First Record of Pandeid Jellyfish, Eutiara decorata Berberian, Michenet and Goy, 2021 (Hydrozoa, Anthoathecata, Pandeidae), from Japan
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Hydromedusa
3.1.1. Systematic Account of Pandeid Species
- Phylum Cnidaria Hatschek, 1888
- Subphylum Medusozoa Petersen, 1979
- Class Hydrozoa Owen, 1843
- Subclass Hydroidolina Collins, 2000
- Order Anthoathecata Cornelius, 1992
- Suborder Filifera Kühn, 1913
- Family Pandeidae Haeckel, 1879
- Genus Eutiara Bigelow, 1918
- Eutiara decorata Berberian, Michenet and Goy, 2021
- New Japanese name: Kuko-no-Mi Kurage (クコノミクラゲ)
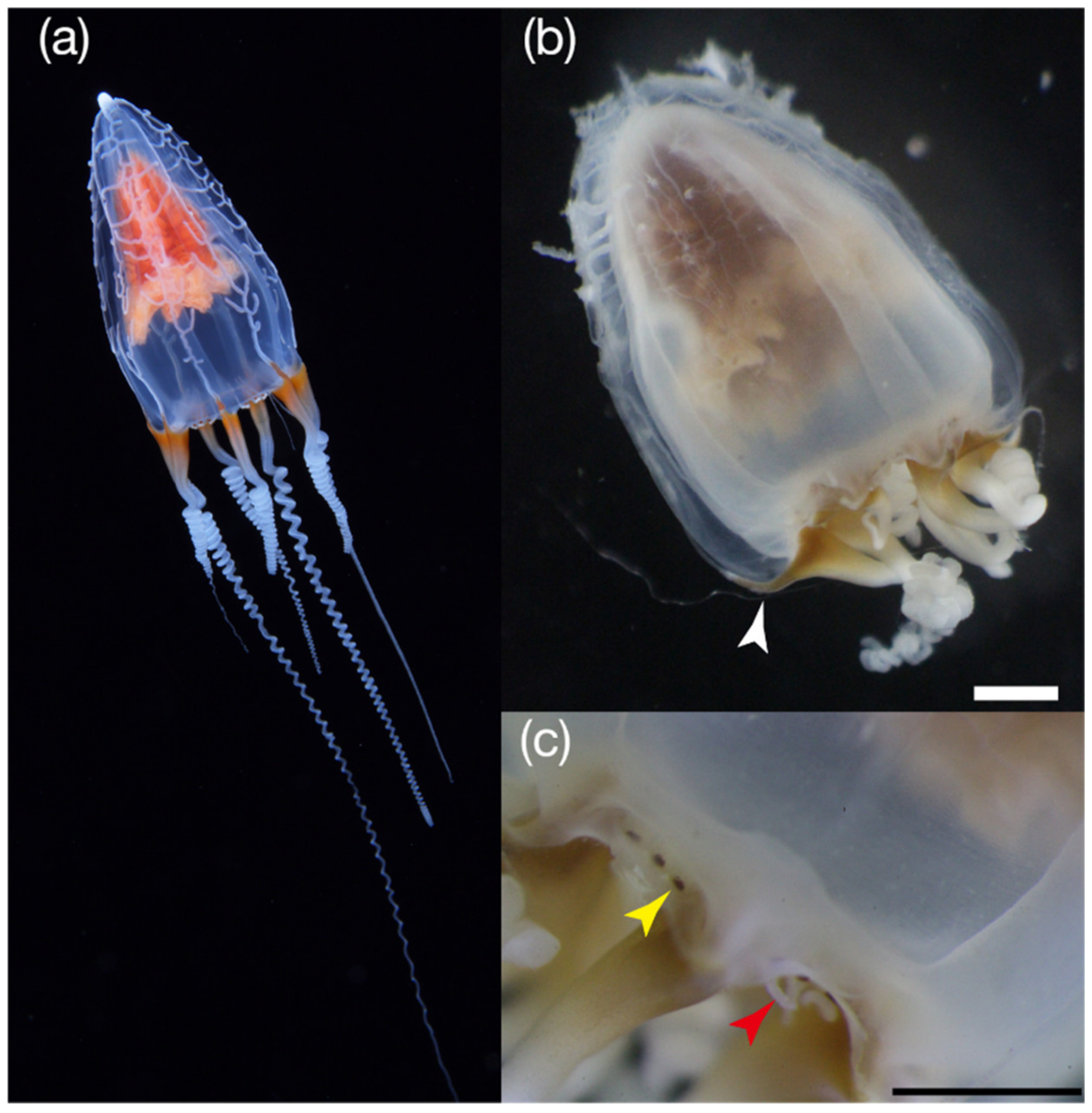
3.1.2. Description of Specimens
- Examined material
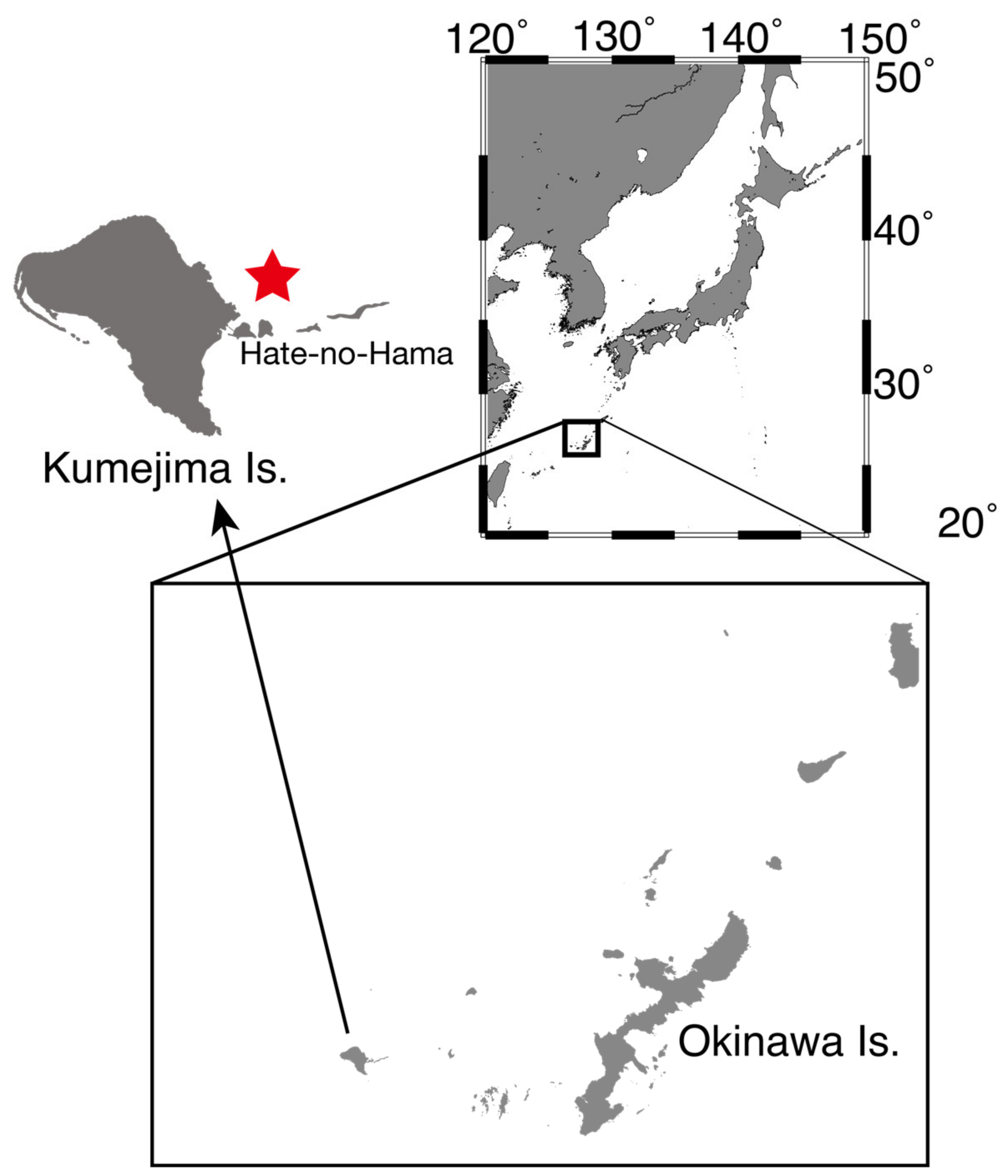
- Sampling date: 5 September 2021
- Sampling site: Off Hate-no-Hama, Kumejima-cho, Shimajiri-gun, Okinawa Prefecture
- GPS coordinate: 26.37488° N 126.844861° E
- Sampling gear: SCUBA
- Depth: 12 m
- Water temperature: 29 °C
- Fixing method: 3% seawater formalin
- Collector: Ryo Minemizu
- P02: UH 6.2 mm, UD 3.1 mm, SUH 5.4 mm, ML 3.2 mm
- P06: UH 15.0 mm, UD 7.9 mm, SUH 14.1 mm, ML 9.1 mm

