Hydrobiological Aspects of Fatty Acids: Unique, Rare, and Unusual Fatty Acids Incorporated into Linear and Cyclic Lipopeptides and Their Biological Activity
Abstract
1. Introduction
2. Bacterial and Cyanobacterial Linear and Cyclic Peptides and Their Fatty Acids
3. Linear and Cyclic Peptides Derived from Seaweeds and Invertebrates
3.1. Fatty Acids Derived from Seaweed Lipopeptides
3.2. Fatty Acids Incorporated into Lipopeptides of Marine Sponges
3.2.1. Saturated, Methyl-Branched, and Unsaturated Fatty Acids
3.2.2. Chlorinated Fatty Acids Derived from Sponge Lipopeptides
3.2.3. Miscellaneous Fatty Acids Incorporated into Sponge Lipopeptides
4. Fatty Acids Derived from Mollusca Lipopeptides
5. Fatty Acids Derived from Tunicate Lipopeptides
6. Fatty Acids Incorporated into Actinomycete and Fungal Lipopeptides
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H. Marine peptides: Bioactivities and applications. Mar. Drugs 2015, 13, 4006–4043. [Google Scholar] [CrossRef]
- Youssef, F.S.; Ashour, M.L.; Singab, A.N.B.; Wink, M. A comprehensive review of bioactive peptides from marine fungi and their biological significance. Mar. Drugs 2019, 17, 559. [Google Scholar] [CrossRef] [PubMed]
- Ngo, D.H.; Vo, T.S.; Ngo, D.N.; Wijesekara, I.; Kim, S.K. Biological activities and potential health benefits of bioactive peptides derived from marine organisms. Int. J. Biol. Macromol. 2012, 51, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Anjum, K.; Abbas, S.Q.; Akhter, N.; Shagufta, B.I.; Shah, S.A.A.; Hassan, S.S.U. Emerging biopharmaceuticals from bioactive peptides derived from marine organisms. Chem. Biol. Drug Des. 2017, 90, 12–30. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ruiz, F.; Mancera-Andrade, E.I.; Iqbal, H.M. Marine-derived bioactive peptides for biomedical sectors: A review. Protein Pept. Lett. 2017, 24, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.K.; Seo, C.H.; Park, Y. Marine peptides and their anti-infective activities. Mar. Drugs 2015, 13, 618–654. [Google Scholar] [CrossRef]
- Kim, S.K.; Kang, K.H. Medicinal effects of peptides from marine microalgae. Adv. Food Nutr. Res. 2011, 64, 313–323. [Google Scholar]
- Harnedy, P.A.; Fitz Gerald, R.J. Bioactive peptides from marine processing waste and shellfish: A review. J. Functional Food. 2012, 4, 6–24. [Google Scholar] [CrossRef]
- Eghtedari, M.; Jafari, S.; Nowruzi, P.B. Anticancer potential of natural peptides from terrestrial and marine environments: A review. Phytochem. Lett. 2021, 42, 87–103. [Google Scholar] [CrossRef]
- Ahmed, S.; Mirzaei, H.; Aschner, M.; Khan, A.; Al-Harrasi, A.; Khan, H. Marine peptides in breast cancer: Therapeutic and mechanistic understanding. Biomed. Pharmacother. 2021, 142, 112038. [Google Scholar] [CrossRef]
- Gogineni, V.; Hamann, M.T. Marine natural product peptides with therapeutic potential: Chemistry, biosynthesis, and pharmacology. Biochim. Biophys. Acta 2018, 1862, 81–196. [Google Scholar] [CrossRef] [PubMed]
- Tardón, M.C.; Allard, M.; Dutoit, V.; Dietrich, P.Y.; Dietrich, P.Y.; Walker, P.R. Peptides as cancer vaccines. Curr. Opin. Pharmacol. 2019, 47, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Klimpel, A.; Lützenburg, T.; Neundorf, I. Recent advances of anti-cancer therapies including the use of cell-penetrating peptides. Curr. Opin. Pharmacol. 2019, 47, 8–13. [Google Scholar] [CrossRef]
- Kurrikoff, K.; Aphkhazava, D.; Lange, Ü. The future of peptides in cancer treatment. Curr. Opin. Pharmacol. 2019, 47, 27–32. [Google Scholar] [CrossRef]
- Skjånes, K.; Aesoy, R.; Herfindal, L.; Skomedal, H. Bioactive peptides from microalgae. Focus on anti-cancer and immunomodulating activity. Physiol. Plantarum 2021, 173, 612–623. [Google Scholar] [CrossRef]
- Shannon, E.; Abu-Ghannam, N. Antibacterial derivatives of marine algae: An overview of pharmacological mechanisms and applications. Mar. Drugs 2016, 14, 81. [Google Scholar] [CrossRef]
- Kastin, A. Handbook of Biologically Active Peptides, 2nd ed.; Academic Press: San Diego, CA, USA, 2013. [Google Scholar]
- Mayer, A.M.S.; Gustafson, K.R. Marine pharmacology in 2003–2004: Antitumour and cytotoxic compounds. Eur. J. Cancer 2006, 42, 2241–2270. [Google Scholar] [CrossRef]
- Jin, L.; Quan, C.; Hou, X.; Fan, S. Potential pharmacological resources: Natural bioactive compounds from marine-derived fungi. Mar. Drugs 2016, 14, 76. [Google Scholar] [CrossRef]
- Biniarz, P.; Łukaszewicz, M.; Janek, T. Screening concepts, characterization, and structural analysis of microbial-derived bioactive lipopeptides: A review. Crit. Rev. Biotechnol. 2017, 37, 393–410. [Google Scholar] [CrossRef]
- Mnif, I.; Ghribi, D. Review lipopeptides biosurfactants: Mean classes and new insights for industrial, biomedical, and environmental applications. Pept. Sci. 2015, 104, 129–147. [Google Scholar] [CrossRef]
- Hutchinson, J.A.; Burholt, S. Peptide hormones and lipopeptides: From self-assembly to therapeutic applications. J. Pept. Sci. 2017, 23, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Meena, K.R.; Sharma, A.; Kanwar, S.S. Microbial lipopeptides and their medical applications. Ann. Pharmacol. Pharm. 2017, 2, 1126. [Google Scholar]
- Anke, H.; Laatsch, H. Cyclic peptides and depsipeptides from fungi. In Physiology and Genetics. The Mycota (A Comprehensive Treatise on Fungi as Experimental Systems for Basic and Applied Research); Anke, T., Schüffler, A., Eds.; Springer: Cham, Switzerland, 2018; Volume 15. [Google Scholar]
- Rangel, M.; José Correia de Santana, C.; Pinheiro, C.; dos Anjos, L.A. Marine depsipeptides as promising pharmacotherapeutic agents. Curr. Protein Pept. Sci. 2017, 18, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Poroikov, V.V. Computer-aided drug design: From discovery of novel pharmaceutical agents to systems pharmacology. Biochemistry 2020, 14, 216–227. [Google Scholar]
- Muratov, E.N.; Bajorath, J.; Sheridan, R.P.; Tetko, I.V.; Filimonov, D.; Poroikov, V.V. QSAR without borders. Chem. Soc. Rev. 2020, 49, 3525–3564. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Antitumor profile of carbon-bridged steroids (CBS) and triterpenoids. Mar. Drugs 2021, 19, 324. [Google Scholar] [CrossRef]
- Fogg, G.E. The comparative physiology and biochemistry of the blue-green algae. Bacteriol. Rev. 1956, 20, 148–156. [Google Scholar] [CrossRef]
- Echlin, P.; Morris, I. The relationship between blue-green algae and bacteria. Biol. Rev. 1965, 40, 143–184. [Google Scholar] [CrossRef]
- Wolk, C.P. Physiology and cytological chemistry blue-green algae. Bacteriol. Rev. 1973, 37, 32–101. [Google Scholar] [CrossRef]
- Whitton, B.A.; Potts, M. Introduction to the Cyanobacteria. In Ecology of Cyanobacteria II: Their Diversity in Space and Time; Whitton, B.A., Ed.; Springer Science & Business Media, B.V.: Berlin/Heidelberg, Germany, 2012; pp. 1–13. [Google Scholar]
- Soo, R.M.; Hemp, J.; Hugenholtz, P. Evolution of photosynthesis and aerobic respiration in the cyanobacteria. Free Radic. Biol. Med. 2019, 140, 200–205. [Google Scholar] [CrossRef]
- Allaby, M. The Concise Dictionary of Botany; Oxford University Press: Oxford, UK, 1992. [Google Scholar]
- Sánchez-Baracaldo, R.; Bianchini, G.; Wilson, J.D.; Knoll, A.H. Cyanobacteria, and biogeochemical cycles through Earth history. Trends Microbiol. 2022, 30, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, S.A.M.; Shedid, E.S.; Saied, E.M.; Jassbi, A.R.; Jamebozorgi, F.H.; Rateb, M.E.; Du, M.; Abdel-Daim, M.M.; Kai, G.-Y.; Al-Hammady, M.A.M. Cyanobacteria—From the oceans to the potential biotechnological and biomedical applications. Mar. Drugs 2021, 19, 241. [Google Scholar] [CrossRef] [PubMed]
- Mondal, A.; Bose, S.; Banerjee, S.; Patra, J.K. Marine cyanobacteria and microalgae metabolites—A rich source of potential anticancer drugs. Mar. Drugs 2020, 18, 476. [Google Scholar] [CrossRef] [PubMed]
- Namikoshi, M.; Rinehart, K. Bioactive compounds produced by cyanobacteria. J. Ind. Microbiol. Biotechnol. 1996, 17, 373–384. [Google Scholar] [CrossRef]
- Burja, A.M.; Banaigs, E.B.; Burgess, J.G.; Wright, P.C. Marine cyanobacteria—A prolific source of natural products. Tetrahedron 2001, 57, 9347–9377. [Google Scholar] [CrossRef]
- Tan, L.T.; Sitachitta, N.; Gerwick, W.H. The guineamides, novel cyclic depsipeptides from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2003, 66, 764–771. [Google Scholar] [CrossRef]
- Singh, S.; Bhushan, N.; Kate, U.C. Banerjee. Bioactive compounds from cyanobacteria and microalgae: An Overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Řezanka, T. Metabolites produced by nitrogen fixing Nostoc species. Folia Microbiol. 2005, 50, 363–391. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Shkrob, I.; Dor, I. Separation and identification of hydrocarbons and other volatile compounds from cultured blue-green alga Nostoc sp. by gas chromatography—Mass spectrometry using serially coupled capillary columns with consecutive nonpolar and semipolar stationary phases. J. Chromatogr. A 1999, 862, 221–229. [Google Scholar] [CrossRef]
- Řezanka, T.; Dembitsky, V.M. Metabolites produced by cyanobacteria belonging to several species of the family Nostocaceae. Folia Microbiol. 2006, 51, 159–182. [Google Scholar] [CrossRef]
- Temina, M.; Rezankova, H.; Rezanka, T.; Dembitsky, V.M. Diversity of the fatty acids of the Nostoc species and their statistical analysis. Microbiol. Res. 2007, 162, 308–321. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.E.; Hirsch, C.F.; Sesin, D.F.; Flor, J.E.; Chartrain, M. Pharmaceuticals from cultured algae. J. Ind. Microbiol. 1990, 5, 113–124. [Google Scholar] [CrossRef]
- Trimurtulu, G.; Ohtani, I.; Patterson, G.M.L.; Moore, R.E.; Corbett, T.H.; Valeriote, F.A.; Demchik, L. Total structures of cryptophycins, potent antitumor depsipeptides from the blue-green alga Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1994, 116, 4729–4737. [Google Scholar] [CrossRef]
- Mooberry, S.L.; Busquets, L.; Tien, G. Induction of apoptosis by cryptophycin 1, a new antimicrotubule agent. Int. J. Cancer 1997, 73, 440–448. [Google Scholar] [CrossRef]
- Hirsch, C.F.; Liesch, J.M.; Salvatore, M.J.; Schwartz, R.E.; Sesin, D.F. Antifungal Fermentation Product and Method. U.S. Patent 4946835, 18 August 1990. [Google Scholar]
- Weiss, C.; Figueras, E.; Borbely, A.N.; Sewald, N. Cryptophycins: Cytotoxic cyclodepsipeptides with potential for tumor targeting. J. Pept. Sci. 2017, 23, 514–531. [Google Scholar] [CrossRef]
- Subbaraju, G.V.; Golakoti, T.; Patterson, G.M.L.; Moore, R.E. Three new cryptophycins from Nostoc sp. GSV 224. J. Nat. Prod. 1997, 60, 302–305. [Google Scholar] [CrossRef]
- Chaganty, S.; Golakoti, T.; Heltzel, C.; Moore, R.E.; Yoshida, W.Y. Isolation and structure determination of cryptophycins 38, 326, and 327 from the terrestrial cyanobacterium Nostoc sp. GSV 224. J. Nat. Prod. 2004, 67, 1403–1406. [Google Scholar] [CrossRef]
- Golakoti, T.; Ogino, J.; Heltzel, C.E.; Husebo, T.L.; Jensen, C.M.; Larsen, L.K.; Patterson, G.M.L.; Moore, R.E.; Mooberry, S.L.; Corbett, T.H.; et al. Structure determination, conformational analysis, chemical stability studies, and antitumor evaluation of the cryptophycins. Isolation of 18 new analogs from Nostoc sp. strain GSV 224. J. Am. Chem. Soc. 1995, 117, 12030–12049. [Google Scholar] [CrossRef]
- Shih, C.; Teicher, B.A. Cryptophycins: A novel class of potent antimitotic antitumor depsipeptides. Curr. Pharm. Des. 2001, 7, 1259–1276. [Google Scholar] [CrossRef]
- Rohr, J. Cryptophycin anticancer drugs revisited. ACS Chem. Biol. 2006, 1, 747–752. [Google Scholar] [CrossRef][Green Version]
- Eggen, M.J.; Georg, G.I. The cryptophycins: Their synthesis and anticancer activity. Med. Res. Rev. 2002, 22, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.; Costa-Rodrigues, J.; Fernandes, M.H.; Barros, P.; Vasconcelos, V.; Martins, R. Marine cyanobacteria compounds with anticancer properties: A review on the implication of apoptosis. Mar. Drugs 2012, 10, 2181–2207. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, A.; Dembitsky, V. Acetylenic anticancer agents. Anti-Cancer Agents Med. Chem. 2008, 8, 132–170. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Tava, A. Rare fatty acids and lipids in plant oilseeds: Occurrence and bioactivity. Phytochem. Rev. 2022, 21, 401–428. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Anticancer activity of natural and synthetic acetylenic lipids. Lipids 2006, 41, 883–924. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Levitsky, D.O. Acetylenic terrestrial anticancer agents. Nat. Prod. Commun. 2006, 1, 405. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T. Distribution of acetylenic acids and polar lipids in some aquatic bryophytes. Phytochemistry 1995, 40, 93–97. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Rezanka, T. Acetylenic fatty acids of the Dicranaceae. Phytochemistry 1994, 36, 685–689. [Google Scholar] [CrossRef]
- Cahoon, E.B.; Li-Beisson, Y. Plant unusual fatty acids: Learning from the less common. Curr. Opin. Plant Biol. 2020, 55, 66–73. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Levitsky, D.O.; Gloriozova, T.A.; Poroikov, V.V. Acetylenic aquatic anticancer agents and related compounds. Nat. Prod. Commun. 2006, 1, 773. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Domb, A.J.; Dembitsky, V.M. Bioactive acetylenic metabolites. Phytomedicine 2013, 20, 1145–1159. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y. Microalgal metabolites. Curr. Opin. Microbiol. 2003, 6, 236. [Google Scholar] [CrossRef]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Apramides A−G, novel lipopeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 1106. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Micromide and guamamide: Cytotoxic alkaloids from a species of the marine cyanobacterium Symploca. J. Nat. Prod. 2004, 67, 49. [Google Scholar] [CrossRef]
- Jimenez, J.I.; Scheuer, P.J. New lipopeptides from the Caribbean cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 200. [Google Scholar] [CrossRef]
- Chen, H.; Feng, Y.; Xu, Z.; Ye, T. The total synthesis and reassignment of stereochemistry of dragonamide. Tetrahedron 2005, 61, 11132. [Google Scholar] [CrossRef]
- Hooper, G.J.; Orjala, J.; Schatzman, R.C.; Gerwick, W.H. Carmabins A and B, new lipopeptides from the caribbean cyanobacterium Lyngbya majuscula. J. Nat. Prod. 1998, 61, 529. [Google Scholar] [CrossRef]
- Nogle, L.M.; Gerwick, W.H. Isolation of four new cyclic depsipeptides, antanapeptins A−D, and dolastatin 16 from a Madagascan collection of Lyngbya majuscula. J. Nat. Prod. 2002, 65, 21. [Google Scholar] [CrossRef]
- Luesch, H.; Pangilinan, R.Y.; Moore, R.E.; Paul, V.J. Pitipeptolides A and B, new cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 304. [Google Scholar] [CrossRef]
- Sitachitta, N.; Williamson, R.T.; Gerwick, W.H. Yanucamides A and B, two new depsipeptides from an assemblage of the marine cyanobacteria Lyngbya majuscula and Schizothrix species. J. Nat. Prod. 2000, 63, 197. [Google Scholar] [CrossRef]
- Xu, Z.; Peng, Y.; Ye, T. The total synthesis and stereochemical revision of yanucamide A. Org. Lett. 2003, 5, 2821. [Google Scholar] [CrossRef] [PubMed]
- Williams, P.G.; Yoshida, W.Y.; Quon, M.K.; Moore, R.E.; Paul, V.J. Ulongapeptin, a cytotoxic cyclic depsipeptide from a Palauan marine cyanobacterium Lyngbya sp. J. Nat. Prod. 2003, 66, 651. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Goeger, D.; Maier, C.S.; Gerwick, W.H. The Wewakpeptins, Cyclic depsipeptides from a Papua New Guinea collection of the marine cyanobacterium Lyngbya semiplena. J. Org. Chem. 2005, 70, 3133. [Google Scholar] [CrossRef] [PubMed]
- Blunt, J.W.; Copp, B.R.; Munro, M.H.G.; Northcote, P.T.; Prinsep, M.R. Marine natural products. Nat. Prod. Rep. 2005, 22, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Wan, F.; Erickson, K.L. Georgamide, a new cyclic depsipeptide with an alkynoic acid residue from an Australian cyanobacterium. J. Nat. Prod. 2001, 64, 143. [Google Scholar] [CrossRef]
- Rodriguez, J.; Fernandez, R.; Quinoa, E.; Riguera, R.; Debitus, C.; Bouchet, P. Onchidin: A cytotoxic depsipeptide with C2 symmetry from a marine mollusc. Tetrahedron Lett. 1994, 35, 9239. [Google Scholar] [CrossRef]
- Fernandez, R.; Rodriguez, J.; Quiñoa, E.; Riguera, R.; Muñoz, L.; Fernandez-Suarez, M.; Debitus, C. Onchidin B: A new cyclodepsipeptide from the mollusc Onchidium sp. J. Am. Chem. Soc. 1996, 118, 11635–11643. [Google Scholar] [CrossRef]
- Reese, M.T.; Gulavita, N.K.; Nakao, Y.; Hamann, M.T.; Yoshida, W.Y.; Coval, S.J.; Scheuer, P.J. Kulolide: A cytotoxic depsipeptide from a cephalaspidean mollusk, Philinopsis speciosa. J. Am. Chem. Soc. 1996, 118, 11081–11084. [Google Scholar] [CrossRef]
- Nakao, Y.; Yoshida, W.Y.; Szabo, C.M.; Baker, B.J.; Scheuer, P.J. More peptides and other diverse constituents of the marine mollusk Philinopsis speciosa. J. Org. Chem. 1998, 63, 3272–3280. [Google Scholar] [CrossRef]
- Liu, W.-T.; Ng, J.; Meluzzi, D.; Bandeira, N.; Gutierrez, M.; Simmons, T.L.; Schultz, A.W.; Linington, R.G.; Moore, B.S.; Gerwick, W.H. Interpretation of tandem mass spectra obtained from cyclic nonribosomal peptides. Anal. Chem. 2009, 81, 4200–4209. [Google Scholar] [CrossRef]
- Montaser, R.; Paul, V.J.; Luesch, H. Pitipeptolides C-F, antimycobacterial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula from Guam. Phytochemistry 2011, 72, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.P.; Owle, C.S.; Montaser, R.; Luesch, H.; Paul, V.J. Malyngamide 3 and cocosamides A and B from the marine cyanobacterium Lyngbya majuscula from Cocos lagoon, Guam. J. Nat. Prod. 2011, 74, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, P.D.; Byrum, T.; Liu, W.T.; Dorrestein, P.C.; Gerwick, W.H. Viequeamide A, a cytotoxic member of the kulolide superfamily of cyclic depsipeptides from a marine button cyanobacterium. J. Nat. Prod. 2012, 75, 1560–1570. [Google Scholar] [CrossRef] [PubMed]
- Almaliti, J.; Malloy, K.L.; Glukhov, E.; Spadafora, C.; Gutierrez, M.; Gerwick, W.H. Dudawalamides A−D, antiparasitic cyclic depsipeptides from the marine cyanobacterium Moorea producers. J. Nat. Prod. 2017, 80, 1827–1836. [Google Scholar] [CrossRef] [PubMed]
- Salvador, L.A.; Biggs, J.S.; Paul, V.J.; Luesch, H. Veraguamides A–G, cyclic hexadepsipeptides from a dolastatin 16-producing cyanobacterium Symploca cf. hydnoides from Guam. J. Nat. Prod. 2011, 74, 917–927. [Google Scholar] [CrossRef]
- Mevers, E.; Liu, W.T.; Engene, N.; Mohimani, H.; Byrum, T.; Pevzner, P.A.; Dorrestein, P.C.; Spadafora, C.; Gerwick, W.H. Cytotoxic veraguamides, alkynyl bromide-containing cyclic depsipeptides from the marine cyanobacterium cf. Oscillatoria margaritifera. J. Nat. Prod. 2011, 74, 928–936. [Google Scholar] [CrossRef]
- Sueyoshi, K.; Kudo, T.; Yamano, A.; Sumimoto, S. Odobromoamide, a terminal alkynyl bromide-containing cyclodepsipeptide from the marine cyanobacterium Okeania sp. Bull. Chem. Soc. Jpn. 2017, 90, 436–440. [Google Scholar] [CrossRef]
- Williams, P.G.; Yoshida, W.Y.; Quon, M.K.; Moore, R.E.; Paul, V.J. The structure of palauamide, a potent cytotoxin from a species of the marine cyanobacterium Lyngbya. J. Nat. Prod. 2003, 66, 1545–1549. [Google Scholar] [CrossRef]
- Bunyajetpong, S.; Yoshida, W.Y.; Sitachitta, N.; Kaya, K. Trungapeptins A−C, cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2006, 69, 1539–1542. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.F.; Tan, L.T. Hantupeptin A, a cytotoxic cyclic depsipeptide from a Singapore collection of Lyngbya majuscula. J. Nat. Prod. 2009, 72, 29–32. [Google Scholar] [CrossRef]
- Arif, J.M.; Farooqui, A.; Siddiqui, M.H.; Al-Karrawi, M. Novel bioactive peptides from cyanobacteria: Functional, biochemical, and biomedical significance. Bioactive Nat. Prod. 2012, 36, 111–161. [Google Scholar]
- Gunasekera, S.P.; Ross, C.; Paul, V.J.; Matthew, S.; Luesch, H. Dragonamides C and D, linear lipopeptides from the marine cyanobacterium brown Lyngbya polychroa. J. Nat. Prod. 2008, 71, 887–890. [Google Scholar] [CrossRef] [PubMed]
- McPhail, K.L.; Correa, J.; Linington, R.G.; González, J.; Ortega-Barría, E.; Capson, T.L.; Gerwick, W.H. Antimalarial linear lipopeptides from a Panamanian strain of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2007, 70, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Balunas, M.J.; Linington, R.G.; Tidgewell, K.; Fenner, A.M.; Ureña, L.D.; Togna, G.D.; Kyle, D.E.; Gerwick, W.H. Dragonamide E, a modified linear lipopeptide from Lyngbya majuscula with antileishmanial activity. J. Nat. Prod. 2010, 73, 60–66. [Google Scholar] [CrossRef]
- Simmons, T.L.; Engene, N.; Ureña, L.D.; Romero, L.I.; Ortega-Barría, E.; Gerwick, L.; Gerwick, W.H. Viridamides A and B, lipodepsipeptides with anti-protozoal activity from the marine cyanobacterium Oscillatoria nigro-viridis. J. Nat. Prod. 2008, 71, 1544–1550. [Google Scholar] [CrossRef]
- Iwasaki, A.; Ohno, O.; Sumimoto, S.; Sud, S.; Suenaga, K. Kurahyne, an acetylene-containing lipopeptide from a marine cyanobacterial assemblage of Lyngbya sp. Commun. RSC Adv. 2014, 4, 12840–12843. [Google Scholar] [CrossRef]
- Dixit, R.B.; Suseela, M.R. Cyanobacteria: Potential candidates for drug discovery. Antonie van Leeuwenhoek 2013, 103, 947–961. [Google Scholar] [CrossRef]
- Quintana, J.; Bayona, L.M.; Castellanos, L.; Puyana, M.; Camargo, P.; Aristizábal, F.; Edwards, C.; Tabudravu, J.N.; Jaspars, M.; Ramos, F.A. Almiramide D, cytotoxic peptide from the marine cyanobacterium Oscillatoria nigroviridis. Bioorg. Med. Chem. 2014, 22, 6789–6795. [Google Scholar] [CrossRef]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Lee, P.P.F.; Tan, L.T. Hantupeptins B and C, cytotoxic cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2010, 71, 307–311. [Google Scholar] [CrossRef]
- Bingnan, H.; Gross, H.; McPhail, K.L.; Goeger, D.; Maier, C.S.; Gerwick, W.H. Wewakamide A and guineamide G, cyclic depsipeptides from the marine cyanobacteria Lyngbya semiplena and Lyngbya majuscula. J. Microbiol. Biotechnol. 2011, 21, 930–936. [Google Scholar]
- Chai, Q.Y.; Yang, Z.; Lin, H.W.; Han, B.N. Alkynyl-containing peptides of marine origin: A review. Mar. Drugs 2016, 14, 216. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Fudou, R.; Jojima, Y.; Ogawa, S.; Yamanaka, S.; Inukai, Y.; Ojika, M. Miuraenamides A and B, novel antimicrobial cyclic depsipeptides from a new slightly halophilic myxobacterium: Taxonomy, production, and biological properties. J. Antibiot. 2006, 59, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Galica, T.; Borbone, N.; Mareš, J.; Kust, A.; Caso, A.; Esposito, G.; Saurav, K. Cyanochelins, an overlooked class of widely distributed cyanobacterial siderophores, discovered by silent gene cluster awakening. Appl. Environ. Microbiol. 2021, 87, e0312820. [Google Scholar] [CrossRef] [PubMed]
- Soria-Mercado, I.E.; Pereira, A.; Cao, Z.; Murray, T.F.; Gerwick, W.H. Alotamide A, a novel neuropharmacological agent from the marine cyanobacterium Lyngbya bouillonii. Org. Lett. 2009, 11, 4704–4707. [Google Scholar] [CrossRef]
- Luesch, H.L.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Corbett, T.H. Total structure determination of apratoxin A, a potent novel cytotoxin from the marine cyanobacterium Lyngbya majuscula. J. Am. Chem. Soc. 2001, 123, 5418–5423. [Google Scholar] [CrossRef]
- Gutiérrez, M.; Suyama, T.L.; Engene, N.; Wingerd, J.S.; Matainaho, T.; Gerwick, W.H. Apratoxin D, a potent cytotoxic cyclodepsipeptide from Papua New Guinea collections of the marine cyanobacteria Lyngbya majuscula and Lyngbya sordida. J. Nat. Prod. 2008, 71, 1099–1103. [Google Scholar] [CrossRef]
- Choi, H.; Pereira, A.R.; Cao, Z.; Shuman, C.F.; Engene, N.; Byrum, T.; Matainaho, T.; Murray, T.F.; Mangoni, A.; Gerwick, W.H. The hoiamides, structurally intriguing neurotoxic lipopeptides from Papua New Guinea marine cyanobacteria. J. Nat. Prod. 2010, 73, 1411–1421. [Google Scholar] [CrossRef]
- Matthew, S.; Schupp, P.J.; Luesch, H. Apratoxin E, a cytotoxic peptolide from a Guamanian collection of the marine cyanobacterium Lyngbya bouillonii. J. Nat. Prod. 2008, 71, 1113–1116. [Google Scholar] [CrossRef]
- Sweeney-Jones, A.M.; Gagaring, K.; Antonova-Koch, J.; Zhou, H.; Mojib, N.; Soapi, K.; Skolnick, J.; McNamara, C.W.; Kubanek, J. Antimalarial peptide and polyketide natural products from the Fijian marine cyanobacterium Moorea producens. Mar. Drugs 2020, 18, 167. [Google Scholar] [CrossRef]
- Matthew, S.; Salvador, L.A.; Schupp, P.J.; Paul, V.J.; Luesch, V.J. Cytotoxic halogenated macrolides and modified peptides from the apratoxin-producing marine cyanobacterium Lyngbya bouillonii from Guam. J. Nat. Prod. 2010, 73, 1544–1552. [Google Scholar] [CrossRef]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J.; Mooberry, S.L. Isolation, structure determination, and biological activity of lyngbyabellin A from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2000, 63, 611–615. [Google Scholar] [CrossRef] [PubMed]
- Fathoni, I.; Petitbois, J.G.; Alarif, W.M.; Abdel-Lateff, A.; Al-Lihaibi, S.S.; Yoshimura, E.; Nogata, Y.; Vairappan, C.S.; Sholikhah, E.N.; Okino, T. Bioactivities of lyngbyabellins from cyanobacteria of Moorea and Okeania genera. Molecules 2020, 25, 3986. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; McPhail, K.L.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Isolation and structure of five lyngbyabellin derivatives from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. Tetrahedron 2005, 61, 11723–11729. [Google Scholar] [CrossRef]
- Marquez, B.L.; Watts, K.S.; Yokochi, A.; Roberts, M.A.; Verdier-Pinard, P.; Jimenez, J.I.; Hamel, E.; Scheuer, P.J.; Gerwick, W.H. Structure and absolute stereochemistry of hectochlorin, a potent stimulator of actin assembly. J. Nat. Prod. 2002, 65, 866–871. [Google Scholar] [CrossRef]
- Petitbois, J.G.; Casalme, L.O.; Lopez, J.A.V.; Alarif, W.M.; Abdel-Late, A.; Al-Lihaibi, S.S.; Yoshimura, E.; Nogata, Y.; Umezawa, T.; Matsuda, F. Serinolamides and lyngbyabellins from an Okeania sp. cyanobacterium collected from the Red Sea. J. Nat. Prod. 2017, 80, 2708–2715. [Google Scholar] [CrossRef]
- Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Structurally diverse new alkaloids from Palauan collections of the apratoxin-producing marine cyanobacterium Lyngbya sp. Tetrahedron 2002, 58, 7958–7959. [Google Scholar] [CrossRef]
- Williams, P.G.; Luesch, H.; Yoshida, W.Y.; Moore, R.E.; Paul, V.J. Continuing studies on the cyanobacterium Lyngbya sp.: Isolation and structure determination of 15-norlyngbyapeptin A and lyngbyabellin D. J. Nat. Prod. 2003, 66, 595–598. [Google Scholar] [CrossRef]
- Choi, H.; Mevers, E.; Byrun, T.; Valeriote, F.A.; Gerwick, W.H. Lyngbyabellins K–N from two Palmyra atoll collections of the marine cyanobacterium Moorea bouillonii. Eur. J. Org. Chem. 2012, 27, 5141–5150. [Google Scholar] [CrossRef]
- Li, W.I.; Berman, F.W.; Okino, T.; Yokokawa, F.; Shioiri, T.; Gerwick, W.H.; Murray, T.F. Antillatoxin is a marine cyanobacterial toxin that potently activates voltage-gated sodium channels. Proc. Natl. Acad. Sci. USA 2001, 98, 7599–7604. [Google Scholar] [CrossRef]
- Nogle, L.M.; Okino, T.; Gerwick, W.H. Antillatoxin B, a neurotoxic lipopeptide from the Marine Cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2001, 64, 983–985. [Google Scholar] [CrossRef]
- Li, W.I.; Marquez, B.L.; Okino, T.; Yokokawa, F.; Shioiri, T.; Gerwick, W.H.; Murray, T.F. Characterization of the preferred stereochemistry for the neuropharmacologic actions of antillatoxin. J. Nat. Prod. 2004, 67, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Okura, K.; Matsuoka, S.; Goto, R.; Inoue, M. The twisted side chain of antillatoxin is important for potent toxicity: Total synthesis and biological evaluation of antillatoxin and analogues. Angew. Chem. Int. Ed. Engl. 2010, 49, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Natural neo acids and neo alkanes: Their analogs and derivatives. Lipids 2006, 41, 309–340. [Google Scholar] [CrossRef]
- Teruya, T.; Sasaki, H.; Fukazawa, H.; Suenaga, K. Bisebromoamide, a potent cytotoxic peptide from the marine cyanobacterium Lyngbya sp.: Isolation, stereostructure, and biological activity. Org. Lett. 2009, 11, 5062–5065. [Google Scholar] [CrossRef]
