Occurrence of Freshwater Cyanobacteria and Bloom Records in Spanish Reservoirs (1981–2017)
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Occurrence of Cyanobacteria and Blooms in Spanish Reservoirs
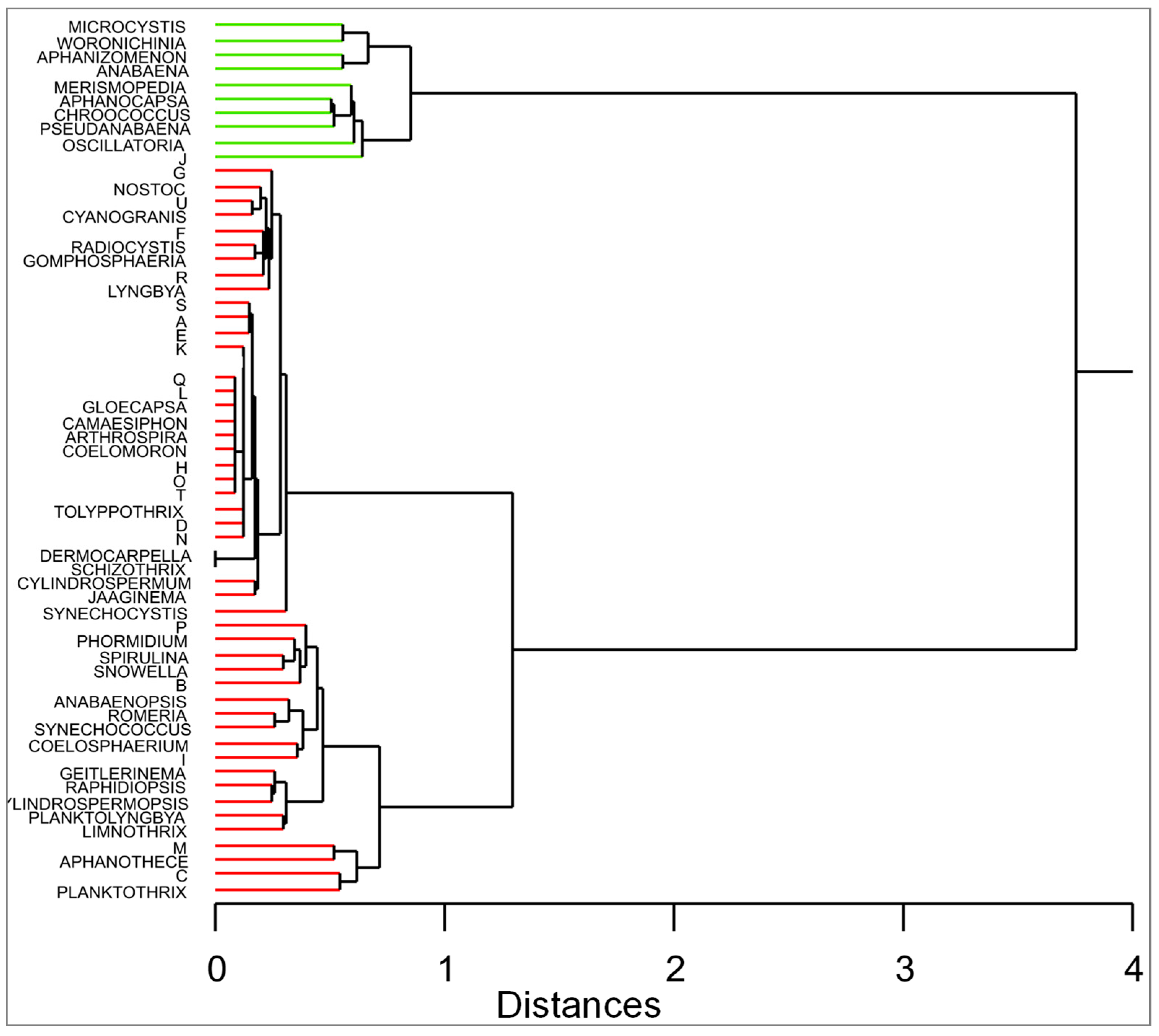
3.2. Species of Cyanobacteria in Spanish Reservoirs
3.3. Toxic Cyanobacteria and Blooms in Spanish Reservoirs
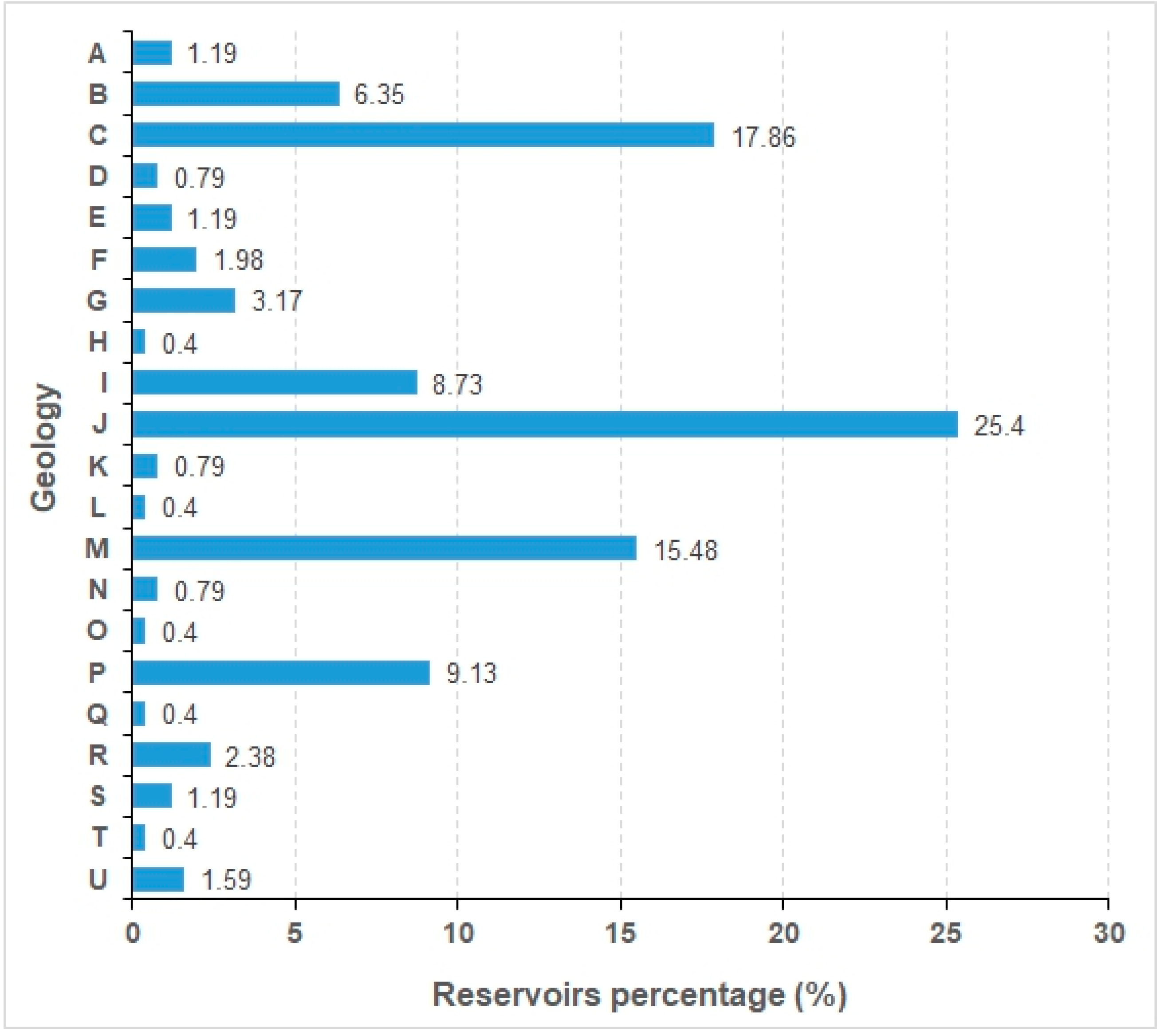
3.4. Cyanobacteria and Geology
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Almodóvar, A.; Nicola, G.G.; Nuevo, M. Effects of a bloom of Planktothrix rubescens on the fish community of a Spanish reservoir. Limnetica 2004, 23, 167–178. [Google Scholar] [CrossRef]
- Cobo, F.; Lago, L.; Barca, S.; Vieira-Lanero, R.; Servia, M.J. Cianobacterias y medioambiente. In Aspectos Ecotoxicológicos De Sus Floraciones En Aguas Continentales; AGAIA (Asociación Galega de Investigadores da Auga): Santiago de Compostela, Spain, 2012; p. 131. [Google Scholar]
- Cobo, F. Métodos de control de las floraciones de cianobacterias en aguas continentals. Limnetica 2015, 34, 247–268. [Google Scholar]
- Brittain, S.; Mohamed, Z.; Wang, J.; Lehmann, V.; Carmichael, W.; Rinehart, K. Isolation and characterization of microcystins from a River Nile strain of Oscillatoria tenuis Agardh ex Gomont. Toxicon 2000, 38, 1759–1771. [Google Scholar] [CrossRef]
- Hitzfeld, B.; Lampert, C.; Spaeth, N.; Mountfort, D.; Kaspar, H.; Dietrich, D. Toxin production in cyanobacterial mats from ponds on the McMurdo Ice Shelf, Antarctica. Toxicon 2000, 38, 1731–1748. [Google Scholar] [CrossRef] [Green Version]
- Freitas de Magalhães, V.F.; Soares, R.M.; Azevedo, S.M. Microcystin contamination in fish from the Jacarepaguá Lagoon (Rio de Janeiro, Brazil): Ecological implication and human health risk. Toxicon 2001, 39, 1077–1085. [Google Scholar] [CrossRef]
- Park, H.; Namikoshi, M.; Brittain, S.; Carmichael, W.; Murphy, T. [d-Leu1] microcystin-LR, a new microcystin isolated from waterbloom in a Canadian prairie lake. Toxicon 2001, 39, 855–862. [Google Scholar] [CrossRef]
