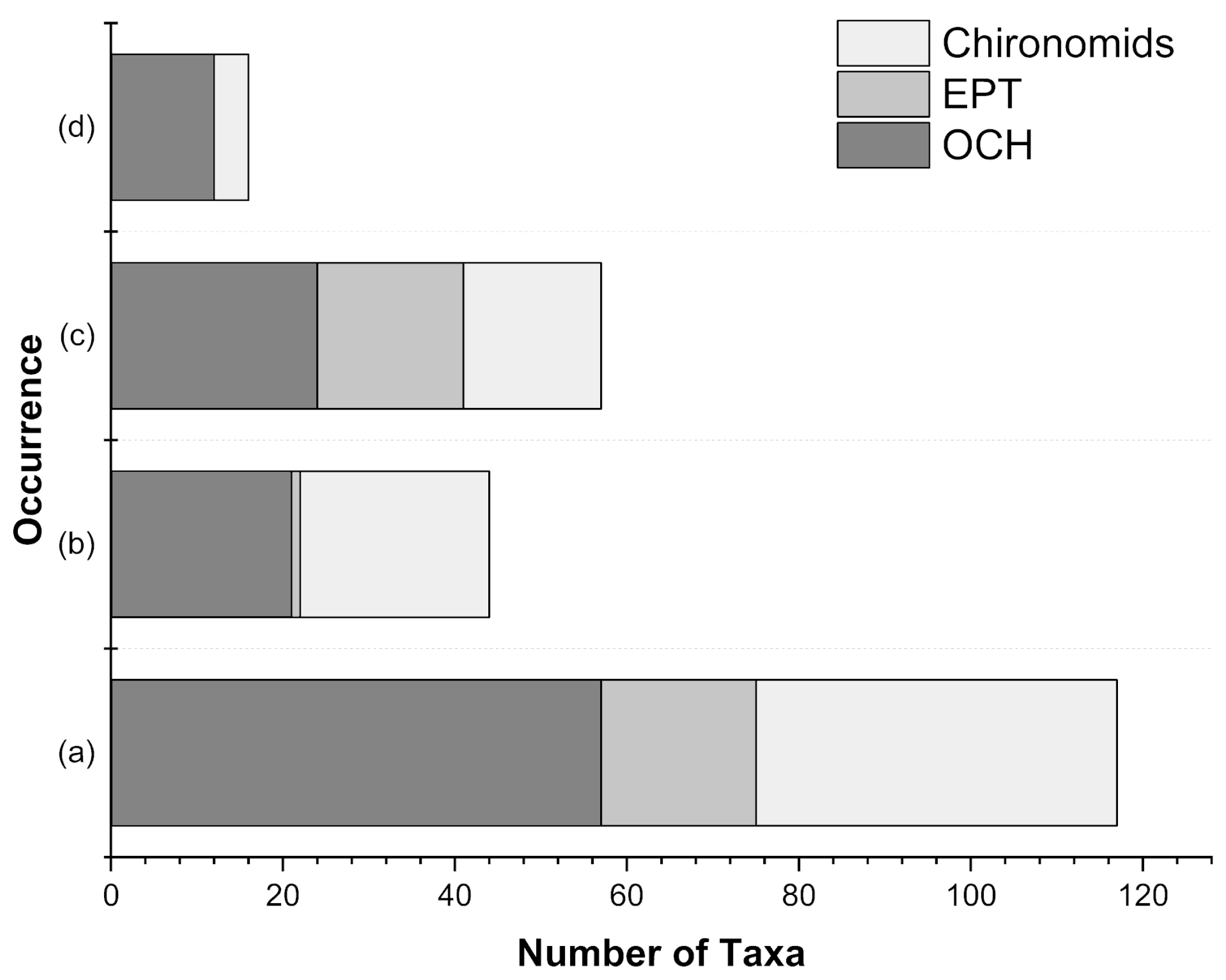
3.2. Macroinvertebrate Assemblages
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QBR | Riparian Quality Index |
| IHF | River Habitat Index |
| MQI | Morphological Quality Index |
| EPT | Ephemeroptera, Plecoptera, Tricoptera |
| OCH | Odonata, Coleoptera, Hemiptera |
| CE | Electrical Conductivity |
| DO | Dissolved Oxygen |
| BOD | Biochemical Oxygen Demand |
| BOD5 | Biochemical Oxygen Demand in 5 Days |
| COD | Chemical Oxygen Demand |
| ABHL | Loukkos Hydraulic Basin Agency |
| UV | Ultra Violet |
| DPSIR | Driving forces, Pressure, State, Impact, Response |
| EEA | European Environmental Agency |
| PCA | Principal Component Analysis |
Appendix A
| Quality Class | T (°C) | pH | EC (S/cm) | DO (%) | BOD5 (mg/L) | COD (mg/L) | QBR | IHF | MQI |
|---|---|---|---|---|---|---|---|---|---|
| Excellent | <20 | 6.5–8.5 | <750 | >7 | <3 | <30 | <95 | <60 | 0.85–1 |
| Good | 20–25 | - | 750–1300 | 7–5 | 3–5 | 30–35 | 90–75 | 40–60 | 0.7–0.85 |
| Average | 25–30 | 8.5–9.2 | 1300–2700 | 5–3 | 5–10 | 35–40 | 70–55 | >40 | 0.5–0.7 |
| Poor | 30–35 | <6.5 or >9.2 | 2700–3000 | 3–1 | 10–25 | 40–80 | 50–30 | - | 0.3–0.5 |
| Very Poor | >35 | - | >3000 | <1 | >25 | >80 | >30 | - | <0.3 |
| Taxa | Previous Findings | CIT. | Stations |
|---|---|---|---|
| Odonata | |||
| Lestes barbarus (Fabricius, 1798) | Benazzouz, 1988 [72] | * | M2 |
| Lestes viridis (Vander Linden, 1825) | *** | M5 | |
| Calopteryx sp. | *** | M5 | |
| Calopteryx exul Selys, 1853 | Benazzouz, 1988 | * | M2 |
| Erythromma lindenii (Selys, 1840) | El Haissoufi et al., 2015 [73] | * | M2 |
| Ischnura graellsii (Rambur, 1842) | Benazzouz, 1988; El Haissoufi et al., 2015 | ** | M2, M3, M4, M5 |
| Aeshna mixta Latreille, 1805 | Benazzouz, 1988 | ** | M1, M2 |
| Anax parthenope Selys, 1839 | Benazzouz, 1988 | * | M2 |
| Anax sp. | *** | M1, M2, M4, M5 | |
| Onychogomphus forcipatus unguiculatus (Vander Linden, 1823) | Benazzouz, 1988 | ** | M2, M3 |
| Paragomphus genei (Selys, 1841) | Benazzouz, 1988 | * | M2 |
| Cordulegaster boltonii (Donovan, 1807) algirica Morton, 1916 | *** | M4 | |
| Crocothemis erythraea (Brullé, 1832) | Benazzouz, 1988; El Haissoufi et al., 2015 | * | M2 |
| Libellulidae | *** | M3, M4 | |
| Sympetrum fonscolombii (Selys, 1840) | Benazzouz, 1988 | * | M2 |
| Trithemis annulata (Palisot de Bauvois, 1807) | El Haissoufi et al., 2015 | * | M2 |
| Coleoptera | |||
| Aulonogyrus striatus (Fabricius, 1792) | Benamar, 2015 [74] | ** | M1, M2, M3, M4, M5 |
| Gyrinus dejeani Brullé, 1832 | Benamar, 2015 | ** | M1, M2, M5 |
| Gyrinus urinator Illiger, 1807 | *** | M1 | |
| Haliplus lineatocollis (Marsham, 1802) | *** | M4 | |
| Noterus laevis Sturm, 1834 | *** | M4 | |
| Agabus brunneus (Fabricius, 1798) | Benamar, 2015 | * | M2 |
| Agabus conspersus (Marsham, 1802) | *** | M5 | |
| Hydroglyphus geminus (Fabricius, 1792) | *** | M5 | |
| Deronectes fairmairei (Leprieur, 1876) | *** | M1, M2 | |
| Stictonectes optatus (Seidlitz, 1887) | Benamar, 2015 | * | M2 |
| Laccophilus minutus (Linnaeus, 1758) | *** | M5 | |
| Hydroporus discretus discretus Fairmaire & Brisout, 1859 | Benamar, 2015 | * | M2 |
| Helophorus atlantis Angus & Aouad, 2009 | *** | M5 | |
| Berosus affinis Brullé, 1835 | *** | M5 | |
| Berosus hispanicus Küster,1847 | *** | M2, M3, M4 | |
| Laccobius atrocephalus Reitter, 1872 | *** | M1, M2 | |
| Hydrochus aljibensis Castro & Delgado, 1999 | Benamar, 2015 | * | M2 |
| Hydraena cordata Schaufuss, 1883 | Benamar, 2015 | * | M2 |
| Ochthebius mediterraneus (Ienistea, 1988) | Bennas et al., 2001 [75] | * | M2 |