- Sasaki, H.; Teruya, T.; Fukazawa, H.; Suenaga, K. Revised structure and structureeactivity relationshipof bisebromoamide and structure of norbisebromoamide from the marine cyanobacterium Lyngbya sp. Tetrahedron 2011, 67, 990–994. [Google Scholar] [CrossRef]
- Nakamura, S.; Chikaike, T.; Karasawa, K.; Tanaka, N.; Yonehara, H.; Umezawa, H. Isolation and characterization of bottromycins A and B. J. Antibiot. 1965, 18, 47–52. [Google Scholar]
- Nakamura, S.; Tanaka, N.; Umezawa, H. Bottromycins A1 and A2 and their structures. J. Antibiot. 1966, 19, 10–12. [Google Scholar]
- Hata, F.; Matsumae, A.; Abe, K.; Sano, Y.; Otani, M.; Omura, S. Fermentative preparation of bottromycin, Japan Tokkyo Koho. Japanese Patent JP 47010036, 22 May 1972. [Google Scholar]
- Mizuno, K.; Muto, N.; Kamata, S.; Asano, K. Antibiotic, bottromycin, Japan Kokai Tokkyo Koho. Japanese Patent JP 49116297 19741106/JP 73–28677 19730312, 12 December 1974. [Google Scholar]
- Nagle, D.G.; Paul, V.J.; Roberts, M.A. Ypaoamide, a new broadly acting feeding deterrent from the marine cyanobacterium Lyngbya majuscula. Tetrahedron Lett. 1996, 37, 6263–6266. [Google Scholar] [CrossRef]
- Nagle, D.G.; Paul, V.J. Chemical defense of a marine cyanobacterial bloom. J. Exp. Mar. Biol. Ecol. 1998, 225, 29–38. [Google Scholar] [CrossRef]
- Nagle, D.G.; Paul, V.J. Production of secondary metabolites by filamentous tropical marine cyanobacteria: Ecological functions of the compounds. J. Phycol. 1999, 35, 1412–1421. [Google Scholar]
- Sueyoshi, K.; Yamada, M.; Yamano, A.; Ozaki, K.; Sumimoto, S.; Iwasaki, A.; Suenaga, K.; Teruya, T. Ypaoamides B and C, linear lipopeptides from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2018, 81, 1103–1107. [Google Scholar] [CrossRef] [PubMed]
- Hamada, T.; Matsunaga, S.; Yano, G.; Fusetani, N. Polytheonamides A and B, highly cytotoxic, linear polypeptides with unprecedented structural features, from the marine sponge Theonella swinhoei. J. Am. Chem. Soc. 2005, 127, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, H.; Iwasaki, A.; Sumimoto, S.; Kanamori, Y.; Ohno, O.; Iwatsuki, M. Janadolide, a cyclic polyketide–peptide hybrid possessing a tert-butyl group from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2016, 79, 1862–1866. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Ermolenko, E.; Savidov, N.; Gloriozova, T.A.; Proroikov, V.V. Antiprotozoal and antitumor activity of natural polycyclic endoperoxides: Origin, structures, and biological activity. Molecules 2021, 26, 686. [Google Scholar] [CrossRef]
- Sikorsky, T.V.; Ermolenko, E.V.; Gloriozova, T.A.; Dembitsky, V.M. Mini Review: Anticancer activity of diterpenoid peroxides. Vietnam, J. Chem. 2020, 58, 273–280. [Google Scholar] [CrossRef]
- Ermolenko, E.V.; Imbs, A.B.; Gloriozova, T.A.; Poroikov, V.V.; Sikorskaya, T.V.; Dembitsky, V.M. Chemical diversity of soft coral steroids and their pharmacological activities. Mar. Drugs 2020, 18, 613. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Antitumor and hepatoprotective activity of natural and synthetic neo steroids. Prog. Lipid Res. 2020, 79, 101048. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Yaremenko, I.A. Stable and unstable 1,2-dioxolanes: Origin, synthesis, and biological activities. Sci. Synth. 2020, 38, 277–321. [Google Scholar]
- Dembitsky, V.M.; Savidov, N.; Poroikov, V.V.; Gloriozova, T.A.; Imbs, A.B. Naturally occurring aromatic steroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 4663–4674. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Savidov, N. Steroid phosphate esters and phosphonosteroids and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 7679–7692. [Google Scholar] [CrossRef]
- Vil, V.A.; Gloriozova, T.A.; Poroikov, V.V.; Terent’ev, A.O.; Savidov, N.; Dembitsky, V.M. Peroxy steroids derived from plant and fungi and their biological activities. Appl. Microbiol. Biotechnol. 2018, 102, 7657–7667. [Google Scholar] [CrossRef] [PubMed]
- Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Dembitsky, V.M. Highly oxygenated isoprenoid lipids derived from fungi and fungal endophytes: Origin and biological activities. Steroids 2018, 140, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.; Terentev, A.O.; Al Quntar, A.A.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Oxetane-containing metabolites: Origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 2449–2467. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Gloriozova, T.A.; Terentev, A.O.; Savidov, N.; Dembitsky, V.M. Hydroperoxides derived from marine sources: Origin and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Gloriozova, T.A.; Poroikov, V.V.; Terentev, A.O.; Savidov, N.; Dembitsky, V.M. Naturally occurring of α,β-diepoxy-containing compounds: Origin, structures, and biological activities. Appl. Microbiol. Biotechnol. 2019, 103, 3249–3264. [Google Scholar] [CrossRef] [PubMed]
- Vil, V.A.; Terent’ev, A.O.; Savidov, N.; Gloriozova, T.A.; Poroikov, V.V.; Pounina, T.A.; Dembitsky, V.M. Hydroperoxy steroids and triterpenoids derived from plant and fungi: Origin, structures, and biological activities. J. Steroid Biochem. Mol. Biol. 2019, 190, 76–87. [Google Scholar] [CrossRef]
- Iwasaki, A.; Tadenuma, T.; Sumimoto, S.; Shiota, I.; Matsubara, T. Hoshinoamides A and B, acyclic lipopeptides from the marine cyanobacterium Caldora penicillata. J. Nat. Prod. 2018, 81, 2545–2552. [Google Scholar] [CrossRef]
- Brumley, D.A.; Gunasekera, S.P.; Chen, Q.Y.; Paul, V.J.; Luesch, H. Discovery, total synthesis, and SAR of anaenamides A and B: Anticancer cyanobacterial depsipeptides with a chlorinated pharmacophore. Org. Lett. 2020, 22, 4235–4239. [Google Scholar] [CrossRef]
- Nowruzi, B.; Wahlsten, M.; Jokela, J. A report on finding a new peptide aldehyde from cyanobacterium Nostoc sp. Iran, J. Biotechnol. 2019, 17, e1853. [Google Scholar] [CrossRef]
- Liu, L.; Jokela, J.; Wahlsten, M.; Nowruzi, B.; Permi, P.; Zhang, Y.Z. Nostosins, trypsin inhibitors isolated from the terrestrial cyanobacterium Nostoc sp. strain FSN. J. Nat. Prod. 2014, 77, 1784–1790. [Google Scholar] [CrossRef]
- Hoffmann, H.; Kogler, H.; Heyse, W.; Matter, H.; Caspers, M.; Schummer, D.; Klemke-Jahn, C.; Bauer, A.; Penarier, G.; Debussche, L. Discovery, structure elucidation, and biological characterization of nannocystin A, a macrocyclic myxobacterial metabolite with potent antiproliferative properties. Angew. Chem. 2015, 54, 10145–10148. [Google Scholar] [CrossRef] [PubMed]
- Krastel, P.; Roggo, S.; Schirle, M.; Ross, N.T.; Perruccio, F.; Aspesi, P., Jr.; Aust, T.; Buntin, K.; Estoppey, D.; Liechty, B. Nannocystin A: An elongation factor 1 inhibitor from Myxobacteria with differential anti-cancer properties. Angew. Chem. 2015, 54, 10149–10154. [Google Scholar] [CrossRef] [PubMed]
- Andrianasolo, E.H.; Goeger, D.; Gerwick, W.H. Mitsoamide: A cytotoxic linear lipopeptide from the Madagascar marine cyanobacterium Geitlerinema sp. Pure Appl. Chem. 2007, 79, 593–602. [Google Scholar] [CrossRef]
- Sumimoto, S.; Kobayashi, M.; Sato, R.; Shinomiya, S.; Iwasaki, A.; Suda, S.; Teruya, T.; Inuzuka, T.; Ohno, O.; Suenaga, K. Minnamide A, a linear lipopeptide from the marine cyanobacterium Okeania hirsuta. Org. Lett. 2019, 21, 1187–1901. [Google Scholar] [CrossRef]
- Pereira, A.; Cao, Z.Y.; Murray, T.F.; Gerwick, W.H. Hoiamide A, a sodium channel activator of unusual architecture from a consortium of two Papua New Guinea cyanobacteria. Chem. Biol. 2009, 16, 893–906. [Google Scholar] [CrossRef]
- Tan, L.T.; Tatsufumi Okino, T.; Gerwick, W.H. Bouillonamide: A mixed polyketide–peptide cytotoxin from the marine cyanobacterium Moorea bouillonii. Mar. Drugs 2013, 11, 3015–3024. [Google Scholar] [CrossRef]
- Neuhof, T.; Schmieder, P.; Seibold, M.; Preussel, K.; Döhrena, H. Hassallidin B—Second antifungal member of the Hassallidin family. Bioorg. Med. Chem. Lett. 2006, 16, 4220–4222. [Google Scholar] [CrossRef]
- Yu, H.-B.; Glukhov, E.; Li, Y.; Iwasaki, A.; Gerwick, L.; Dorrestein, P.C.; Jiao, B.-H.; Gerwick, W.H. Cytotoxic microcolin lipopeptides from the marine cyanobacterium Moorea producens. J. Nat. Prod. 2019, 82, 2608–2619. [Google Scholar] [CrossRef]
- Tareq, F.S.; Lee, M.A.; Lee, H.S.; Lee, J.S.; Lee, Y.J.; Shin, H.J. Gageostatins A–C, antimicrobial linear lipopeptides from a marine Bacillus subtilis. Mar. Drugs 2014, 12, 871–885. [Google Scholar] [CrossRef]
- Iwasaki, K.; Iwasaki, A.; Sumimoto, S.; Matsubara, T.; Sato, T.; Nozaki, T.; Saito-Nakano, Y.; Suenaga, K. Ikoamide, an antimalarial lipopeptide from an Okeania sp. marine cyanobacterium. J. Nat. Prod. 2020, 83, 481–488. [Google Scholar] [CrossRef]
- Jiang, W.; Zhou, W.; Othman, R.; Uchida, H.; Watanabe, R.; Suzuki, T. A new malyngamide from the marine cyanobacterium Moorea producens. Nat. Prod. Res. 2018, 32, 97–104. [Google Scholar] [CrossRef]
- Yamanaka, K.; Reynolds, K.A.; Kerstena, R.D.; Ryan, K.S.; Gonzalez, D.J. Direct cloning and refactoring of a silent lipopeptide biosynthetic gene cluster yields the antibiotic taromycin A. Proc. Natl. Acad. Sci. USA 2014, 111, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, K.A.; Luhavaya, H.; Li, J.; Dahesh, S.; Nizet, V.; Yamanaka, K.; Moore, B.S. Isolation and structure elucidation of lipopeptide antibiotic taromycin B from the activated taromycin biosynthetic gene cluster. J. Antibiot. 2018, 71, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Omoboye, O.O.; Geudens, N.; Duban, M.; Chevalier, M.; Flahaut, C.; Martins, J.C.; Leclère, V.; Oni, F.E.; Höfte, M. Pseudomonas sp. COW3 produces new bananamide type cyclic lipopeptides with antimicrobial activity against Pythium myriotylum and Pyricularia oryzae. Molecules 2019, 24, 4170. [Google Scholar] [CrossRef]
- Tareq, F.S.; Hasan, C.M.; Lee, H.S.; Lee, Y.J.; Lee, J.S.; Surovy, M.Z.; Islam, M.T.; Shin, H.J. Gageopeptins A and B, new inhibitors of zoospore motility of the phytopathogen Phytophthora capsici from a marine-derived bacterium Bacillus sp. 109GGC020. Bioorg. Med. Chem. Lett. 2015, 28, 3325–3329. [Google Scholar] [CrossRef]
- Chakraborty, M.; Mahmud, N.U.; Gupta, D.R.; Tareq, F.S.; Shin, H.J.; Islam, T. Inhibitory effects of linear lipopeptides from a marine Bacillus subtilis on the wheat blast fungus Magnaporthe oryzae triticum. Front. Microbiol. 2020, 11, 665. [Google Scholar] [CrossRef]
- Matsui, K.; Kan, Y.; Kikuchi, J.; Matsushima, K.; Takemura, M.; Maki, H.; Kozono, I.; Ueda, T.; Minagawa, K. Stalobacin: Discovery of novel lipopeptide antibiotics with potent antibacterial activity against multidrug-resistant bacteria. J. Med. Chem. 2020, 63, 6090–6095. [Google Scholar] [CrossRef]
- Taniguchi, M.; Nunnery, J.K.; Engene, N.; Esquenazi, E.; Byrum, T.; Dorrestein, P.C.; Gerwick, W.H. Palmyramide A, a cyclic depsipeptide from a Palmyra atoll collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 393–398. [Google Scholar] [CrossRef]
- Ueoka, R.; Shinzato, N.; Kagaya, N. Pseudoalteropeptide A, a novel lipopeptide from the marine bacterium Pseudoalteromonas piscicida SWA4_PA4 isolated from marine seaweed. J. Antibiot. 2021, 74, 105–110. [Google Scholar] [CrossRef]
- Moss, N.A.; Seiler, G.; Leão, T.F.; Castro-Falcón, G.; Gerwick, L.; Hughes, C.C.; Gerwick, W.H. Nature’s combinatorial biosynthesis produces vatiamides A–F. Angew. Chem. Int. Ed. 2019, 58, 9027–9031. [Google Scholar] [CrossRef]
- Jiménez, J.I.; Vansach, T.; Yoshida, W.Y.; Sakamoto, B.; Pörzgen, P.; Horgen, F.D. Halogenated fatty acid amides and cyclic depsipeptides from an eastern Caribbean collection of the cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2009, 72, 1573–1578. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.; Canuto, K.M.; Liu, C.; Suzuki, B.M.; Almaliti, J. Tutuilamides A–C: Vinyl-chloride-containing cyclodepsipeptides from marine cyanobacteria with potent elastase inhibitory properties. ACS Chem. Biol. 2020, 15, 751–757. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Paul, V.J.; Luesch, H. Structure and activity of largazole, a potent antiproliferative agent from the Floridian marine cyanobacterium Symploca sp. J. Am. Chem. Soc. 2008, 130, 1806–1807. [Google Scholar] [CrossRef] [PubMed]
- Taori, K.; Matthew, S.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatins 5–7, potent elastase inhibitors from Floridian marine cyanobacteria, Lyngbya spp. J. Nat. Prod. 2007, 70, 1593–1600. [Google Scholar] [CrossRef] [PubMed]
- Matthew, S.; Ross, C.; Rocca, J.R.; Paul, V.J.; Luesch, H. Lyngbyastatin 4, a dolastatin 13 analog with elastase and chymotrypsin inhibitory activity from the marine cyanobacterium Lyngbya confervoides. J. Nat. Prod. 2007, 70, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Bodanszky, M.; Sigler, G.F.; Bodanszky, A. Structure of the peptide antibiotic amphomycin. J. Am. Chem. Soc. 1973, 95, 2352–2357. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, B.; Kaplan, M.A.; Muir, R.D.; Hooper, I.R. Amphomycin, a new antibiotic. Antibiot. Chemother. 1953, 3, 1239–1242. [Google Scholar]
- Shoji, J.; Otsuka, H. Studies on tsushimycin. II. J. Antibiot. 1969, 22, 473–479. [Google Scholar] [CrossRef][Green Version]
- Heinzelmann, E.; Berger, S.; Muller, C.; Hartner, T.; Poralla, K.; Wohlleben, W.; Schwartz, D. An acyl-CoA dehydrogenase is involved in the formation of the Dcis3 double bond in the acyl residue of the lipopeptide antibiotic friulimicin in Actinoplanes friuliensis. Microbiology 2005, 151, 1963–1974. [Google Scholar] [CrossRef]
- Vertesy, L.; Ehlers, E.; Kogler, H.; Kurz, M.; Meiwes, J.; Seibert, G.; Vogel, M.; Hammann, P. Friulimicins: Novel lipopeptide antibiotics with peptidoglycan synthesis inhibiting activity from Actinoplanes friuliensis sp. nov. II. Isolation and structural characterization. J. Antibiot. 2000, 53, 816–827. [Google Scholar] [CrossRef]
- Kong, F.; Carter, G.T. Structure determination of glycinocins A to D, further evidence for the cyclic structure of the amphomycin antibiotics. J. Antibiot. 2003, 56, 557–564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, B.; Gross, H.; Goeger, D.E.; Mooberry, S.L.; Gerwick, W.H. Aurilides B and C, cancer cell toxins from a Papua New Guinea collection of the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2006, 69, 572–575. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Tan, L.T. Lagunamides A and B: Cytotoxic and antimalarial cyclodepsipeptides from the marine cyanobacterium Lyngbya majuscula. J. Nat. Prod. 2010, 73, 1810–1814. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, A.; Puddick, J.; Prinsep, M.R.; Rottmann, M.; Chan, K.P.; Chen, D.K.K.; Tan, L.T. Lagunamide C, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Lyngbya majuscula. Phytochemistry 2011, 72, 2369–2375. [Google Scholar] [CrossRef]
- Luo, D.; Putra, M.Y.; Ye, T.; Paul, V.J.; Luesch, H. Isolation, structure elucidation and biological evaluation of lagunamide D: A new cytotoxic macrocyclic depsipeptide from marine cyanobacteria. Mar. Drugs 2019, 17, 83. [Google Scholar] [CrossRef]
- Sueyoshi, K.; Kaneda, M.; Sumimoto, S.; Oishi, S.; Fujii, N.; Suenaga, K.; Teruya, T. Odoamide, a cytotoxic cyclodepsipeptide from the marine cyanobacterium Okeania sp. Tetrahedron 2016, 72, 5472–5478. [Google Scholar] [CrossRef]
- Bui, T.-H.; Wray, V.; Nimtz, M.; Fossen, T.; Preisitsch, M.; Schröder, G.; Wende, K.; Heiden, S.E.; Mundt, S. Balticidins A–D, antifungal hassallidin-like lipopeptides from the Baltic Sea cyanobacterium Anabaena cylindrica Bio33. J. Nat. Prod. 2014, 77, 1287–1296. [Google Scholar] [CrossRef]
- Shah, S.A.A.; Akhter, N.; Auckloo, B.N.; Khan, I.; Lu, Y.; Wang, K.; Wu, B.; Guo, Y.-W. Structural diversity, biological properties and applications of natural products from cyanobacteria. A Review. Mar. Drugs 2017, 15, 354. [Google Scholar] [CrossRef]
- Schmidt, Y.; van der Voort, M.; Crsemann, M.; Piel, J. Biosynthetic origin of the antibiotic cyclocarbamate brabantamide A (SB-253514) in plant-associated Pseudomonas. ChemBioChem. 2014, 15, 259–266. [Google Scholar] [CrossRef]
- Neuhof, T.; Schmieder, P.; Preussel, K.; Dieckmann, R.; Pham, H.; Bartl, F.; von Dohren, H. Hassallidin A, a glycosylated lipopeptide with antifungal activity from the cyanobacterium Hassallia sp. J. Nat. Prod. 2005, 68, 695–700. [Google Scholar] [CrossRef]
- Humisto, A.; Jokela, J.; Teigen, K.; Wahlsten, M.; Permi, P.; Sivonen, K.; Herfindal, L. Characterization of the interaction of the antifungal and cytotoxic cyclic glycolipopeptide hassallidin with sterol-containing lipid membranes. BBA–Biomembr. 2019, 1861, 1510–1521. [Google Scholar] [CrossRef] [PubMed]
- Ackman, R.A. Marine Biogenic Lipids, Fats & Oils; CRC Press: Boca Raton, FL, USA, 1989; Volume I. [Google Scholar]
- Dembitsky, V.M. Lipids of Bryophytes. Prog. Lipid Res. 1993, 32, 281–356. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Betaine ether-linked glycerolipids: Chemistry and biology. Prog. Lipid Res. 1996, 35, 1–51. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Levitsky, D.O. Arsenolipids. Prog. Lipid Res. 2004, 43, 403–448. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M.; Srebnik, M. Natural halogenated fatty acids: Their analogues and derivatives. Prog. Lipid Res. 2002, 41, 315–367. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Maoka, T. Allenic and cumulenic lipids. Prog. Lipid Res. 2007, 46, 328–375. [Google Scholar] [CrossRef]
- Kuklev, D.V.; Dembitsky, V.M. Epoxy acetylenic lipids: Their analogs and derivatives. Prog. Lipid Res. 2014, 56, 67–91. [Google Scholar] [CrossRef]
- Hashizume, H.; Nishimura, Y. Cyclic lipopeptide antibiotics. Stud. Nat. Prod. Chem. 2008, 35, 693–751. [Google Scholar]
- Dembitsky, V.M. Astonishing diversity of natural surfactants. 1. Glycosides of fatty acids and alcohols. Lipids 2004, 39, 933–953. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants. 2. Polyether glycosidic ionophores and macrocyclic glycosides. Lipids 2005, 40, 219–248. [Google Scholar] [CrossRef]
- Dembitsky, V.M. Astonishing diversity of natural surfactants. 3. Carotenoid glycosides and isoprenoid glycolipids. Lipids 2005, 40, 535–557. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants. 4. Fatty acid amide glycosides, their analogues, and derivatives. Lipids 2005, 40, 641–660. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants. 5. Biological active glycosides of aromatic metabolites. Lipids 2005, 40, 869–900. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants. 6. Biological active marine and terrestrial alkaloid glycosides. Lipids 2005, 40, 1081–1105. [Google Scholar] [CrossRef] [PubMed]
- Dembitsky, V.M. Astonishing diversity of natural surfactants. 7. Biological active hemi- and monoterpenoid glycosides. Lipids 2006, 41, 1–27. [Google Scholar] [CrossRef]
- Guschina, I.A.; Harwood, J.L. Lipids, and lipid metabolism in eukaryotic algae. Prog. Lipid Res. 2006, 45, 160–186. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Quntar, A.; Srebnik, M. Natural and synthetic small boron-containing molecules as potential inhibitors of bacterial and fungal quorum sensing. Chem. Rev. 2011, 111, 112–143. [Google Scholar] [CrossRef]
- Dembitsky, V.M. In silico prediction of steroids and triterpenoids as potential regulators of lipid metabolism. Mar. Drugs 2021, 19, 650. [Google Scholar] [CrossRef]
- Miyashita, K.; Mikami, N.; Hosokawa, H. Chemical, and nutritional characteristics of brown seaweed lipids: A review. J. Funct. Foods 2013, 5, 1507–1517. [Google Scholar] [CrossRef]
- Pounina, T.A.; Gloriozova, T.A.; Savidov, N.; Dembitsky, V.M. Sulfated and sulfur-containing steroids and their pharmacological profile. Mar. Drugs 2021, 19, 240. [Google Scholar] [CrossRef]
- Admassu, H.; Gasmalla, M.A.A.; Yang, R.; Zhao, W. Bioactive peptides derived from seaweed protein and their health benefits: Antihypertensive, antioxidant, and antidiabetic properties. J. Food Sci. 2018, 83, 6–16. [Google Scholar] [CrossRef] [PubMed]
- Lafarga, T.; Acién-Fernández, F.G.; Garcia-Vaquero, M. Bioactive peptides and carbohydrates from seaweed for food applications: Natural occurrence, isolation, purification, and identification. Algal Res. 2020, 48, 101909. [Google Scholar] [CrossRef]
- Mehdi, A.; Ali, H.; Mehdi, A. Seaweed proteins as a source of bioactive peptides. Curr. Pharm. Des. 2021, 27, 1342–1352. [Google Scholar]
- Bizzaro, G.; Vatland, A.K.; Pampanin, D.M. The One-Health approach in seaweed food production. Environ. Internat. 2022, 158, 106948. [Google Scholar] [CrossRef]
- Fusetani, N.; Matsunaga, S. Bioactive sponge peptides. Chem. Rev. 1993, 93, 1793–1806. [Google Scholar] [CrossRef]
- Agrawal, S.; Adholeya, A.; Deshmukh, S.K.; Agrawal, S. The pharmacological potential of non-ribosomal peptides from marine sponge and tunicates. Front. Pharmacol. 2016, 7, 333. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N. Nonribosomal peptides from marine sponges. Curr. Org. Chem. 2003, 7, 945–966. [Google Scholar] [CrossRef]
- Williams, S.L.; Smith, J.E. A global review of the distribution, taxonomy, and impacts of introduced seaweeds. Ann. Rev. Ecol. Syst. 2007, 38, 327–359. [Google Scholar] [CrossRef]
- Pérez, M.J.; Falqué, E.; Domínguez, H. Antimicrobial action of compounds from marine seaweed. Mar. Drugs 2016, 14, E52. [Google Scholar] [CrossRef]
- Maciel, E.; Costa Leal, M.; Lillebø, A.I.; Domingues, P.; Domingues, M.R.; Calado, R. Bioprospecting of marine macrophytes using MS based lipidomics as a new approach. Mar. Drugs 2016, 14, E49. [Google Scholar] [CrossRef]
- Plouguerné, E.; da Gama, B.A.; Pereira, R.C.; Barreto Bergter, E. Glycolipids from seaweeds and their potential biotechnological applications. Front. Cell Infect. Microbiol. 2014, 4, 174–189. [Google Scholar] [CrossRef] [PubMed]
- Moussavou, G.; Kwak, D.H.; Obiang Obonou, B.W. Anticancer effects of different seaweeds on human colon and breast cancers. Mar. Drugs 2014, 12, 4898–4911. [Google Scholar] [CrossRef] [PubMed]
- Yende, S.R.; Harle, U.N.; Chaugule, B.B. Therapeutic potential and health benefits of Sargassum species. Pharmacogn. Rev. 2014, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Ohno, O.; Sumimoto, S. Mebamamides A and B, cyclic lipopeptides isolated from the green alga Derbesia marina. J. Nat. Prod. 2015, 78, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Pelay-Gimeno, M.; Tulla-Puche, J.; Albericio, F. “Head-to-Side-Chain” cyclodepsipeptides of marine origin. Mar. Drugs 2013, 11, 1693–1717. [Google Scholar] [CrossRef]
- Hamann, M.T.; Scheuer, P.J. Kahalalide F: A bioactive depsipeptide from the sacoglossan mollusk Elysia rufescens and the green alga Bryopsis sp. J. Am. Chem. Soc. 1993, 115, 5825–5826. [Google Scholar] [CrossRef]
- Hamann, M.T.; Otto, C.S.; Scheuer, P.J.; Dunbar, D.C. Kahalalides: Bioactive peptides from a marine mollusk Elysia rufescens and its algal diet Bryopsis sp. J. Org. Chem. 1996, 61, 6594–6600. [Google Scholar] [CrossRef]
- Goetz, G.; Nakao, Y.; Scheuer, P.J. Two acyclic kahalalides from the sacoglossan mollusk Elysia rufescens. J. Nat. Prod. 1997, 60, 562–567. [Google Scholar] [CrossRef]
- Dmitrenok, A.; Iwashita, T.; Nakajima, T.; Sakamoto, B.; Namikoshi, M.; Nagai, H. New cyclic depsipeptides from the green alga Bryopsis species; application of a carboxypeptidase hydrolysis reaction to the structure determination. Tetrahedron 2006, 62, 1301–1308. [Google Scholar] [CrossRef]
- Nakao, Y.; Fujita, M.; Warabi, K. Miraziridine A, a novel cysteine protease inhibitor from the marine sponge Theonella aff. mirabilis. J. Am. Chem. Soc. 2000, 122, 10462–10463. [Google Scholar] [CrossRef]
- Schaschke, N. Miraziridine A: Natures blueprint towards protease class-spanning inhibitors. Bioorg. Med. Chem. Lett. 2004, 14, 855–857. [Google Scholar] [CrossRef] [PubMed]
- Naganawa, H.; Usui, N.; Takita, T. S-2,3-dicarboxy-aziridine: A new metabolite from a Streptomyces. J. Antibiot. 1975, 28, 828–829. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tabares, P.; Degel, B.; Schaschke, N. Identification of the protease inhibitor miraziridine A in the Red Sea sponge Theonella swinhoei. Pharmacogn. Res. 2012, 4, 63–66. [Google Scholar]
- Ismail, F.M.D.; Levitsky, D.O.; Dembitsky, V.M. Aziridine alkaloids as potential therapeutic agents. Eur. J. Med. Chem. 2009, 44, 3373–3387. [Google Scholar] [CrossRef]
- Oettel, H.; Wilhelm, G. Wege zur chemotherapic des krebses. Arzneim.-Forsch. 1954, 4, 681–703. [Google Scholar]
- Jermy, A. Ribosomal origin for polytheonamides. Nat. Rev. Microbiol. 2012, 10, 802. [Google Scholar] [CrossRef]
- Ueoka, R.; Ise, Y.; Ohtsuka, S. Yaku’amides A and B, cytotoxic linear peptides rich in dehydroamino acids from the marine sponge Ceratopsion sp. J. Am. Chem. Soc. 2010, 132, 17692–17694. [Google Scholar] [CrossRef]
- Takada, K.; Choi, B.W.; Rashid, M.A. Structural assignment of poecillastrins B and C, macrolide lactams from the deep-water Caribbean sponge Poecillastra species. J. Nat. Prod. 2007, 70, 428–431. [Google Scholar] [CrossRef]
- Rashid, M.A.; Cantrell, C.L.; Gustafson, K.R.; Boyd, M.R. Chondropsin D, a new 37-membered-ring macrolide lactam from the marine sponge Chondropsis species. J. Nat. Prod. 2001, 64, 1341–1344. [Google Scholar] [CrossRef]
- Takemoto, D.; Takekawa, Y.; van Soest, R.W.M. Poecillastrin D: A new cytotoxin of the chondropsin class from marine sponge Jaspis serpentina. Biosci. Biotechnol. Biochem. 2007, 71, 2697–2700. [Google Scholar] [CrossRef]
- Tan, K.C.; Wakimoto, T.; Abe, I. Lipodiscamides A–C, new cytotoxic lipopeptides from Discodermia kiiensis. Org. Lett. 2014, 16, 3256–3259. [Google Scholar] [CrossRef] [PubMed]
- Martín, M.J.; Rodríguez-Acebes, R.; García-Ramos, Y. Stellatolides, a new cyclodepsipeptide family from the sponge Ecionemia acervus: Isolation, solid-phase total synthesis, and full structural assignment of stellatolide A. J. Am. Chem. Soc. 2014, 136, 6754–6762. [Google Scholar] [CrossRef] [PubMed]
- Oku, N.; Gustafson, K.R.; Cartner, L.K. Neamphamide A, a new HIV-inhibitory depsipeptide from the Papua New Guinea marine sponge Neamphius huxleyi. J. Nat. Prod. 2004, 67, 1407–1411. [Google Scholar] [CrossRef] [PubMed]
- Randazzo, A.; Bifulco, G.; Giannini, C. Halipeptins A and B: Two novel potent anti-inflammatory cyclic depsipeptides from the Vanuatu marine sponge Haliclona species. J. Am. Chem. Soc. 2001, 123, 10870–10876. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, C.; Tanaka, J.; Bolland, R.F.; Marriott, G.; Higa, T. Seragamides A–F, new actin-targeting depsipeptides from the sponge Suberites japonicus Thiele. Tetrahedron 2006, 62, 3536–3542. [Google Scholar] [CrossRef]
- Scott, V.R.; Boehme, R.; Matthews, T.R. New class of antifungal agents: Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis species. Antimicrob. Agents Chemother. 1988, 32, 1154–1157. [Google Scholar] [CrossRef]
- Molinski, T.F. Anti-infective agents. Curr. Med. Chem. 2004, 3, 197–220. [Google Scholar]
- Bishara, A.; Rudi, A.; Aknin, M. Taumycins A and B, two bioactive lipodepsipeptides from the Madagascar sponge Fascaplysinopsis sp. Org. Lett. 2008, 10, 4307–4309. [Google Scholar] [CrossRef]
- Zampella, A.; Sepe, V.; Bellotta, F.; Luciano, P. Homophymines B E and A1 E1, a family of bioactive cyclodepsipeptides from the sponge Homophymia sp. Org. Biomol. Chem. 2009, 7, 4037–4044. [Google Scholar] [CrossRef]
- Zampella, A.; Sepe, V.; Luciano, P.; Bellotta, F. Homophymine A, an anti-HIV cyclodepsipeptide from the sponge Homophymia sp. J. Org. Chem. 2008, 73, 5319–5327. [Google Scholar] [CrossRef]
- Trevisi, L.; Bova, S.; Cargnelli, G.; Danieli Betto, D. Callipeltin A, a cyclic depsipeptide inhibitor of the cardiac sodium calcium exchanger and positive inotropic agent. Biochem. Biophys. Res. Commun. 2000, 279, 219–222. [Google Scholar] [CrossRef] [PubMed]
- Trevisi, L.; Cargnelli, G.; Ceolotto, G.; Papparella, I. Callipeltin A: Sodium ionophore effect and tension development in vascular smooth muscle. Biochem. Pharmacol. 2004, 68, 1331–1338. [Google Scholar] [CrossRef] [PubMed]
- Noro, J.C.; Kalaitzis, J.A.; Neilan, B.A. Bioactive natural products from Papua New Guinea marine sponges. Chem. Biodivers. 2012, 9, 2077–2095. [Google Scholar] [CrossRef]