- Humpage, A. Toxin Types, Toxicokinetics and Toxicodynamics. In Cyanobacterial Harmful Algal Blooms: State of the Science and Research Needs; Springer: Berlin/Heidelberg, Germany, 2008; pp. 383–415. [Google Scholar]
- Bláha, L.; Babica, P.; Maršálek, B. Toxins produced in cyanobacterial water blooms—Toxicity and risks. Interdiscip. Toxicol. 2009, 2, 36. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Otten, T.G. Harmful Cyanobacterial Blooms: Causes, Consequences, and Controls. Microb. Ecol. 2013, 65, 995–1010. [Google Scholar] [CrossRef]
- Svirčev, Z.; Lalić, D.; Savić, G.B.; Tokodi, N.; Backović, D.D.; Chen, L.; Meriluoto, J.; Codd, G.A. Global geographical and historical overview of cyanotoxin distribution and cyanobacterial poisonings. Arch. Toxicol. 2019, 93, 2429–2481. [Google Scholar] [CrossRef]
- Sivonen, K.; Jones, G. Chapter 3: Cyanobacterial toxins. In Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Chorus, I., Bartram, J., Eds.; Taylor & Francis: Abingdon, UK, 1999; pp. 14–111. [Google Scholar]
- Jones, G.J. Cyanobacterial Research in Australia; CSIRO: Melbourne, Australia, 1994; p. 193. [Google Scholar]
- Chorus, I.; Bartram, J. Toxic Cyanobateria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; World Health Organization: London, UK, 1999; p. 416. [Google Scholar]
- Niamien-Ebrottie, J.E.; Bhattacharyya, S.; Deep, P.R.; Nayak, B. Cyanobacteria and cyanotoxins in the World: Review. Int. J. Appl. Res. 2015, 1, 563–569. [Google Scholar]
- Chorus, I.; Welker, M. Toxic Cyanobacteria in Water: A Guide to Their Public Health Consequences, Monitoring and Management; Taylor & Francis: Abingdon, UK, 2021; p. 858. [Google Scholar]
- Huisman, J.; Codd, G.A.; Paerl, H.W.; Ibelings, B.W.; Verspagen, J.M.; Visser, P.M. Cyanobacterial blooms. Nat. Rev. Microbiol. 2018, 16, 471–483. [Google Scholar] [CrossRef]
- Lucena, E. Aspectos sanitarios de las cianotoxinas. Hig. Sanid. Ambient. 2008, 8, 291–302. [Google Scholar]