| Elmis maugetii velutina (Reiche, 1879) | *** | M5 | |
| Oulimnius troglodytes (Gyllenhal, 1827) | Benamar, 2015 | * | M2 |
| Dryops algiricus (Lucas, 1849) | Benamar, 2015 | * | M2 |
| Dryops sulcipennis (Costa, 1883) | Benamar, 2015 | * | M2 |
| Hemiptera | |||
| Aquarius cinereus (Puton, 1869) | *** | M1 | |
| Aquarius najas (de Geer, 1773) | *** | M1 | |
| Gerris gibbifer Schummel, 1832 | L’Mohdi, 2015 [76] | ** | M4, M5 |
| Gerris thoracicus Schummel, 1832 | *** | M1, M4, M5 | |
| Hydrometra stagnorum (Linnaeus, 1758) | L’Mohdi, 2015 | ** | M1, M2, M3 |
| Mesovelia vittigera Horváth, 1895 | *** | M5 | |
| Velia ioannis Tamanini, 1971 | L’Mohdi, 2015 | ** | M2 |
| Corixa affinis Leach, 1817 | L’Mohdi, 2015 | ** | M2 |
| Hesperocorixa furtiva (Horváth, 1907) | L’Mohdi, 2015 | * | M2 |
| Sigara lateralis (Leach, 1817) | L’Mohdi, 2015 | ** | M2 |
| Micronecta scholtzi (Fieber, 1860) | L’Mohdi, 2015 | ** | M2 |
| Naucoris maculatus Fabricius, 1798 | ** | M4 | |
| Nepa cinerea Linnaeus, 1758 | L’Mohdi, 2015 | * | M2 |
| Anisops sardeus sardeus Henrrich-Schaeffer, 1849 | *** | M1, M2, M3, M4, M5 | |
| Notonecta maculata Fabricius, 1794 | *** | M5 | |
| Notonecta meridionalis Poisson, 1926 | L’Mohdi, 2015 | * | M2 |
| Plea minutissima Leach, 1818 | *** | M4, M5 | |
| Ephemeroptera | |||
| Acentrella almohades (Alba-Tercedor & El Alami, 1999) | *** | M2 | |
| Baetis fuscatus (Linné, 1761) | *** | M4 | |
| Baetis pavidus (Grandi, 1949) | *** | M1, M2, M3, M4, M5 | |
| Baetis rhodani (Pictet, 1984) | *** | M1, M2, M3, M4, M5 | |
| Cloeon dipterum (Linné, 1761) | *** | M1, M2, M3, M4, M5 | |
| Cloeon simile (Eaton, 1870) | *** | M1, M2, M4 | |
| Procloean concinnum (Eaton, 1885) | *** | M1 | |
| Ecdyonurus rotschildi (Navás, 1929) | *** | M4 | |
| Chroterpes volubilis (Thomas & Vitte, 1988) | *** | M1, M2, M3, M5 | |
| Chroterpes atlas (Soldan & Thomas, 1983) | *** | M1, M5 | |
| Ephoron virgo (Olivier, 1791) | *** | M1 | |
| Serratella ignita (Poda, 1791) | *** | M1 | |
| Caenis luctuosa (Burmeister, 1839) | *** | M1, M2, M3, M4, M5 | |
| Plecoptera | |||
| Capnioneura sp. | *** | M1 | |
| Hemimelaena flaviventris (Pictet, 1841) | *** | M1 | |
| Trichoptera | |||
| Hydropsyche iberomaroccana (González & Malicky, 1999) | *** | M1, M5 | |
| Hydropsyche lobata (McLachlan, 1884) | *** | M2, M3 | |
| Hydropsyche pellucidula (Curtis, 1834) | *** | M3 | |
| Chimarra marginata (Linnaeus, 1767) | Hajji, 2017 [77] | * | M2 |
| Diptera (Chironomidae) | |||
| Tanypus punctipennis (Maigen, 1818) | *** | M2 | |
| Cricotopus bicintus (Meigen, 1818) | Kettani et al., 1995 [78] | * | M2 |
| Cricotopus fuscus (Kieffer, 1909) | *** | M3 | |
| Cricotopus pallidipes (Edwards, 1929) | Kettani et al., 1995 | * | M2 |
| Orthocladius ashei (Soponis, 1990) | *** | M3 | |
| Orthocladius obumbratus (Johannsen, 1905) | Kettani et al., 1995 | * | M3 |
| Diptera (Chironomidae) | |||
| Orthocladius rubicundus (Meigen,1818) | Kettani et al., 1995 | ** | M2 |
| Rheocricotopus atripes (Kieffer, 1913) | Kettani et al., 1996 [79] | ** | M2, M3 |
| Rheocricotopus chalybeatus (Edwards, 1929) | Kettani et al., 1995 | * | M2 |
| Rheocricotopus tirolus (Lehmann, 1969) | *** | M3 | |
| Chironomus barbarensis (Theowald & Oosterbroek, 1980) | *** | M2 | |
| Chironomus dorsalis (Meigen, 1818) | *** | M4, M5 | |
| Chironomus luridus (Strenzke, 1959) | Kettani & Langton, 2011 [80] | * | M2 |
| Chironomus nuditarsis (Keyl, 1961) | *** | M5 | |
| Chironomus plumosus (Linnaeus) | *** | M2, M3, M4, M5 | |
| Chironomus riparius (Meigen, 1804) | *** | M5 | |