- Ford, P.W.; Gustafson, K.R.; McKee, T.C.; Shigematsu, N. Papuamides A−D, HIV inhibitory and cytotoxic depsipeptides from the sponges Theonella mirabilis and Theonella swinhoei collected in Papua New Guinea. J. Am. Chem. Soc. 1999, 121, 5899–5909. [Google Scholar] [CrossRef]
- Prasad, P.; Aalbersberg, W.; Feussner, K.D.; Van Wagoner, R.M. Papuamides E and F, cytotoxic depsipeptides from the marine sponge Melophlus sp. Tetrahedron 2011, 67, 8529–8531. [Google Scholar] [CrossRef]
- Plaza, A.; Bifulco, G.; Keffer, J.L.; Lloyd, J.R. Celebesides A C and theopapuamides B D, depsipeptides from an Indonesian sponge that inhibit HIV 1 entry. J. Org. Chem. 2009, 74, 504–512. [Google Scholar] [CrossRef] [PubMed]
- Plaza, A.; Gustchina, E.; Baker, H.L.; Kelly, M.; Bewley, C.A. Mirabamides A D, depsipeptides from the sponge Siliquariaspongia mirabilis that inhibit HIV 1 fusion. J. Nat. Prod. 2007, 70, 1753–1760. [Google Scholar] [CrossRef]
- Tran, T.D.; Pham, N.B.; Fechner, G.; Zencak, D. Cytotoxic cyclic depsipeptides from the Australian marine sponge Neamphius huxleyi. J. Nat. Prod. 2012, 75, 2200–2208. [Google Scholar] [CrossRef]
- Rinehart, K.L.; Kishore, V.; Bible, K.C.; Sakai, R. Didemnins and tunichlorin: Novel natural products from the marine tunicate Trididemnum solidum. J. Nat. Prod. 1988, 51, 1–21. [Google Scholar] [CrossRef]
- Tsukimoto, M.; Nagaoka, M.; Shishido, Y.; Junji Fujimoto, J.; Fukiko Nishisaka, F. Bacterial production of the tunicate-derived antitumor cyclic depsipeptide didemnin B. J. Nat. Prod. 2011, 74, 2329–2331. [Google Scholar] [CrossRef]
- Ahir, U.N.; Vyas, T.K.; Gandhi, K.D.; Faldu, P.R.; Patel, K.G. In vitro efficacy for chlorpyrifos degradation by novel isolate Tistrella sp. AUC10 isolated from chlorpyrifos contaminated field. Curr. Microbiol. 2020, 77, 2226–2232. [Google Scholar] [CrossRef] [PubMed]
- Ratnayake, A.S.; Bugni, T.S.; Feng, X.; Harper, M.K.; Skalicky, J.J.; Mohammed, K.A.; Andjelic, C.D.; Barrows, L.R.; Ireland, C.M. Theopapuamide, a cyclic depsipeptide from a Papua New Guinea lithistid sponge Theonella swinhoei. J. Nat. Prod. 2006, 69, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Bewley, C.A.; He, H.; Williams, D.H.; Faulkner, D.J. Aciculitins A−C: Cytotoxic and antifungal cyclic peptides from the lithistid sponge Aciculites orientalis. J. Am. Chem. Soc. 1996, 118, 4314–4321. [Google Scholar] [CrossRef]
- Uesugi, S.; Watanabe, T.; Imaizumi, T.; Ota, Y.; Yoshida, K. Total synthesis and biological evaluation of irciniastatin A (a.k.a. Psymberin) and irciniastatin B. Org. Chem. 2015, 80, 12333–123350. [Google Scholar] [CrossRef] [PubMed]
- Crimmins, M.T.; Stevens, J.M.; Schaaf, G.M. Total synthesis of irciniastatin A (Psymberin). Org. Lett. 2009, 11, 3990–3993. [Google Scholar] [CrossRef][Green Version]
- Fisch, K.M.; Gurgui, C.; Heycke, N.; van der Sar, S.A. Polyketide assembly lines of uncultivated sponge symbionts from structure-based gene targeting. Nat. Chem. Biol. 2009, 5, 494–501. [Google Scholar] [CrossRef]
- Cardani, C.; Ghiringhelli, D.; Mondelli, R.; Quilico, A. The structure of pederin. Tetrahedron Lett. 1965, 6, 2537–2545. [Google Scholar] [CrossRef]
- Cichewicz, R.H.; Valeriote, F.A.; Crews, P. Psymberin, a potent sponge derived cytotoxin from Psammocinia distantly related to the pederin family. Org. Lett. 2004, 6, 1951–1954. [Google Scholar] [CrossRef]
- An, C.; Jurica, J.A.; Walsh, S.P.; Hoye, A.T.; Smith, A.B., 3rd. Total synthesis of (+) irciniastatin A (a.k.a. psymberin) and irciniastatin B. J. Org. Chem. 2013, 78, 4278–4296. [Google Scholar] [CrossRef]
- García Ruiz, C.; Sarabia, F. Chemistry and biology of bengamides and bengazoles, bioactive natural products from Jaspis sponges. Mar. Drugs 2014, 12, 1580–1622. [Google Scholar] [CrossRef]
- Quinoa, E.; Adamczeski, M.; Crews, P.; Bakus, G.J. Bengamides, heterocyclic anthelmintics from a Jaspidae marine sponge. J. Org. Chem. 1986, 51, 4494–4497. [Google Scholar] [CrossRef]
- White, K.N.; Tenney, K.; Crews, P. The bengamides: A mini-review of natural sources, analogues, biological properties, biosynthetic origins, and future prospects. J. Nat. Prod. 2017, 80, 740–755. [Google Scholar] [CrossRef] [PubMed]
- Groweiss, A.; Newcomer, J.J.; O’Keefe, B.R. Cytotoxic metabolites from an Australian collection of the sponge Jaspis species. J. Nat. Prod. 1999, 62, 1691–1693. [Google Scholar] [CrossRef]
- Galitz, A.; Nakao, Y.; Schupp, P.J.; Wörheide, G.; Erpenbeck, D. A soft spot for chemistry—Current taxonomic and evolutionary implications of sponge secondary metabolite distribution. Mar. Drugs 2021, 19, 448. [Google Scholar] [CrossRef] [PubMed]
- Porras-Alcalá, C.; Moya-Utrera, F.; García-Castro, M.; Sánchez-Ruiz, A.; López-Romero, J.M.; Pino-González, M.S.; Díaz-Morilla, A.; Kitamura, S.; Wolan, D.W.; Prados, J.; et al. The development of the bengamides as new antibiotics against drug-resistant bacteria. Mar. Drugs 2022, 20, 373. [Google Scholar] [CrossRef]
- Plaza, A.; Baker, H.L.; Bewley, C.A. Mirabalin, an antitumor macrolide lactam from the marine sponge Siliquariaspongia mirabilis. J. Nat. Prod. 2008, 71, 473–477. [Google Scholar] [CrossRef]
- Winder, P.L.; Pomponi, S.A.; Wright, A.E. Natural products from the Lithistida: A review of the literature since 2000. Mar. Drugs 2011, 9, 2643–2682. [Google Scholar] [CrossRef]
- Cornil, J.; Echeverria, P.G.; Reymond, S. Synthetic studies toward the C14–C29 fragment of mirabalin. Org. Lett. 2016, 18, 4534–4537. [Google Scholar] [CrossRef]
- Chhetri, B.K.; Lavoie, S.; Sweeney-Jones, A.M.; Kubanek, J. Recent trends in the structural revision of natural products. Nat. Prod. Rep. 2018, 35, 514–531. [Google Scholar] [CrossRef]
- Huss, M.; Wieczorek, H. Inhibitors of V-ATPases: Old and new players. J. Exp. Biol. 2009, 212, 341–346. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Gustafson, K.R.; Cecere, M.R. Chondropsins A and B: Novel tumor cell growth-inhibitory macrolide lactams from the marine sponge Chondropsis sp. J. Am. Chem. Soc. 2000, 122, 8825–8829. [Google Scholar] [CrossRef]
- Molinski, T.F. Cyclic azole-homologated peptides from marine sponges. Org. Biomol. Chem. 2018, 16, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Talpir, R.; Benayahu, Y.; Kashman, Y. Hemiasterlin and geodiamolide TA.; two new cytotoxic peptides from the marine sponge Hemiasterella minor (Kirkpatrick). Tetrahedron Lett. 1994, 35, 4453–4456. [Google Scholar] [CrossRef]
- Coello, L.; Reyes, F.; Martín, M.J. Isolation and structures of pipecolidepsins A and B, cytotoxic cyclic depsipeptides from the Madagascan sponge Homophymia lamellose. J. Nat. Prod. 2014, 77, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Matsunaga, S.; van Soest, R.W.; Fusetani, N. Nagahamide A, an antibacterial depsipeptide from the marine sponge Theonella swinhoei. Org. Lett. 2002, 4, 3039–3042. [Google Scholar] [CrossRef]
- MacMillan, J.B.; Molinski, T.F. Herbacic acid, a simple prototype of 5,5,5-trichloroleucine metabolites from the sponge Dysidea herbacea. J. Nat. Prod. 2000, 63, 155–157. [Google Scholar] [CrossRef]
- Lee, G.M.; Molinski, T.F. Herbaceamide, a chlorinated N-acyl amino ester from the marine sponge, Dysidea herbacea. Tetrahedron Lett. 1992, 33, 7671–7674. [Google Scholar] [CrossRef]
- Kobayashi, M.; Aoki, S.; Ohyabu, N. Arenastatin A, a potent cytotoxic depsipeptide from the okinawan marine sponge Dysidea arenaria. Tetrahedron Lett. 1994, 35, 7969–7972. [Google Scholar] [CrossRef]
- Kobayashi, M.; Wang, W.; Ohyabu, N. Improved total synthesis and structure-activity relationship of arenastatin A, a potent cytotoxic spongean depsipeptide. Chem. Pharm. Bull. 1995, 43, 1598–1600. [Google Scholar] [CrossRef]
- Koiso, Y.; Morita, K.; Kobayashi, M. Effects of arenastatin A and its synthetic analogs on microtubule assembly. Chem. Biol. Interact. 1996, 102, 183–191. [Google Scholar] [CrossRef]
- Morita, K.; Koiso, Y.; Hashimoto, Y. Interaction of arenastatin A with porcine brain tubulin. Biol. Pharm. Bull. 1997, 20, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Tamura, S.; Koyama, K. New analogue of arenastatin A, a potent cytotoxic spongean depsipeptide, with anti-tumor activity. Bioorg. Med. Chem. Lett. 2004, 14, 2597–2601. [Google Scholar] [CrossRef] [PubMed]
- Murakami, N.; Wang, W.; Tamura, S.; Kobayashi, M. Synthesis, and biological property of carba and 20-deoxo analogues of arenastatin A. Bioorg. Med. Chem. Lett. 2000, 10, 1823–1826. [Google Scholar] [CrossRef]
- Andavan, G.S.; Lemmens Gruber, R. Cyclodepsipeptides from marine sponges: Natural agents for drug research. Mar. Drugs 2010, 8, 810–834. [Google Scholar] [CrossRef]
- Singh, R.; Sharma, M.; Joshi, P.; Rawat, D.S. Clinical status of anti-cancer agents derived from mar one sources. Anticancer Agents Med. Chem. 2008, 8, 603–617. [Google Scholar] [CrossRef]
- Sakemi, S.; Ichiba, T.; Kohmoto, S.; Saucy, G.; Higa, T. Isolation and structure elucidation of onnamide A, a new bioactive metabolite of a marine sponge, Theonella sp. J. Am. Chem. Soc. 1988, 110, 4851–4853. [Google Scholar] [CrossRef]
- Matsunaga, S.; Fusetani, N.; Nakao, Y. Eight new cytotoxic metabolites closely related to onnamide A from two marine sponges of the genus Theonella. Tetrahedron 1992, 48, 8369–8376. [Google Scholar] [CrossRef]
- Lee, K.H.; Nishimura, S.; Matsunaga, S.; Fusetani, N. Inhibition of protein synthesis and activation of stress activated protein kinases by onnamide A and theopederin B, antitumor marine natural products. Cancer Sci. 2005, 96, 357–364. [Google Scholar] [CrossRef]
- Chill, L.; Kashman, Y.; Schleyer, M. Oriamide, a new cytotoxic cyclic peptide containing a novel amino acid from the marine sponge Theonella sp. Tetrahedron 1997, 53, 16147–16152. [Google Scholar] [CrossRef]
- Carroll, A.R.; Pierens, G.K.; Fechner, G. Dysinosin A: A novel inhibitor of Factor VIIa and thrombin from a new genus and species of Australian sponge of the family Dysideidae. J. Am. Chem. Soc. 2002, 124, 13340–13341. [Google Scholar] [CrossRef]
- Carroll, A.R.; Buchanan, M.S.; Edser, A. Dysinosins B-D, inhibitors of factor VIIa and thrombin from the Australian sponge Lamellodysidea chlorea. J. Nat. Prod. 2004, 67, 1291–1294. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, E.W.; Raventos-Suarez, C.; Bifano, M. Scleritodermin A, a cytotoxic cyclic peptide from the lithistid sponge Scleritoderma nodosum. J. Nat. Prod. 2004, 67, 475–478. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, J.; Sato, M.; Ishibashi, M. Keramamide A, a novel peptide from the Okinawan marine sponge Theonella sp. J. Chem. Soc. Perkin Trans 1 1991, 21, 2609–2611. [Google Scholar] [CrossRef]
- Toda, H.; Tozyo, T.; Terui, Y.; Hayashi, F. Discokiolides. Cytotoxic cyclic depsipeptides from the marine sponge Discodermia kiiensis. Chem. Lett. 1992, 2, 431–434. [Google Scholar] [CrossRef]
- Odeleye, T.; White, W.L.; Lu, J. Extraction techniques and potential health benefits of bioactive compounds from marine molluscs: A review. Food Funct. 2019, 10, 2278–2289. [Google Scholar] [CrossRef]
- Russo, P.; Nastrucci, C.; Cesario, A. From the sea to anticancer therapy. Curr. Med. Chem. 2011, 18, 3551–3562. [Google Scholar] [CrossRef] [PubMed]
- Avila, C.; Angulo-Preckler, C. Bioactive compounds from marine heterobranchs. Mar. Drugs 2020, 18, 657. [Google Scholar] [CrossRef]
- Bingham, J.P.; Andrews, E.A.; Kiyabu, S.M. Drugs from slugs. Part II—Conopeptide bioengineering. Chem. Biolog. Interact. 2012, 200, 92–113. [Google Scholar] [CrossRef]
- Ribeiro, R.; Pinto, E.; Fernandes, C.; Sousa, E. Marine cyclic peptides: Antimicrobial activity and synthetic strategies. Mar. Drugs 2022, 20, 397. [Google Scholar] [CrossRef]
- Ahmed, S.; Alam, W.; Jeandet, P.; Aschner, M.; Alsharif, K.F.; Saso, L.; Khan, H. Therapeutic potential of marine peptides in prostate cancer: Mechanistic insights. Mar. Drugs 2022, 20, 466. [Google Scholar] [CrossRef]
- Katz, L.; Baltz, R.H. Natural product discovery: Past, present, and future. J. Ind. Microbiol. Biotechnol. 2016, 43, 155–176. [Google Scholar] [CrossRef] [PubMed]
- Nakao, Y.; Yoshida, W.Y.; Takada, Y. Kulokekahilide-2, a cytotoxic depsipeptide from a cephalaspidean mollusk Philinopsis speciose. J. Nat. Prod. 2004, 67, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.-H.; Wang, Y.-J.; Sheng, J.; Wang, F.; Zheng, Y.; Lin, X.-K.; Sun, M. Antitumor peptides from marine organisms. Mar. Drugs 2011, 9, 1840–1859. [Google Scholar] [CrossRef] [PubMed]
- Hilla, R.A. Marine natural products. Annu. Rep. Prog. Chem. Sect. B: Org. Chem. 2005, 101, 124–136. [Google Scholar] [CrossRef]
- Liu, Y. Mechanism of Action of Seven Marine-Derived Natural Products. Ph.D. Thesis, University of Hawaii, Honolulu, HI, USA, 2014; p. 47. [Google Scholar]
- Singh, K.S. Pyrone-derived marine natural products: A review on isolation, bioactivities, and synthesis. Curr. Org. Chem. 2020, 24, 354–401. [Google Scholar]
- Wang, B.; Chen, C.; Yu, M.; Liu, Y.; Liu, P.; Zhang, X. A review on metabolites from Onchidium genus: Chemistry and bioactivity. Chem. Biodiver. 2021, 18, e2000580. [Google Scholar] [CrossRef]
- Tilvi, S.; Naik, C.G. Tandem mass spectrometry of kahalalides: Identification of two new cyclic depsipeptides, kahalalide R and S from Elysia grandifolia. J. Mass. Spectr. 2007, 42, 70–80. [Google Scholar] [CrossRef]
- Faircloth, G.; del Carmen Cuevas Marchante, M. Kahalalide F and ES285: Potent anticancer agents from marine molluscs. Molluscs 2006, 43, 363–379. [Google Scholar]
- Suenaga, K.; Mutou, T.; Shibata, T. Isolation and stereostructure of aurilide, a novel cyclodepsipeptide from the Japanese sea hare Dolabella Auricularia. Tetrahedron Lett. 1996, 37, 6771–6774. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L. Antineoplastic agent. 174. Isolation and structure of the cytostatic depsipeptide dolastatin 13 from the sea hare Dolabella Auricularia. J. Am. Chem. Soc. 1989, 111, 5015–5017. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Kizu, H. Isolation and structure of the cell growth inhibitory depsipeptides dolastatins 11 and 12. Heterocycles 1989, 28, 553–558. [Google Scholar] [CrossRef]
- Pettit, G.R.; Kamano, Y.; Herald, C.L. Antineoplastic agents. 190. Isolation and structure of the cyclodepsipeptide dolastatin 14. J. Org. Chem. 1990, 55, 2989–2990. [Google Scholar] [CrossRef]
- Sone, H.; Nemoto, T.; Ojika, M.; Yamada, K. Isolation, structure, and synthesis of dolastatin C, a new depsipeptide from the sea hare Dolabella auricularia. Tetrahedron Lett. 1993, 34, 8445–8448. [Google Scholar] [CrossRef]
- Sone, H.; Shibata, T.; Fujita, T.; Ojika, M.; Yamada, K. Dolastatin H and isodolastatin H, potent cytotoxic peptides from the sea hare Dolabella auricularia: Isolation, stereostructures, and synthesis. J. Am. Chem. Soc. 1996, 118, 1874–1880. [Google Scholar] [CrossRef]
- Mutou, T.; Kondo, T.; Ojika, M.; Yamada, K. Isolation and stereostructures of dolastatin G and nordolastatin G, cytotoxic 35-membered cyclodepsipeptides from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 1996, 61, 6340–6345. [Google Scholar] [CrossRef]
- Oda, T.; Crane, Z.D.; Dicus, C.W. Dolastatin 11 connects two long-pitch strands in F-actin to stabilize microfilaments. J. Mol. Biol. 2003, 328, 319–324. [Google Scholar] [CrossRef]
- Bai, R.; Verdier-Pinard, P.; Gangwar, S. Dolastatin 11, a marine depsipeptide, arrests cells at cytokinesis and induces hyperpolymerization of purified actin. Mol. Pharmacol. 2001, 59, 462–469. [Google Scholar] [CrossRef]
- Pettit, G.R.; Xu, J.-P.; Hogan, F.; Schmidt, J.M. Antineoplastic agents 370. Isolation and structure of dolastatin 18. Bioorg. Med. Chem. Lett. 1997, 7, 827–832. [Google Scholar] [CrossRef]
- Ishiwata, H.; Nemoto, T.; Ojika, M.; Yamada, K. Isolation and stereostructure of doliculide, a cytotoxic cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 1994, 59, 4710–4711. [Google Scholar] [CrossRef]
- Ishiwata, H.; Sone, H.; Kigoshi, H.; Yamada, K. Total synthesis of doliculide, a potent cytotoxic cyclodepsipeptide from the Japanese sea hare Dolabella auricularia. J. Org. Chem. 1994, 59, 4712–4713. [Google Scholar] [CrossRef]
- Bai, R.; Covell, D.G.; Liu, C. (−)-Doliculide, a new macrocyclic depsipeptide enhancer of actin assembly. J. Biol. Chem. 2002, 277, 32165–32171. [Google Scholar] [CrossRef]
- Casertano, M.; Menna, M.; Imperatore, C. The ascidian-derived metabolites with antimicrobial properties. Antibiotics 2020, 9, 510. [Google Scholar] [CrossRef]
- Arumugam, V.; Venkatesan, M.; Ramachandran, S. Bioactive peptides from marine ascidians and future drug development—A review. Int. J. Pept. Res. Ther. 2018, 24, 13–18. [Google Scholar] [CrossRef]
- Phyo, Y.Z.; Ribeiro, J.; Fernandes, C.; Kijjoa, A.; Pinto, M.M.M. Marine natural peptides: Determination of absolute configuration using liquid chromatography methods and evaluation of bioactivities. Molecules 2018, 23, 306. [Google Scholar] [CrossRef]
- Laurent, D.; Pietra, F. Natural-product diversity of the New Caledonian marine ecosystem compared to other ecosystems: A pharmacologically oriented view. Chem. Biodiver. 2004, 1, 539–594. [Google Scholar] [CrossRef]
- Tincu, J.A.; Taylor, S.W. Antimicrobial peptides from marine invertebrates. Antimicrob. Agents Chemother. 2004, 48, 3645–3654. [Google Scholar] [CrossRef]
- Crews, P.; Manes, L.V.; Boehler, M. Jasplakinolide, a cyclodepsipeptide from the marine sponge, Jaspis sp. Tetrahedron Lett. 1986, 27, 2797–2800. [Google Scholar] [CrossRef]
- Rinehart, K.L. Antitumor compounds from tunicates. Med. Res. Rev. 2000, 20, 1–27. [Google Scholar] [CrossRef]
- Chun, H.G.; Davies, B.; Hoth, D.; Suffness, M.; Plowman, J.; Didemnin, B. The first marine compound entering clinical trials as an antineoplastic agent. Investig. New Drugs 1986, 4, 279–284. [Google Scholar]
- Weiss, G.R.; Arteaga, C.L.; Brown, T.D.; Craig, J.B.; Harman, G.S. New anticancer agents. Cancer Chemother. Biol. Response Modif. 1988, 10, 85–116. [Google Scholar]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Chlorinated plant steroids and their biological activities. Int. J. Curr. Res. Biosci. Plant Biol. 2017, 4, 70–85. [Google Scholar] [CrossRef]
- Le, V.H.; Inai, M.; Williams, R.M.; Kan, T. Ecteinascidins. A review of the chemistry, biology and clinical utility of potent tetrahydroisoquinoline antitumor antibiotics. Nat. Prod. Rep. 2015, 32, 328–347. [Google Scholar] [CrossRef]
- Dembitsky, V.M.; Gloriozova, T.A.; Poroikov, V.V. Naturally occurring plant isoquinoline N-oxide alkaloids: Their pharmacological and SAR activities. Phytomedicine 2015, 22, 183–202. [Google Scholar] [CrossRef]
- Zewail Foote, M.; Hurley, L.H. Ecteinascidin 743: A minor groove alkylator that bends DNA toward the major groove. J. Med. Chem. 1999, 42, 2493–2497. [Google Scholar] [CrossRef]
- Takebayashi, Y.; Pourquier, P.; Zimonjic, D.B.; Nakayama, K. Antiproliferative activity of ecteinascidin 743 is dependent upon transcription coupled nucleotide excision repair. Nat. Med. 2001, 7, 961–966. [Google Scholar] [CrossRef]
- Lievens, S.C.; Molinski, T.F. Sagittamides A and B. Polyacetoxy long chain acyl amino acids from a Didemnid ascidian. Org. Lett. 2005, 7, 2281–2284. [Google Scholar] [CrossRef]
- Schuetz, A.; Junker, J.; Leonov, A.; Lange, O.F.; Molinski, T.F.; Griesinger, C. Stereochemistry of sagittamide A from residual dipolar coupling enhanced NMR. J. Am. Chem. Soc. 2007, 129, 15114–15115. [Google Scholar] [CrossRef]
- Humbert, A.; Plé, K.; Harakat, D.; Martinez, A.; Haudrechy, A. A further contribution to the study of sagittamide: Synthesis of a pivotal intermediate belonging to a rare L series. Molecules 2012, 17, 7709–7721. [Google Scholar] [CrossRef]
- Biard, J.F.; Roussakis, C.; Kornprobst, J.M.; Gouiffes Barbin, D.; Verbist, J.F. Bistramides A, B, C, D, and K: A new class of bioactive cyclic polyethers from Lissoclinum bistratum. J. Nat. Prod. 1994, 57, 1336–1345. [Google Scholar]
- Riou, D.; Roussakis, C.; Robillard, N.; Biard, J.F.; Verbist, J.F. Bistramide A induced irreversible arrest of cell proli feration in a non-small cell bronchopulmonary carcinoma is similar to induction of terminal maturation. Biol. Cell 1993, 77, 261–264. [Google Scholar] [CrossRef]
- Statsuk, A.V.; Bai, R.; Baryza, J.L.; Verma, V.A.; Hamel, E.; Wender, P.A.; Kozmin, S.A. Actin is the primary cellular receptor of bistramide A. Nat. Chem. Biol. 2005, 1, 383–388. [Google Scholar] [CrossRef]
- Rinehart, K.L., Jr.; Gloer, J.B.; Hughes, R.G., Jr.; Renis, H.E. Didemnins: Antiviral and antitumor depsipeptides from a caribbean tunicate. Science 1981, 212, 933–935. [Google Scholar] [CrossRef]
- Crampton, S.L.; Adams, E.G.; Kuentzel, S.L.; Li, L.H. Biochemical and cellular effects of didemnins A and B. Cancer Res. 1984, 44, 1796–1801. [Google Scholar]
- Lee, J.; Currano, J.N.; Carroll, P.J.; Joullié, M.M. Didemnins, tamandarins and related natural products. Nat. Prod. Rep. 2012, 29, 404–424. [Google Scholar] [CrossRef]
- Whitson, E.L.; Ratnayake, A.S.; Bugni, T.S.; Harper, M.K.; Ireland, C.M. Isolation, structure elucidation and synthesis of eudistomides A and B, lipopeptides from a Fijian ascidian Eudistoma sp. J. Org. Chem. 2009, 74, 1156–1162. [Google Scholar] [CrossRef]
- Gutiérrez-Chávez, C.; Benaud, N.; Ferrari, B.C. The ecological roles of microbial lipopeptides: Where are we going? Comp. Struct. Biotechnol. J. 2021, 19, 1400–1413. [Google Scholar] [CrossRef]
- Zhao, P.; Xue, Y.; Li, X.; Li, J.; Zhao, Z.; Quan, C.; Gao, W.; Zu, X. Fungi-derived lipopeptide antibiotics developed since 2000. Peptides 2019, 113, 52–65. [Google Scholar] [CrossRef]
- Kashif, A.; Rehman, R.; Fuwad, A.; Shahid, M.K. Current advances in the classification, production, properties and applications of microbial biosurfactants – A critical review. Adv. Colloid Interface Sci. 2022, 306, 102718. [Google Scholar] [CrossRef]
- Inès, M.; Dhouha, G. Lipopeptide surfactants: Production, recovery and pore forming capacity. Peptides 2015, 71, 100–112. [Google Scholar] [CrossRef]
- Ratnayake, R.; Fremlin, L.J.; Lacey, E.; Gill, J.H.; Capon, R.J. Acremolides A–D, lipodepsipeptides from an Australian marine-derived fungus, Acremonium sp. J. Nat. Prod. 2008, 71, 403–408. [Google Scholar] [CrossRef]
- Shiono, Y.; Tsuchinari, M.; Shimanuki, K.; Miyajima, T.; Murayama, T.; Koseki, T.; Laatsch, H. Fusaristatins A and B, 2 new cyclic lipopeptides from an endophytic Fusarium sp. J. Antibiot. 2007, 60, 309–316. [Google Scholar] [CrossRef]
- Sugawara, T.; Tanaka, A.; Tanaka, K.; Nagai, K.; Suzuki, K.; Suzuki, T. YM-170320, a novel lipopeptide antibiotic inducing morphological change of colonies in a mutant of Candida tropicalis pK233. J. Antibiot. 1998, 51, 435–438. [Google Scholar] [CrossRef]
- McBrien, K.D.; Berry, R.L.; Lowe, S.E.; Neddermann, K.M.; Bursuker, I. Rakicidins, new cytotoxic lipopeptides from Micromonospora sp. Fermentation, isolation and characterization. J. Antibiot. 1995, 48, 1446–1452. [Google Scholar] [CrossRef]
- Hu, J.-F.; Wunderlich, D.; Sattler, I.; Feng, X.Z.; Grabley, S.; Thiericke, R. Rakicidin C, a new cyclic depsipeptide from Streptomyces sp. Eur. J. Org. Chem. 2000, 28, 3353–3356. [Google Scholar] [CrossRef]
- Igarashi, Y.; Shimasaki, R.; Miyanaga, S.; Oku, N.; Onaka, H.; Sakurai, H. Rakicidin D, an inhibitor of tumor cell invasion from marine-derived Streptomyces sp. J. Antibiot. 2010, 63, 563–565. [Google Scholar] [CrossRef]
- Motohashi, K.; Toda, T.; Sue, M.; Furihata, K.; Shizuri, Y.; Matsuo, Y.; Kasai, H. Isolation and structure elucidation of tumesce amides A and B, two peptides produced by Streptomyces tumescens YM23–260. J. Antibiot. 2010, 63, 549–552. [Google Scholar] [CrossRef][Green Version]
- Kishimoto, S.; Tsunematsu, Y.; Nishimura, S.; Hayashi, Y.; Hattori, A.; Kakeya, H. Tumescenamide C, an antimicrobial cyclic lipodepsipeptide from Streptomyces sp. Tetrahedron 2012, 68, 5572–5578. [Google Scholar] [CrossRef]
- Oh, D.C.; Kauffman, C.A.; Jensen, P.R.; Fenical, W. Induced production of emericellamides A and B from the marine-derived fungus Emericella sp. in competing co-culture. J. Nat. Prod. 2007, 70, 515–520. [Google Scholar] [CrossRef]
- Asolkar, R.N.; Freel, K.C.; Jensen, P.R.; Fenical, W. Arenamides A–C, cytotoxic NFκB inhibitors from the marine actinomycete Salinispora arenicola. J. Nat. Prod. 2009, 72, 396–402. [Google Scholar] [CrossRef]
- Trischman, J.A.; Tapiolas, D.M.; Jensen, P.R.; Dwight, R.; Fenical, W.; McKee, T.C.; Ireland, C.M.; Stout, T.J.; Clardy, J. Salinamides A and B: Anti-inflammatory depsipeptides from a marine streptomycete. J. Am. Chem. Soc. 1994, 116, 757–758. [Google Scholar] [CrossRef]
- Igarashi, M.; Shida, T.; Sasaki, Y.; Kinoshita, N.; Naganawa, H.; Hamada, M.; Takeuchi, T. Vinylamycin, a new depsipeptide antibiotic, from Streptomyces sp. J. Antibiot. 1999, 52, 873–879. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Nishide, M.; Kurotaki, M.; Watanabe, Y. New Antibiotic na30851a, Its Production and Use Thereof. Japan Patent 1999049768, 23 February 1999. [Google Scholar]
- Watanabe, Y.; Kurotaki, M.; Nishide, M.; Yoshida, H. Antibiotic NA30851A, Its Manufacture with Streptomyces, Insecticides or Microbicides Containing It, and Streptomyces sp. NA30851A. Kokai Tokkyo Koho. Japanese Patent JP 11049768 A 19990223, 18 May 1999. [Google Scholar]
- Ueda, J.Y.; Nagai, A.; Izumikawa, M.; Chijiwa, S.; Takagi, M.; Shin-ya, K. A novel antimycin-like compound, JBIR-06, from Streptomyces sp. ML55. J. Antibiot. 2008, 61, 241–244. [Google Scholar] [CrossRef] [PubMed]
- Kozone, I.; Ueda, J.Y.; Takagi, M.; Shin-Ya, K. JBIR-52, a new antimycin-like compound, from Streptomyces sp. ML55. J. Antibiot. 2009, 62, 593–595. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Vodanovic-Jankovic, S.; Kron, M.; Shen, B. New WS9326A congeners from Streptomyces sp. 9078 Inhibiting Brugia malayi asparaginyl-tRNA synthetase. Org. Lett. 2012, 14, 4946–4949. [Google Scholar] [CrossRef]
- Mayer, C.; Sass, P.; Brötz-Oesterhelt, H. Consequences of dosing and timing on the antibacterial effects of ADEP antibiotics. Int. J. Med. Microbiol. 2019, 309, 151329. [Google Scholar] [CrossRef]
- Yin, X.; Chen, Y.; Zhang, L.; Wang, Y.; Zabriskie, T.M. Enduracidin analogues with altered halogenation patterns produced by genetically engineered strains of Streptomyces fungicidicus. J. Nat. Prod. 2010, 73, 583–589. [Google Scholar] [CrossRef][Green Version]
- Cavalleri, B.; Pagani, H.; Volpe, G.; Selva, E.; Parenti, F. A-16686, a new antibiotic from Actinoplanes, I. Fermentation, isolation and preliminary physico-chemical characteristics. J. Antibiot. 1984, 37, 309–317. [Google Scholar] [CrossRef]
- Pallanza, R.; Berti, M.; Scotti, R.; Randisi, E.; Arioli, E.V. A-16686, a new antibiotic from Actinoplanes II. Biological properties. J. Antibiot. 1984, 37, 318–324. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Chen, J.S.; Edmonds, D.J.; Estrada, A.A. Recent advances in the chemistry and biology of naturally occurring antibiotics. Angew. Chem. Int. Ed. Engl. 2009, 48, 660–719. [Google Scholar] [CrossRef]
- Meyers, E.; Weisenborn, F.L.; Pansy, F.E.; Slusarchyk, D.S.; Von Saltza, M.H.; Rathnum, M.L.; Parker, W.L. Janiemycin, a new peptide antibiotic. J. Antibiot. 1970, 23, 502–507. [Google Scholar] [CrossRef]
- Hommel, U.; Weber, H.P.; Oberer, L.; Naegeli, H.U.; Oberhauser, B.; Foster, C.A. The 3D-structure of a natural inhibitor of cell adhesion molecule expression. FEBS Lett. 1996, 379, 69–73. [Google Scholar] [CrossRef]
- Foster, C.A.; Dreyfuss, M.; Mandak, B.; Merngassner, J.G.; Naegeli, H.U.; Nussbaumer, A.; Oberer, L.; Schell, G.; Swoboda, E.M. Pharmacological modulation of endothelial cell-associated adhesion molecule expression: Implications for future treatment of dermatological diseases. J. Dermatol. 1994, 21, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Oberhauser, B.; Grohmann, B.; Sperner, H. Selective epimerisation of a fungal cyclopeptolide via an 2-amino-oxazole intermediate—Conformational consequences. In Proceedings of the 2nd International Electronic Conference on Synthetic Organic Chemistry (ECSOC-2), Basel, Switzerland, 1–30 September 1998; MDPI: Basel, Switzerland, 1999. Available online: https://www.mdpi.org/ecsoc/ (accessed on 7 August 2022).