3.2. Symbionts on the Hydromedusae
3.2.1. Systematic Account of the Hyperiid Amphipod on Eutiara decorata, Voucher Specimen P06
- Phylum Arthropoda von Siebold, 1848
- Subphylum Crustacea Brünnich, 1772
- Superclass Multicrustacea Regier, Shultz, Zwick, Hussey, Ball, Wetzer, Martin and Cunningham, 2010
- Class Malacostraca Latreille, 1802
- Subclass Eumalacostraca Grobben, 1892
- Superorder Peracarida Calman, 1904
- Order Amphipoda Latreille, 1816
- Suborder Hyperiidea H. Milne Edwards, 1830
- Infraorder Physocephalata Bowman and Gruner, 1973
- Parvorder Physocephalatidira Bowman and Gruner, 1973
- Superfamily Platysceloidea Spence Bate, 1862
- Family Brachyscelidae Stephensen, 1923
- Genus Brachyscelus Spence Bate, 1861
- Species Brachyscelus crusculum Spence Bate, 1861
- Japanese name: Nokoba-Umi-Nomi (ノコバウミノミ)
- Voucher number: P06-Amphi (Figure 4)
3.2.2. Description of Specimen
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bigelow, H.B. Some medusae and siphonophorae from the western Atlantic. Bull. Mus. Comp. Zool. Harv. 1918, 62, 365–442. [Google Scholar]