| Chironomus salinarius (Kieffer, 1915) | *** | M2, M5 | |
| Chironomus Pe 3 (Langton, 1991) | *** | M4 | |
| Cryptochironomus rostratus (Kieffer, 1921) | *** | M2 | |
| Cryptochironomus Pe 5 (Langton, 1991) | *** | M2 | |
| Dicrotendipes modestus (Say, 1823) | Kettani & Langton, 2011 | * | M2 |
| Dicrotendipes septemmaculatus (Becker, 1908) | Kettani et al., 1995 | ** | M2 |
| Glyptotendipes barbatipes (Staeger, 1911) | *** | M2 | |
| Glyptotendipes gripekoveni (Kieffer, 1913) | *** | M2, M5 | |
| Glyptotendipes pallens (Meigen, 1804) | *** | M2 | |
| Harnischia curtilamellata (Malloch, 1915) | Kettani et al., 1995 | * | M2 |
| Microtendipes britteni (Edwards, 1929) | Kettani et al., 1995 | * | M2 |
| Nubensia nubens (Edwards, 1929) | Kettani et al., 1995 | * | M2 |
| Parachironomus frequens (Johannsen, 1905) | Kettani et al., 1995 | * | M2 |
| Paracladopelma camptolabis (Kieffer, 1913) | Kettani et al., 1995 | * | M2 |
| Paratendipes albimanus (Meigen, 1818) | Kettani et al., 1995 | * | M2 |
| Polypedilum aegyptium (Kieffer, 1925) | Kettani et al., 1995 | * | M2 |
| Polypedilum separabilis (Brundin, 1947) | Kettani et al., 1995 | * | M2 |
| Polypedilum sordens (Van der Wulp, 1875)) | Kettani & Langton, 2011 | ** | M2, M5 |
| Polypedilum Pe 1 (Langton, 1991) | Kettani et al., 1995 | * | M2 |
| Stictochironomus maculipennis (Meigen, 1818) | Kettani et al., 1995 | * | M2 |
| Cladotanytarsus vanderwulpi (Edwards, 1929) | Kettani et al., 1995 | * | M2 |
| Paratanytarsus bituberculatus (Edwards, 1929) | Kettani et al., 1995 | * | M2 |
| Paratanytarsus inopertus (Walker, 1856) | Kettani & Langton 2011 | * | M2 |
| Rheotanytarsus reissi (Lehman, 1970) | Kettani et al., 1995 | * | M2 |
| Tanytarsus medius (Reiss & Fittkau, 1971) | Kettani et al., 1995 | * | M2 |
| Virgatanytarsus albisutus (Santos Abréu, 1918) | Kettani et al., 1995 | * | M2 |
References
- Besacier-Monbertrand, A.L.; Paillex, A.; Castella, E. Short-term impacts of lateral hydrological connectivity restoration on aquatic macroinvertebrates. River Res. Appl. 2012, 30, 557–570. [Google Scholar] [CrossRef]
- Schirmer, M.; Luster, J.; Linde, N.; Perona, P.; Mitchell, E.A.; Barry, D.A.; Hollender, J.; Cirpka, O.A.; Schneider, P.; Vogt, T.; et al. Morphological, hydrological, biogeochemical and ecological changes and challenges in river restoration–the Thur River case study. Hydrol. Earth Syst. Sci. 2014, 18, 2449–2462. [Google Scholar] [CrossRef]
- Sofi, M.S.; Bhat, S.U.; Rashid, I.; Kuniyal, J.C. The natural flow regime: A master variable for maintaining river ecosystem health. Ecohydrology 2020, 13, e2247. [Google Scholar] [CrossRef]
- Palmer, M.A.; Bernhardt, E.S. Hydroecology and river restoration: Ripe for research and synthesis. Water Resour. Res. 2006, 42, W03S07. [Google Scholar] [CrossRef]
- Nakano, D.; Nakamura, F. Responses of macroinvertebrate communities to river restoration in a channelized segment of the Shibetsu River, Northern Japan. River Res. Appl. 2006, 22, 681–689. [Google Scholar] [CrossRef]
- Ling, T.Y.; Soo, C.L.; Heng, T.L.E.; Nyanti, L.; Sim, S.F.; Grinang, J. Physicochemical characteristics of river water downstream of a large tropical hydroelectric dam. J. Chem. 2016, 2016, 7895234. [Google Scholar] [CrossRef]
- Rubin, Z.; Kondolf, G.M.; Rios-Touma, B. Evaluating stream restoration projects: What do we learn from monitoring? Water 2017, 9, 174. [Google Scholar] [CrossRef]
- Mitsch, W.J.; Jørgensen, S.E. Ecological engineering: A field whose time has come. Ecol. Eng. 2003, 20, 363–377. [Google Scholar] [CrossRef]