- Fukuchi, N.; Furihata, K.; Nakayama, J.; Goudo, T.; Takayama, S.; Isogai, A.; Suzuki, A. Rotihibins, novel plant growth regulators from Streptomyces graminofaciens. J. Antibiot. 1995, 48, 1004–1010. [Google Scholar] [CrossRef]
- Um, S.; Park, S.H.; Kim, J.; Park, H.J.; Ko, K.; Bang, H.S.; Lee, S.K.; Shin, J.; Oh, D.C. Coprisamides A and B, new branched cyclic peptides from a gut bacterium of the dung beetle Copris tripartitus. Org. Lett. 2015, 17, 1272–1275. [Google Scholar] [CrossRef]
- Borders, D.B.; Leese, R.A.; Jarolmen, H.; Francis, N.D.; Fantini, A.A.; Falla, T.; Fiddes, J.C.; Aumelas, A. Laspartomycin, an acidic lipopeptide antibiotic with a unique peptide core. J. Nat. Prod. 2007, 70, 443–446. [Google Scholar] [CrossRef]
- Vervoort, H.C.; Drašković, M.; Crews, P. Histone deacetylase inhibitors as a tool to up-regulate new fungal biosynthetic products: Isolation of EGM-556, a cyclodepsipeptide, from Microascus sp. Org. Lett. 2011, 13, 410–413. [Google Scholar] [CrossRef]
- Li, D.; Carr, G.; Zhang, Y.; Williams, D.E.; Amlani, A.; Bottriell, H.; Mui, A.L.; Andersen, R.J. Turnagainolides A and B, cyclic depsipeptides produced in culture by a Bacillus sp.: Isolation, structure elucidation, and synthesis. J. Nat. Prod. 2011, 74, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Cooper, E.C.; Curtis, N.; Cranswick, N.; Gwee, A. Pristinamycin: Old drug, new tricks? J. Antimicrob. Chemother. 2014, 69, 2319–2325. [Google Scholar] [CrossRef]
- Weber, P. Streptococcus pneumoniae: Lack of emergence of pristinamycin resistance. Pathol. Biol. 2001, 49, 840–845. [Google Scholar] [CrossRef]
- De Crecy-Lagard, V.; Blanc, V.; Gil, P.; Naudin, L.; Lorenzon, S.; Famechon, A.; Bamas-Jacques, N.; Crouzet, J.; Thibaut, D. Pristinamycin I biosynthesis in Streptomyces pristinaespiralis: Molecular characterization of the first two structural peptide synthetase genes. J. Bacteriol. 1997, 179, 705–713. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Feng, R.; Zheng, G.; Tian, J.; Ruan, L.; Ge, M.; Jiang, W.; Lu, Y. Involvement of the TetR-type regulator PaaR in the regulation of pristinamycin I biosynthesis through an effect on precursor supply in Streptomyces pristinaespiralis. J. Bacteriol. 2015, 197, 2062–2071. [Google Scholar] [CrossRef] [PubMed]
- Kamiyama, T.; Satoh, T.; Umino, T.; Katoh, H.; Uemura, F.; Nakamura, Y.; Yokose, K. Monamidocin, a novel fibrinogen receptor antagonist. J. Antibiot. 1995, 48, 1226–1229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kajimura, Y.; Kaneda, M. Fusaricidins B, C and D, new depsipeptide antibiotics produced by Bacillus polymyxa KT-8: Isolation, structure elucidation and biological activity. J. Antibiot. 1997, 50, 220–228. [Google Scholar] [CrossRef]
- Harman, R.E.; Ham, E.A.; Bolhofer, W.A.; Brink, N.G. The structure of eulicin, a new antifungal agent. J. Am. Chem. Soc. 1958, 80, 5173–5178. [Google Scholar] [CrossRef]
- Hedge, V.R.; Silver, J.E.; Patel, M.G.; Gullo, V.P.; Das, P.R.; Puar, M.S. Isolation and structure of two novel muscarinic receptor antagonists. J. Nat. Prod. 1995, 58, 843–848. [Google Scholar] [PubMed]
- Nakamura, M.; Yoshida, K.; Yoshida, M.; Kunimoto, S.; Takeuchi, T.; Ohno, T. Eulicin inhibits human immunodeficiency virus infection and replication. J. Antibiot. 1995, 48, 1362–1363. [Google Scholar] [CrossRef][Green Version]
- Mast, Y.; Wohlleben, W. Streptogramins—Two are better than one! Int. J. Med. Microbiol. 2014, 304, 44–50. [Google Scholar] [CrossRef]
- Patel, R.; Gallagher, J.C. Vancomycin-resistant enterococcal bacteremia pharmacotherapy. Ann. Pharmacother. 2015, 49, 69–85. [Google Scholar] [CrossRef]
- Nomura, H.; Kimura, K.; Sasao, K.; Okabe, M.; Ishikura, T. A Method for Enhancing the Yield of Depsipeptide Antibiotics by Fermentation. Jpn. Kokai Tokkyo Koho. Japanese Patent 62198617 A 19870902, 6 December 1987. [Google Scholar]
- Lang, G.; Mitova, M.I.; Cole, A.L.; Din, L.B.; Vikineswary, S.; Abdullah, N.; Blunt, J.W.; Munro, M.H. Pterulamides I-VI, linear peptides from a Malaysian Pterula sp. J. Nat. Prod. 2006, 69, 1389–1393. [Google Scholar] [CrossRef]
- Smith, P.A.; Roberts, T.C.; Romesberg, F.E. Broad-spectrum antibiotic activity of the arylomycin natural products is masked by natural target mutations. Chem. Biol. 2010, 17, 1223–1231. [Google Scholar] [CrossRef]
- Isono, K.; Osada, H.; Etsuno, H. Manufacture of Depsipeptide A and B with Streptomyces as Antibacterial and Antiviral Agents. Jpn. Kokai Tokkyo Koho. Japanese Patent 05117298 A 19930514, 9 April 1993. [Google Scholar]
- Osada, H.; Yano, T.; Koshino, H.; Isono, K. Enopeptin A, a novel depsipeptide antibiotic with anti-bacteriophage activity. J. Antibiot. 1991, 44, 1463–1466. [Google Scholar] [CrossRef] [PubMed]
- Ortíz-López, F.J.; Monteiro, M.C.; González-Menéndez, V.; Tormo, J.R.; Genilloud, O.; Bills, G.F. Cyclic colisporifungin and linear cavinafungins, antifungal lipopeptides isolated from Colispora cavincola. J. Nat. Prod. 2015, 78, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Blunt, J.W.; Cole, A.L.; Munro, M.H. A novel cyclodepsipeptide, HA23, from a Fusarium sp. Org. Lett. 2002, 4, 2095–2096. [Google Scholar] [CrossRef] [PubMed]
- Gribble, G.W. Recently discovered naturally occurring heterocyclic organohalogen compounds. Heterocycles 2012, 84, 157–207. [Google Scholar] [CrossRef]
- Koshino, S.; Koshino, H.; Matsuura, N.; Kobinata, K.; Onose, R.; Isono, K.; Osada, H. A new cyclic lipopeptide antibiotic, enamidonin. J. Antibiot. 1995, 48, 185–187. [Google Scholar] [CrossRef][Green Version]
- Lelais, G.; Seebach, D. α-Amino acids—Syntheses, occurrence in natural products, and components of β-peptides. Biopolymers 2004, 76, 206–243. [Google Scholar] [CrossRef]
- Tomoda, H.; Ōmura, S. Potential therapeutics for obesity and atherosclerosis: Inhibitors of neutral lipid metabolism from microorganisms. Pharmacol. Therapeut. 2007, 115, 375–389. [Google Scholar] [CrossRef]
- Namatame, I.; Tomoda, H.; Matsuda, D.; Tabata, N.; Kobayashi, S.; Omura, S. K97–0239 A and B, new inhibitors of macrophage foam cell formation, produced by Streptomyces sp. K97–0239. Proc. Jpn. Acad. Ser. B. 2002, 78, 45–50. [Google Scholar] [CrossRef]
- Son, S.; Ko, S.K.; Jang, M.; Lee, J.K.; Ryoo, I.J.; Lee, J.S.; Lee, K.H.; Soung, N.K.; Oh, H. Ulleungamides A and B, modified α,β-dehydropipecolic acid containing cyclic depsipeptides from Streptomyces sp. KCB13F003. Org. Lett. 2015, 17, 4046–4069. [Google Scholar] [CrossRef]
- Michel, K.H.; Kastner, R.E. AS4556 Antibiotics and Process for Production Thereof. U.S. Patent 4492650, 28 December 1985. [Google Scholar]
- Lin, Z.; Flores, M.; Forteza, I.; Henriksen, N.; Concepcion, G.P.; Rosenberg, G.; Haygood, M.G. Totopotensamides, polyketide-cyclic peptide hybrids from a mollusk-associated bacterium Streptomyces sp. J. Nat. Prod. 2012, 75, 644–649. [Google Scholar] [CrossRef]
- Jalal, M.A.; Hossain, M.B.; van der Helm, D.; Barnes, C.L. Structure of ferrichrome-type siderophores with dissimilar N delta-acyl groups: Asperchrome B1, B2, B3, D1, D2 and D3. Biol. Met. 1988, 1, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Sasaki, K.; Nagai, K.; Shin-ya, K.; Furihata, K. Structure of thioviridamide, a novel apoptosis inducer from Streptomyces olivoviridis. J. Antibiot. 2006, 59, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Kanasaki, R.; Kobayashi, M.; Fujine, K.; Sato, I.; Hashimoto, M.; Takase, S. FR227673 and FR190293, novel antifungal lipopeptides from Chalara sp. No. 22210 and Tolypocladium parasiticum No. 16616. J. Antibiot. 2006, 59, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.X.; Jin, X.J.; Draskovic, M.; Crews, M.S.; Tenney, K.; Valeriote, F.A.; Yao, X.J.; Crews, P. Unraveling the numerous biosynthetic products of the marine sediment-derived fungus, Aspergillus insulicola. Phytochem. Lett. 2012, 5, 114–117. [Google Scholar] [CrossRef]
- Wang, S.Y.; Xu, Z.L.; Wang, H.; Li, C.R.; Fu, L.W. Total synthesis, absolute configuration, and biological activity of xyloallenoide A. Helvetica Chim. Acta 2012, 95, 973–982. [Google Scholar] [CrossRef]
- Abbanat, D.; Leighton, M.; Maiese, W.; Jones, E.B.; Pearce, C.; Greenstein, M. Cell wall active antifungal compounds produced by the marine fungus Hypoxylon oceanicum LL-15G256. I. Taxonomy and fermentation. J. Antibiot. 1998, 51, 296–302. [Google Scholar] [CrossRef]
- Schlingmann, G.; Milne, L.; Williams, D.R.; Carter, G.T. Cell wall active antifungal compounds produced by the marine fungus Hypoxylon oceanicum LL-15G256. II. Isolation and structure determination. J. Antibiot. 1998, 51, 303–316. [Google Scholar] [CrossRef]
- Lira, S.P.; Vita-Marques, A.M.; Seleghim, M.H.; Bugni, T.S.; La Barbera, D.V.; Sette, L.D. New destruxins from the marine-derived fungus Beauveria felina. J. Antibiot. 2006, 59, 553–563. [Google Scholar] [CrossRef]
- Cai, P.; Smith, D.; Katz, B.; Pearce, C.; Venables, D.; Houck, D. Destruxin-A4 chlorohydrin, a novel destruxin from fungus OS-F68576: Isolation, structure determination, and biological activity as an inducer of erythropoietin. J. Nat. Prod. 1998, 61, 290–293. [Google Scholar] [CrossRef]
- Odier, F.; Vey, A.; Bureau, J.P. In vitro effect of fungal cyclodepsipeptides on leukemic cells: Study of destruxins A, B and E. Biol. Cell. 1992, 74, 267–271. [Google Scholar] [CrossRef]
- Jegorov, A.; Sedmera, P.; Havlicek, V.; Mat’ha, V. Destruxin Ed1 a cyclopeptide from the fungus Metarhizium anisopliae. Phytochemistry 1999, 49, 1815–1817. [Google Scholar] [CrossRef]
- Hanada, K.; Tamai, M.; Yamagishi, M.; Ohmura, S.; Sawada, J.; Tanaka, I. Isolation and characterization of E–64, a new thiol protease inhibitor. Agric. Biol. Chem. 1978, 42, 523–528. [Google Scholar]
- Barrett, A.; Kembhavi, A.; Brown, M.; Kirschke, H.; Knight, C.; Tamai, M.; Hanada, K. L-trans-Epoxysuccinyl-leucylamido-(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. J. Biochem. 1982, 201, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Gill, K.A.; Berrué, F.; Arens, J.C.; Kerr, R.G. Isolation and structure elucidation of cystargamide, a lipopeptide from Kitasatospora cystarginea. J. Nat. Prod. 2014, 77, 1372–1376. [Google Scholar] [CrossRef]
- Vilhena, C.; Bettencourt, A. Daptomycin: A review of properties, clinical use, drug delivery and resistance. Mini Rev. Med. Chem. 2012, 12, 202–209. [Google Scholar] [CrossRef]
- Baltz, R.H.; Miao, V.; Wrigley, S.K. Natural products to drugs: Daptomycin and related lipopeptide antibiotics. Nat. Prod. Rep. 2005, 22, 717–741. [Google Scholar] [CrossRef]
- Mahlert, C.; Kopp, F.; Thirlway, J.; Micklefield, J.; Marahiel, M.A. Stereospecific enzymatic transformation of α-ketoglutarate to (2S,3R)-3-methyl glutamate during acidic lipopeptide biosynthesis. J. Am. Chem. Soc. 2007, 129, 12011–12018. [Google Scholar] [CrossRef]
- Hata, T.; Koga, F.; Sano, Y.; Kanamori, K.; Matsumae, A.; Sugawara, R. Carzinophilin, a new tumor inhibitory substance produced by Streptomyces. I. J Antibiot. 1954, 7, 107–112. [Google Scholar]
- Carr, G.; Poulsen, M.; Klassen, J.L.; Hou, Y.; Wyche, T.P.; Bugni, T.S.; Currie, C.R.; Clardy, J. Microtermolides A and B from termite-associated Streptomyces sp. and structural revision of vinylamycin. Org. Lett. 2012, 14, 2822–2825. [Google Scholar] [CrossRef]
- Bycroft, B.W. Dictionary of Antibiotics and Related Substances; Chapman and Hall: London, UK, 1988; p. 962. [Google Scholar]
- Sohda, K.; Takebayashi, Y.; Nagai, K.; Yokoi, T.; Nanya, T.; Kuromitsu, S.; Shindo, N. Novel Antitumor Hexadepsipeptide Compounds of Streptomyces nobilis. WO 9845320 A1 19981015, 4 April 1998. [Google Scholar]
- Sakai, Y.; Yoshida, T.; Tsujita, T.; Ochiai, K.; Agatsuma, T.; Saitoh, Y.; Tanaka, F.; Akiyama, T.; Akinaga, S.; Mizukami, T. GE3, a novel hexadepsipeptide antitumor antibiotic, produced by Streptomyces sp. I. Taxonomy, production, isolation, physico-chemical properties, and biological activities. J. Antibiot. 1997, 50, 659–664. [Google Scholar] [CrossRef]
- Umezawa, K.; Nakazawa, K.; Ikeda, Y.; Naganawa, H.; Kondo, S. Polyoxypeptins A and B produced by Streptomyces: Apoptosis-inducing cyclic depsipeptides containing the novel amino acid (2S,3R)-3-hydroxy-3-methylproline. J. Org. Chem. 1999, 64, 3034–3038. [Google Scholar] [CrossRef] [PubMed]
- Graefe, U.; Schlegel, R.; Ritzau, M.; Ihn, W.; Dornberger, K.; Stengel, C.; Fleck, W.F.; Gutsche, W.; Haertl, A.; Paulus, E.F. Aurantimycins, new depsipeptide antibiotics from Streptomyces aurantiacus IMET 43917: Production, isolation, structure elucidation, and biological activity. J. Antibiot. 1995, 48, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Hayakawa, Y.; Nakagawa, M.; Toda, Y.; Seto, H. A new depsipeptide antibiotic, citropeptin. Agric. Biol. Chem. 1990, 54, 1007–1011. [Google Scholar] [PubMed]
- Maehr, H.; Liu, C.M.; Palleroni, N.J.; Smallheer, J.; Todaro, L.; Williams, T.H.; Blount, J.F. Microbial products. VIII. Azinothricin, a novel hexadepsipeptide antibiotic. J. Antibiot. 1986, 39, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Smitka, T.A.; Deeter, J.B.; Hunt, A.H.; Mertz, F.P.; Ellis, R.M.; Boeck, L.D.; Yao, R.C. A83586C, a new depsipeptide antibiotic. J. Antibiot. 1988, 41, 726–733. [Google Scholar] [CrossRef]
- Raju, R.; Zeinab, G.; Piggott, A.M.; Blumenthal, A.; Gardiner, D.L.; Skinner-Adams, T.S.; Capon, R.J. Mollemycin A: An antimalarial and antibacterial glyco-hexadepsipeptide-polyketide from an Australian marine-derived Streptomyces sp. (CMB-M0244). Org. Lett. 2014, 16, 1716–1719. [Google Scholar] [CrossRef]
- Mishra, P.D.; Eyyammadichiyil, S.S.; George, S.D.; Sonawane, S.; Chakor, N.S.; Roychowdhury, A.; Sharma, R. Hexadepsipeptide Analogues as Anticancer Compounds. Wipo Patent WO2013168075A1, 14 November 2013. [Google Scholar]
- Uchihata, Y.; Ando, N.; Ikeda, Y.; Kondo, S.; Hamada, M.; Umezawa, K. Isolation of a novel cyclic hexadepsipeptide pipalamycin from Streptomyces as an apoptosis-inducing agent. J. Antibiot. 2002, 55, 1–5. [Google Scholar] [CrossRef]
- Nakagawa, M.; Hayakawa, Y.; Adachi, K.; Seto, H. A new depsipeptide antibiotic, variapeptin. Agric. Biol. Chem. 1990, 54, 791–794. [Google Scholar]
- Nakagawa, M.; Hayakawa, Y.; Furihata, K.; Seto, H. Structural studies on new depsipeptide antibiotics, variapeptin and citropeptin. J. Antibiot. 1990, 43, 477–484. [Google Scholar] [CrossRef]
- Raju, R.; Gromyko, O.; Fedorenko, A.B.; Luzhetsky, A.V.; Müller, R. Oleamycins A and B: New antibacterial cyclic hexadepsipeptides isolated from a terrestrial Streptomyces sp. J. Antibiot. 2014, 67, 339–343. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Sugawara, K.; Tomita, K.; Yamamoto, H.; Kamei, H.; Oki, T. Verucopeptin, a new antitumor antibiotic active against B16 melanoma. I. Taxonomy, production, isolation, physico-chemical properties and biological activity. J. Antibiot. 1993, 46, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Nishimura, S.; Otsuka, S.; Hattori, A.; Kakeya, H. Structure elucidation of verucopeptin, a HIF-1 inhibitory polyketide-hexapeptide hybrid metabolite from an Actinomycete. Org. Lett. 2015, 17, 5364–5367. [Google Scholar] [CrossRef] [PubMed]