- Cameán Fernandez, A.M.; Moreno Navarro, I.; Repetto Kuhn, G.; Pichardo Sánchez, S.; Prieto Ortega, A.I. Ciano-bacterias y cianotoxinas: Necesidad de su control en el agua de consumo humano. Rev. Salud Ambient. 2005, 5, 137–141. [Google Scholar]
- Kapsalis, V.C.; Kalavrouziotis, I.K. Eutrophication—A worldwide water quality issue. In Chemical Lake Restoration; Springer: Cham, Switzerland, 2021; pp. 1–21. [Google Scholar]
- Sivonen, K. Cyanobacterial toxins and toxin production. Phycology 1996, 35, 12–24. [Google Scholar] [CrossRef]
- Dasí, M.J.; Miracle, M.R.; Camacho, A.; Soria, J.M.; Vicente, E. Summer phytoplankton assemblages across trophic gradients in hard-water reservoirs. Phytoplankton Trophic Gradients 1998, 369, 27–43. [Google Scholar] [CrossRef]
- Sanchis, D.; Padilla, C.; Fernández-Valiente, E.; Del Campo, F.F.; Carrasco, D.; Leganés, F.; Quesada, A. Spatial and temporal heterogeneity in succession of cyanobacterial blooms in a Spanish reservoir. Ann. Limnol. Int. J. Limnol. 2002, 38, 173–183. [Google Scholar] [CrossRef] [Green Version]
- Cook, C.M.; Vardaka, E.; Lanaras, T. Toxic Cyanobacteria in Greek Freshwaters, 1987—2000: Occurrence, Toxicity, and Impacts in the Mediterranean Region. Acta Hydrochim. Hydrobiol. 2004, 32, 107–124. [Google Scholar] [CrossRef]
- Carvalho, L.; Poikane, S.; Solheim, A.L.; Phillips, G.; Borics, G.; Catalan, J.; De Hoyos, C.; Drakare, S.; Dudley, B.J.; Jarvinen, M.; et al. Strength and uncertainty of phytoplankton metrics for assessing eutrophication impacts in lakes. Hydrobiologia 2013, 704, 127–140. [Google Scholar] [CrossRef] [Green Version]
- Xia, R.; Zhang, Y.; Critto, A.; Wu, J.; Fan, J.; Zheng, Z.; Zhang, Y. The Potential Impacts of Climate Change Factors on Freshwater Eutrophication: Implications for Research and Countermeasures of Water Management in China. Sustainability 2016, 8, 229. [Google Scholar] [CrossRef] [Green Version]
- Paerl, H.W.; Huisman, J. Blooms Like It Hot. Science 2008, 320, 57–58. [Google Scholar] [CrossRef] [Green Version]