- Louhi, P.; Mykrä, H.; Paavola, R.; Huusko, A.; Vehanen, T.; Mäki-Petäys, A.; Muotka, T. Twenty years of stream restoration in Finland: Little response by benthic macroinvertebrate communities. Ecol. Appl. 2011, 21, 1950–1961. [Google Scholar] [CrossRef]
- Pedersen, M.L.; Friberg, N.; Skriver, J.; Baattrup-Pedersen, A.; Larsen, S.E. Restoration of Skjern River and its valley—Short-term effects on river habitats, macrophytes and macroinvertebrates. Ecol. Eng. 2007, 30, 145–156. [Google Scholar] [CrossRef]
- Pedersen, M.L.; Kristensen, K.K.; Friberg, N. Re-meandering of lowland streams: Will disobeying the laws of geomorphology have ecological consequences? PLoS ONE 2014, 9, e108558. [Google Scholar] [CrossRef] [PubMed]
- Negishi, J.; Inoue, M.; Nunokawa, M. Effects of channelisation on stream habitat in relation to a spate and flow refugia for macroinvertebrates in northern Japan. Freshw. Biol. 2002, 47, 1515–1529. [Google Scholar] [CrossRef]
- Pretty, J.; Harrison, S.; Shepherd, D.; Smith, C.; Hildrew, A.; Hey, R. River rehabilitation and fish populations: Assessing the benefit of instream structures. J. Appl. Ecol. 2003, 40, 251–265. [Google Scholar] [CrossRef]
- Brooks, A.P.; Gehrke, P.C.; Jansen, J.D.; Abbe, T.B. Experimental reintroduction of woody debris on the Williams River, NSW: Geomorphic and ecological responses. River Res. Appl. 2004, 20, 513–536. [Google Scholar] [CrossRef]
- Nakano, D.; Nagayama, S.; Kawaguchi, Y.; Nakamura, F. River restoration for macroinvertebrate communities in lowland rivers: Insights from restorations of the Shibetsu River, north Japan. Landsc. Ecol. Eng. 2008, 4, 63–68. [Google Scholar] [CrossRef]
- Kail, J.; Brabec, K.; Poppe, M.; Januschke, K. The effect of river restoration on fish, macroinvertebrates and aquatic macrophytes: A meta-analysis. Ecol. Indic. 2015, 58, 311–321. [Google Scholar] [CrossRef]
- Nilsson, C.; Polvi, L.E.; Gardeström, J.; Hasselquist, E.M.; Lind, L.; Sarneel, J.M. Riparian and in-stream restoration of boreal streams and rivers: Success or failure? Ecohydrology 2015, 8, 753–764. [Google Scholar] [CrossRef]
- Francis, R.A. Positioning urban rivers within urban ecology. Urban Ecosyst. 2012, 15, 285–291. [Google Scholar] [CrossRef]
- Vugteveen, P.; Lenders, R.; Van den Besselaar, P. The dynamics of interdisciplinary research fields: The case of river research. Scientometrics 2014, 100, 73–96. [Google Scholar] [CrossRef]
- Lepori, F.; Palm, D.; Brännäs, E.; Malmqvist, B. Does restoration of structural heterogeneity in streams enhance fish and macroinvertebrate diversity? Ecol. Appl. 2005, 15, 2060–2071. [Google Scholar] [CrossRef]
- Kumarasamy, P.; Arthur James, R.; Dahms, H.U.; Byeon, C.W.; Ramesh, R. Multivariate water quality assessment from the Tamiraparani river basin, Southern India. Environ. Earth Sci. 2014, 71, 2441–2451. [Google Scholar] [CrossRef]
- Storey, A.W.; Lynas, J. Application of the functional habitat concept to the regulated Lower Ord River, Western Australia, Part I, macroinvertebrate assemblages. Hydrobiologia 2007, 592, 499–512. [Google Scholar] [CrossRef]
- Baumgartner, S.D.; Robinson, C.T. Short-term colonization dynamics of macroinvertebrates in restored channelized streams. Hydrobiologia 2017, 784, 321–335. [Google Scholar] [CrossRef]
- Lake, P.S. On the maturing of restoration: Linking ecological research and restoration. Ecol. Manag. Restor. 2001, 2, 110–115. [Google Scholar] [CrossRef]
- Ministère de l’Urbanisme et de l’Aménagement du Territoire, Direction de l’Urbanisme. Référentiel de L’urbanisme Durable; Groupement d’Expertises et d’Études Fquih Berrada Chraf-Eddine & Mikou Khalid: Rabat, Morocco, 2016. [Google Scholar]