- Imamura, N.; Nishijima, M.; Adachi, K.; Sano, H. Novel antimycin antibiotics, urauchimycins A and B, produced by marine actinomycete. J. Antibiot. 1993, 46, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, Q. Regulatory mechanisms of lipid biosynthesis in microalgae. Biol. Rev. 2021, 96, 2373–2391. [Google Scholar] [CrossRef] [PubMed]
- Hegde, V.R.; Dai, P.; Patel, M.; Das, P.R.; Wang, S.; Puar, M.S. A depsipeptide fungal metabolite inhibitor of cholesteryl ester transfer protein. Bioorg. Med. Chem. Lett. 1998, 8, 1277–1280. [Google Scholar] [CrossRef]
- Hedge, V.R.; Puar, M.S.; Dai, P.; Pu, H.; Patel, M.; Anthes, J.C. A family of depsi-peptide fungal metabolites, as selective and competitive human tachykinin receptor (NK2) antagonists: Fermentation, isolation, physico-chemical properties, and biological activity. J. Antibiot. 2001, 54, 125–135. [Google Scholar]
- Churchill, A.C.; Dunkle, L.D.; Silbert, W.; Kennedy, K.J.; Macko, V. Differential synthesis of peritoxins and precursors by pathogenic strains of the fungus Periconia circinata. Appl. Environ. Microbiol. 2001, 67, 5721–5728. [Google Scholar] [CrossRef]
- Macko, V.; Stimmel, M.B.; Wolpert, T.J.; Dunkle, L.D.; Acklin, W.; Bänteli, R.; Jaun, B.; Arigoni, D. Structure of the host-specific toxins produced by the fungal pathogen Periconia circinata. Proc. Natl. Acad. Sci. USA 1992, 89, 9574–9578. [Google Scholar] [CrossRef]
- Jiao, P. Chemical Investigations of Freshwater and Fungicolous Fungi. PhD Thesis, University of Iowa, Iowa City, IA, USA, 2006. [Google Scholar]
- Donadio, S.; Maffioli, S.; Paolo Monciardini, P.; Sosio, M.; Jabes, D. Sources of novel antibiotics—aside the common roads. Appl. Microbiol. Biotechnol. 2010, 88, 1261–1267. [Google Scholar] [CrossRef]
- Yount, N.Y.; Yeaman, M.R. Peptide antimicrobials: Cell wall as a bacterial target. Ann. N. Y. Acad. 2013, 1277, 127–138. [Google Scholar] [CrossRef]
- Hammann, P.; Babenhausen, J.M.; Seibert, G.; Vertesy, L.; Wink, J.; Markus, A. Lipopeptides from Actinoplanes sp. with Pharmacological Action, Process for Their Production and The Use Thereof. U.S. Patent 6,194,383 B1, 27 February 2001. [Google Scholar]
- Dini, C. MraY inhibitors as novel antibacterial agents. Current Topics Med. Chem. 2005, 5, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Boeck, L.D.; Fukuda, D.S.; Abbott, B.J.; Debono, M. Deacylation of A21978C, an acidic lipopeptide antibiotic complex, by Actinoplanes utahensis. J. Antibiot. 1988, 41, 1085–1092. [Google Scholar] [CrossRef] [PubMed]
- Debono, M.; Abbott, B.J.; Molloy, R.M.; Fukuda, D.S.; Hunt, A.H.; Daupert, V.M. Enzymatic and chemical modifications of lipopeptide antibiotic A21978C: The synthesis and evaluation of daptomycim (LY146032). J. Antibiot. 1988, 41, 1093–1105. [Google Scholar] [CrossRef] [PubMed]
- Fujino, M. On glumamycin, a new antibiotic. VI. An approach to the amino acid sequence. Bull. Chem. Soc. Jpn. 1965, 38, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Hausmann, W.K.; Struck, A.H.; Martin, J.H.; Barritt, R.H.; Bohonos, N. Structure determination of fatty acids from the antibiotic Aspartocin. Antimicrob. Agents Chemother. 1963, 33, 352–359. [Google Scholar]
- Hausmann, W.K.; Borders, D.B.; Lancaster, J.E.; Heinemann, B. Alpha, beta-diaminobutyric acid obtained from aspartocin. J. Antibiot. 1969, 22, 207–210. [Google Scholar] [CrossRef][Green Version]
- Bodanszky, M.; Chaturvedi, N.C.; Scozzie, J.A. The structure of fatty acids from the antibiotic amphomycin. J. Antibiot. 1969, 22, 399–408. [Google Scholar] [CrossRef][Green Version]
- Hinuma, Y. Zaomycin, a new antibiotic from a Streptomyces sp. J. Antibiot. 1954, 7A, 134–136. [Google Scholar]
- Huber, F.M.; Pieper, R.L.; Tietz, A.J. The formation of daptomycin by supplying decanoic acid to Strptomyces roseosporus cultures producing the antibiotic complex A21978C. J. Biotechnol. 1988, 7, 283–292. [Google Scholar] [CrossRef]
- Martin, J.H.; Hausmann, W.K. Isolation and identification of D-α-pepecolic acid, a,b-methylaspartic acid and a,b-diaminobutyric acid from the polypeptide antibiotic aspartocin. J. Am. Chem. Soc. 1960, 82, 2079. [Google Scholar] [CrossRef]
- Naganawa, H.; Takita, T.; Maeda, K.; Umezawa, H. A novel fatty acid from laspartomycin. J. Antibiot. 1970, 23, 423–424. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naganawa, H.; Hamada, M.; Maeda, K.; Okami, Y.; Takeushi, T. Laspartomycin, a new anti-staphylococcal peptide. J. Antibiot. 1968, 21, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Shay, A.J.; Adam, J.; Martin, J.H.; Hausmann, W.K.; Shu, P.; Bohonos, N. Aspartocin. I. Production, isolation, and characteristics. Antibiot. Ann. 1959, 2, 194–198. [Google Scholar]
- Shoji, J.-I. Studies on tsushimycin. I. Isolation and characterization of an acidic acylpeptide containing a new fatty acid. J. Antibiot. 1968, 21, 439–443. [Google Scholar] [CrossRef]
- Wooda, T.M.; Martin, N.I. The calcium-dependent lipopeptide antibiotics: Structure, mechanism, & medicinal chemistry. Med. Chem. Commun. 2019, 10, 634–646. [Google Scholar]
- Engstrom, G.W.; De Lance, J.V.; Richard, J.L.; Baetz, A.L. Purification and characterization of roseotoxin b, a toxic cyclodepsipeptide from Trichothecium roseum. J. Agric. Food Chem. 1975, 23, 244–253. [Google Scholar] [CrossRef]
- Springer, J.P.; Cole, R.J.; Dorner, J.W.; Cox, R.H.; Richard, J.L.; Barnes, C.L.; Van der Helm, D. Structure and conformation of roseotoxin B. J. Am. Chem. Soc. 1984, 106, 2388–2392. [Google Scholar] [CrossRef]
- Silber, J.; Ohlendorf, B.; Labes, A.; Näther, C.; Imhoff, J.F. Calcaripeptides A–C, cyclodepsipeptides from a Calcarisporium strain. J. Nat. Prod. 2013, 76, 1461–1467. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, A.; Gao, J.; Chen, W.; Wang, S.; Wu, X.; Shen, Y. Trichomide A, a natural cyclodepsipeptide, exerts immunosuppressive activity against activated T lymphocytes by upregulating SHP2 activation to overcome contact dermatitis. J. Invest. Dermatol. 2014, 134, 2737–2746. [Google Scholar] [CrossRef]
- Wang, N.; Dong, Y.; Yang, Y.; Xu, R.; Li, C.W.; Cui, C.B. Penicimutanin C, a new alkaloidal compound, isolated from a neomycin-resistant mutant 3-f-31of Penicillium purpurogenum G59. Chem. Biodivers. 2020, 17, e2000241. [Google Scholar] [CrossRef]
- Suzuki, K.; Yamaizumi, M.; Tateishi, S.; Monnai, Y.; Uyeda, M. Topostatin, a novel inhibitor of topoisomerases I and II produced by Thermomonospora alba strain No. 1520. III. Inhibitory properties. J. Antibiot. 1999, 52, 460–465. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bills, G.F.; Platas, G.; Peláez, F.; Masurekar, P. Reclassification of a pneumocandin-producing anamorph, Glarea lozoyensis gen. et sp. nov., previously identified as Zalerion arboricola. Mycolog. Res. 1999, 103, 179–192. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Sesin, D.F.; Joshua, H.; Wilson, K.E. Pneumocandins from Zalerion arboricola I. Discovery and isolation. J. Antibiot. 1992, 45, 1853–1866. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Schmatz, D.M.; Abruzzo, G.; Powles, M.A. Pneumocandins from Zalerion arboricola. IV. Biological evaluation of natural and semisynthetic pneumocandins for activity against Pneumocystis carinii and Candida species. J. Antibiot. 1992, 45, 1886–1891. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, A.; Broberg, A.; Johansson, M.; Kenne, L.; Levenfors, J. Pseudotrienic acids A and B, two bioactive metabolites from Pseudomonas sp. MF381-IODS. J. Nat. Prod. 2005, 68, 1380–1385. [Google Scholar] [CrossRef]
- Pettit, R.K. Mixed fermentation for natural product drug discovery. Appl. Microbiol. Biotechnol. 2009, 83, 19–25. [Google Scholar] [CrossRef]
- Kunze, B.; Jansen, R.; Sasse, F.; Hofle, G. Chondramides A–D, new antifungal and cytostatic depsipeptides from Chondromyces crocatus (Myxobacteria). J. Antibiot. 1995, 48, 1262–1266. [Google Scholar] [CrossRef]
- Furumai, R.; Matsuyama, A.; Kobashi, N.; Lee, K.H.; Nishiyama, M.; Nakajima, H. FK228 (Depsipeptide) as a natural prodrug that inhibits class I. Histone deacetylases. Cancer Res. 2002, 62, 4916–4921. [Google Scholar]
- Ueda, H.; Nakajima, H.; Hori, Y.; Fujita, T.; Nishimura, M.; Goto, T.; Okuhara, M. FR901228, a novel antitumor bicyclic depsipeptide produced by Chromobacterium violaceum No. 968. I. Taxonomy, fermentation, isolation, physico-chemical and biological properties, and antitumor activity. J. Antibiot. 1994, 47, 301–310. [Google Scholar] [CrossRef]
- Kjaerulff, L.; Nielsen, A.; Mansson, M.; Gram, L.; Larsen, T.O. Identification of four new agr quorum sensing-interfering cyclodepsipeptides from a marine Photobacterium. Mar. Drugs 2013, 11, 5051–5062. [Google Scholar] [CrossRef]
- Adachi, K.; Kawabata, Y.; Kasai, H.; Katsuta, M.; Shizuri, Y. New Antibiotic. Jpn. Pat. Appl. JP 2007230911A, 13 September 2007. [Google Scholar]
- Plaza, A.; Garcia, R.; Bifulco, G.; Martinez, J.P. Aetheramides A and B, potent HIV-inhibitory depsipeptides from a Myxobacterium of the new genus “Aetherobacter”. Org. Lett. 2012, 14, 2854–2857. [Google Scholar] [CrossRef] [PubMed]
- Weissmana, K.J.; Müller, R. Myxobacterial secondary metabolites: Bioactivities and modes-of-action. Nat. Prod. Rep. 2010, 27, 1276–1295. [Google Scholar] [CrossRef] [PubMed]
- Leibold, T.; Sasse, F.; Reichenbach, H. Cyrmenins, novel antifungal peptides containing a nitrogen-linked β-methoxyacrylate pharmacophore: Isolation and structural elucidation. Eur. J. Org. Chem. 2004, 2, 431–435. [Google Scholar] [CrossRef]
- Sasse, F.; Leibold, T.; Kunze, B.; Höfle, G. Cyrmenins, new β-methoxyacrylate inhibitors of the electron transport production, isolation, physico-chemical and biological properties. J. Antibiot. 2003, 56, 827–831. [Google Scholar] [CrossRef][Green Version]
- Oka, M.; Konishi, M. BU-2867T Peptide Antibiotics. U.S. Patent 4777160A, 18 September 1986. [Google Scholar]
- Oka, M.; Numata, K.; Konishi, M. Semi-Synthetic Peptide Antibiotics. U.S. Patent 4789731, 6 December 1988. [Google Scholar]
- Ray, L.; Yamanaka, K.; Moore, B.S. A peptidyl-transesterifying type I thioesterase in salinamide biosynthesis. Angew. Chem. Int. Ed. Engl. 2016, 55, 364–367. [Google Scholar] [CrossRef]
- Ohlendorf, B.; Kehraus, S.; König, G.M. Myxochromide B3, a new member of the myxochromide family of secondary metabolites. J. Nat. Prod. 2008, 71, 1708–1713. [Google Scholar] [CrossRef]
- PASS Online URL. Available online: http://www.way2drug.com/passonline/ (accessed on 24 February 2022).
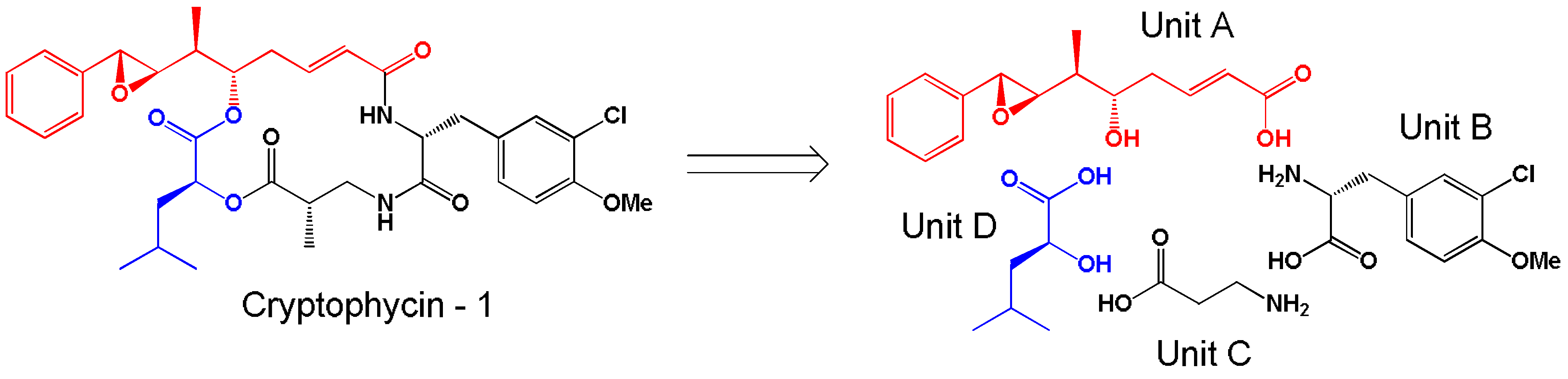

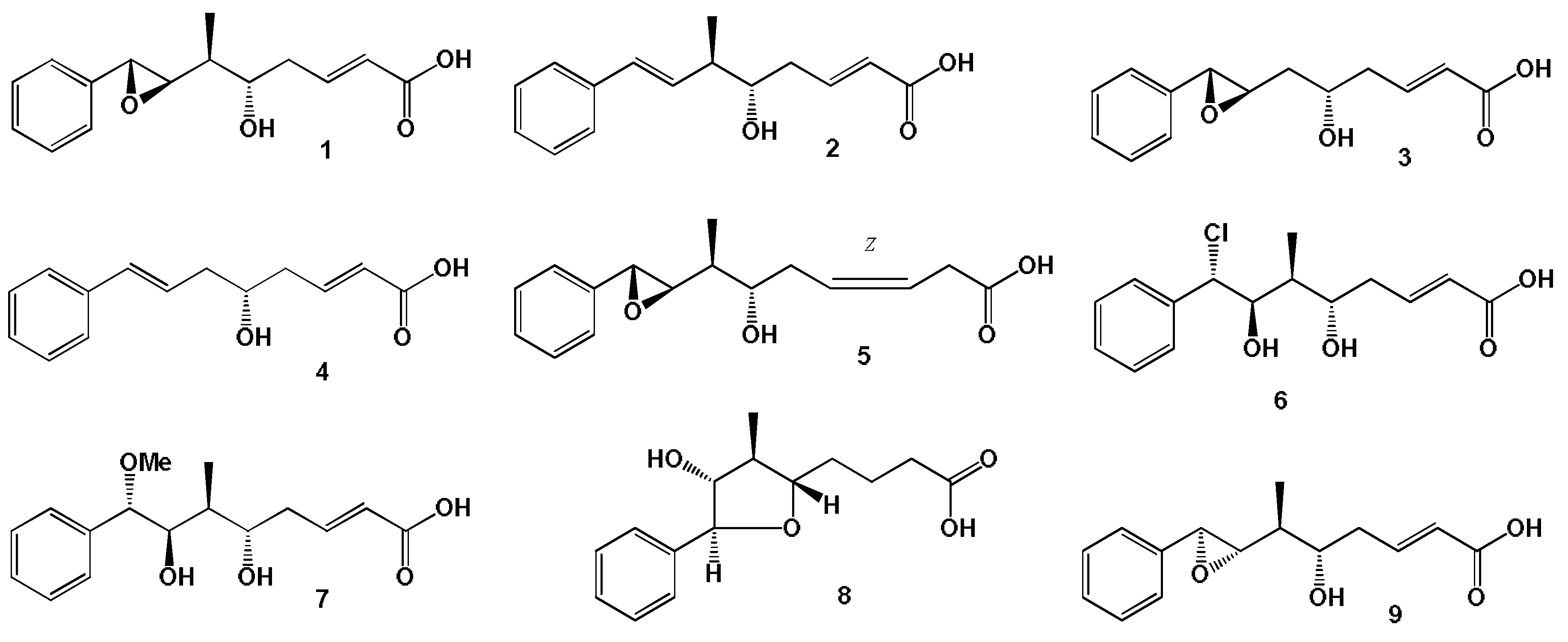

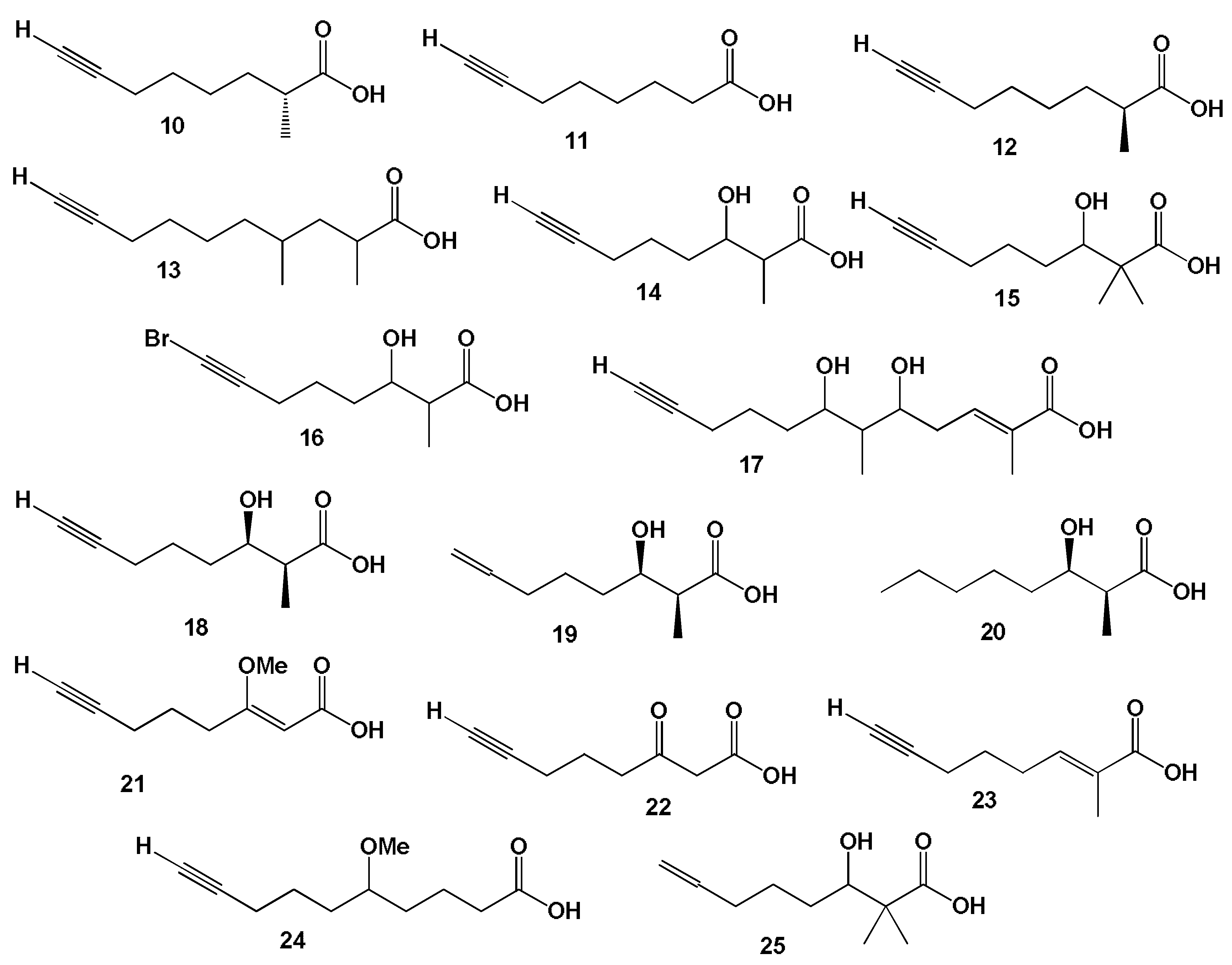
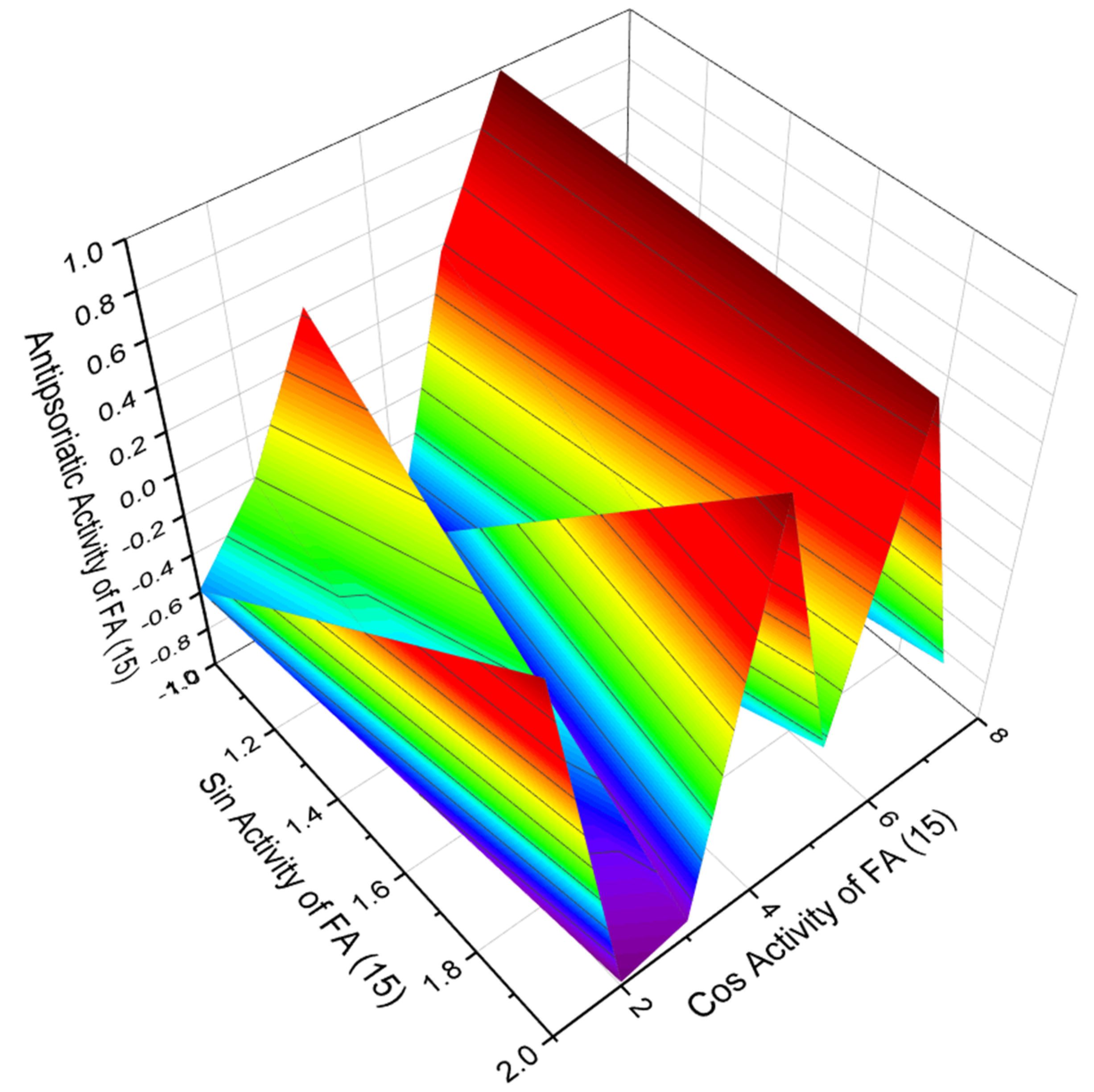
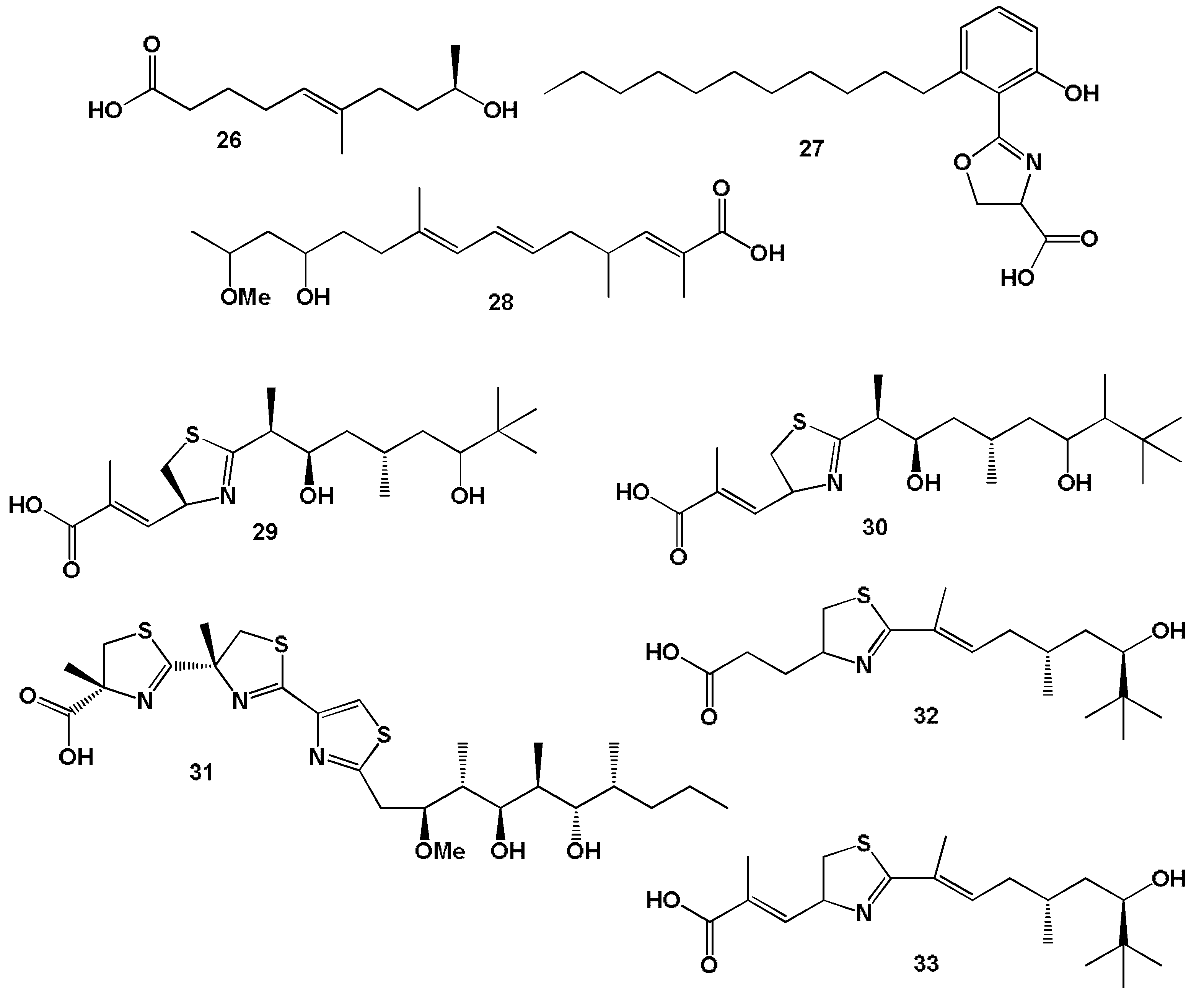
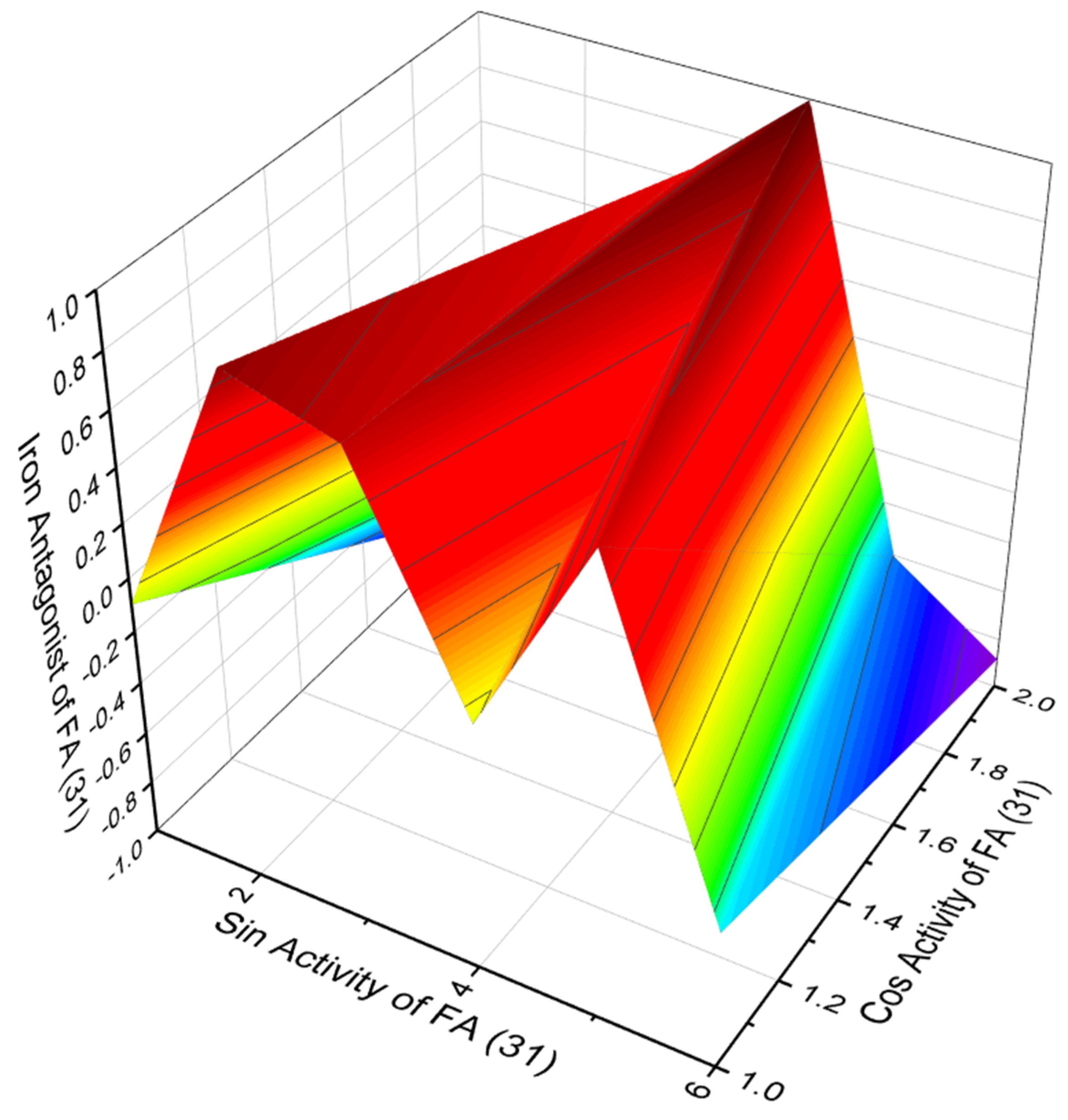
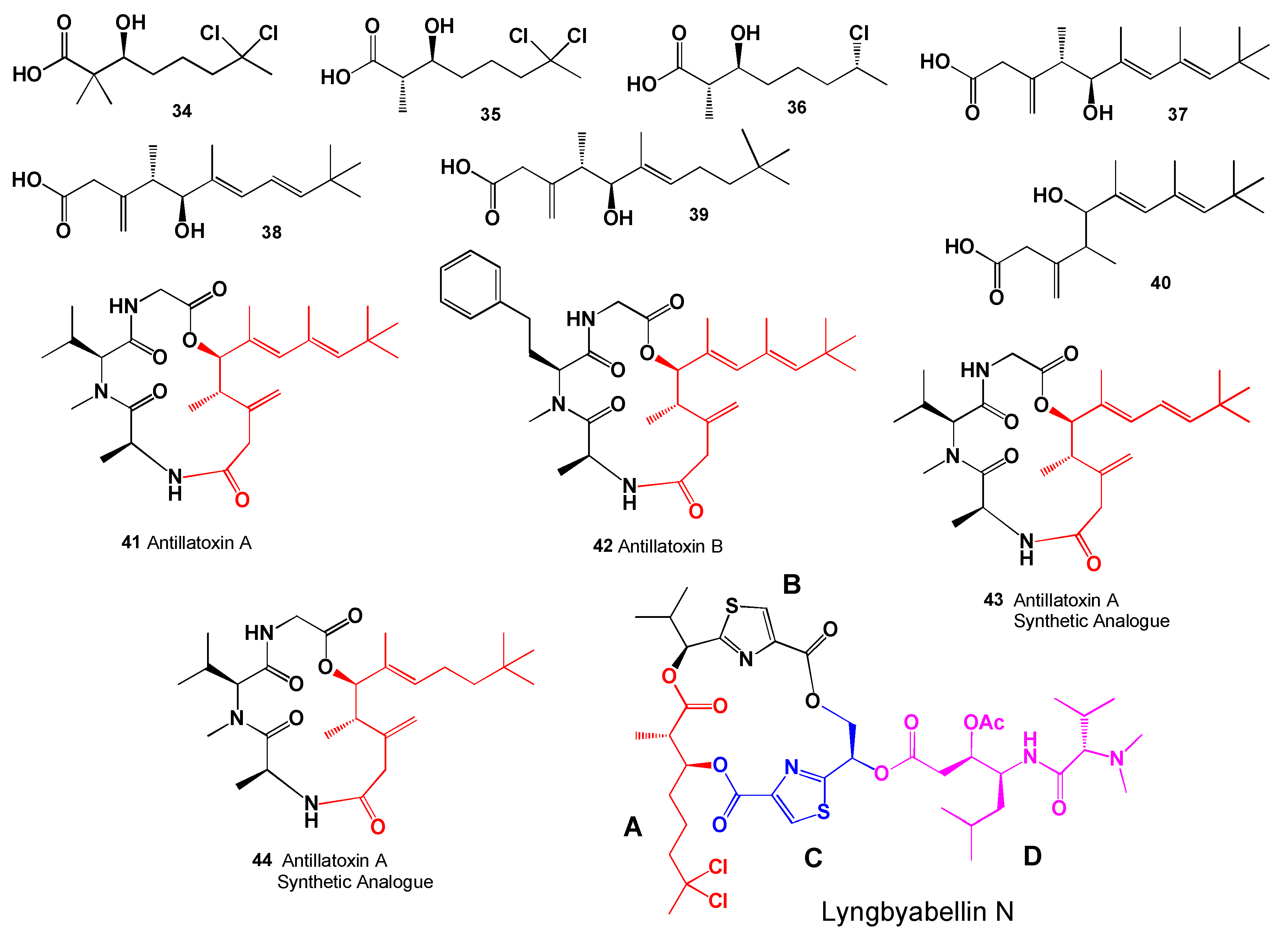
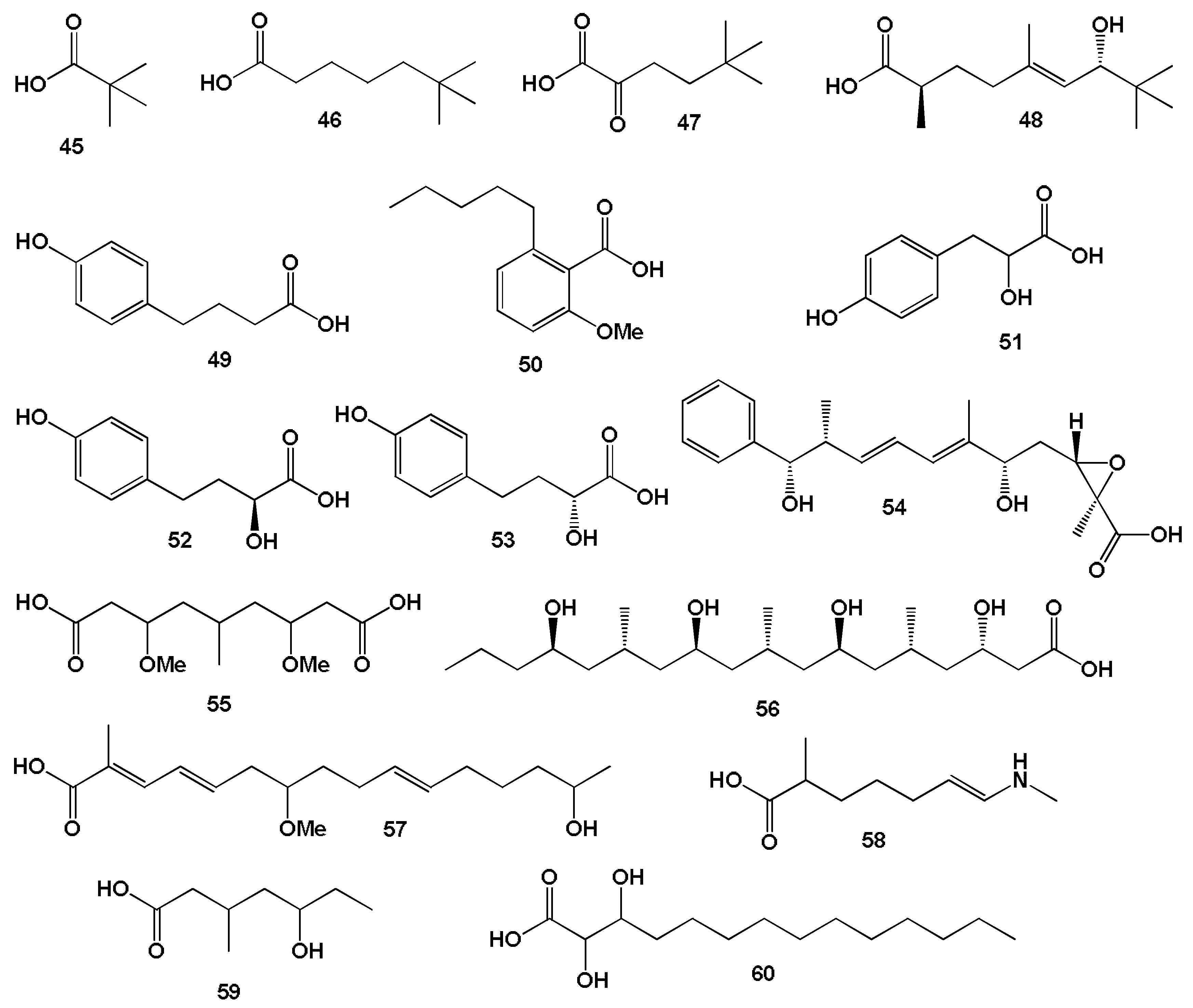
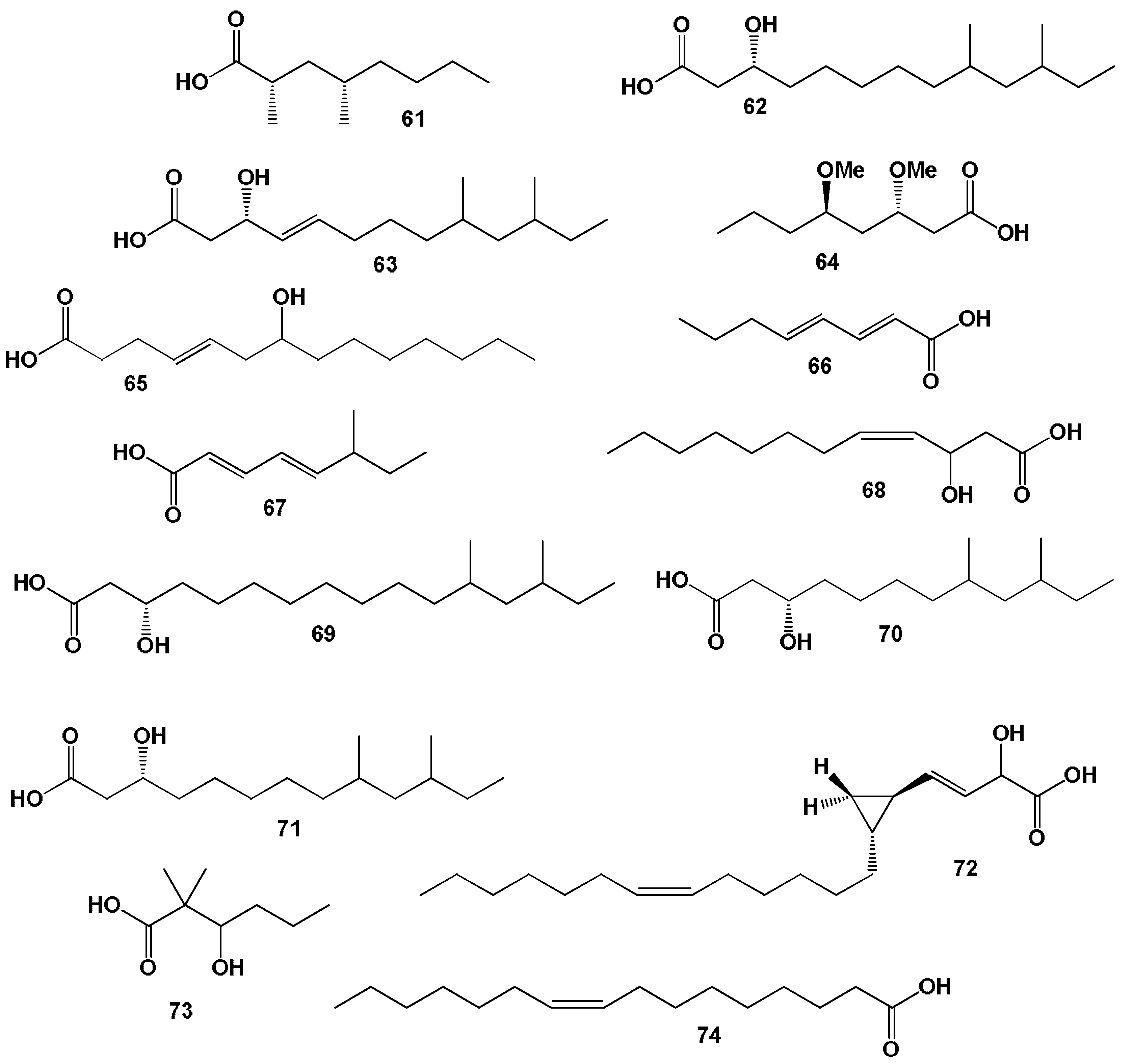
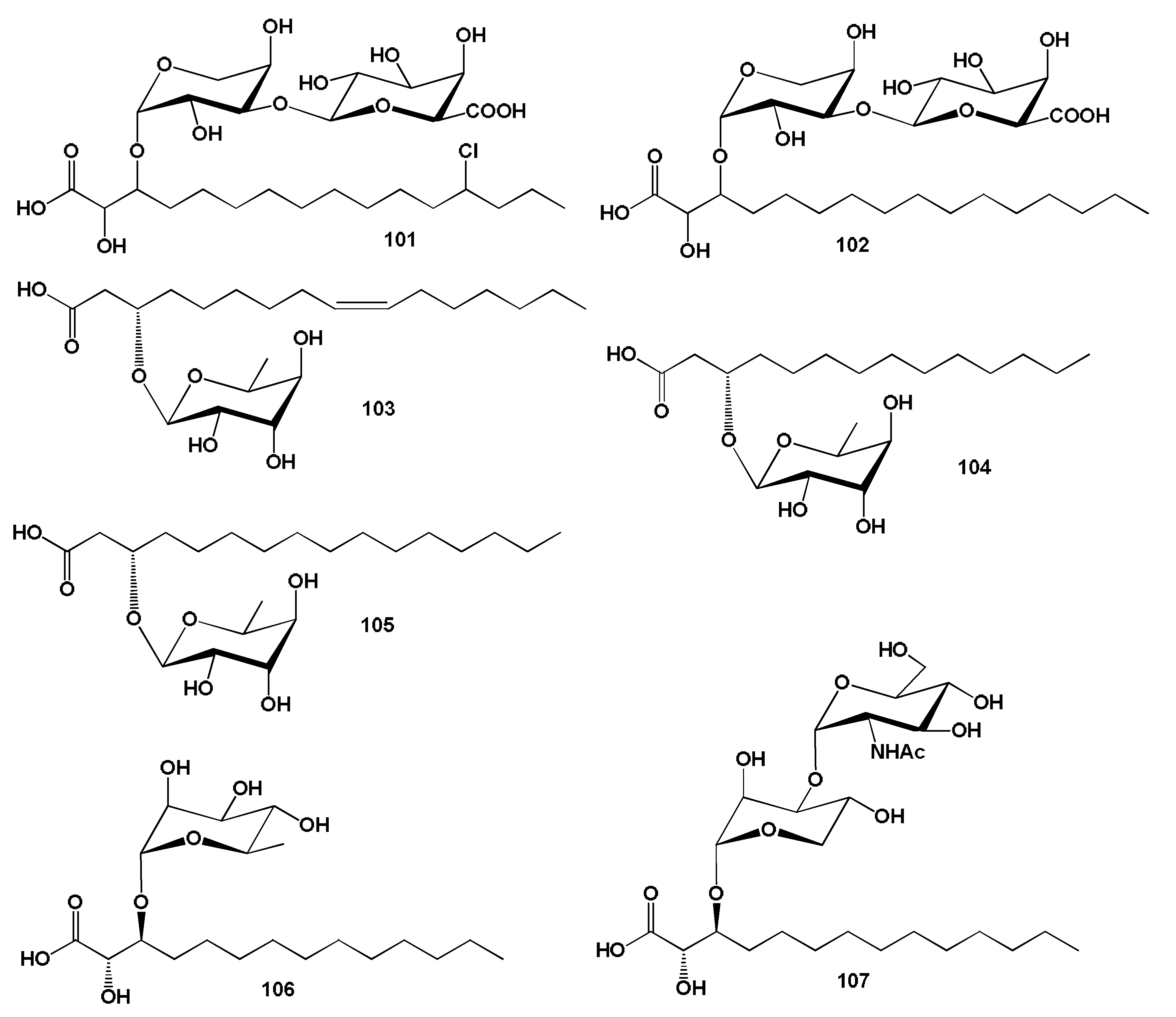
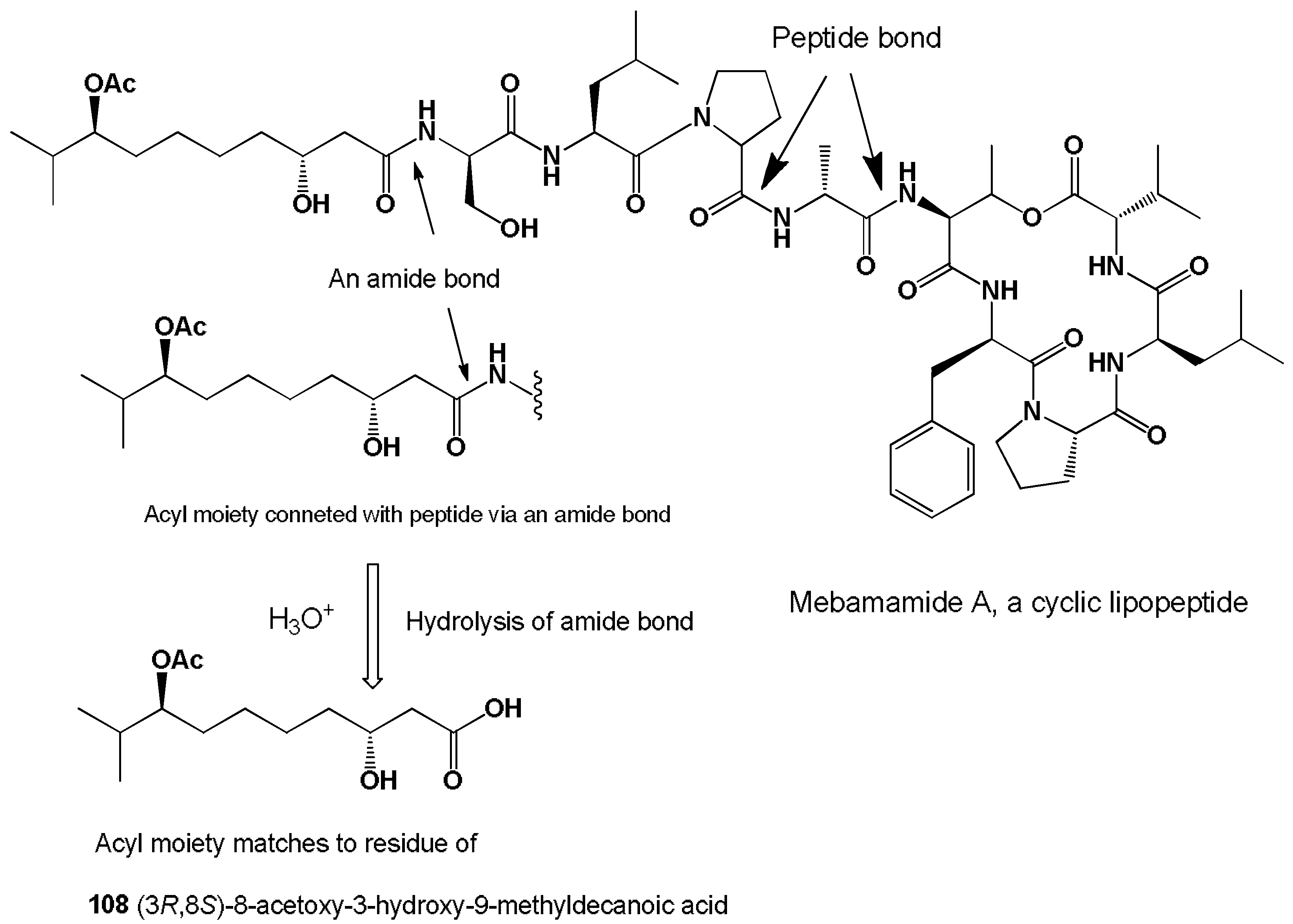
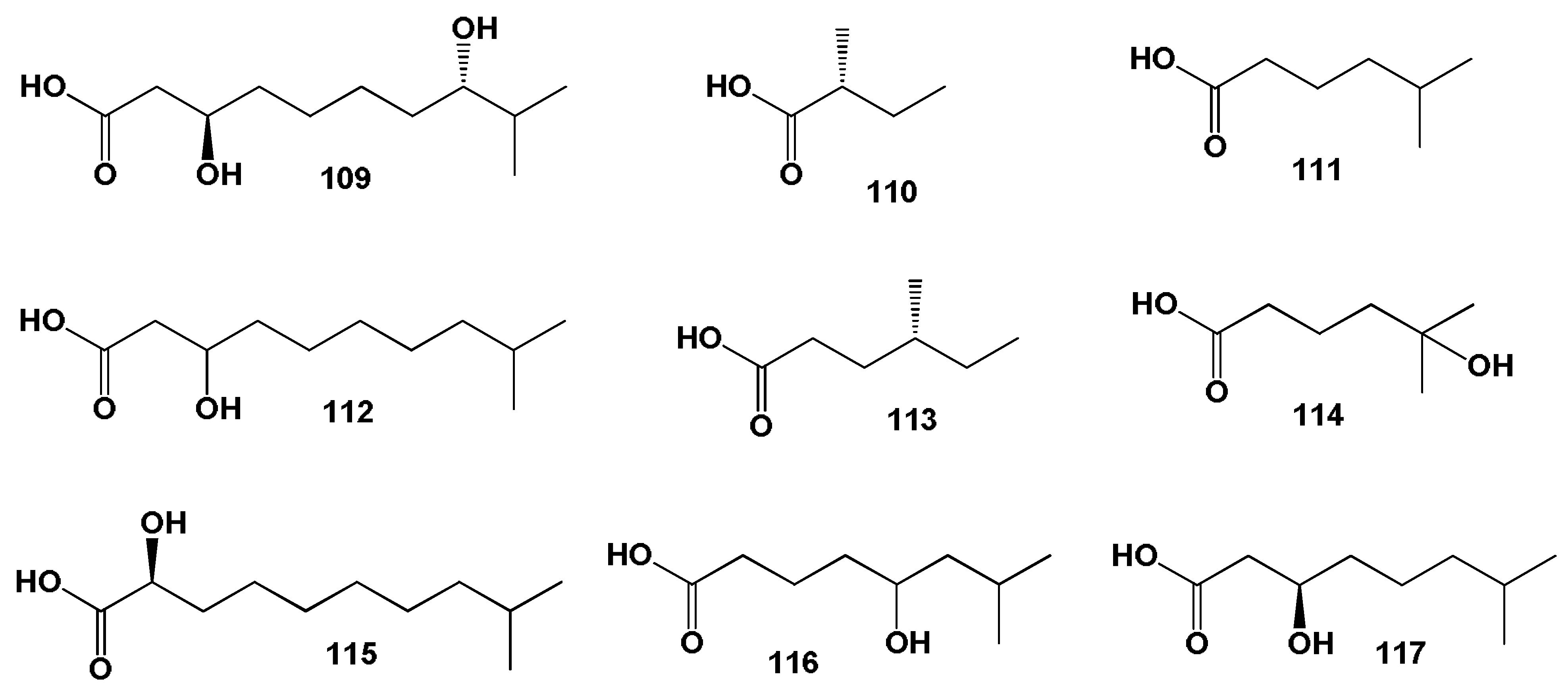
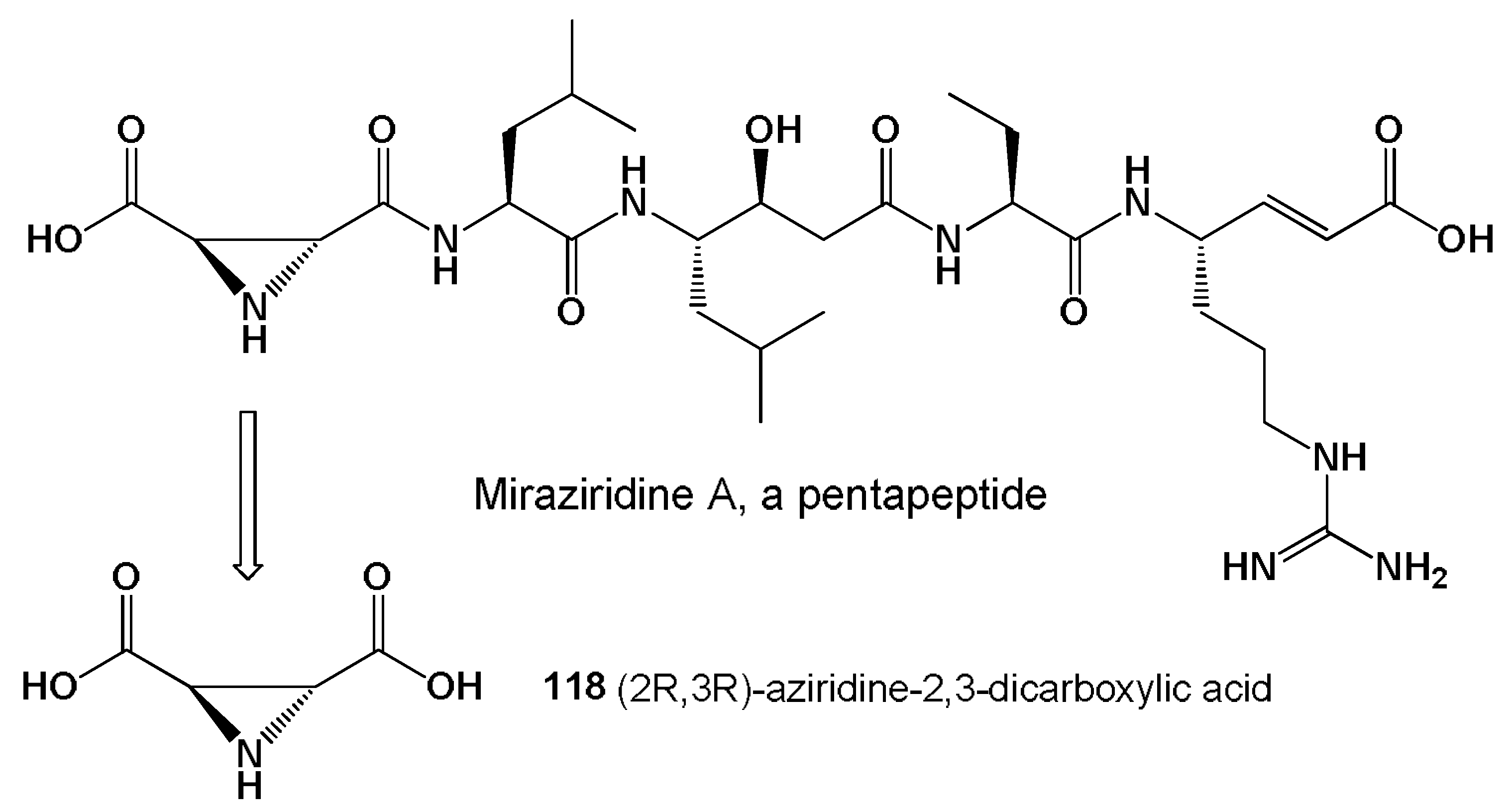

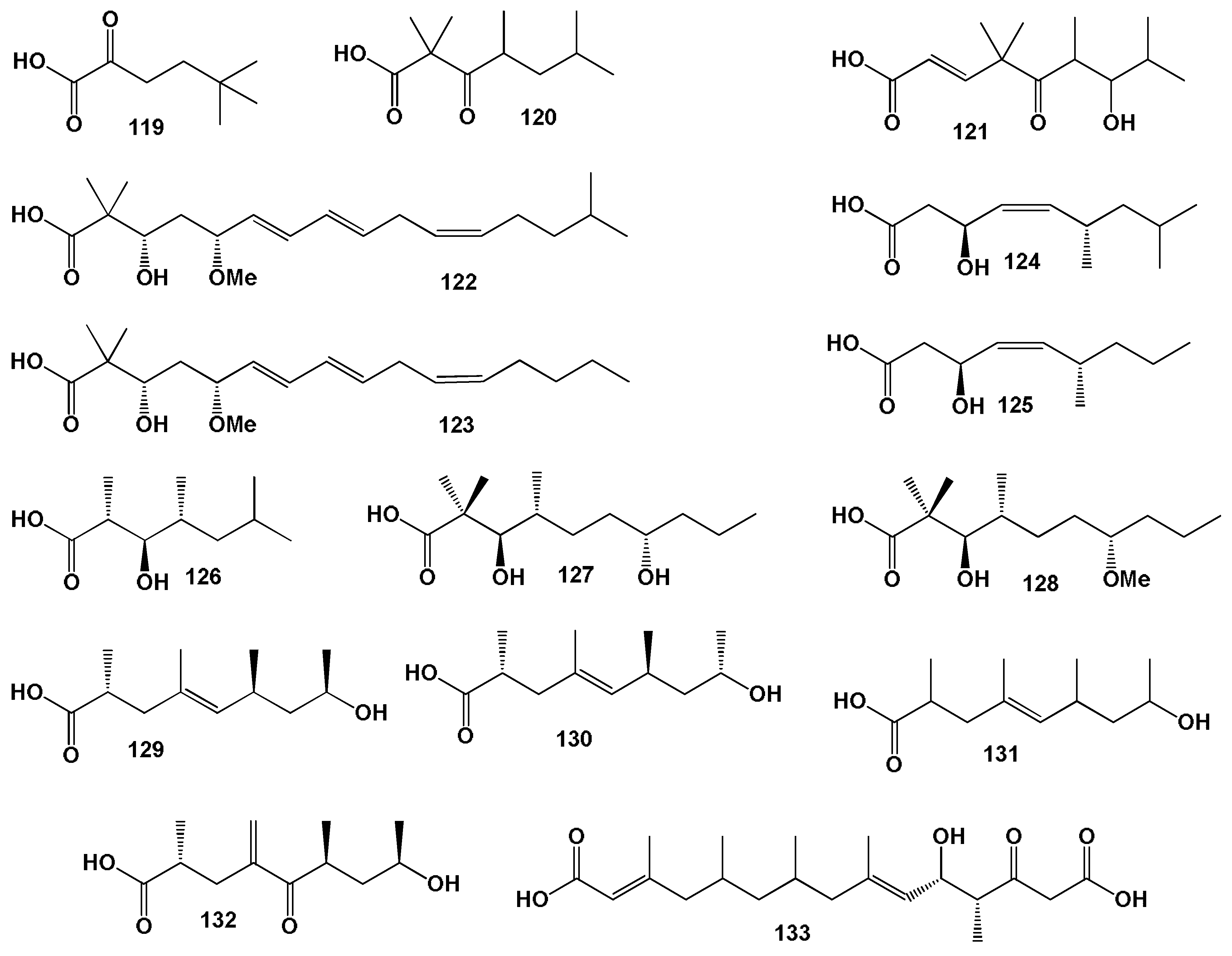

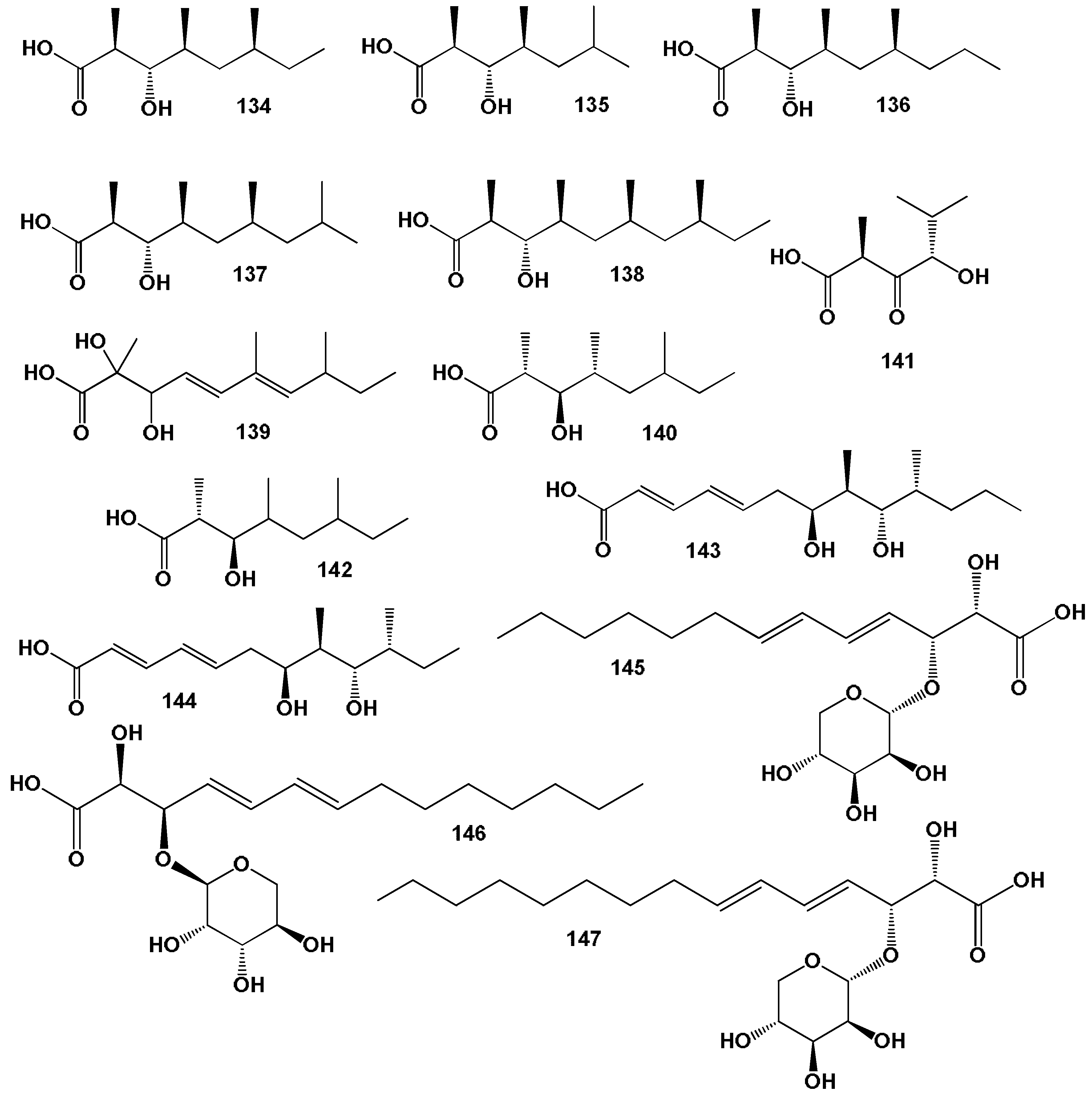

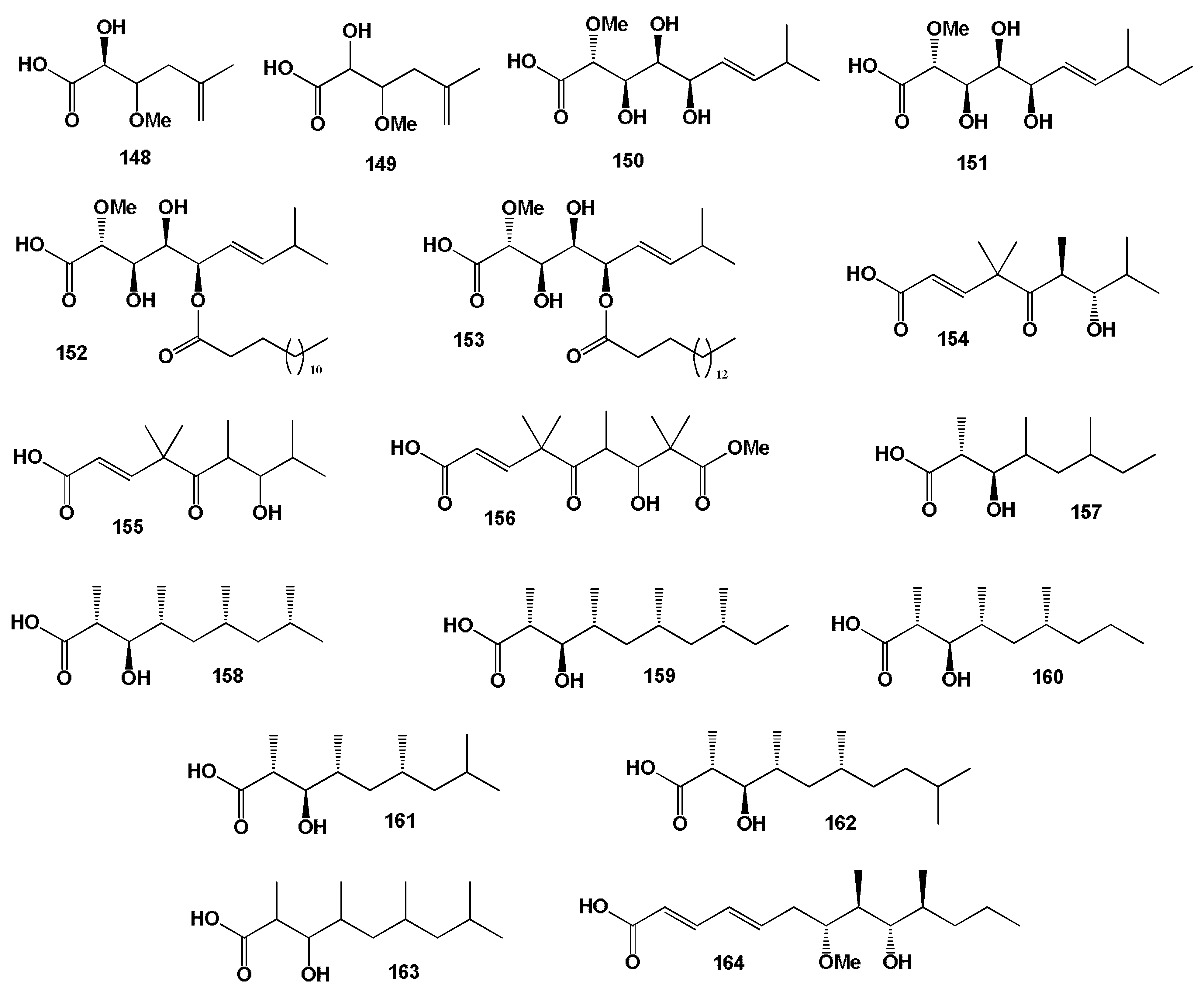
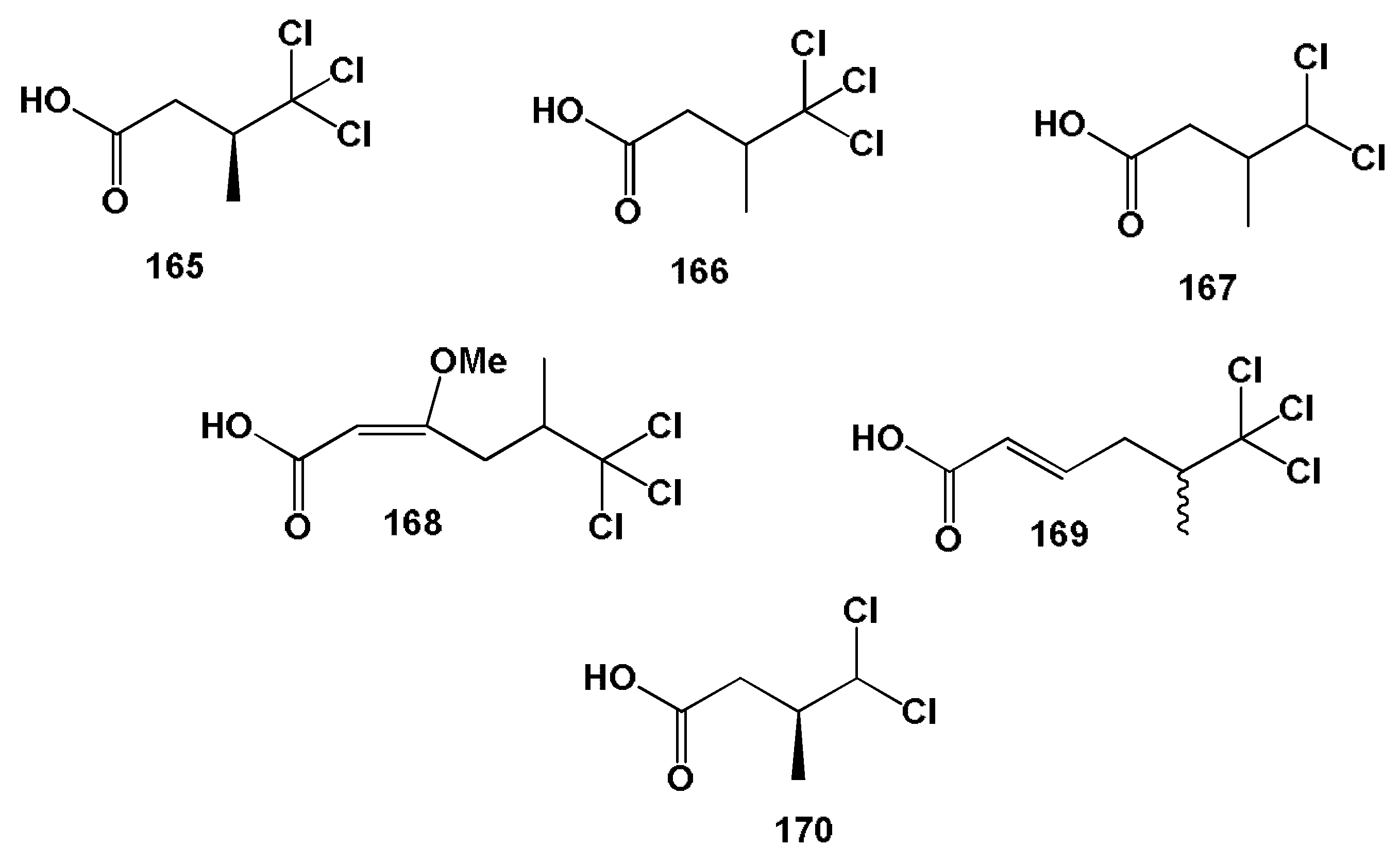
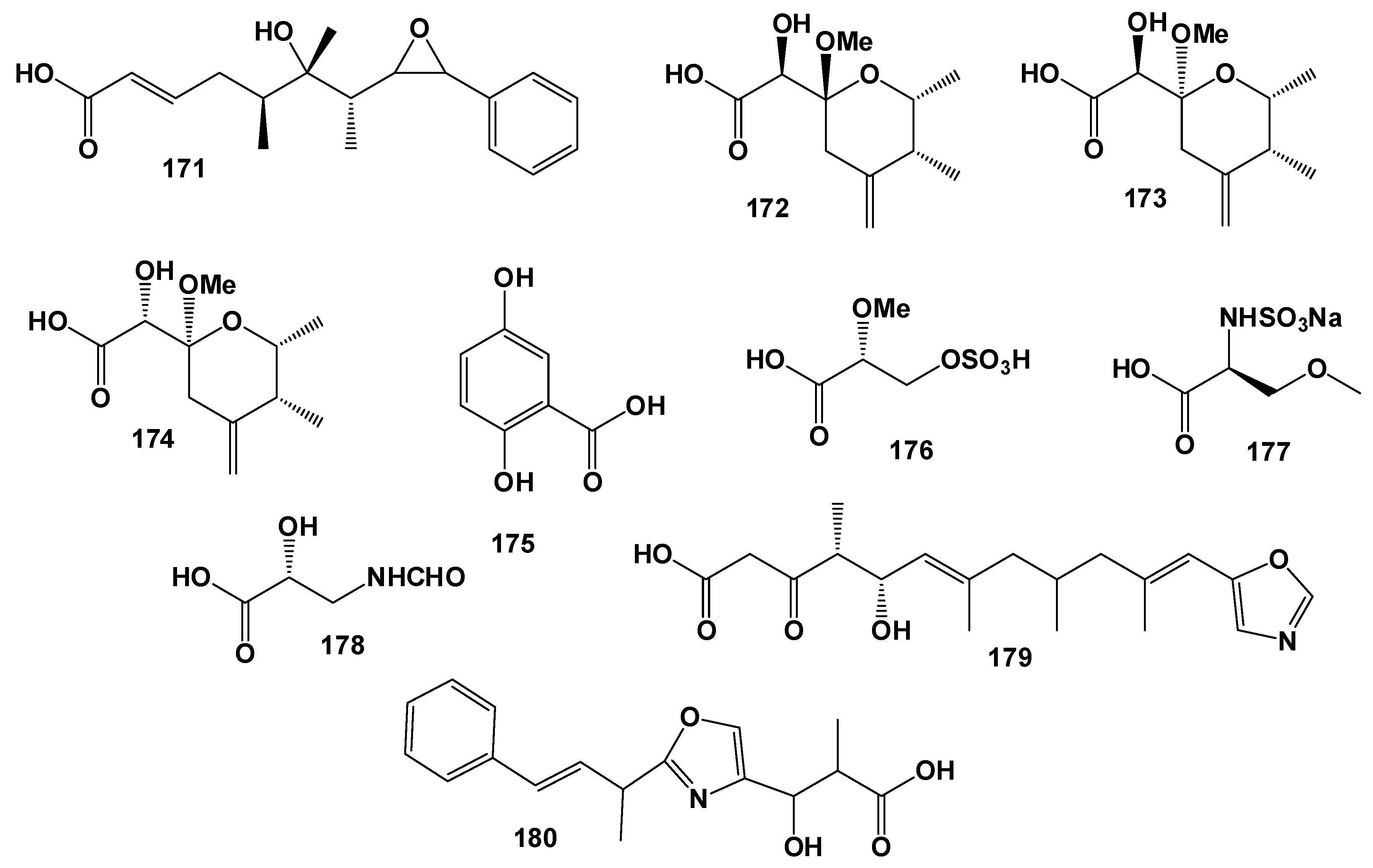
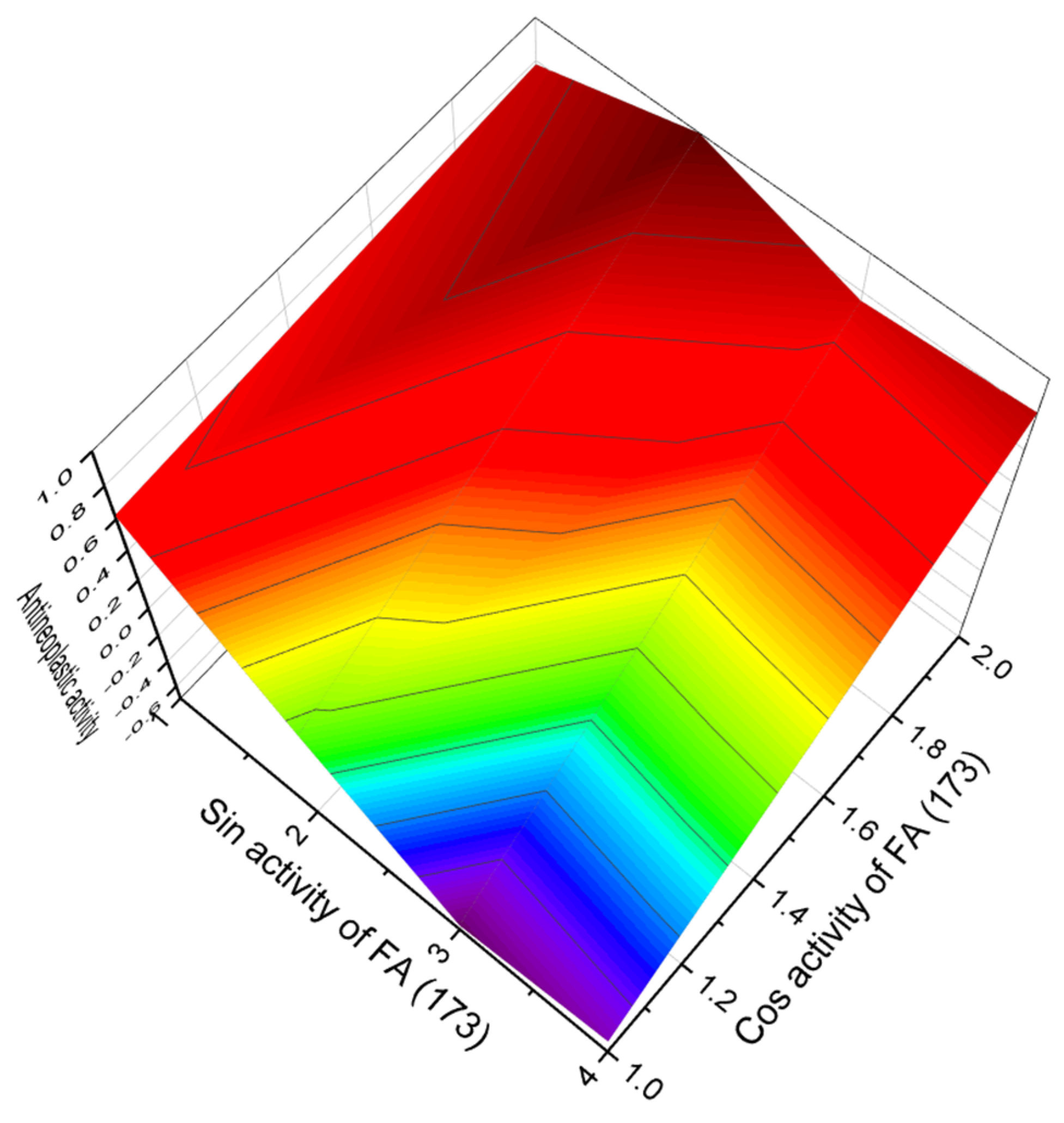
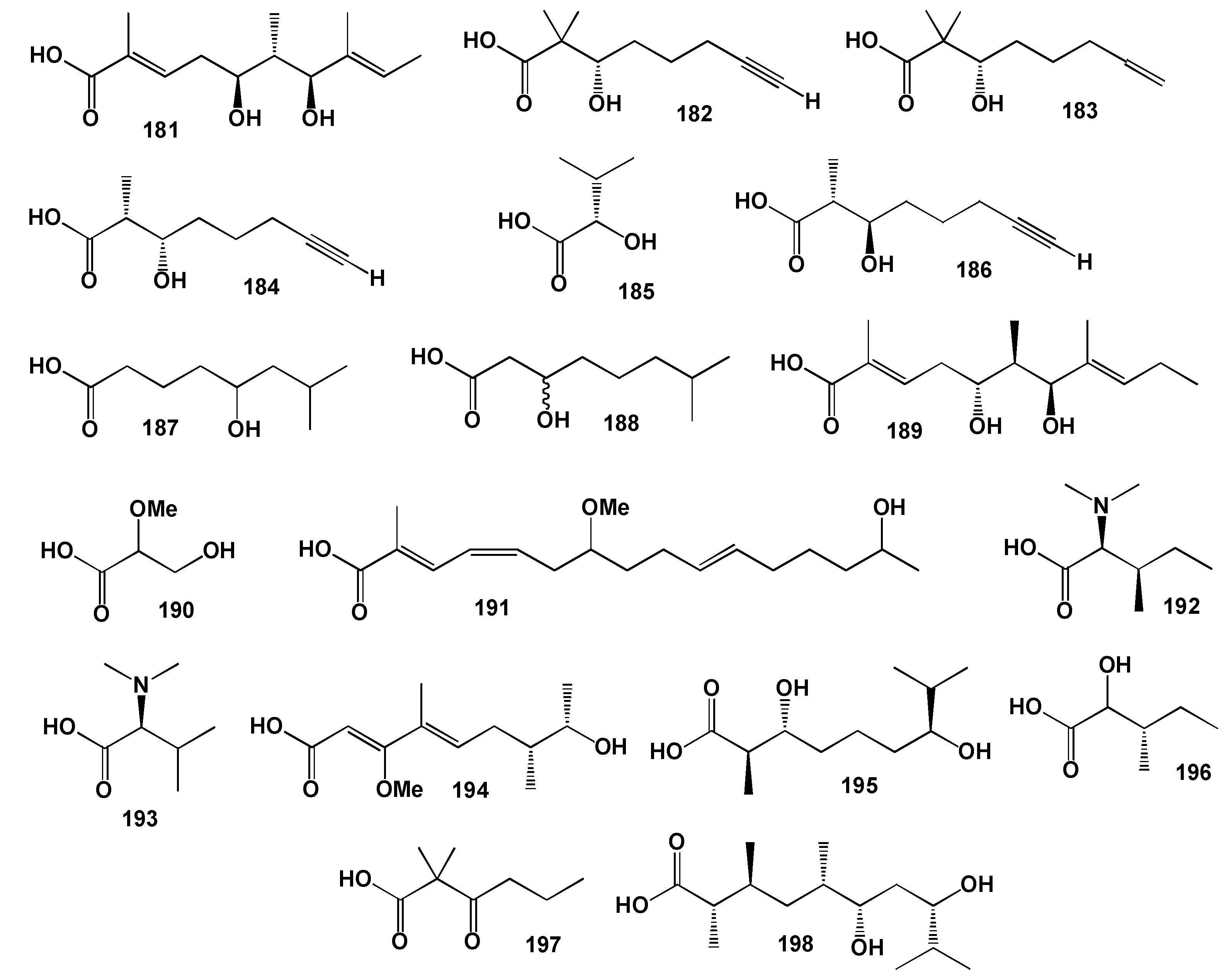

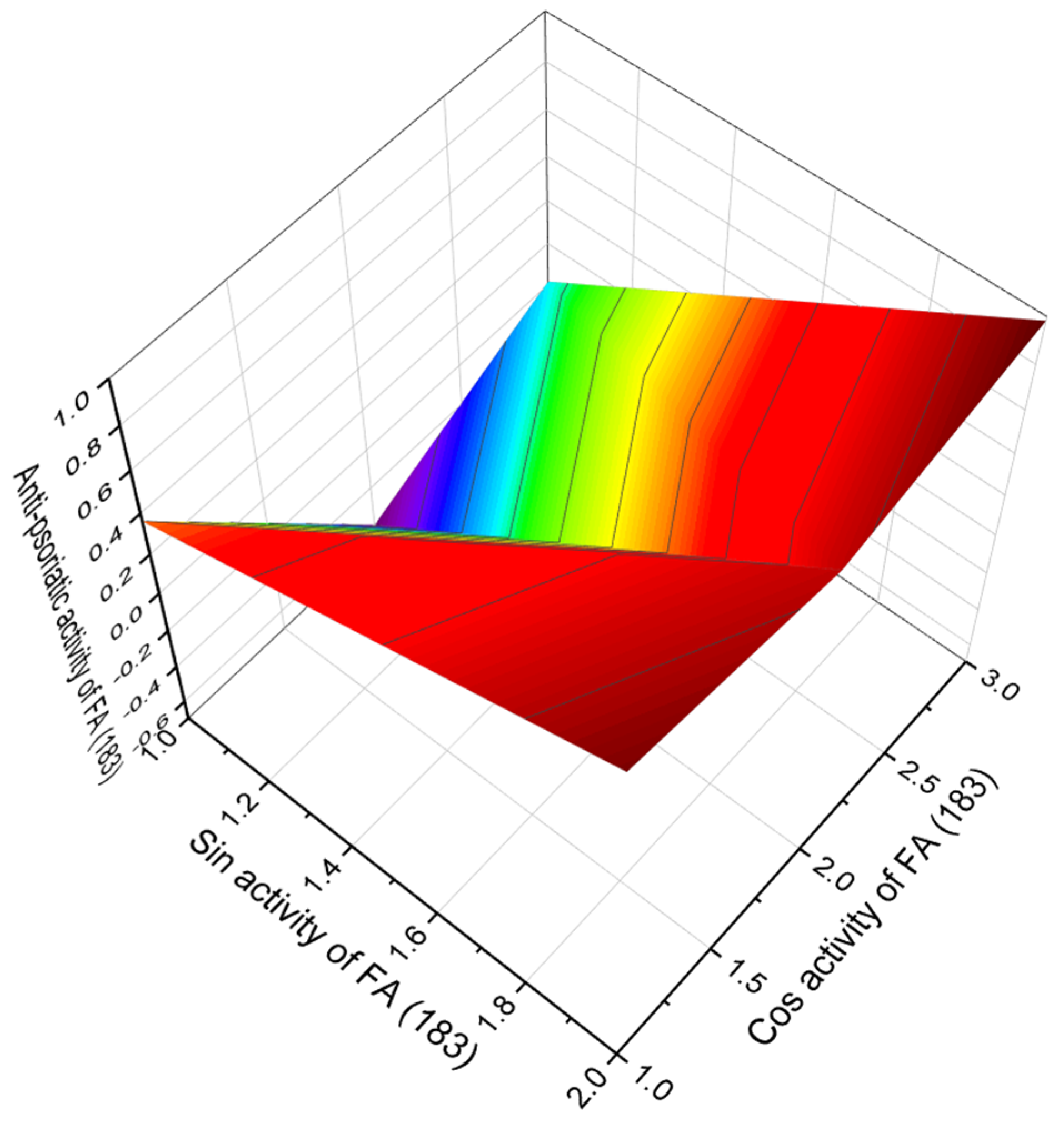
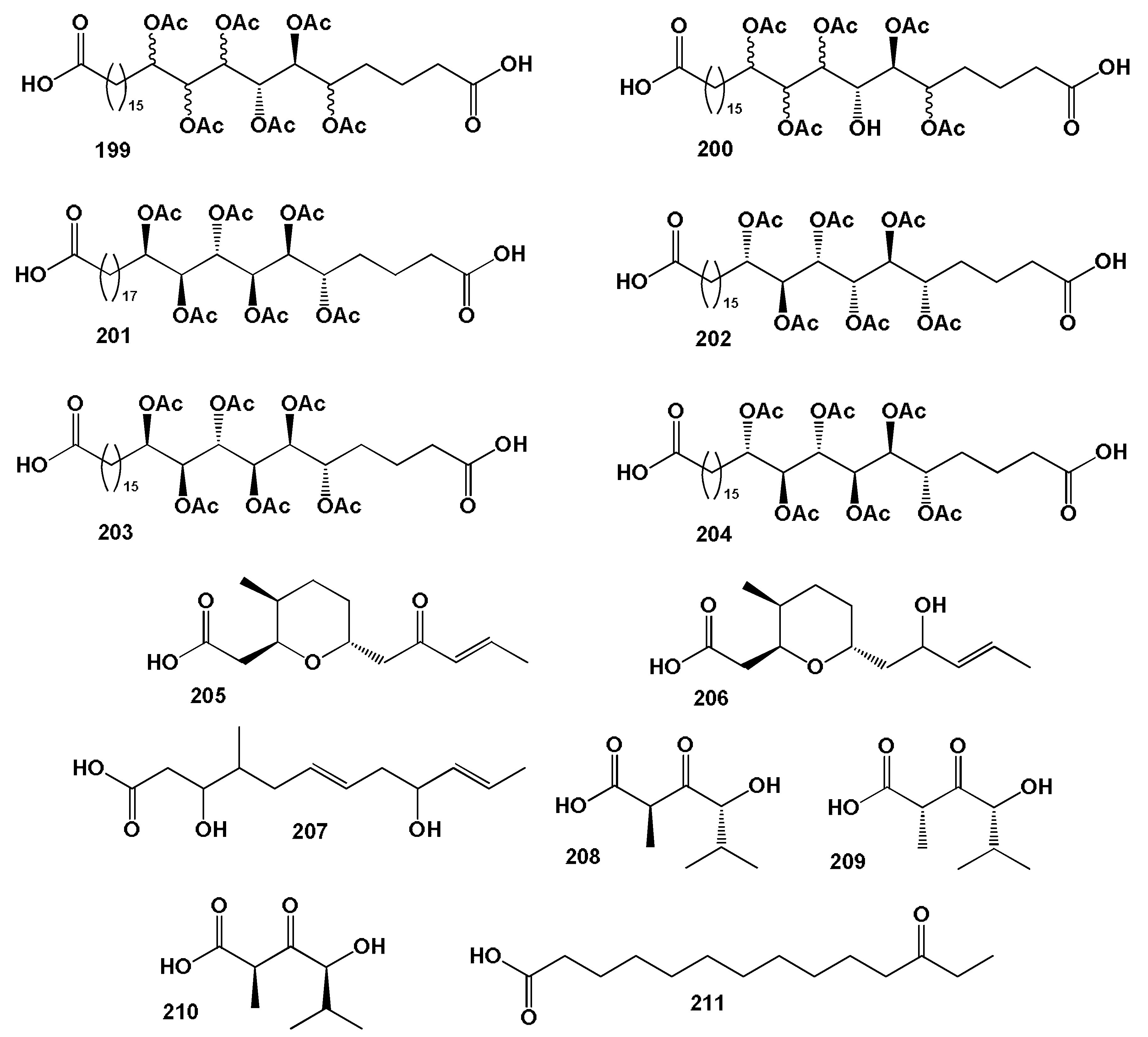
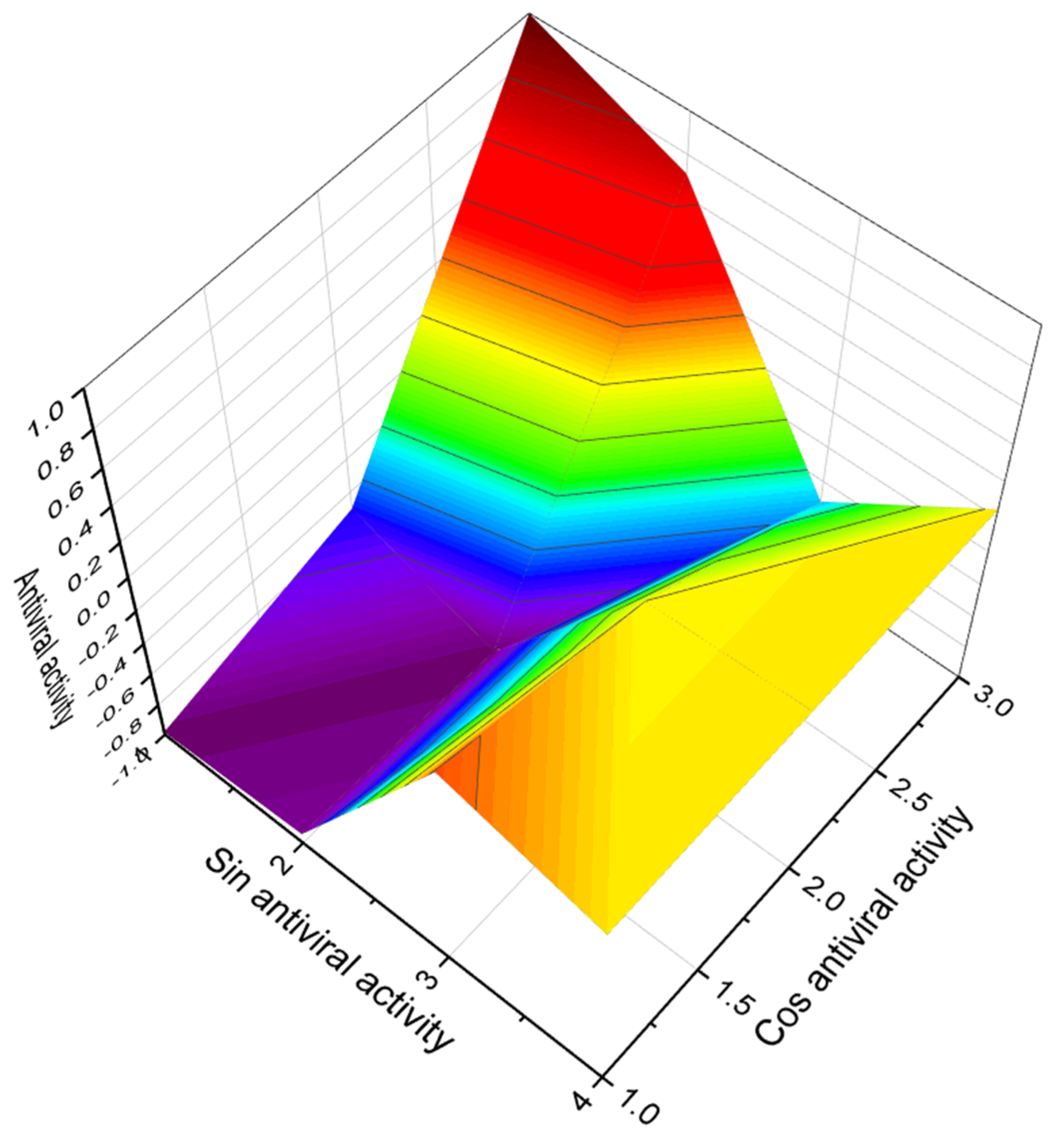
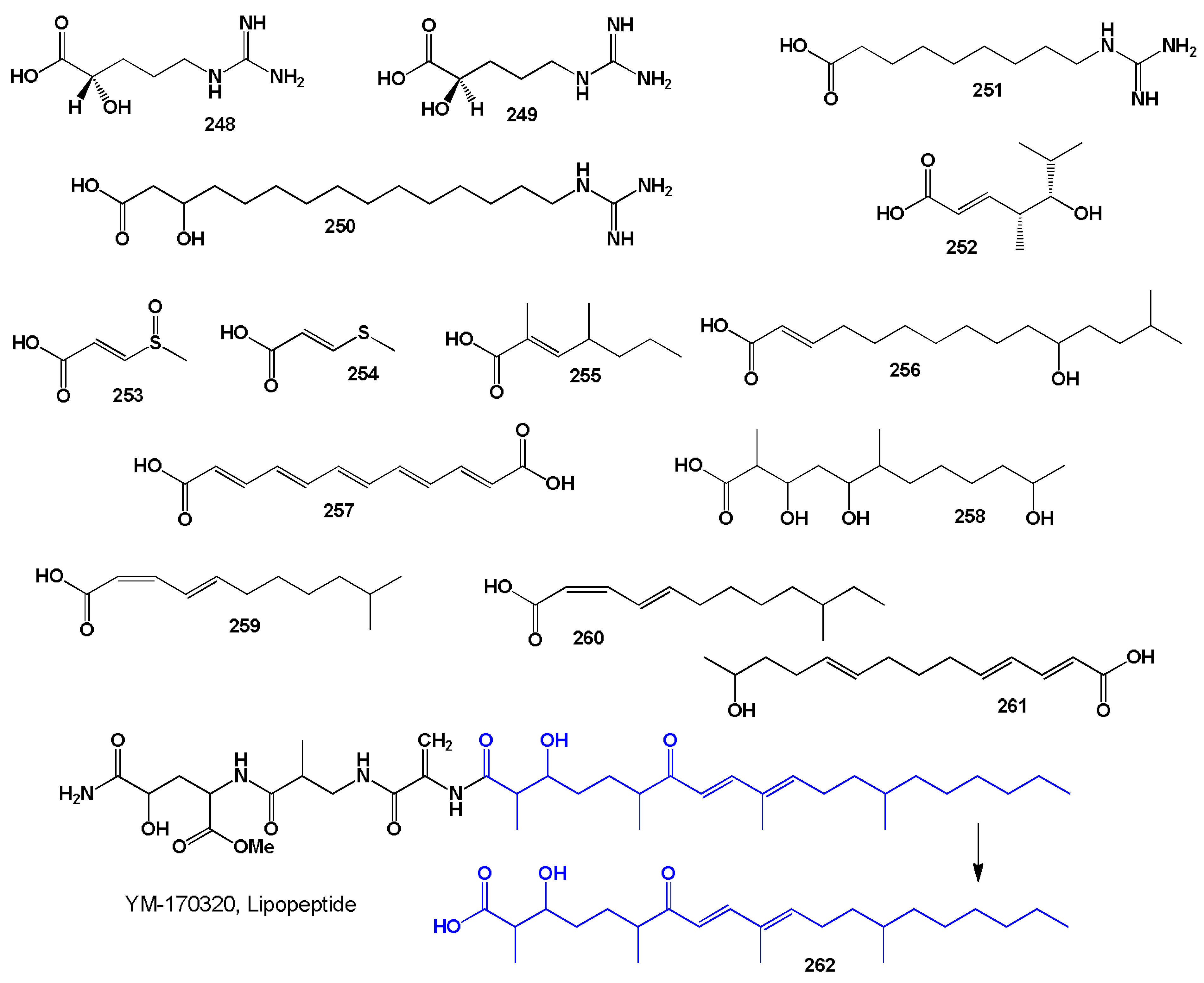
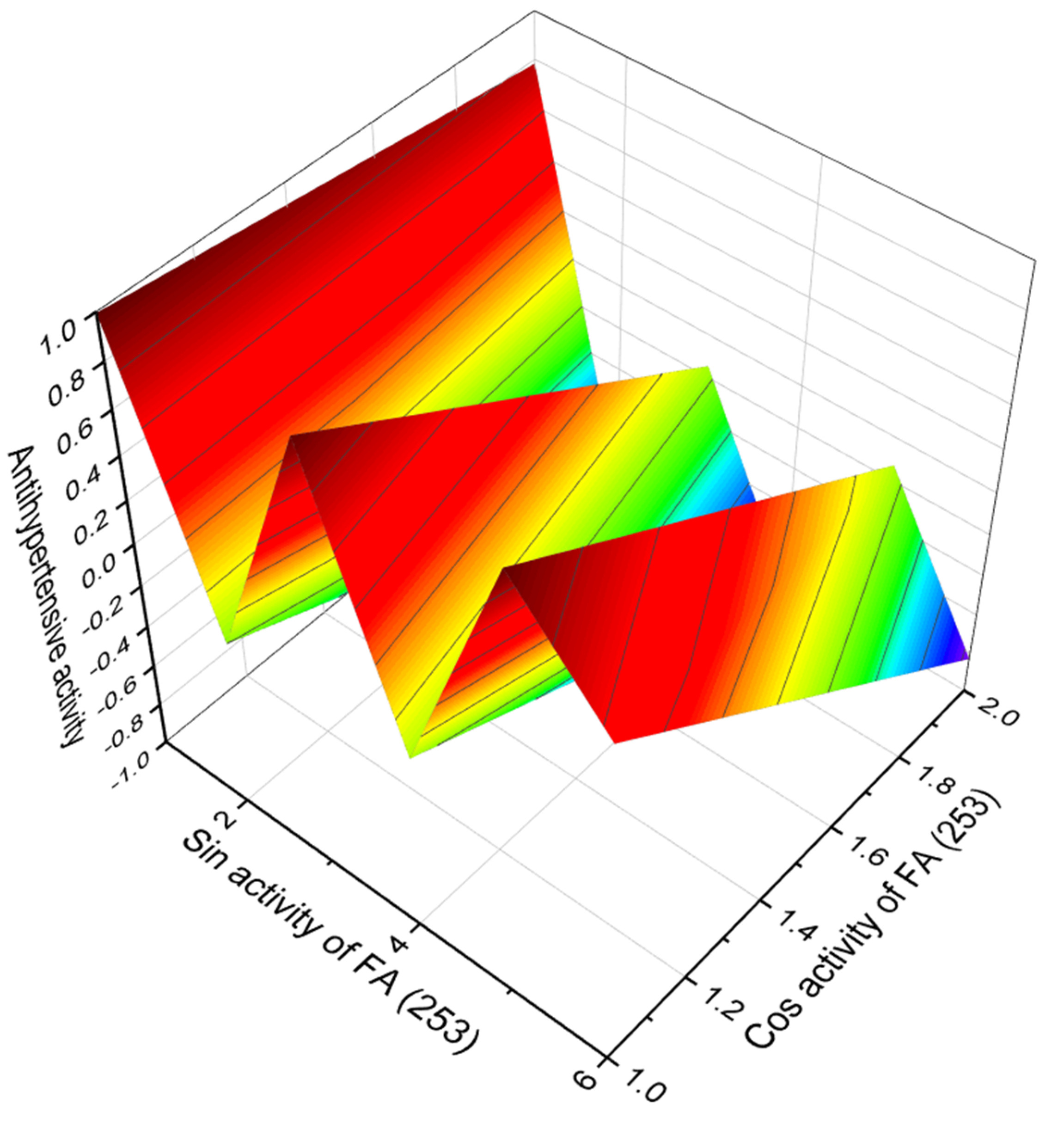
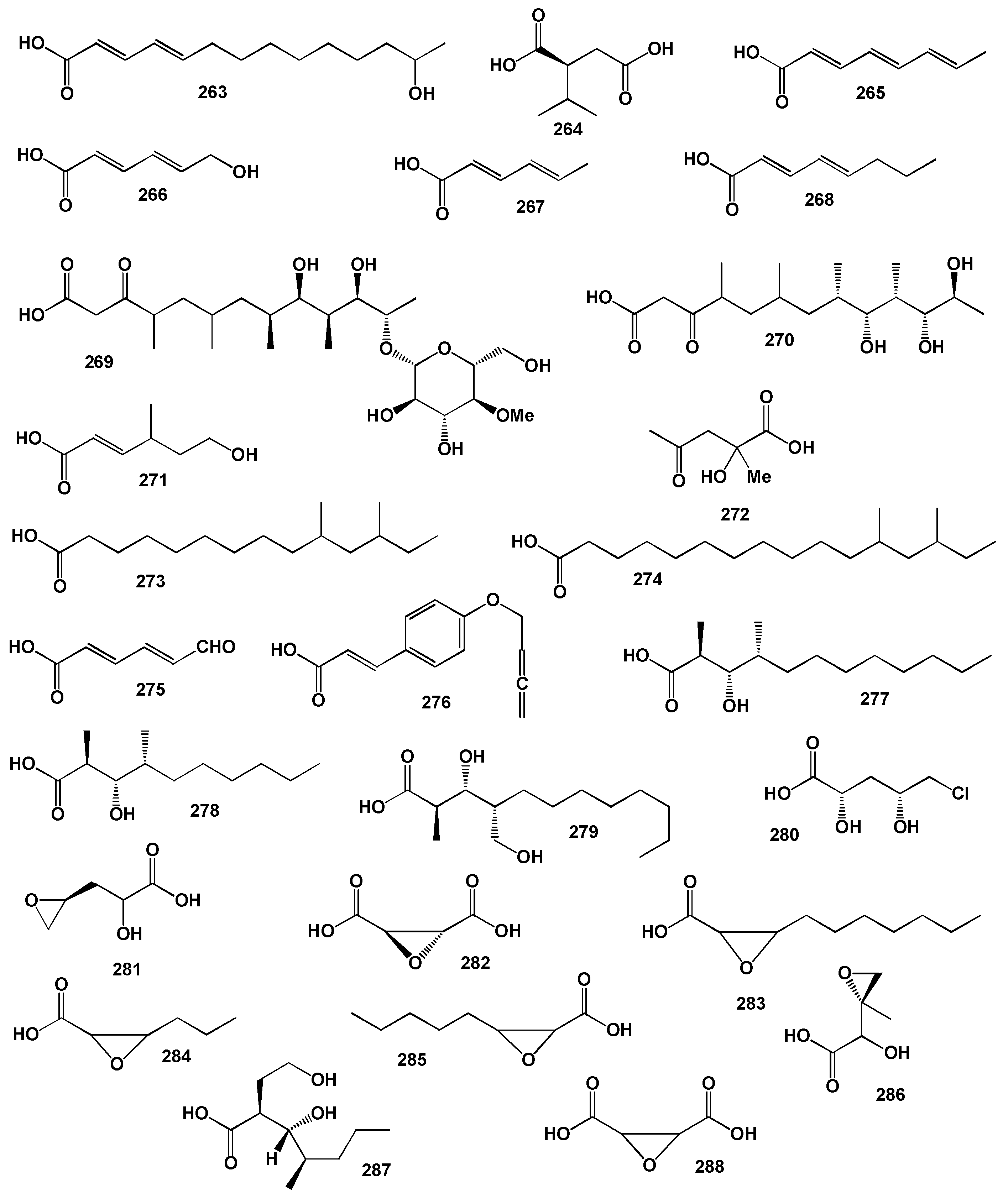
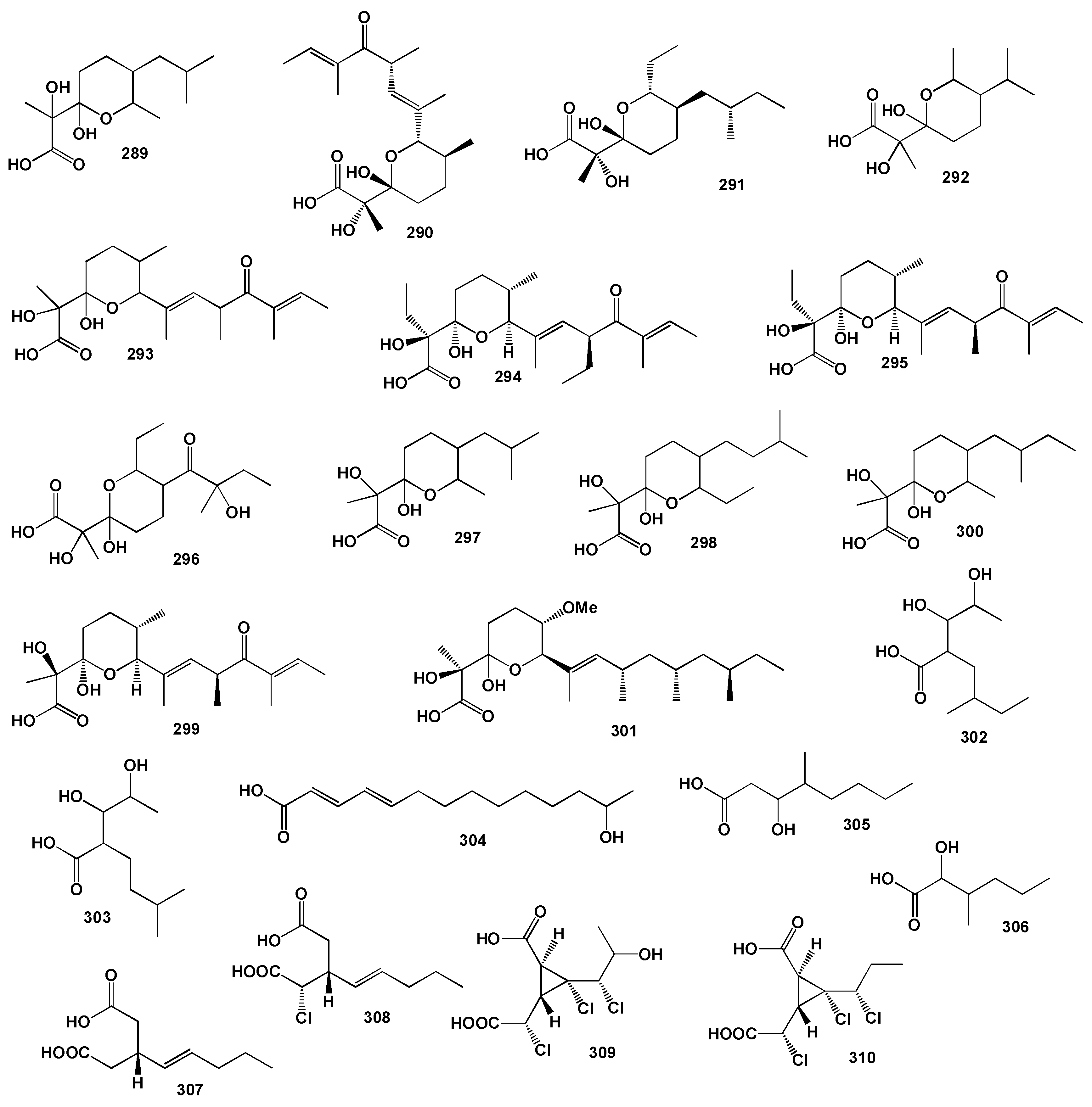
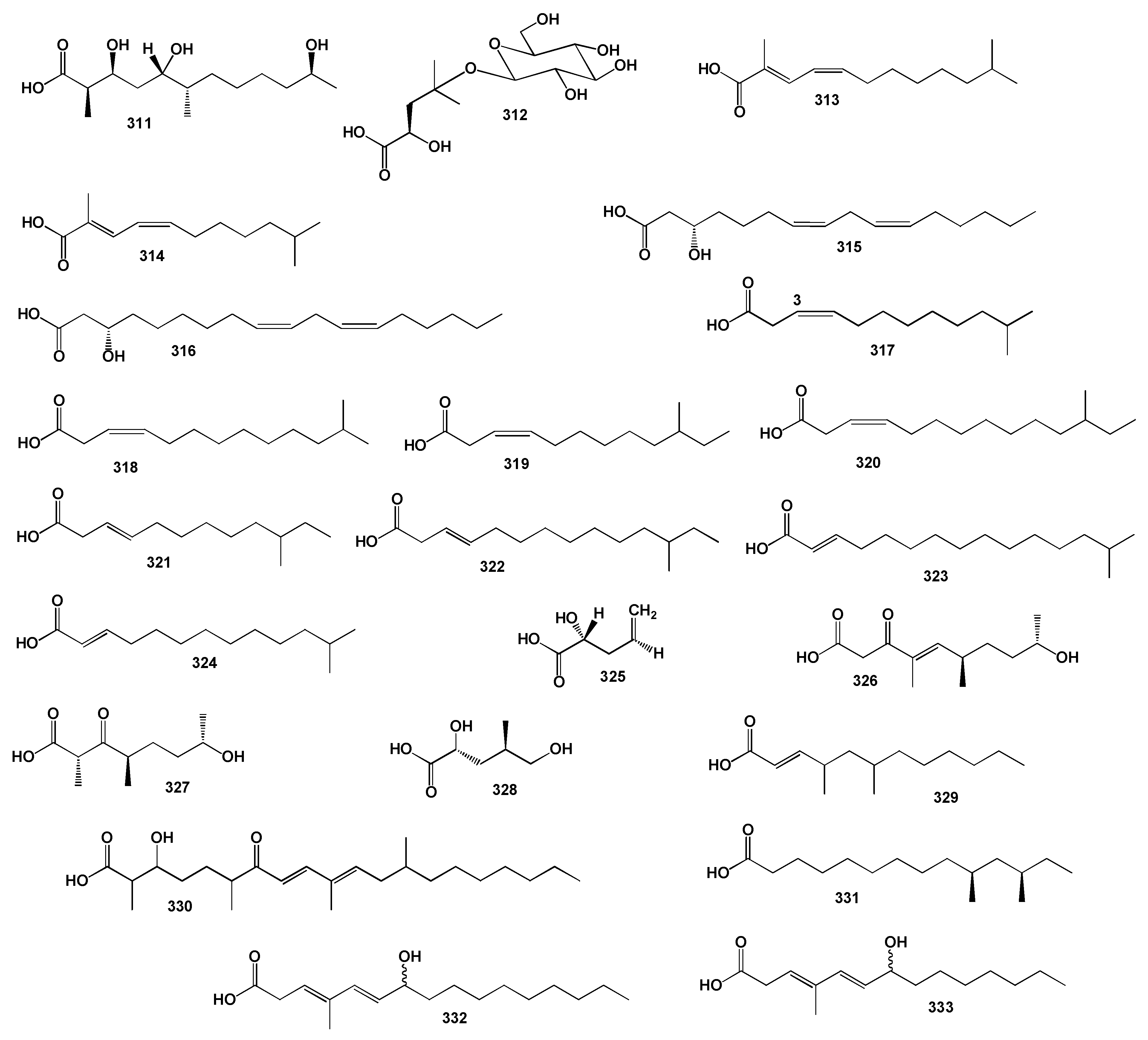



| Fragment Name | Predicted Biological Activity, Pa * | Reported Activity [46,47,48,49] |
|---|---|---|
| Cryptophycin-1 | Antifungal (0.845) Antimitotic (0.784) Antineoplastic (0.771) Antineoplastic (solid tumors) (0.631) Apoptosis agonist (0.625) | Antifungal Anticancer Apoptosis |
| Subunit A (1) | Antineoplastic (0.856) Antileukemic (0.783) Antiviral (Arbovirus) (0.775) Antifungal (0.773) Cytoprotectant (0.675) Apoptosis agonist (0.669) Fibrinolytic (0.664) Antimitotic (0.634) Antithrombotic (0.624) | No published data |
| Subunit B | Preneoplastic conditions treatment (0.836) Antiviral (Arbovirus) (0.774) Acute neurologic disorders treatment (0.759) Antiviral (Picornavirus) (0.715) | No published data |
| Subunit C | Fibrinolytic (0.814) Preneoplastic conditions treatment (0.803) Antiviral (Arbovirus) (0.760) Antimutagenic (0.737) Anticonvulsant (0.676) Antiviral (Picornavirus) (0.662) | No published data |
| Subunit D | Anti-ischemic, cerebral (0.921) Sclerosant (0.871) Antihypertensive (0.756) Anti-hypoxic (0.741) Antiviral (Arbovirus) (0.740) | No published data |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 2 | Antiviral (Arbovirus) (0.780); Antineoplastic (0.766); Antifungal (0.707) Lipid metabolism regulator (0.705); Hypolipemic (0.694) Preneoplastic conditions treatment (0.667); Apoptosis agonist (0.664) |
| 3 | Antiviral (Arbovirus) (0.804); Antineoplastic (0.795); Antidiabetic (0.701) Antifungal (0.695); Anti-inflammatory (0.680); Cytoprotectant (0.661) Immunosuppressant (0.634); Anti-hypercholesterolemic (0.629) Antithrombotic (0.611) |
| 4 | Antiviral (Arbovirus) (0.833); Lipid metabolism regulator (0.827) Anti-inflammatory (0.765); Hypolipemic (0.759); Cytoprotectant (0.729) Anti-hypercholesterolemic (0.715); Antineoplastic (0.697) |
| 5 | Antineoplastic (0.850); Antileukemic (0.781); Antifungal (0.727) Anti-hypoxic (0.702); Antiviral (Arbovirus) (0.683); Cytoprotectant (0.638) |
| 6 | Antineoplastic (0.764); Apoptosis agonist (0.762) Antiviral (Arbovirus) (0.728); Antimitotic (0.664); Antifungal (0.560) |
| 7 | Antiviral (Arbovirus) (0.730); Antifungal (0.658); Antineoplastic (0.611) |
| 8 | Antineoplastic (0.776); Antifungal (0.694); Anti-helmintic (0.691) Antidiabetic (0.660); Acute neurologic disorders treatment (0.625) Antibacterial (0.624); Antiviral (Arbovirus) (0.621) Antiviral (Picornavirus) (0.608) |
| 9 | Antineoplastic (0.856); Antileukemic (0.783); Antiviral (Arbovirus) (0.775) Antifungal (0.773); Anti-hypercholesterolemic (0.742); Apoptosis agonist (0.669) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 10 | Neuroprotector (0.806); Sclerosant (0.779); Anticonvulsant (0.734) Acute neurologic disorders treatment (0.684); Anti-inflammatory (0.681) Antineoplastic (0.631); Preneoplastic conditions treatment (0.628); Anti-neurogenic pain (0.610) |
| 11 | Neuroprotector (0.827); Antineoplastic (0.708); Preneoplastic conditions treatment (0.672) Anticonvulsant (0.672); Antiviral (Arbovirus) (0.647); Psychostimulant (0.643) |
| 12 | Neuroprotector (0.806); Sclerosant (0.779); Acute neurologic disorders treatment (0.684) Antineoplastic (0.631); Preneoplastic conditions treatment (0.628) |
| 13 | Anticonvulsant (0.797); Hypolipemic (0.762); Acute neurologic disorders treatment (0.759) Neuroprotector (0.739); Sclerosant (0.727); Antineoplastic (0.638) |