- Confederación Hidrográfica del Ebro. Diagnóstico y Gestión Ambiental de Embalses en el Ámbito de la Cuenca Hidrográfica del Ebro. Embalse de Ullivarri. 1996; pp. 602–612. Available online: https://hispagua.cedex.es/documentacion/documento/32331 (accessed on 11 October 2017).
- Jaurlaritza, E.; Vasco, G. Caracterización de las Masas de Aguas Superficiales de la CAPV. Tomo III: Caracterización de los Embalses de la CAPV. 2002; p. 197. Available online: http://www.uragentzia.euskadi.eus/contenidos/libro/caracterizacion_masas_agua/es_12298/adjuntos/T03_1EmbalsesMemoria.pdf (accessed on 11 October 2017).
- Confederación Hidrográfica del Duero. Informe Sobre Estado Trófico de los Embalses de la Cuenca del Duero. 2006. Available online: http://www.chduero.es/descarga.aspx?fich=/CalidadAguas/Estado_trofico_embalses_2006.pdf (accessed on 11 October 2017).
- Infraeco & Confederación Hidrográfica del Ebro. Ejecución de Trabajos Relacionados con los Requisitos de la Directiva Marco (2000/60/CE) en el Ámbito de la Confederación Hidrográfica del Ebro Referidos a: Elaboración del Registro de Zonas Protegidas, Determinación del Potencial Ecológico de los Embalses, Desarrollo de Programas Específicos de Investigación. Documento I: Memoria. 2006; p. 234. Available online: https://www.chebro.es/es/web/guest/search?q=2006_Embalses_Memoria.pdf (accessed on 11 October 2017).
- Infraeco & Confederación Hidrográfica del Ebro. Ejecución de Trabajos Relacionados con los Requisitos de la Directiva Marco (2000/60/CE) en el Ámbito de la Confederación Hidrográfica del Ebro Referidos a: Elaboración del Registro de Zonas Protegidas, Determinación del Potencial Ecológico de los Embalses, Desarrollo de Programas Específicos de Investigación. Embalse de Urrúnaga. 2006; p. 41. Available online: https://www.chebro.es/documents/20121/49621/Informe_Final_Embalse_de_Urrunaga_2004_2005.pdf (accessed on 11 October 2017).
- UTE Red Biológica Ebro & Confederación Hidrográfica del Ebro. Informe Final del Embalse de Mequinenza, Pena y Rialp, año 2007. Available online: http://195.55.247.234/webcalidad/estudios/embalses/2007embalsesbio/2007_reservoir_name.pdf (accessed on 28 September 2017).