- Karrouchi, M.; Touhami, M.O.; Oujidi, M.; Chourak, M. Cartographie des zones à risque d’inondation dans la région Tanger-Tétouan: Cas du bassin versant de Martil (Nord du Maroc)/[Mapping of flooding risk areas in the Tangier-Tetouan region: Case of Martil Watershed (Northern Morocco)]. Int. J. Innov. Appl. Stud. 2016, 14, 1019. [Google Scholar]
- Bidaoui, H.; El Abbassi, I.; El Bouardi, A.; Darcherifc, A.M. Heating and Cooling Power Demand of Residential Building with Different Envelope Design under Moroccan Conditions. In Proceedings of the International Conference on Materials and Energy, Tetouan, Morocco, 17–18 May 2015; Available online: https://www.researchgate.net/publication/277529150_HEATING_AND_COOLING_POWER_DEMAND_OF_RESIDENTIAL_BUILDING_WITH_DIFFERENT_ENVELOPE_DESIGN_UNDER_MOROCCAN_CONDITIONS (accessed on 29 June 2023).
- Salhi, A.; Martin-Vide, J.; Benhamrouche, A.; Benabdelouahab, S.; Himi, M.; Benabdelouahab, T.; Casas Ponsati, A. Rainfall distribution and trends of the daily precipitation concentration index in northern Morocco: A need for an adaptive environmental policy. SN Appl. Sci. 2019, 1, 277. [Google Scholar] [CrossRef]
- Haut Commissariat au Plan. Monographie Régionale De Tanger-Tétouan. Regional Direction of Tangier-Tetouan; HCP: Tangier, Morocco, 2015. [Google Scholar]
- Rodier, J.; Legube, B.; Merlet, N.; Brunet, R. L’analyse de l’eau-9e éd. In Eaux Naturelles, Eaux Résiduaires, eau de mer; DUNOD: Ile-de-France, France, 2009; pp. 564–571. [Google Scholar]
- Fornells, N.P.; Solá, C.; Munné, A. QBR: Un índice rápido para la evaluación de la calidad de los ecosistemas de ribera. Tecnol. Agua 1998, 175, 20–37. [Google Scholar]
- Pardo, I.; Álvarez, M.; Casas, J.; Moreno, J.L.; Vivas, S.; Bonada, N.; Alba-Tercedor, J.; Jáimez-Cuéllar, P.; Moyà, G.; Prat, N.; et al. El hábitat de los ríos mediterráneos. Diseño de un índice de diversidad de hábitat. Limnetica 2002, 21, 115–133. [Google Scholar] [CrossRef]
- Rinaldi, M.; Surian, N.; Comiti, F.; Bussettini, M. A method for the assessment and analysis of the hydromorphological condition of Italian streams: The Morphological Quality Index (MQI). Geomorphology 2013, 180, 96–108. [Google Scholar] [CrossRef]
- Sansoni, G. Atlante per il Riconoscimento Dei Macroinvertebrati Dei Corsi d’acqua Italiani; Centro Italiano Studi di Biologia Ambientale: Trento, Italy, 1992. [Google Scholar]
- Tachet, H.; Richoux, P.; Bournaud, M.; Usseglio-Polatera, P. Invertébrés d’eau Douce: Systématique, Biologie, Ecologie; CNRS Editions: Paris, France, 2010; Volume 15. [Google Scholar]
- Smeets, E.; Weterings, R. Environmental Indicators: Typology and Overview; European Environment Agency: Copenhagen, Denmark, 1999; Volume 19. [Google Scholar]
- Lalande, N.; Cernesson, F.; Decherf, A.; Tournoud, M.G. Implementing the DPSIR framework to link water quality of rivers to land use: Methodological issues and preliminary field test. Int. J. River Basin Manag. 2014, 12, 201–217. [Google Scholar] [CrossRef]
- Malekmohammadi, B.; Jahanishakib, F. Vulnerability assessment of wetland landscape ecosystem services using driver-pressure-state-impact-response (DPSIR) model. Ecol. Indic. 2017, 82, 293–303. [Google Scholar] [CrossRef]
- Lu, W.; Xu, C.; Wu, J.; Cheng, S. Ecological effect assessment based on the DPSIR model of a polluted urban river during restoration: A case study of the Nanfei River, China. Ecol. Indic. 2019, 96, 146–152. [Google Scholar] [CrossRef]
- James, F.C.; McCulloch, C.E. Multivariate analysis in ecology and systematics: Panacea or Pandora’s box? Annu. Rev. Ecol. Syst. 1990, 21, 129–166. [Google Scholar] [CrossRef]