| 14 | Sclerosant (0.767); Neuroprotector (0.748); Antineoplastic (0.666) Acute neurologic disorders treatment (0.645); Antiviral (Arbovirus) (0.595) |
| 15 | Anti-psoriatic (0.923); Antineoplastic (0.883); Neuroprotector (0.675) Antiviral (Arbovirus) (0.635); Neurodegenerative diseases treatment (0.620) Alzheimer’s disease treatment (0.591) |
| 16 | Sclerosant (0.767); Antifungal (0.735); Antineoplastic (0.730) |
| 17 | Antineoplastic (0.812); Anti-inflammatory (0.763); Apoptosis agonist (0.691) |
| 18 | Sclerosant (0.767); Antineoplastic (0.666); Acute neurologic disorders treatment (0.645) |
| 19 | Acute neurologic disorders treatment (0.795); Sclerosant (0.754) Lipid metabolism regulator (0.749); Antiviral (Arbovirus) (0.704) |
| 20 | Sclerosant (0.910); Antiviral (Arbovirus) (0.784); Acute neurologic disorders treatment (0.747) Preneoplastic conditions treatment (0.714); Lipid metabolism regulator (0.667) |
| 21 | Antineoplastic (0.758); Neuroprotector (0.752); Antiviral (Arbovirus) (0.636) |
| 22 | Neuroprotector (0.756); Periodontitis treatment (0.744); Antineoplastic (0.680) Preneoplastic conditions treatment (0.655); Psychostimulant (0.545) |
| 23 | Antineoplastic (0.765); Neuroprotector (0.724); Lipid metabolism regulator (0.720) Apoptosis agonist (0.625); Acute neurologic disorders treatment (0.566) |
| 24 | Neuroprotector (0.800); Antineoplastic (0.738); Anticonvulsant (0.678) |
| 25 | Anti-psoriatic (0.924); Lipid metabolism regulator (0.889); Antineoplastic (0.867) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 26 | Antineoplastic (0.855); Lipid metabolism regulator (0.842) Apoptosis agonist (0.803) |
| 27 | Sclerosant (0.871); Acute neurologic disorders treatment (0.870) Antineoplastic (0.714) |
| 28 | Antineoplastic (0.906); Hypolipemic (0.902); Lipid metabolism regulator (0.879) Apoptosis agonist (0.811); Acute neurologic disorders treatment (0.670) Atherosclerosis treatment (0.662); Proliferative diseases treatment (0.562) |
| 29 | Cholesterol antagonist (0.649); Antianginal (0.628) Lipid metabolism regulator (0.532) |
| 30 | Cholesterol antagonist (0.649); Antianginal (0.628) Lipid metabolism regulator (0.532) |
| 31 | Iron antagonist (0.952); Antineoplastic (0.736) Microtubule formation stimulant (0.578) |
| 32 | Neurodegenerative diseases treatment (0.688); Bone diseases treatment (0.528) |
| 33 | Dermatologic (0.667); Anti-psoriatic (0.663) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 34 | Hypolipemic (0.921); Anti-psoriatic (0.913); Antidiabetic (0.897); Antineoplastic (0.799); Anti-obesity (0.783); Antihypertriglyceridemic (0.766); Lipid metabolism regulator (0.633) |
| 35 | Hypolipemic (0.910); Antidiabetic (0.902); Antihypertriglyceridemic (0.761) |
| 36 | Sclerosant (0.741); Inflammatory Bowel disease treatment (0.607); Antibacterial (0.588) |
| 37 | Antineoplastic (0.880); Hypolipemic (0.858); Lipid metabolism regulator (0.608) |
| 38 | Antineoplastic (0.822); Hypolipemic (0.801); Lipid metabolism regulator (0.700) |
| 39 | Hypolipemic (0.890); Lipid metabolism regulator (0.813); Anti-hypercholesterolemic (0.704) Atherosclerosis treatment (0.657); Cholesterol synthesis inhibitor (0.542) |
| 40 | Hypolipemic (0.858); Antineoplastic (0.810); Lipid metabolism regulator (0.608) |
| 41 | Antineoplastic (0.897); Antifungal (0.787); Apoptosis agonist (0.696) |
| 42 | Antineoplastic (0.869); Antifungal (0.728); Apoptosis agonist (0.650) |
| 43 | Antineoplastic (0.895); Antifungal (0.802); Apoptosis agonist (0.734) |
| 44 | Antineoplastic (0.854); Antifungal (0.801); Antibacterial (0.708); Apoptosis agonist (0.653) |
| A | Hypolipemic (0.910); Antidiabetic (0.902); Antihypertriglyceridemic (0.761) |
| B | Anti-ischemic, cerebral (0.756); Neuroprotector (0.718); Antiviral (Arbovirus) (0.687); Genital warts treatment (0.648); Antineoplastic (liver cancer) (0.582); Antimetastatic (0.540) |
| C | Antiviral (Arbovirus) (0.732); Neuroprotector (0.726); Antineoplastic (liver cancer) (0.633); Acute neurologic disorders treatment (0.568); Antimitotic (0.567) |
| D | Antineoplastic (liver cancer) (0.923); Antineoplastic (0.685); Anti-ischemic, cerebral (0.664) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 45 | Lipid metabolism regulator (0.928); Anti-hypercholesterolemic (0.738) Preneoplastic conditions treatment (0.722); Antihypoxic (0.711) Hypolipemic (0.676) |
| 46 | Psychostimulant (0.768); Antiviral (0.766) Preneoplastic conditions treatment (0.678) |
| 47 | Psychostimulant (0.731); Antiviral (0.731) Preneoplastic conditions treatment (0.718) |
| 48 | Lipid metabolism regulator (0.885); Hypolipemic (0.772) Anti-hypercholesterolemic (0.735) |
| 49 | Lipid metabolism regulator (0.843); Preneoplastic conditions treatment (0.832) Antimutagenic (0.832); Acute neurologic disorders treatment (0.691) |
| 50 | Fibrinolytic (0.893); Preneoplastic conditions treatment (0.804) |
| 51 | Antidiabetic (0.886); Inflammatory Bowel disease treatment (0.852) |
| 52 | Antidiabetic (0.886); Inflammatory Bowel disease treatment (0.852) |
| 53 | Antidiabetic (0.886); Inflammatory Bowel disease treatment (0.852) |
| 54 | Antidiabetic (0.916); Antineoplastic (0.831); Immunosuppressant (0.681) |
| 55 | Hypolipemic (0.790); Lipid metabolism regulator (0.738) Atherosclerosis treatment (0.679) |
| 56 | Lipid metabolism regulator (0.908); Hypolipemic (0.881) Anti-hypercholesterolemic (0.765) Acute neurologic disorders treatment (0.734); Atherosclerosis treatment (0.722) |
| 57 | Antineoplastic (0.834); Lipid metabolism regulator (0.831) Apoptosis agonist (0.818) Acute neurologic disorders treatment (0.795); Hypolipemic (0.725); Atherosclerosis treatment (0.617) |
| 58 | Antineoplastic (0.703); Lipid metabolism regulator (0.678) Antiviral (Arbovirus) (0.643) |
| 59 | Lipid metabolism regulator (0.886); Hypolipemic (0.823) Anti-hypercholesterolemic (0.742) |
| 60 | Anti-infective (0.877); Lipid metabolism regulator (0.866) Antiviral (Arbovirus) (0.827) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 61 | Hypolipemic (0.861); Antineoplastic (0.854); Lipid metabolism regulator (0.849) |
| 62 | Lipid metabolism regulator (0.934); Hypolipemic (0.903); Sclerosant (0.869) Acute neurologic disorders treatment (0.845); Anti-hypercholesterolemic (0.831) Atherosclerosis treatment (0.705); Cholesterol synthesis inhibitor (0.519) |
| 63 | Lipid metabolism regulator (0.959); Anti-hypercholesterolemic (0.900); Hypolipemic (0.895) Acute neurologic disorders treatment (0.893); Atherosclerosis treatment (0.696) Multiple sclerosis treatment (0.548); Antibacterial (0.537); Cholesterol synthesis inhibitor (0.526) |
| 64 | Hypolipemic (0.878); Acute neurologic disorders treatment (0.780) Lipid metabolism regulator (0.771); Atherosclerosis treatment (0.675) |
| 65 | Lipid metabolism regulator (0.941); Hypolipemic (0.829); Anti-hypercholesterolemic (0.792) Acute neurologic disorder treatment (0.697); Atherosclerosis treatment (0.647) |
| 66 | Antiviral (Arbovirus) (0.947); Lipid metabolism regulator (0.884); Antimutagenic (0.793) Antiviral (Picornavirus) (0.782); Hypolipemic (0.724); Anti-hypercholesterolemic (0.695) |
| 67 | Hypolipemic (0.835); Lipid metabolism regulator (0.830); Antiviral (Arbovirus) (0.821) Antiviral (Picornavirus) (0.761); Preneoplastic conditions treatment (0.722) |
| 68 | Lipid metabolism regulator (0.953); Antiviral (Arbovirus) (0.885) Hypolipemic (0.884); Anti-hypercholesterolemic (0.856); Leukopoiesis stimulant (0.826); Atherosclerosis treatment (0.692) |
| 69 | Lipid metabolism regulator (0.934); Hypolipemic (0.903); Anti-hypercholesterolemic (0.831) Atherosclerosis treatment (0.705); Cholesterol synthesis inhibitor (0.519) |
| 70 | Lipid metabolism regulator (0.934); Hypolipemic (0.903); Anti-hypercholesterolemic (0.831) Atherosclerosis treatment (0.705); Cholesterol synthesis inhibitor (0.519) |
| 71 | Lipid metabolism regulator (0.934); Hypolipemic (0.903); Anti-hypercholesterolemic (0.831) Atherosclerosis treatment (0.705); Cholesterol synthesis inhibitor (0.519) |
| 72 | Vasoprotector (0.890); Lipid metabolism regulator (0.884); Antiviral (Arbovirus) (0.824) |
| 73 | Antipsoriatic (0.957); Antineoplastic (0.886); Antiviral (Arbovirus) (0.796) Lipid metabolism regulator (0.792); Alzheimer’s disease treatment (0.613) |
| 74 | Lipid metabolism regulator (0.929); Antiviral (Arbovirus) (0.891); Antimutagenic (0.866) Anti-hypercholesterolemic (0.786); Hypolipemic (0.721); Atherosclerosis treatment (0.629) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 75 | Antifungal (0.791); Antineoplastic (0.744) Lipid metabolism regulator (0.680) |
| 76 | Antieczematic (0.869); Anesthetic general (0.722) Neuroprotector (0.714) |
| 77 | Antieczematic (0.900); Lipid metabolism regulator (0.859) Antifungal (0.756) |
| 78 | Antineoplastic (0.960); Preneoplastic conditions treatment (0.661) Antiviral (Arbovirus) (0.618); Antiviral (Picornavirus) (0.520) |
| 79 | Cystic fibrosis treatment (0.861); Antiviral (Arbovirus) (0.717); Anesthetic general (0.702) |
| 80 | Cystic fibrosis treatment (0.850); Anesthetic general (0.733) Antiviral (Arbovirus) (0.687) |
| 81 | Anti-hypercholesterolemic (0.902); Apoptosis agonist (0.779); Antineoplastic (0.779) |
| 82 | Lipid metabolism regulator (0.888); Angiogenesis stimulant (0.869); Expectorant (0.715) |
| 83 | Acute neurologic disorders treatment (0.870); Anti-inflammatory (0.776) |
| 84 | Acute neurologic disorders treatment (0.870); Anti-inflammatory (0.776) |
| 85 | Lipid metabolism regulator (0.930); Anti-hypercholesterolemic (0.842) Hypolipemic (0.830) |
| 86 | Lipid metabolism regulator (0.848); Anti-hypercholesterolemic (0.770) Hypolipemic (0.760) |
| 87 | Lipid metabolism regulator (0.848); Anti-hypercholesterolemic (0.770) |
| 88 | Lipid metabolism regulator (0.930); Anti-hypercholesterolemic (0.842) Hypolipemic (0.830) |
| 89 | Lipid metabolism regulator (0.848); Anti-hypercholesterolemic (0.770) Hypolipemic (0.760) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 90 | Lipid metabolism regulator (0.930); Anti-hypercholesterolemic (0.842) Hypolipemic (0.830) Atherosclerosis treatment (0.635); Multiple sclerosis treatment (0.507) |
| 91 | Lipid metabolism regulator (0.848); Anti-hypercholesterolemic (0.770); Hypolipemic (0.760) Acute neurologic disorders treatment (0.696); Atherosclerosis treatment (0.551) |
| 92 | Lipid metabolism regulator (0.848); Anti-hypercholesterolemic (0.770); Hypolipemic (0.760) Acute neurologic disorders treatment (0.696); Atherosclerosis treatment (0.551) |
| 93 | Lipid metabolism regulator (0.930); Anti-hypercholesterolemic (0.842); Hypolipemic (0.830) Acute neurologic disorders treatment (0.690); Atherosclerosis treatment (0.635) |
| 94 | Antineoplastic (0.859); Apoptosis agonist (0.765); Antimitotic (0.689) |
| 95 | Antineoplastic (0.778); Apoptosis agonist (0.724); Antiviral (Arbovirus) (0.695) Preneoplastic conditions treatment (0.551) |
| 96 | Antineoplastic (0.800); Apoptosis agonist (0.794); Lipid metabolism regulator (0.780) Antifungal (0.750); Preneoplastic conditions treatment (0.622); Spasmolytic (0.540) |
| 97 | Antineoplastic (0.877); Apoptosis agonist (0.818); Hypolipemic (0.809); Antifungal (0.775) Anti-inflammatory (0.748); Preneoplastic conditions treatment (0.540) |
| 98 | Antineoplastic (0.881); Apoptosis agonist (0.814); Hypolipemic (0.810) Antifungal (0.767); Anti-inflammatory (0.746); Lipid metabolism regulator (0.706) |
| 99 | Antineoplastic (0.871); Hypolipemic (0.815); Antifungal (0.775); Apoptosis agonist (0.764) Anti-inflammatory (0.729); Lipid metabolism regulator (0.643) |
| 100 | Antineoplastic (0.812); Anti-inflammatory (0.763); Immunosuppressant (0.712) Antifungal (0.695); Apoptosis agonist (0.691); Hypolipemic (0.667) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 101 | Anti-hypercholesterolemic (0.936); Antifungal (0.848); Antibacterial (0.798) Hypolipemic (0.723); Anti-infective (0.718); Atherosclerosis treatment (0.522) |
| 102 | Anti-infective (0.936); Anti-hypercholesterolemic (0.928); Antifungal (0.853) Antioxidant (0.848); Antineoplastic (0.833); Antidiabetic (0.807) Antibacterial (0.774); Hypolipemic (0.774) Acute neurologic disorders treatment (0.702) Proliferative diseases treatment (0.690); Atherosclerosis treatment (0.603) |
| 103 | Vasoprotector (0.970); Anti-infective (0.966); Hemostatic (0.950) Neuroprotector (0.942) Anti-hypercholesterolemic (0.915); Lipid metabolism regulator (0.903) Acute neurologic disorders treatment (0.845); Hypolipemic (0.770) Atherosclerosis treatment (0.624); DNA synthesis inhibitor (0.584) Dementia treatment (0.582) |
| 104 | Anti-infective (0.961); Vasoprotector (0.960); Neuroprotector (0.905) Anti-hypercholesterolemic (0.875); Antithrombotic (0.850) Hypolipemic (0.757); Atherosclerosis treatment (0.627); DNA synthesis inhibitor (0.590) |
| 105 | Anti-infective (0.961); Vasoprotector (0.960); Neuroprotector (0.905); Sclerosant (0.891) Anti-hypercholesterolemic (0.875); Antithrombotic (0.850); Lipid metabolism regulator (0.807) Hypolipemic (0.757); Atherosclerosis treatment (0.627); DNA synthesis inhibitor (0.590) |
| 106 | Anti-infective (0.966); Vasoprotector (0.953); Anti-hypercholesterolemic (0.890) Antihypoxic (0.881); Lipid metabolism regulator (0.853); Antineoplastic (0.835) Hypolipemic (0.751); Acute neurologic disorders treatment (0.684); DNA synthesis inhibitor (0.566) |
| 107 | Vasodilator (0.868); Anti-infective (0.865); Antifungal (0.829); Antineoplastic (0.809) Anti-hypercholesterolemic (0.609); Hypolipemic (0.566); Antimycobacterial (0.545) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 108 | Hypolipemic (0.858); Lipid metabolism regulator (0.835); Anti-hypercholesterolemic (0.634) Antifungal (0.648); Atherosclerosis treatment (0.603) |
| 109 | Hypolipemic (0.858); Lipid metabolism regulator (0.835); Anti-hypercholesterolemic (0.634) Antifungal (0.648); Atherosclerosis treatment (0.603) |
| 110 | Preneoplastic conditions treatment (0.779); Hypolipemic (0.775); Anesthetic general (0.772) Lipid metabolism regulator (0.768); Acute neurologic disorders treatment (0.694) |
| 111 | Preneoplastic conditions treatment (0.805); Acute neurologic disorders treatment (0.723) Antiviral (Arbovirus) (0.716); Anti-inflammatory (0.650); Antiviral (Picornavirus) (0.649) |
| 112 | Lipid metabolism regulator (0.890); Hypolipemic (0.870); Anti-hypercholesterolemic (0.802) Atherosclerosis treatment (0.692); Cholesterol synthesis inhibitor (0.511) |
| 113 | Lipid metabolism regulator (0.895); Preneoplastic conditions treatment (0.778) Anti-hypercholesterolemic (0.777); Hypolipemic (0.758); Atherosclerosis treatment (0.683) |
| 114 | Mucositis treatment (0.886); Anesthetic general (0.852); Lipid metabolism regulator (0.842) Autoimmune disorders treatment (0.798); Transplant rejection treatment (0.795) |