- Confederación Hidrográfica del Júcar. Explotación de la Red de Control Biológico de Lagos y Embalses de la Confederación Hidrográfica del Júcar. Informe Anual de Embalses 2016. Available online: http://www.chj.es/es-es/medioambiente/redescontrol/InformesAguasSuperficiales/Biol%C3%B3gico%20Embalses%20anual%202016.pdf (accessed on 2 October 2017).
- Junta de Andalucía. Programa de Seguimiento del Estado de Calidad de las Aguas Continentales de las Cuencas Intra-Comunitarias de la Comunidad Autónoma de Andalucía. Demarcación Hidrográfica de las Cuencas Mediterráneas Andaluzas. Diseño y Explotación del Programa de Control de Calidad Biológica e Hidromorfológica de las Aguas Superficiales en la Demarcación de las Cuencas Mediterráneas Andaluzas. Informe Resultados Segunda Campaña 2014. Available online: https://www.juntadeandalucia.es/medioambiente/portal_web/web/temas_ambientales/agua/2_calidad/superficiales_continentales/datos/informes_calidad_dmed/2014/sp_md_2c_bhm_2014.pdf (accessed on 2 October 2017).
- Confederación Hidrográfica del Tajo. Resultados/Informes: Aguas Superficiales Potencial Ecológico en Empalses. 2017; p. 68. Available online: http://www.chtajo.es/LaCuenca/CalidadAgua/Resultados_Informes/Paginas/RISupPotencialEmbalses.aspx (accessed on 5 October 2017).
- Xunta de Galicia. SIAM: Seguimento de Cianobacterias nos Encoros. 2017. Available online: http://siam.xunta.gal/resultados-do-seguimento-de-cianobacterias (accessed on 10 October 2017).
- Forján-Lozano, E.; Domínguez Vargas, M.J.; Vilchez Lobato, C.; Miguel, R.; Costa, C.; Reis, M.P. Cianoalerta: Estra-tegia para predecir el desarrollo de Cianobacterias tóxicas en embalses. Ecosistemas 2008, 17, 37–45. [Google Scholar]
- Lago, L. Tratamientos de Inhibición de las Floraciones de Cianobacterias en Condiciones Controladas Mediante el uso de Limnocorrales. Ph.D. Thesis, University of Santiago de Compostela, Galicia, Spain, 2015. (In Spanish). [Google Scholar]
- Planas, D. Distribution and productivity of the phytoplankton in Spanish reservoirs. SIL Proc. 1975, 19, 1860–1870. [Google Scholar] [CrossRef]
- Quesada, A.; Carrasco, D.; Sanchis, D.; De Hoyos, C. Abundancia de cianobacterias y cianotoxinas en embalses españoles. Rev. Salud Ambient. 2005, 1, 99. [Google Scholar]
- Bennasar, G.; Frau, C.; García, L.; Gómez, M.; Moyá, G.; Ramón, G. Composición cualitativa del fitoplancton de los embalses de Cúber y Gorg Blau (Serra de Tramuntana, Mallorca) I. Cyanophyta Dinophyta. Bolletí Soc. D’història Nat. Balear. 1990, 33, 87–98. [Google Scholar]
- Carrasco, D.; Moreno, E.; Sanchis, D.; Wörmer, L.; Paniagua, T.; Del Cueto, A.; Quesada, A. Cyanobacterial abundance and microcystin occurrence in Mediterranean water reservoirs in Central Spain: Microcystins in the Madrid area. Eur. J. Phycol. 2006, 41, 281–291. [Google Scholar] [CrossRef]
- Caputo, L.; Naselli-Flores, L.; Ordoñez, J.; Armengol, J. Phytoplankton distribution along trophic gradients within and among reservoirs in Catalonia (Spain). Freshw. Biol. 2008, 53, 2543–2556. [Google Scholar] [CrossRef]
- Negro, A.I.; De Hoyos, C.; Del Rio, A.; Le Cohu, R. Comparación de las comunidades fitoplanctónicas en dos embalses de reciente creación: Riaño y Valparaíso (España). Limnetica 1994, 10, 115–121. [Google Scholar] [CrossRef]
- López, P.; Marcé, R.; Ordóñez, J.; Urrutia, I.; Armengol, J. Sedimentary phosphorus in a cascade of five reservoirs (Lozoya River, Central Spain). Lake Reserv. Manag. 2009, 25, 39–48. [Google Scholar] [CrossRef] [Green Version]
- Alonso-Fernández, J.R.A.; Nieto, P.J.G.; Muñiz, C.D.; Antón, J.C.Á. Modeling eutrophication and risk prevention in a reservoir in the Northwest of Spain by using multivariate adaptive regression splines analysis. Ecol. Eng. 2014, 68, 80–89. [Google Scholar] [CrossRef]