- Team, R Developement Core. A Language and Environment for Statistical Computing. 2009. Available online: http://www.R-project.org (accessed on 29 June 2023).
- Oualad Mansour, N.; Targuisti, K.; Stitou, J. Evaluation de la qualité des eaux dans les systèmes fluviaux du Rif (cas de la rivière Martil) et étude de la biodiversité des communautés des macroinvertébrés. UTRILLAS-2009 2009, 8, 95–114. [Google Scholar]
- Belhaj, H.; Kettani, K. Evaluation de la qualité physico-chimique de l’oued Martil (Rif Occidental, Maroc) Evaluation of the physico-chemical quality of oued Martil (Western Rif, Morocco). GENVIRON-5 2013, 3, 31–38. [Google Scholar]
- Boulton, A.J.; Findlay, S.; Marmonier, P.; Stanley, E.H.; Valett, H.M. The functional significance of the hyporheic zone in streams and rivers. Annu. Rev. Ecol. Syst. 1998, 29, 59–81. [Google Scholar] [CrossRef]
- Fernald, A.G.; Landers, D.H.; Wigington, P.J., Jr. Water quality changes in hyporheic flow paths between a large gravel bed river and off-channel alcoves in Oregon, USA. River Res. Appl. 2006, 22, 1111–1124. [Google Scholar] [CrossRef]
- Martín, E.J.; Ryo, M.; Doering, M.; Robinson, C.T. Evaluation of restoration and flow interactions on river structure and function: Channel widening of the thur river, switzerland. Water 2018, 10, 439. [Google Scholar] [CrossRef]
- Southwood, T.R. Habitat, the templet for ecological strategies? J. Anim. Ecol. 1977, 46, 337–365. [Google Scholar] [CrossRef]
- Martin, D.M.; Mazzotta, M.; Bousquin, J. Combining ecosystem services assessment with structured decision making to support ecological restoration planning. Environ. Manag. 2018, 62, 608–618. [Google Scholar] [CrossRef]
- Herbst, D.B.; Kane, J.M. Responses of aquatic macroinvertebrates to stream channel reconstruction in a degraded rangeland creek in the Sierra Nevada. Ecol. Restor. 2009, 27, 76–88. [Google Scholar] [CrossRef]
- Selego, S.M.; Rose, C.L.; Merovich, G.T.; Welsh, S.A.; Anderson, J.T. Community-level response of fishes and aquatic macroinvertebrates to stream restoration in a third-order tributary of the Potomac River, USA. Int. J. Ecol. 2012, 2012, 753634. [Google Scholar] [CrossRef]
- Mérigoux, S.; Forcellini, M.; Dessaix, J.; Fruget, J.F.; Lamouroux, N.; Statzner, B. Testing predictions of changes in benthic invertebrate abundance and community structure after flow restoration in a large river (French Rhône). Freshw. Biol. 2015, 60, 1104–1117. [Google Scholar] [CrossRef]
- Paillex, A.; Castella, E.; zu Ermgassen, P.S.; Aldridge, D.C. Testing predictions of changes in alien and native macroinvertebrate communities and their interaction after the restoration of a large river floodplain (French Rhône). Freshw. Biol. 2015, 60, 1162–1175. [Google Scholar] [CrossRef]
- Li, M.; Fan, J.; Zhang, Y.; Guo, F.; Liu, L.; Xia, R.; Xu, Z.; Wu, F. A systematic approach for watershed ecological restoration strategy making: An application in the Taizi River Basin in northern China. Sci. Total Environ. 2018, 637, 1321–1332. [Google Scholar] [CrossRef]
- Mundahl, N.D.; Hunt, A.M. Recovery of stream invertebrates after catastrophic flooding in southeastern Minnesota, USA. J. Freshw. Ecol. 2011, 26, 445–457. [Google Scholar] [CrossRef]
- Smith, J.G.; Brandt, C.C.; Christensen, S.W. Long-term benthic macroinvertebrate community monitoring to assess pollution abatement effectiveness. Environ. Manag. 2011, 47, 1077–1095. [Google Scholar] [CrossRef] [PubMed]