- Berberian, A.; Michenet, F.; Goy, J. A New species of Eutiara, in the Pandeidae family (Cnidaria: Hydrozoa: Anthoathecata) from Tahiti Island, French Polynesia. Pac. Sci. 2021, 74, 257–268. [Google Scholar] [CrossRef]
- Bouillon, J. A new species of the genus Eutiara, Eutiara russelli n. sp. (Anthomedusae, Hydrozoa, Cnidaria). Steenstrupia 1981, 7, 233–236. [Google Scholar]
- Bouillon, J.; Gravili, C.; Pages, F.; Gili, J.M.; Boero, F. An Introduction to Hydrozoa; Publications Scientifiques du Museum: Paris, France, 2006; Volume 194, p. 591. [Google Scholar]
- Kramp, P.L. Synopsis of the medusae of the world. J. Mar. Biol. Assoc. 1961, 40, 1–469. [Google Scholar] [CrossRef]
- Kramp, P.L. The hydromedusae of the Atlantic Ocean and adjacent waters. Dana Rep. 1959, 46, 1–283. [Google Scholar]
- Shiga, M. Available online: https://www.instagram.com/p/B01mDqNhjXf/?utm_medium (accessed on 24 January 2022).
- Minemizu, R. Available online: https://www.instagram.com/p/B4TCkPLhuh2/ (accessed on 24 January 2022).
- Minemizu, R. Available online: https://www.instagram.com/ryominemizu/p/B1ZogKlBfQs/?utm_medium (accessed on 25 January 2022).