| 115 | Anti-ischemic, cerebral (0.943); Acute neurologic disorders treatment (0.797) Anticonvulsant (0.702); Anti-hypercholesterolemic (0.642); Antihypertensive (0.627) |
| 116 | Lipid metabolism regulator (0.822); Vasodilator, peripheral (0.803); Vasoprotector (0.793) Hypolipemic (0.757); Anti-hypercholesterolemic (0.677); Atherosclerosis treatment (0.647) |
| 117 | Lipid metabolism regulator (0.890); Hypolipemic (0.870); Anti-hypercholesterolemic (0.802) Atherosclerosis treatment (0.692); Cholesterol synthesis inhibitor (0.511) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 118 | Antineoplastic (0.883); Lipid metabolism regulator (0.836); Anti-inflammatory (0.845) Apoptosis agonist (0.847); Acute neurologic disorders treatment (0.795); Antifungal (0.793) |
| 119 | Phobic disorders treatment (0.859); Psychostimulant (0.731); Antiviral (0.731) Acute neurologic disorders treatment (0.586); Neuroprotector (0.574) |
| 120 | Antiarthritic (0.805); Preneoplastic conditions treatment (0.730); Sclerosant (0.726) Acute neurologic disorders treatment (0.696); Anti-inflammatory (0.641) |
| 121 | Antineoplastic (0.813); Antiviral (Arbovirus) (0.748); Lipid metabolism regulator (0.693) Cytoprotectant (0.668); Antiviral (Picornavirus) (0.585); Hypolipemic (0.575) |
| 122 | Lipid metabolism regulator (0.880); Antineoplastic (0.863); Hypolipemic (0.816) Anti-hypercholesterolemic (0.672); Atherosclerosis treatment (0.590) |
| 123 | Lipid metabolism regulator (0.924); Antineoplastic (0.873); Hypolipemic (0.839) Anti-hypercholesterolemic (0.642); Atherosclerosis treatment (0.592) |
| 124 | Hypolipemic (0.911); Lipid metabolism regulator (0.829); Anti-inflammatory (0.765) Anti-hypercholesterolemic (0.718); Acute neurologic disorders treatment (0.715) |
| 125 | Lipid metabolism regulator (0.929); Hypolipemic (0.908) Anti-hypercholesterolemic (0.825); Atherosclerosis treatment (0.680) |
| 126 | Antineoplastic (0.788); Hypolipemic (0.754); Acute neurologic disorders treatment (0.687) |
| 127 | Dermatologic (0.909); Anti-psoriatic (0.888); Anti-eczematic (0.856); Antifungal (0.605) |
| 128 | Anti-psoriatic (0.862); Antineoplastic (0.846); Antifungal (0.625); Anti-eczematic (0.601) |
| 129 | Hypolipemic (0.880); Antineoplastic (0.821); Antifungal (0.707); Antiviral (Arbovirus) (0.654) |
| 130 | Hypolipemic (0.880); Antineoplastic (0.821); Antifungal (0.707); Antibacterial (0.555) |
| 131 | Hypolipemic (0.880); Antineoplastic (0.821); Antifungal (0.707); Antibacterial (0.555) |
| 132 | Antifungal (0.688); Antiprotozoal (Plasmodium) (0.570); Antibacterial (0.514) |
| 133 | Antineoplastic (0.876); Antifungal (0.771); Lipid metabolism regulator (0.763); Hypolipemic (0.707) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 134 | Sclerosant (0.834); Hypolipemic (0.825); Antineoplastic (0.779); Anti-inflammatory (0.731) |
| 135 | Sclerosant (0.835); Antineoplastic (0.788); Hypolipemic (0.754); Anti-inflammatory (0.716) |
| 136 | Sclerosant (0.853); Hypolipemic (0.824); Antineoplastic (0.775); Anti-inflammatory (0.734) |
| 137 | Sclerosant (0.815); Hypolipemic (0.807); Antineoplastic (0.781); Anti-inflammatory (0.730) |
| 138 | Sclerosant (0.834); Hypolipemic (0.825); Antineoplastic (0.779); Anti-inflammatory (0.731) |
| 139 | Lipid metabolism regulator (0.903); Hypolipemic (0.848); Antineoplastic (0.805); Antifungal (0.782) |
| 140 | Sclerosant (0.834); Hypolipemic (0.825); Antineoplastic (0.779); Anti-inflammatory (0.731) |
| 141 | Restenosis treatment (0.827); Sclerosant (0.738); Neurodegenerative diseases treatment (0.722) |
| 142 | Sclerosant (0.834); Hypolipemic (0.825); Antineoplastic (0.779); Anti-inflammatory (0.731) |
| 143 | Antineoplastic (0.880); Antiviral (Arbovirus) (0.829); Apoptosis agonist (0.804) Hypolipemic (0.794); Antiprotozoal (Coccidial) (0.684); Antiviral (Picornavirus) (0.599) |
| 144 | Antineoplastic (0.885); Antiviral (Arbovirus) (0.814); Apoptosis agonist (0.800) Hypolipemic (0.794); Antiprotozoal (Coccidial) (0.621); Antiviral (Picornavirus) (0.598) |
| 145 | Anti-infective (0.934); Anti-hypercholesterolemic (0.916); Vasodilator (0.915) Antineoplastic (0.911); Vasoprotector (0.864); Lipid metabolism regulator (0.856) |
| 146 | Anti-infective (0.934); Anti-hypercholesterolemic (0.916); Vasodilator (0.915) Antineoplastic (0.911); Vasoprotector (0.864); Lipid metabolism regulator (0.856) |
| 147 | Anti-infective (0.934); Anti-hypercholesterolemic (0.916); Vasodilator (0.915) Antineoplastic (0.911); Vasoprotector (0.864); Lipid metabolism regulator (0.856) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 148 | Antineoplastic (0.752); Lipid metabolism regulator (0.715); Antiviral (Arbovirus) (0.642) |
| 149 | Antineoplastic (0.752); Lipid metabolism regulator (0.715); Antiviral (Arbovirus) (0.642) |
| 150 | Cell adhesion molecule inhibitor (0.883); Lipid metabolism regulator (0.801); Apoptosis agonist (0.752) |
| 151 | Cell adhesion molecule inhibitor (0.866); Hypolipemic (0.816); Antineoplastic (0.780) |
| 152 | Lipid metabolism regulator (0.931); Antineoplastic (0.826); Apoptosis agonist (0.634) |
| 153 | Lipid metabolism regulator (0.931); Antineoplastic (0.826); Apoptosis agonist (0.634) |
| 154 | Antineoplastic (0.813); Antiviral (Arbovirus) (0.748); Lipid metabolism regulator (0.693) |
| 156 | Antineoplastic (0.813); Antiviral (Arbovirus) (0.748); Lipid metabolism regulator (0.693) |
| 157 | Antineoplastic (0.779); Acute neurologic disorders treatment (0.681); Antiviral (Arbovirus) (0.680) |
| 158 | Sclerosant (0.815); Antineoplastic (0.781); Acute neurologic disorders treatment (0.722) |
| 159 | Sclerosant (0.834); Antineoplastic (0.799); Acute neurologic disorders treatment (0.725) |
| 160 | Sclerosant (0.835); Antineoplastic (0.795); Acute neurologic disorders treatment (0.731) |
| 161 | Sclerosant (0.815); Antineoplastic (0.781); Acute neurologic disorders treatment (0.722) |
| 162 | Sclerosant (0.835); Antineoplastic (0.781); Acute neurologic disorders treatment (0.764) |
| 163 | Sclerosant (0.815); Antineoplastic (0.781); Acute neurologic disorders treatment (0.722) |
| 164 | Antineoplastic (0.881); Hypolipemic (0.797); Antifungal (0.793); Antimitotic (0.787) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 165 | Antineoplastic (0.841); Preneoplastic conditions treatment (0.689); Antiprotozoal (0.586) |
| 166 | Antineoplastic (0.841); Preneoplastic conditions treatment (0.689); Antiprotozoal (0.586) |
| 167 | Antineoplastic (0.960); Anti-infective (0.613); Acute neurologic disorders treatment (0.572) |
| 168 | Antineoplastic (0.774); Preneoplastic conditions treatment (0.690); Antiprotozoal (0.557) |
| 169 | Antineoplastic (0.850); Antiviral (Arbovirus) (0.765); Acute neurologic disorders treatment (0.574) |
| 170 | Antineoplastic (0.965); Anti-infective (0.628); Acute neurologic disorders treatment (0.589) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 171 | Anti-hypercholesterolemic (0.873); Lipid metabolism regulator (0.713); Atherosclerosis treatment (0.559) |
| 172 | Antineoplastic (0.962); Apoptosis agonist (0.955); Antiparasitic (0.703); Antiprotozoal (0.590) |
| 173 | Antineoplastic (0.962); Apoptosis agonist (0.955); Antiparasitic (0.703); Antiprotozoal (0.590) |
| 174 | Antineoplastic (0.962); Apoptosis agonist (0.955); Antiparasitic (0.703); Antiprotozoal (0.590) |
| 175 | Antiseptic (0.945); Antiinfective (0.900); Preneoplastic conditions treatment (0.818) |
| 176 | Acute neurologic disorders treatment (0.858); Antidiabetic (0.818); Antidiabetic (type 2) (0.645) |
| 177 | Atherosclerosis treatment (0.857); Sweetener (0.635); Restenosis treatment (0.602) |
| 178 | Antihypertensive (0.765); Antidiabetic (0.757); Antithrombotic (0.522) |
| 179 | Antineoplastic (0.855); Transplant rejection treatment (0.591); Autoimmune disorders treatment (0.574) |
| 180 | Antineoplastic (0.667); Angiogenesis stimulant (0.566); Antidiabetic (0.531) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 181 | Antineoplastic (0.871); Apoptosis agonist (0.764); Anti-inflammatory (0.729) |
| 182 | Anti-psoriatic (0.923); Anti-eczematic (0.721); Alzheimer’s disease treatment (0.591) |
| 183 | Anti-psoriatic (0.924); Anti-eczematic (0.879); Antifungal (0.603) |
| 184 | Anti-inflammatory (0.751); Antiviral (Arbovirus) (0.595); Antifungal (0.579) |
| 185 | Anti-hypoxic (0.768); Antihypertensive (0.730); Antiviral (Picornavirus) (0.613) |
| 186 | Anti-inflammatory (0.751); Antiviral (Arbovirus) (0.595); Antifungal (0.579) |
| 187 | Lipid metabolism regulator (0.822); Hypolipemic (0.757); Anti-hypercholesterolemic (0.677) |
| 188 | Lipid metabolism regulator (0.889); Hypolipemic (0.685); Anti-hypercholesterolemic (0.644) |
| 189 | Antineoplastic (0.859); Apoptosis agonist (0.765); Atherosclerosis treatment (0.640) |
| 190 | Antidiabetic (0.916); Leukopoiesis stimulant (0.822); Atherosclerosis treatment (0.568) |
| 191 | Antineoplastic (0.834); Apoptosis agonist (0.818); Acute neurologic disorders treatment (0.795) |
| 192 | Anesthetic general (0.759); Antiviral (Arbovirus) (0.737); Antitoxic (0.728); Antihypoxic (0.726) |
| 193 | Antihypoxic (0.758); Antiviral (Arbovirus) (0.735); Antiviral (Picornavirus) (0.671) |
| 194 | Antineoplastic (0.796); Apoptosis agonist (0.719); Antiparasitic (0.711); Antifungal (0.675) |
| 195 | Acute neurologic disorders treatment (0.754); Antiviral (Arbovirus) (0.733); Antineurotic (0.555) |
| 196 | Lipid metabolism regulator (0.809); Hypolipemic (0.758); Antihypertensive (0.741) Anti-hypercholesterolemic (0.616); Atherosclerosis treatment (0.535) |
| 197 | Hypolipemic (0.796); Antineoplastic (0.614); Preneoplastic conditions treatment (0.547) |
| 198 | Hypolipemic (0.804); Antineoplastic (0.689); Preneoplastic conditions treatment (0.588) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 199 | Neuroprotector (0.748); Immunosuppressant (0.710); Acute neurologic disorders treatment (0.645) |
| 200 | Lipid metabolism regulator (0.901); Macrophage stimulant (0.829); Antineoplastic (0.777) |
| 201 | Neuroprotector (0.748); Immunosuppressant (0.710); Acute neurologic disorders treatment (0.645) |
| 202 | Lipid metabolism regulator (0.850); Antidiabetic symptomatic (0.740) Atherosclerosis treatment (0.654); Hypolipemic (0.653); Antidiabetic (0.605) |
| 203 | Lipid metabolism regulator (0.850); Antidiabetic symptomatic (0.740) Atherosclerosis treatment (0.654); Hypolipemic (0.653); Antidiabetic (0.605) |
| 204 | Lipid metabolism regulator (0.850); Antidiabetic symptomatic (0.740) Atherosclerosis treatment (0.654); Hypolipemic (0.653); Antidiabetic (0.605) |
| 205 | Lipid metabolism regulator (0.857); Apoptosis agonist (0.729); Immunosuppressant (0.689) |
| 206 | Lipid metabolism regulator (0.879); Immunosuppressant (0.760); Apoptosis agonist (0.744) |
| 207 | Hypolipemic (0.880); Lipid metabolism regulator (0.861); Anti-hypercholesterolemic (0.683) Atherosclerosis treatment (0.634); Neurodegenerative diseases treatment (0.610) |
| 208 | Restenosis treatment (0.827); Neurodegenerative diseases treatment (0.722); Antidiabetic (0.655) |
| 209 | Restenosis treatment (0.827); Neurodegenerative diseases treatment (0.722); Antidiabetic (0.655) |
| 210 | Restenosis treatment (0.827); Neurodegenerative diseases treatment (0.722); Antidiabetic (0.655) |
| 211 | Mucositis treatment (0.803); Antimutagenic (0.727); Cytoprotectant (0.719) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 212 | Hypolipemic (0.845); Antihypertensive (0.622); Lipid metabolism regulator (0.621) |
| 213 | Lipid metabolism regulator (0.791); Hypolipemic (0.778); Antihypertensive (0.643) |
| 214 | Acute neurologic disorders treatment (0.944); Lipid metabolism regulator (0.868) |
| 215 | Natural killer cell stimulant (0.785); Antineoplastic (0.785); Leukopoiesis stimulant (0.733) Acute neurologic disorders treatment (0.701); Immunosuppressant (0.697); Neuroprotector (0.664) |
| 216 | Natural killer cell stimulant (0.785); Antineoplastic (0.785); Leukopoiesis stimulant (0.733) Acute neurologic disorders treatment (0.701); Immunosuppressant (0.697); Neuroprotector (0.664) |
| 217 | Hypolipemic (0.807); Acute neurologic disorders treatment (0.722); Immunosuppressant (0.710) Leukopoiesis stimulant (0.687); Natural killer cell stimulant (0.685); Erythropoiesis stimulant (0.551) |
| 218 | Natural killer cell stimulant (0.795); Leukopoiesis stimulant (0.784); Hypolipemic (0.765) Immunosuppressant (0.702); Acute neurologic disorders treatment (0.696); Neuroprotector (0.684) |
| 219 | Hypolipemic (0.851); Acute neurologic disorders treatment (0.844); Anticonvulsant (0.733) Natural killer cell stimulant (0.715); Leukopoiesis stimulant (0.713) |
| 220 | Hypolipemic (0.851); Acute neurologic disorders treatment (0.844); Anticonvulsant (0.733) Natural killer cell stimulant (0.715); Leukopoiesis stimulant (0.713) |
| 221 | Natural killer cell stimulant (0.795); Leukopoiesis stimulant (0.784); Hypolipemic (0.765) Immunosuppressant (0.702); Acute neurologic disorders treatment (0.696); Neuroprotector (0.684) |
| 222 | Hypolipemic (0.828); Acute neurologic disorders treatment (0.774); Natural killer cell stimulant (0.746) Leukopoiesis stimulant (0.736); Immunosuppressant (0.726); Lipid metabolism regulator (0.685) |
| 223 | Lipid metabolism regulator (0.881); Hypolipemic (0.851); Leukopoiesis stimulant (0.822) Natural killer cell stimulant (0.795); Anti-hypercholesterolemic (0.728) |
| 224 | Lipid metabolism regulator (0.881); Hypolipemic (0.851); Leukopoiesis stimulant (0.822) Natural killer cell stimulant (0.795); Anti-hypercholesterolemic (0.728) |
| 225 | Platelet antagonist (0.800); Acute neurologic disorders treatment (0.701); Fibrinolytic (0.700) Natural killer cell stimulant (0.696); Erythropoiesis stimulant (0.619); Immunosuppressant (0.612) |
| 226 | Leukopoiesis stimulant (0.780); Antineoplastic (0.773); Hypolipemic (0.748) Natural killer cell stimulant (0.744); Lipid metabolism regulator (0.650) |
| 227 | Lipid metabolism regulator (0.809); Leukopoiesis stimulant (0.776); Hypolipemic (0.758) Antihypertensive (0.741); Natural killer cell stimulant (0.730); Antineoplastic (0.683) |
| 228 | Antidiabetic (0.781); Leukopoiesis stimulant (0.725); Bone diseases treatment (0.679) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 229 | Lipid metabolism regulator (0.861); Leukopoiesis stimulant (0.840); Hypolipemic (0.685) |
| 230 | Fibrinolytic (0.760); Natural killer cell stimulant (0.746); Leukopoiesis stimulant (0.746) |
| 231 | Antiviral (Arbovirus) (0.834); Antimutagenic (0.827); Mucositis treatment (0.812) Preneoplastic conditions treatment (0.766); Lipid metabolism regulator (0.718) |
| 232 | Antiviral (Arbovirus) (0.861); Lipid metabolism regulator (0.844); Antiviral (Picornavirus) (0.776) |
| 233 | Antiviral (Arbovirus) (0.861); Lipid metabolism regulator (0.844); Antiviral (Picornavirus) (0.776) |
| 234 | Antiviral (Arbovirus) (0.947); Lipid metabolism regulator (0.884); Antiviral (Picornavirus) (0.782) |
| 235 | Antiviral (Arbovirus) (0.891); Lipid metabolism regulator (0.743); Antiviral (Picornavirus) (0.730) |