- Ratia, H.; Vuori, K.M.; Oikari, A. Caddis larvae (Trichoptera, Hydropsychidae) indicate delaying recovery of a watercourse polluted by pulp and paper industry. Ecol. Indic. 2012, 15, 217–226. [Google Scholar] [CrossRef]
- Choe, L.J.; Jung, S.W.; Kim, D.G.; Baek, M.J.; Kang, H.J.; Lee, C.Y.; Bae, Y.J. Temporal changes in benthic macroinvertebrates and their interactions with fish predators after restoration in the Cheonggyecheon, a downtown stream in Seoul, Korea. Entomol. Res. 2014, 44, 338–348. [Google Scholar] [CrossRef]
- Verdonschot, R.C.; Kail, J.; McKie, B.G.; Verdonschot, P.F. The role of benthic microhabitats in determining the effects of hydromorphological river restoration on macroinvertebrates. Hydrobiologia 2016, 769, 55–66. [Google Scholar] [CrossRef]
- White, J.; Hill, M.J.; Bickerton, M.; Wood, P. Macroinvertebrate taxonomic and functional trait compositions within lotic habitats affected by river restoration practices. Environ. Manag. 2017, 60, 513–525. [Google Scholar] [CrossRef] [PubMed]
- Poulos, H.M.; Miller, K.E.; Heinemann, R.; Kraczkowski, M.L.; Whelchel, A.W.; Chernoff, B. Dam removal effects on benthic macroinvertebrate dynamics: A New England stream case study (Connecticut, USA). Sustainability 2019, 11, 2875. [Google Scholar] [CrossRef]
- Sundermann, A.; Stoll, S.; Haase, P. River restoration success depends on the species pool of the immediate surroundings. Ecol. Appl. 2011, 21, 1962–1971. [Google Scholar] [CrossRef] [PubMed]
- Müller-Peddinghaus, E.; Hering, D. The wing morphology of limnephilid caddisflies in relation to their habitat preferences. Freshw. Biol. 2013, 58, 1138–1148. [Google Scholar] [CrossRef]
- Buczyńska, E.; Szlauer-Łukaszewska, A.; Czachorowski, S.; Buczyński, P. Human impact on large rivers: The influence of groynes of the River Oder on larval assemblages of caddisflies (Trichoptera). Hydrobiologia 2018, 819, 177–195. [Google Scholar] [CrossRef]
- Statzner, B.; Bonada, N.; Dolédec, S. Biological attributes discriminating invasive from native European stream macroinvertebrates. Biol. Invasions 2008, 10, 517–530. [Google Scholar] [CrossRef]
- Jungwirth, M.; Muhar, S.; Schmutz, S. Re-establishing and assessing ecological integrity in riverine landscapes. Freshw. Biol. 2002, 47, 867–887. [Google Scholar] [CrossRef]
- Elosegi, A.; Díez, J.; Mutz, M. Effects of hydromorphological integrity on biodiversity and functioning of river ecosystems. Hydrobiologia 2010, 657, 199–215. [Google Scholar] [CrossRef]
- Nakamura, F.; Ishiyama, N.; Sueyoshi, M.; Negishi, J.N.; Akasaka, T. The significance of meander restoration for the hydrogeomorphology and recovery of wetland organisms in the Kushiro River, a lowland river in Japan. Restor. Ecol. 2014, 22, 544–554. [Google Scholar] [CrossRef]
- Song, X.; Frostell, B. The DPSIR framework and a pressure-oriented water quality monitoring approach to ecological river restoration. Water 2012, 4, 670–682. [Google Scholar] [CrossRef]
- Pan, B.; Yuan, J.; Zhang, X.; Wang, Z.; Chen, J.; Lu, J.; Yang, W.; Li, Z.; Zhao, N.; Xu, M. A review of ecological restoration techniques in fluvial rivers. Int. J. Sediment Res. 2016, 31, 110–119. [Google Scholar] [CrossRef]
- Xu, Y.; Cai, Y.; Sun, T.; Tan, Q. A multi-scale integrated modeling framework to measure comprehensive impact of coastal reclamation activities in Yellow River estuary, China. Mar. Pollut. Bull. 2017, 122, 27–37. [Google Scholar] [CrossRef] [PubMed]
- SEEE (Secrétariat d’Etat auprès du ministère de l’Energie des Mines, de l’Eau et de l’Environnement). Fiche Sur le Nouveau Système d’évaluation de la Qualité des Eaux; Direction de la Recherche et de la Planification de l’Eau: Rabat, Morocco, 2008.