- Lago, L.; Barca, S.; Vieira-Lanero, R.; Cobo, F. Floraciones de cianobacterias y valores de microcistina-LR sestónica y disuelta en embalses de la cuenca hidrográfica del Miño-Sil (NW-España). MOL 2016, 16, 113–125. [Google Scholar]
- Vidal-Celma, A. Evolution d’un lac de barrage dans le NE. de l’Espagne pendant les quatre premières années de service. SIL Proc. 1969, 17, 191–200. [Google Scholar] [CrossRef]
- Toja, J.; Casco, M.A. Contribution of phytoplankton and periphyton to the production in a reservoir of S.W. Spain. Oecologia Aquat. 1991, 10, 61–76. [Google Scholar]
- Negro, A.I.; De Hoyos, C.; Vega, J.C. Phytoplankton structure and dynamics in Lake Sanabria and Valparaíso reservoir (NW Spain). Trophic Spectr. Revisit. 2000, 424, 25–37. [Google Scholar] [CrossRef]
- Gomá, J. El fitopláncton de l´embassament de Foix: Estudi del cicle annual (1996), Trobada d´Estudiosos del Foix; Barcelona Provincial Council: Barcelona, Spain, 2005; pp. 35–41.
- Moreno, I.M.; Pereira, P.; Franca, S.; Cameán, A. Toxic cyanobacteria strains isolated from blooms in the Guadiana river (southwestern Spain). Biol. Res. 2004, 37, 405–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, I.; Repetto, G.; Carballal, E.; Gago, A.; Cameán, A.M. Cyanobacteria and microcystins occurrence in the Guadiana River (SW Spain). Int. J. Environ. Anal. Chem. 2005, 85, 461–474. [Google Scholar] [CrossRef]
- Moreno-Ostos, E.; Elliott, J.A.; Cruz-Pizarro, L.; Escot, C.; Basanta, A.; George, G. Using a numerical model (PRO-TECH) to examine the impact of water transfers on phytoplankton dynamics in a Mediterranean reservoir. Limnetica 2007, 26, 1–11. [Google Scholar] [CrossRef]
- Moreno-Ostos, E.; Palomino-Torres, R.L.; Escot, C.; Blanco, J.M. Planktonic metabolism in a Mediterranean res-ervoir during a near-surface cyanobacterial bloom. Limnetica 2016, 35, 117–130. [Google Scholar]
- Padilla, C.; Sanz-Alférez, S.; Del Campo, F.F. Toxin characterisation and identification of a Microcystis flos-aquae strain from a Spanish drinking-water reservoir. Arch. Für Hydrobiol. 2006, 165, 383–399. [Google Scholar] [CrossRef]
- Quesada, A.; Moreno, E.; Carrasco, D.; Paniagua, T.; Wörmer, L.; De Hoyos, C.; Sukenik, A. Toxicity of Aphanizomenon ovalisporum(Cyanobacteria) in a Spanish water reservoir. Eur. J. Phycol. 2006, 41, 39–45. [Google Scholar] [CrossRef]
- Becker, V.; Caputo, L.; Ordóñez, J.; Marcé, R.; Armengol, J.; Crossetti, L.O.; Huszar, V.L. Driving factors of the phytoplankton functional groups in a deep Mediterranean reservoir. Water Res. 2010, 44, 3345–3354. [Google Scholar] [CrossRef]
- Barco, M.; Flores, C.; Rivera, J.; Caixach, J. Determination of microcystin variants and related peptides present in a water bloom of Planktothrix (Oscillatoria) rubescens in a Spanish drinking water reservoir by LC/ESI-MS. Toxicon 2004, 44, 881–886. [Google Scholar] [CrossRef]
- Lago, L.; Barca, S.; Vieira-Lanero, R.; Cobo, F. Características ambientales, composición del fitoplancton y variación temporal de microcistina-LR disuelta en el embalse de As Forcadas (Galicia, NW España). Limnetica 2015, 34, 187–204. [Google Scholar]
- De Hoyos, C.; Negro, A.I.; Avilés, J. Las Cianobacterias en los embalses españoles: Situación actual. Centro de estudios hidrográficos del CEDEX. Ing. Civ. 2003, 129, 93. [Google Scholar]
- Quesada, A.; Sanchis, D.; Carrasco, D. Cyanobacteria in Spanish reservoirs. How frequently are they toxic? Limnetica 2004, 23, 109–118. [Google Scholar] [CrossRef]
- Aboal, M. Aportación al conocimiento de las algas epicontinentales del sudeste de España. III. Cianofíceas (Cyanop-hyceae, Schaffner, 1909). An. Del Jardín Botánico De Madr. 1988, 45, 3–46. [Google Scholar]