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671. [Google Scholar] [CrossRef] [PubMed]
- Vinogradov, M.E.; Volkov, A.F.; Semenova, T.A.N.N. Hyperiid Amphipods (Amphipoda, Hyperiidea) of the World Oceans; Baba Barkha Nath Printers: New Delhi, India, 1996; p. 632. [Google Scholar]
- Chihara, M.; Murano, M. An Illustrated Guide to Marine Plankton in Japan; Tokai University Press: Tokyo, Japan, 1997; p. 1574. [Google Scholar]
- Mori, M.; Suzuki, Y.; Yamaki, A.; Lindsay Dhugal, J. A checklist of hyperiid amphipods (Amphipoda: Hyperiidea) from Japanese waters, including new records from 1996–2007 for Sagami Bay and outlying areas. Bull. Plankton Soc. Jpn. 2010, 57, 41–54, (In Japanese with English abstract). [Google Scholar]
- Minemizu, R.; Kubota, S.; Hirano, Y.; Lindsay, D. A Photographic Guide to the Jellyfishes of Japan; Heibonsha: Tokyo, Japan, 2015; p. 360. [Google Scholar]
- Shinoda, A.; Aoyama, J.; Miller, M.J.; Otake, T.; Mochioka, N.; Watanabe, S.; Minegishi, Y.; Kuroki, M.; Yoshinaga, T.; Yokouchi, K.; et al. Evaluation of the larval distribution and migration of the Japanese eel in the western North Pacific. Rev. Fish Biol. Fisher. 2011, 21, 591–611. [Google Scholar] [CrossRef]
- Lawley, J.W.; Ames, C.L.; Bentlage, B.; Yanagihara, A.; Goodwill, R.; Kayal, E.; Hurwitz, K.; Collins, A.G. Box jellyfish Alatina alata has a circumtropical distribution. Biol. Bull. 2016, 231, 152–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Toshino, S.; Kotsuka, H. New record of Alatina moseri (Cubozoa, Carybdeida) from Sagami Bay, eastern Japan. Bull. Biogeogr. Soc. Jpn. 2020, 75, 123–125, (In Japanese with English abstract). [Google Scholar]
- Garcia-Soto, C.; Seys, J.J.C.; Zielinski, O.; Busch, J.A.; Luna, S.I.; Baez, J.C.; Domegan, C.; Dubsky, K.; Kotynska-Zielinska, I.; Loubat, P.; et al. Marine citizen science: Current state in Europe and new technological developments. Front. Mar. Sci. 2021, 8, 621472. [Google Scholar] [CrossRef]
- Nordstrom, B.; James, M.C.; Martin, K.; Worm, B. Tracking jellyfish and leatherback sea turtle seasonality through citizen science observers. Mar. Ecol. Prog. Ser. 2019, 620, 15–32. [Google Scholar] [CrossRef]
- Langeneck, J.; Crocetta, F.; Doumpas, N.; Giovos, I.; Piraino, S.; Boero, F. First record of the non-native jellyfish Chrysaora cf. achlyos (Cnidaria: Pelagiidae) in the Mediterranean Sea. BioInvasions Rec. 2019, 8, 608–613. [Google Scholar] [CrossRef]
- Guerrero, E.; Kienberger, K.; Villaescusa, A.; Gili, J.M.; Navarro, G.; Prieto, L. First record of beaching events for a calycophoran siphonophore: Abylopsis tetragona (Otto, 1823) at the Strait of Gibraltar. Mar. Biodivers. 2019, 49, 1587–1593. [Google Scholar] [CrossRef]
- Purcell, J.E. Successes and challenges in jellyfish ecology: Examples from Aequorea spp. Mar. Ecol. Prog. Ser. 2018, 591, 7–27. [Google Scholar] [CrossRef]
- Pires, R.F.T.; Cordeiro, N.; Dubert, J.; Marraccini, A.; Relvas, P.; dos Santos, A. Untangling Velella velella (Cnidaria: Anthoathecatae) transport: A citizen science and oceanographic approach. Mar. Ecol. Prog. Ser. 2018, 591, 241–251. [Google Scholar] [CrossRef]
- Milisen, J.W.; Matye, S.A.; Kobayashi, D.R. Nocturnal visual census of pelagic fauna using scuba near Kona, Hawai’i. Pac. Sci. 2018, 72, 399–410. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Watabe, M.; Minemizu, R.; Miyake, H. First Record of Pandeid Jellyfish, Eutiara decorata Berberian, Michenet and Goy, 2021 (Hydrozoa, Anthoathecata, Pandeidae), from Japan. Hydrobiology 2022, 1, 139-145. https://doi.org/10.3390/hydrobiology1020011
Watabe M, Minemizu R, Miyake H. First Record of Pandeid Jellyfish, Eutiara decorata Berberian, Michenet and Goy, 2021 (Hydrozoa, Anthoathecata, Pandeidae), from Japan. Hydrobiology. 2022; 1(2):139-145. https://doi.org/10.3390/hydrobiology1020011
Chicago/Turabian StyleWatabe, Mai, Ryo Minemizu, and Hiroshi Miyake. 2022. "First Record of Pandeid Jellyfish, Eutiara decorata Berberian, Michenet and Goy, 2021 (Hydrozoa, Anthoathecata, Pandeidae), from Japan" Hydrobiology 1, no. 2: 139-145. https://doi.org/10.3390/hydrobiology1020011
APA StyleWatabe, M., Minemizu, R., & Miyake, H. (2022). First Record of Pandeid Jellyfish, Eutiara decorata Berberian, Michenet and Goy, 2021 (Hydrozoa, Anthoathecata, Pandeidae), from Japan. Hydrobiology, 1(2), 139-145. https://doi.org/10.3390/hydrobiology1020011