- Benazzouz, B. Etude du Cycle Biologique et du Polymorphisme Larvaire et Imaginal d’ischnura Graellsi (Rambur, 1842) au Maroc. Ph.D. Thesis, Mohamed V University, Faculté des Sciences, Rabat, Morocco, 1988. 183p. [Google Scholar]
- El Haissoufi, M.; Knijf, G.D.; Bosch, J.V.; Bennas, N.; Millán, A. Contribution to the knowledge of the Moroccan Odonata, with first records of Orthetrum sabina, and an overview of first and last dates for all species. Odonatologica 2015, 44, 225–254. [Google Scholar]
- Benamar, L. Les Coléoptères Aquatiques du Maroc: Atlas, biogéOgraphie et degré de Vulnérabilité. Ph.D. Thesis, Université Abdelmalek Essaâdi, Faculté des Sciences, Tétouan, Morocco, 2015. 538p. [Google Scholar]
- Bennas, N.; Sáinz-Cantero, C.E.; Ouarour, A. Nouvelles données sur les Coléoptères aquatiques du Maroc: Les Hydraenidae Mulsant, 1844 du Rif, faunistique et biogéographie. Zool. Baetica 2001, 12, 135–168. [Google Scholar]
- L’Mohdi, O. Les Hémiptères aquatiques du Maroc: Atlas, biogéographie et degré de vulnérabilité. Ph.D. Thesis, Université Abdelmalek Essaâdi, Faculté des Sciences, Tétouan, Morocco, 2015. 262p. [Google Scholar]
- Hajji, K. Les Trichoptères du Maroc: Atlas, Biogéographie et Degré de Vulnérabilité. Ph.D. Thesis, Université Abdelmalek Essaâdi, Faculté des Sciences, Tétouan, Morocco, 2017. 309p. [Google Scholar]
- Kettani, K.; Vilchez, A.; Calle, D.; El Ouazzani, T. Nouvelles récolles de (Diptera) du Maroc: Les Chironomidae de l’Oued Martil (Rif). Ann. Limnol. 1995, 31, 253–261. [Google Scholar] [CrossRef]
- Kettani, K.; Calle, D.; El Ouazzani, T. Données faunistiques actuelles sur les Chironomidés. (Diptera) du Rif (Maroc). Bull. l’Institut Sci. 1996, 20, 131–141. [Google Scholar]
- Kettani, K.; Langton, P.H. New data on the Chironomidae (Diptera) of the Rif (northern Morocco). Pol. J. Entomol. 2011, 80, 587–599. [Google Scholar] [CrossRef]


| Factors | M1 | M2 | M3 | M4 | M5 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Physiochemical | ||||||||||
| T (C) | ||||||||||
| PH | ||||||||||
| EC (S/cm) | ||||||||||
| DO (mg/L) | ||||||||||
| BOD (mg/L) | ||||||||||
| COD (mg/L) | ||||||||||
| Hydrological | ||||||||||
| Current Speed (m/s) | ||||||||||
| Water Depth (m) | ||||||||||
| Stream Width (m) | ||||||||||
| Habitat Indices | ||||||||||
| QBR | ||||||||||
| IHF | ||||||||||
| MQI | ||||||||||
| Biological Metrics | ||||||||||
| EPT (%) | ||||||||||
| OCH (%) | ||||||||||
| Chironomids (%) | ||||||||||
| Driving Forces | Population growth; Urbanization; Industry; Agriculture; Tourism |
| Pressure | Water resources use; Domestic water use; Chemical fertilizer use; Land use; Floodplain changes; Construction of dams |
| State | Water pollution; Habitat degradation; Hydrological control; Loss of wetland habitats; Ecological disturbances; Decline in species richness |
| Impacts | Water quality; Hydromorphology; Vegetation coverage; Ecosystem functions; Biodiversity |
| Responses | Martil River rehabilitation project |
| Work in progress: | Reduce Flood Risks; River re-meandering; Creation of leisure zone; Creation of attractive economic zones |
| Approaches to take into consideration: | Ecological integrity; Habitat and hydrological connectivity; Protection of biodiversity; Sensibilisation and law enforcement |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guellaf, A.; Kassout, J.; Boselli, V.A.; Bennas, N.; El Alami, M.; Errochdi, S.; Kettani, K. Short-Term Responses of Aquatic Ecosystem and Macroinvertebrate Assemblages to Rehabilitation Actions in Martil River (North-Western Morocco). Hydrobiology 2023, 2, 446-462. https://doi.org/10.3390/hydrobiology2030029
Guellaf A, Kassout J, Boselli VA, Bennas N, El Alami M, Errochdi S, Kettani K. Short-Term Responses of Aquatic Ecosystem and Macroinvertebrate Assemblages to Rehabilitation Actions in Martil River (North-Western Morocco). Hydrobiology. 2023; 2(3):446-462. https://doi.org/10.3390/hydrobiology2030029
Chicago/Turabian StyleGuellaf, Achraf, Jalal Kassout, Vladimiro Andrea Boselli, Nard Bennas, Majida El Alami, Sanae Errochdi, and Kawtar Kettani. 2023. "Short-Term Responses of Aquatic Ecosystem and Macroinvertebrate Assemblages to Rehabilitation Actions in Martil River (North-Western Morocco)" Hydrobiology 2, no. 3: 446-462. https://doi.org/10.3390/hydrobiology2030029
APA StyleGuellaf, A., Kassout, J., Boselli, V. A., Bennas, N., El Alami, M., Errochdi, S., & Kettani, K. (2023). Short-Term Responses of Aquatic Ecosystem and Macroinvertebrate Assemblages to Rehabilitation Actions in Martil River (North-Western Morocco). Hydrobiology, 2(3), 446-462. https://doi.org/10.3390/hydrobiology2030029