- Armengol, J.; Catalán, J.; Gabellone, N.; Jaume, D.; de Manuel, J.; Martí, E.; Morguí, J.A.; Nolla, J.; Peñuelas, J.; Real, M.; et al. A comparative limnological study of the Guadalhorce reservoirs system (Málaga, S.E. Spain). Sci. Gerund. 1990, 16, 27–41. [Google Scholar]
- Álvarez Cobelas, M.; Arauzo, M. Phytoplankton responses to varying time scales in a eutrophic reservoir. Arch. Für Hydrobiol.-Monogr. Beiträge 1994, 40, 69–80. [Google Scholar]
- Arauzo, M.; Álvarez Cobelas, M. Respuesta de la comunidad fitoplanctónica a la estacionalidad en un embalse eutrófico. Limnetica 1994, 10, 37–42. [Google Scholar] [CrossRef]
- Aboal, M.; Puig, M. Intracellular and dissolved microcystin in reservoirs of the river Segura basin, Murcia, SE Spain. Toxicon 2005, 45, 509–518. [Google Scholar] [CrossRef]
- Cirés, S.; Quesada, A. Catálogo de Cianobacterias Planctónicas Potencialmente Tóxicas de las Aguas Continentales Españolas; Ministerio de Medio Ambiente y Medio Rural y Marino, Secretaría General Técnica Centro de Publicaciones: Madrid, Spain, 2011; ISBN 978-84-491-1072-6S. [Google Scholar]
- Agha Frías, R. Chemotypical Subpopulations in Planktonic Cyanobacteria. Ecology and Application in Advanced Monitoring Tools. Doctoral Thesis, Universidad Autónoma de Madrid, Madrid, Spain, 2013. (In Spanish). [Google Scholar]
- Cirés, S.; Wörmer, L.; Carrasco, D.; Quesada, A. Sedimentation Patterns of Toxin-Producing Microcystis Morphospecies in Freshwater Reservoirs. Toxins 2013, 5, 939–957. [Google Scholar] [CrossRef] [Green Version]
- Cirés, S.; Wörmer, L.; Agha, R.; Quesada, A. Overwintering populations of Anabaena, Aphanizomenon and Microcystis as potential inocula for summer blooms. J. Plankton Res. 2013, 35, 1254–1266. [Google Scholar] [CrossRef] [Green Version]
- Mehnert, G.; Leunert, F.; Cirés, S.; Jöhnk, K.; Rücker, J.; Nixdorf, B.; Wiedner, C. Competitiveness of invasive and native cyanobacteria from temperate freshwaters under various light and temperature conditions. J. Plankton Res. 2010, 32, 1009–1021. [Google Scholar] [CrossRef]
- KomárekJ. A polyphasic approach for the taxonomy of cyanobacteria: Principles and applications. Eur. J. Phycol. 2016, 51, 346–353. [Google Scholar] [CrossRef]
- Margalef, R.; Planas, D.; Armengol, J.; Vidal, A.; Prat, N.; Guisset, A.; Toja, J.; Estrada, M. Limnología de los embalses españoles. In Dirección General de Obras Hidraúlicas; M.O.P.: Madrid, Spain, 1976; p. 123. [Google Scholar]
- Sabater, S.; Nolla, J. Distributional patterns of phytoplankton in Spanish reservoirs: First results and comparison after fifteen years. SIL Proc. 1991, 24, 1371–1375. [Google Scholar] [CrossRef]
- Riera, J.L.; Jaume, D.; de Manuel, J.; Morgui, J.A.; Armengol, J. Patterns of variation in the limnology of Spanish reservoirs: A regional study. Limnetica 1992, 8, 111–123. [Google Scholar] [CrossRef]
- Negro, A.I.; De Hoyos, C. Relationships between diatoms and the environment in Spanish reservoirs. Limnetica 2005, 24, 133–144. [Google Scholar] [CrossRef]
- Junta de Andalucía. Programa de Seguimiento del Estado de Calidad de las Aguas Continentales de las Cuencas Intra-Comunitarias de la Comunidad Autónoma de Andalucía. Demarcación Hidrográfica de las Cuencas Mediterráneas Andaluzas. Control de la Calidad de las Aguas Superficiales. Evaluación de Organismos y de Índices Biológicos e Hidromorfológicos. Segundo Semestre de 2012 (Julio-Diciembre). 2012. Available online: https://www.juntadeandalucia.es/medioambiente/portal_web/gestion_integral_agua/actuaciones/mejora_calidad_agua/calidad_medio_hidrico/contenido_calidad_continentales/calidad_aguas%20superficiales/informes_calidad_dmed/2012/sp_md_2s_bhm_2012.pdf (accessed on 2 October 2017).