| 236 | Apoptosis agonist (0.886); Antineoplastic (0.763); Chemoprotective (0.700) |
| 237 | Antiviral (Arbovirus) (0.904); Lipid metabolism regulator (0.828); Antiviral (Picornavirus) (0.688) |
| 238 | Antiviral (Arbovirus) (0.886); Lipid metabolism regulator (0.874); Antiviral (Picornavirus) (0.744) |
| 239 | Lipid metabolism regulator (0.942); Anti-hypercholesterolemic (0.855); Hypolipemic (0.793) |
| 240 | Antiviral (Arbovirus) (0.853); Antiviral (Picornavirus) (0.760); Apoptosis agonist (0.731) |
| 241 | Antiviral (Arbovirus) (0.873); Lipid metabolism regulator (0.850); Antiviral (Picornavirus) (0.735) |
| 242 | Anti-ischemic, cerebral (0.910); Leukopoiesis stimulant (0.708); Antidiabetic (0.698) |
| 243 | Antiviral (Arbovirus) (0.944); Lipid metabolism regulator (0.882); Antiviral (Picornavirus) (0.766) |
| 244 | Anti-inflammatory (0.785); Antineoplastic (0.782); Preneoplastic conditions treatment (0.661) |
| 245 | Lipid metabolism regulator (0.846); Anti-hypercholesterolemic (0.827); Atherosclerosis treatment (0.538) |
| 246 | Lipid metabolism regulator (0.943); Hypolipemic (0.860); Anti-hypercholesterolemic (0.844) |
| 247 | Antineoplastic (0.844); Hypolipemic (0.782); Lipid metabolism regulator (0.699) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 248 | Anti-ischemic, cerebral (0.919); Mucositis treatment (0.913); Lipid metabolism regulator (0.885) Antithrombotic (0.745); Platelet antagonist (0.685); Antihypertensive (0.555) |
| 249 | Anti-ischemic, cerebral (0.919); Mucositis treatment (0.913); Lipid metabolism regulator (0.885) Antithrombotic (0.745); Platelet antagonist (0.685); Antihypertensive (0.555) |
| 250 | Lipid metabolism regulator (0.923); Mucositis treatment (0.910); Antithrombotic (0.805) Anti-ischemic, cerebral (0.801); Platelet antagonist (0.751); Fibrinolytic (0.684) |
| 251 | Mucositis treatment (0.960); Lipid metabolism regulator (0.869); Platelet antagonist (0.836) Antithrombotic (0.773); Anti-ischemic, cerebral (0.740); Fibrinolytic (0.717) |
| 252 | Antineoplastic (0.844); Antiviral (Arbovirus) (0.790); Antiviral (Picornavirus) (0.659) |
| 253 | Antihypertensive (0.962); Anti-Helicobacter pylori (0.839); Chemoprotective (0.718) Antineoplastic (myeloid leukemia) (0.707); Apoptosis agonist (0.674); Antineoplastic (0.656) |
| 254 | Anti-Helicobacter pylori (0.744); Antiviral (Arbovirus) (0.715); Antiviral (Picornavirus) (0.547) |
| 255 | Hypolipemic (0.795); Antineoplastic (0.791); Antiviral (Arbovirus) (0.742) Preneoplastic conditions treatment (0.740); Lipid metabolism regulator (0.739) |
| 256 | Lipid metabolism regulator (0.902); Anti-hypercholesterolemic (0.871) Hypolipemic (0.812); Atherosclerosis treatment (0.603) |
| 257 | Antiviral (Arbovirus) (0.890); Antiviral (Picornavirus) (0.817); Mucositis treatment (0.743) |
| 258 | Hypolipemic (0.845); Antihypertensive (0.622); Lipid metabolism regulator (0.621) Atherosclerosis treatment (0.590); Antiprotozoal (Coccidial) (0.569); Antibacterial (0.562) |
| 259 | Lipid metabolism regulator (0.874); Anti-hypercholesterolemic (0.785); Hypolipemic (0.707) |
| 260 | Lipid metabolism regulator (0.942); Anti-hypercholesterolemic (0.855); Hypolipemic (0.793) Atherosclerosis treatment (0.635); Multiple sclerosis treatment (0.521) |
| 261 | Antiviral (Arbovirus) (0.927); Lipid metabolism regulator (0.887); Antiviral (Picornavirus) (0.669) |
| YM-170320 | Antifungal (0.867); Antibacterial (0.744); Antineoplastic (0.711); Chemoprotective (0.594) |
| 262 | Acute neurologic disorders treatment (0.944); Lipid metabolism regulator (0.868) Antineoplastic (0.814); Antifungal (0.829); Antibacterial (0.698) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 263 | Antiviral (Arbovirus) (0.927); Lipid metabolism regulator (0.887); Antiviral (Picornavirus) (0.669) |
| 264 | Antitoxic (0.733); Leukopoiesis stimulant (0.730); Preneoplastic conditions treatment (0.721) |
| 265 | Antiviral (Arbovirus) (0.861); Lipid metabolism regulator (0.844); Antiviral (Picornavirus) (0.776) |
| 266 | Antiviral (Arbovirus) (0.860); Antiviral (Picornavirus) (0.751); Antidiabetic (0.713) |
| 267 | Antiviral (Arbovirus) (0.861); Lipid metabolism regulator (0.844); Antiviral (Picornavirus) (0.776) |
| 268 | Antiviral (Arbovirus) (0.947); Lipid metabolism regulator (0.884); Antiviral (Picornavirus) (0.782) |
| 269 | Antineoplastic (0.902); DNA synthesis inhibitor (0.675); Angiogenesis inhibitor (0.668) |
| 270 | Antineoplastic (0.872); Apoptosis agonist (0.653); Antiarthritic (0.611) |
| 271 | Hypolipemic (0.808); Anti-hypercholesterolemic (0.695); Lipid metabolism regulator (0.691) |
| 272 | Lipid metabolism regulator (0.887); Atherosclerosis treatment (0.690); Hypolipemic (0.647) |
| 273 | Lipid metabolism regulator (0.886); Hypolipemic (0.788); Anti-hypercholesterolemic (0.755) |
| 274 | Lipid metabolism regulator (0.886); Hypolipemic (0.788); Anti-hypercholesterolemic (0.755) |
| 275 | Apoptosis agonist (0.886); Antineoplastic (0.763); Chemoprotective (0.700) |
| 276 | Apoptosis agonist (0.781); Antimutagenic (0.777); Preneoplastic conditions treatment (0.600) |
| 277 | Natural killer cell stimulant (0.795); Leukopoiesis stimulant (0.784); Antineoplastic (0.778) |
| 278 | Natural killer cell stimulant (0.795); Leukopoiesis stimulant (0.784); Antineoplastic (0.778) |
| 279 | Leukopoiesis stimulant (0.802); Natural killer cell stimulant (0.769); Immunosuppressant (0.686) |
| 280 | Lipid metabolism regulator (0.929); Hypolipemic (0.786); Lymphocytopoiesis inhibitor (0.726) |
| 281 | Antidiabetic (0.763); Antihypoxic (0.736); Natural killer cell stimulant (0.570) |
| 282 | Myasthenia Gravis treatment (0.962); Cell adhesion molecule inhibitor (0.863); Antidiabetic (0.757) |
| 283 | Angiogenesis stimulant (0.915); Lipid metabolism regulator (0.913); Myasthenia Gravis treatment (0.779) Cell adhesion molecule inhibitor (0.745); Antidiabetic (0.664) |
| 284 | Angiogenesis stimulant (0.914); Myasthenia Gravis treatment (0.844) Cell adhesion molecule inhibitor (0.788); Antidiabetic (0.700) |
| 285 | Angiogenesis stimulant (0.915); Lipid metabolism regulator (0.913); Myasthenia Gravis treatment (0.779) Cell adhesion molecule inhibitor (0.745); Antidiabetic (0.664) |
| 286 | Antihypertensive (0.824); DNA intercalator (0.707); Antidiabetic (0.692) |
| 287 | Leukopoiesis stimulant (0.763); Natural killer cell stimulant (0.721); Antidiabetic symptomatic (0.659) |
| 288 | Myasthenia Gravis treatment (0.962); Cell adhesion molecule inhibitor (0.863); Antidiabetic (0.757) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 289 | Antibiotic Glycopeptide-like (0.908); Antineoplastic (0.877); Apoptosis agonist (0.798) |
| 290 | Antineoplastic (0.942); Apoptosis agonist (0.897); Antibiotic Glycopeptide-like (0.785) |
| 291 | Antibiotic Glycopeptide-like (0.892); Antineoplastic (0.850); Antiprotozoal (Plasmodium) (0.834) |
| 292 | Antineoplastic (0.890); Antibiotic Glycopeptide-like (0.883); Apoptosis agonist (0.802) |
| 293 | Antineoplastic (0.942); Apoptosis agonist (0.897); Antibiotic Glycopeptide-like (0.785) |
| 294 | Antineoplastic (0.913); Apoptosis agonist (0.882); Antibiotic Glycopeptide-like (0.771) |
| 295 | Antineoplastic (0.938); Apoptosis agonist (0.881); Antibiotic Glycopeptide-like (0.769) |
| 296 | Antibiotic Glycopeptide-like (0.813); Antineoplastic (0.800); Apoptosis agonist (0.684) |
| 297 | Antibiotic Glycopeptide-like (0.908); Antineoplastic (0.877); Apoptosis agonist (0.798) |
| 298 | Antibiotic Glycopeptide-like (0.879); Antineoplastic (0.857); Apoptosis agonist (0.803) |
| 299 | Antineoplastic (0.942); Apoptosis agonist (0.897); Antibiotic Glycopeptide-like (0.785) |
| 300 | Antibiotic Glycopeptide-like (0.906); Antineoplastic (0.865); Apoptosis agonist (0.763) |
| 301 | Antineoplastic (0.913); Antibiotic Glycopeptide-like (0.782); Apoptosis agonist (0.779) |
| 302 | Antidiabetic symptomatic (0.732); Leukopoiesis stimulant (0.671); Multiple sclerosis treatment (0.663) |
| 303 | Natural killer cell stimulant (0.739); Antidiabetic symptomatic (0.733); Leukopoiesis stimulant (0.687) |
| 304 | Antiviral (Arbovirus) (0.927); Lipid metabolism regulator (0.887); Antiviral (Picornavirus) (0.669) |
| 305 | Lipid metabolism regulator (0.881); Hypolipemic (0.851); Anti-hypercholesterolemic (0.728) |
| 306 | Lipid metabolism regulator (0.819); Natural killer cell stimulant (0.797); Hypolipemic (0.759) |
| 307 | Lipid metabolism regulator (0.919); Anti-hypercholesterolemic (0.803); Hypolipemic (0.763) |
| 308 | Anti-hypercholesterolemic (0.897); Lipid metabolism regulator (0.819); Antimutagenic (0.784) |
| 309 | Anti-inflammatory (0.842); Analgesic (0.766); Leukopoiesis stimulant (0.584) |
| 310 | Anti-inflammatory (0.814); Analgesic (0.746); Erythropoiesis stimulant (0.611) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 311 | Acute neurologic disorders treatment (0.793); Natural killer cell stimulant (0.711) |
| 312 | Anti-infective (0.945); Antitoxic (0.908); Natural killer cell stimulant (0.900) |
| 313 | Lipid metabolism regulator (0.923); Apoptosis agonist (0.849); Antineoplastic (0.803)Acute neurologic disorders treatment (0.799); Preneoplastic conditions treatment (0.649) |
| 314 | Lipid metabolism regulator (0.923); Apoptosis agonist (0.849); Antineoplastic (0.803)Acute neurologic disorders treatment (0.799); Preneoplastic conditions treatment (0.649) |
| 315 | Lipid metabolism regulator (0.960); Hypolipemic (0.898); Anti-hypercholesterolemic (0.886) |
| 316 | Lipid metabolism regulator (0.960); Hypolipemic (0.898); Anti-hypercholesterolemic (0.886) |
| 317 | Lipid metabolism regulator (0.848); Anti-hypercholesterolemic (0.770); Hypolipemic (0.760) |
| 318 | Lipid metabolism regulator (0.848); Anti-hypercholesterolemic (0.770); Hypolipemic (0.760) |
| 319 | Lipid metabolism regulator (0.930); Anti-hypercholesterolemic (0.842); Hypolipemic (0.830) |
| 320 | Lipid metabolism regulator (0.930); Anti-hypercholesterolemic (0.842); Hypolipemic (0.830) |
| 321 | Lipid metabolism regulator (0.930); Anti-hypercholesterolemic (0.842); Hypolipemic (0.830) |
| 322 | Lipid metabolism regulator (0.930); Anti-hypercholesterolemic (0.842); Hypolipemic (0.830) |
| 323 | Lipid metabolism regulator (0.846); Anti-hypercholesterolemic (0.827) |
| 324 | Lipid metabolism regulator (0.846); Anti-hypercholesterolemic (0.827) |
| 325 | Anti-ischemic, cerebral (0.907); Cell adhesion molecule inhibitor (0.876); Antidiabetic (0.702) |
| 326 | Antineoplastic (0.857); Apoptosis agonist (0.746); Preneoplastic conditions treatment (0.517) |
| 327 | Anti-ischemic, cerebral (0.835); Acute neurologic disorders treatment (0.783); Hypolipemic (0.749) |
| 328 | Anti-ischemic, cerebral (0.845); Leukopoiesis stimulant (0.783); Antitoxic (0.675) |
| 329 | Hypolipemic (0.879); Lipid metabolism regulator (0.825); Anti-hypercholesterolemic (0.769) |
| 330 | Acute neurologic disorders treatment (0.947); Antineoplastic (0.816); Apoptosis agonist (0.771) |
| 331 | Preneoplastic conditions treatment (0.770); Acute neurologic disorders treatment (0.757) |
| 332 | Lipid metabolism regulator (0.937); Acute neurologic disorders treatment (0.832) |
| 333 | Lipid metabolism regulator (0.937); Hypolipemic (0.866)Acute neurologic disorders treatment (0.832); Atherosclerosis treatment (0.653) |
| No. | Predicted Biological Activity, Pa * |
|---|---|
| 334 | Natural killer cell stimulant (0.795); Leukopoiesis stimulant (0.784); Antineurotic (0.700) |
| 335 | Hypolipemic (0.828); Acute neurologic disorders treatment (0.774); Leukopoiesis stimulant (0.736) |
| 336 | Hypolipemic (0.880); Antineoplastic (0.858); Lipid metabolism regulator (0.732) |
| 337 | Lipid metabolism regulator (0.859); Acute neurologic disorders treatment (0.811) Hypolipemic (0.800); Mucositis treatment (0.756); Antidiabetic symptomatic (0.696) |
| 338 | Lipid metabolism regulator (0.891); Hypolipemic (0.861); Anti-hypercholesterolemic (0.784) |
| 339 | Lipid metabolism regulator (0.888); Acute neurologic disorders treatment (0.761) |
| 340 | Hypolipemic (0.816); Lipid metabolism regulator (0.793); Mucositis treatment (0.779) |
| 341 | Antifungal (0.836); Antibacterial (0.653); Antiparasitic (0.614) |
| 342 | Antifungal (0.836); Antibacterial (0.653); Antiparasitic (0.614) |
| 343 | Lipid metabolism regulator (0.952); Antineoplastic (liver cancer) (0.909) Anti-hypercholesterolemic (0.815); Antineoplastic (0.777); Atherosclerosis treatment (0.649) |
| 344 | Lipid metabolism regulator (0.923); Apoptosis agonist (0.849); Antineoplastic (0.803) Hypolipemic (0.712); Atherosclerosis treatment (0.651); Preneoplastic conditions treatment (0.649) |
| 345 | Lipid metabolism regulator (0.942); Antiviral (Arbovirus) (0.903); Hypolipemic (0.741) Acute neurologic disorders treatment (0.728); Antiviral (Picornavirus) (0.676) |
| 346 | Antiviral (Arbovirus) (0.952); Lipid metabolism regulator (0.903); Antiviral (Picornavirus) (0.790) |
| 347 | Antiviral (Arbovirus) (0.952); Lipid metabolism regulator (0.903); Antiviral (Picornavirus) (0.790) |
| 348 | Antiviral (Arbovirus) (0.952); Lipid metabolism regulator (0.903); Antiviral (Picornavirus) (0.790) |
| 349 | Antiviral (Arbovirus) (0.952); Lipid metabolism regulator (0.903); Antiviral (Picornavirus) (0.790) |
| 350 | Platelet antagonist (0.800); Anticoagulant (0.702); Fibrinolytic (0.700) |
| 351 | Apoptosis agonist (0.879); Antineoplastic (0.878); Preneoplastic conditions treatment (0.618) |
| 352 | Antiviral (Arbovirus) (0.861); Lipid metabolism regulator (0.844); Antiviral (Picornavirus) (0.776) |
| 353 | Antiviral (Arbovirus) (0.917); Antiviral (Picornavirus) (0.781); Antimutagenic (0.699) |
| 354 | Antiviral (Arbovirus) (0.861); Lipid metabolism regulator (0.844); Antiviral (Picornavirus) (0.776) |
| 355 | Antiviral (Arbovirus) (0.917); Antiviral (Picornavirus) (0.781); Antimutagenic (0.699) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dembitsky, V.M. Hydrobiological Aspects of Fatty Acids: Unique, Rare, and Unusual Fatty Acids Incorporated into Linear and Cyclic Lipopeptides and Their Biological Activity. Hydrobiology 2022, 1, 331-432. https://doi.org/10.3390/hydrobiology1030024
Dembitsky VM. Hydrobiological Aspects of Fatty Acids: Unique, Rare, and Unusual Fatty Acids Incorporated into Linear and Cyclic Lipopeptides and Their Biological Activity. Hydrobiology. 2022; 1(3):331-432. https://doi.org/10.3390/hydrobiology1030024
Chicago/Turabian StyleDembitsky, Valery M. 2022. "Hydrobiological Aspects of Fatty Acids: Unique, Rare, and Unusual Fatty Acids Incorporated into Linear and Cyclic Lipopeptides and Their Biological Activity" Hydrobiology 1, no. 3: 331-432. https://doi.org/10.3390/hydrobiology1030024
APA StyleDembitsky, V. M. (2022). Hydrobiological Aspects of Fatty Acids: Unique, Rare, and Unusual Fatty Acids Incorporated into Linear and Cyclic Lipopeptides and Their Biological Activity. Hydrobiology, 1(3), 331-432. https://doi.org/10.3390/hydrobiology1030024