- Confederación Hidrográfica Guadiana. Red Biológica de Embalses y Lagos 2007–2008. Available online: http://www.chguadiana.es/corps/chguadiana/data/resources/file/red_ica/red_bio_embalses_lagos_2007_2008/resultados.pdf (accessed on 20 October 2017).
- Confederación Hidrográfica Guadiana. Elementos de Calidad Fisicoquímicos y Elementos de Calidad Biológica: Fitoplancton de los Años 2007 y 2008. Available online: http://www.chguadiana.es/corps/chguadiana/data/resources/file/red_ica/red_bio_embalses_lagos_2007_2008/resultados_por_embalse/reservoir_name.pdf (accessed on 20 October 2017).
- Confederación Hidrográfica del Júcar. Explotación de la red de Control Biológico de Lagos y Embalses de la Confede-Ración Hidrográfica del Júcar. Informe Anual de Embalses 2015. Available online: http://www.chj.es/es-es/medioambiente/redescontrol/InformesAguasSuperficiales/Biol%C3%B3gico%20Embalses%20anual%202015.pdf (accessed on 2 October 2017).
- Junta de Andalucía. Servicio para la Explotación de los Programas de Control de Calidad Biológicos e Hidromorfológricos de las Aguas Superficiales en las Demarcaciones de las Cuencas de los ríos Guadalete y Barbate y Tinto, Odiel y Piedras. Demarcación Hidrográfica Tinto, Odiel y Piedras. Datos Obtenidos en la Identificación de Muestras y Resultados de los Indicadores Biológicos e Idromorfológicos. Informe de Resultados de la 2º Campaña 2014. 2015. Available online: http://www.juntadeandalucia.es/medioambiente/portal_web/web/temas_ambientales/agua/2_calidad/superficiales_continentales/datos/informes_calidad_top/2014/sp_top_2c_bhm_2014.pdf (accessed on 2 October 2017).
- De Hoyos, C.; Negro, A.; Aldasoro, J.J. Cianobacteria distribution and abundance in the Spanish water reservoirs during thermal stratification. Limnetica 2004, 23, 119–132. [Google Scholar] [CrossRef]
- Confederación Hidrográfica Miño-Sil. Capítulo 8. Diagnóstico del Cumplimiento de los Objetivos Medioambientales. Plan Hidrológico del Ciclo 2009–2015. 2015, p. 187. Available online: https://www.chminosil.es/es/chms/planificacionhidrologica/planhidrologico (accessed on 11 October 2017).
- Confederación Hidrográfica Miño-Sil. Capítulo 8. Objetivos Medioambientales y Exenciones. Plan Hidrológico del ciclo 2016–2021. 2015; p. 391. Available online: https://www.chminosil.es/es/ide-mino-sil/80-chms/1359-plan-hidrologico-2015-2021-rd-1-2016 (accessed on 11 October 2017).
- García-Nieto, P.; Fernández, J.A.; Juez, F.D.C.; Lasheras, F.S.; Muñiz, C.D. Hybrid modelling based on support vector regression with genetic algorithms in forecasting the cyanotoxins presence in the Trasona reservoir (Northern Spain). Environ. Res. 2013, 122, 1–10. [Google Scholar] [CrossRef]
- Cobo, F. Floracións de Cianobacterias tóxicas en augas continentais. CERNA 2008, 54, 24–28. [Google Scholar]
- Komarek, J. Cyanobacterial taxonomy: Current problems and prospects for the integration of traditional and molecular approaches. Algae 2006, 21, 349–375. [Google Scholar] [CrossRef] [Green Version]
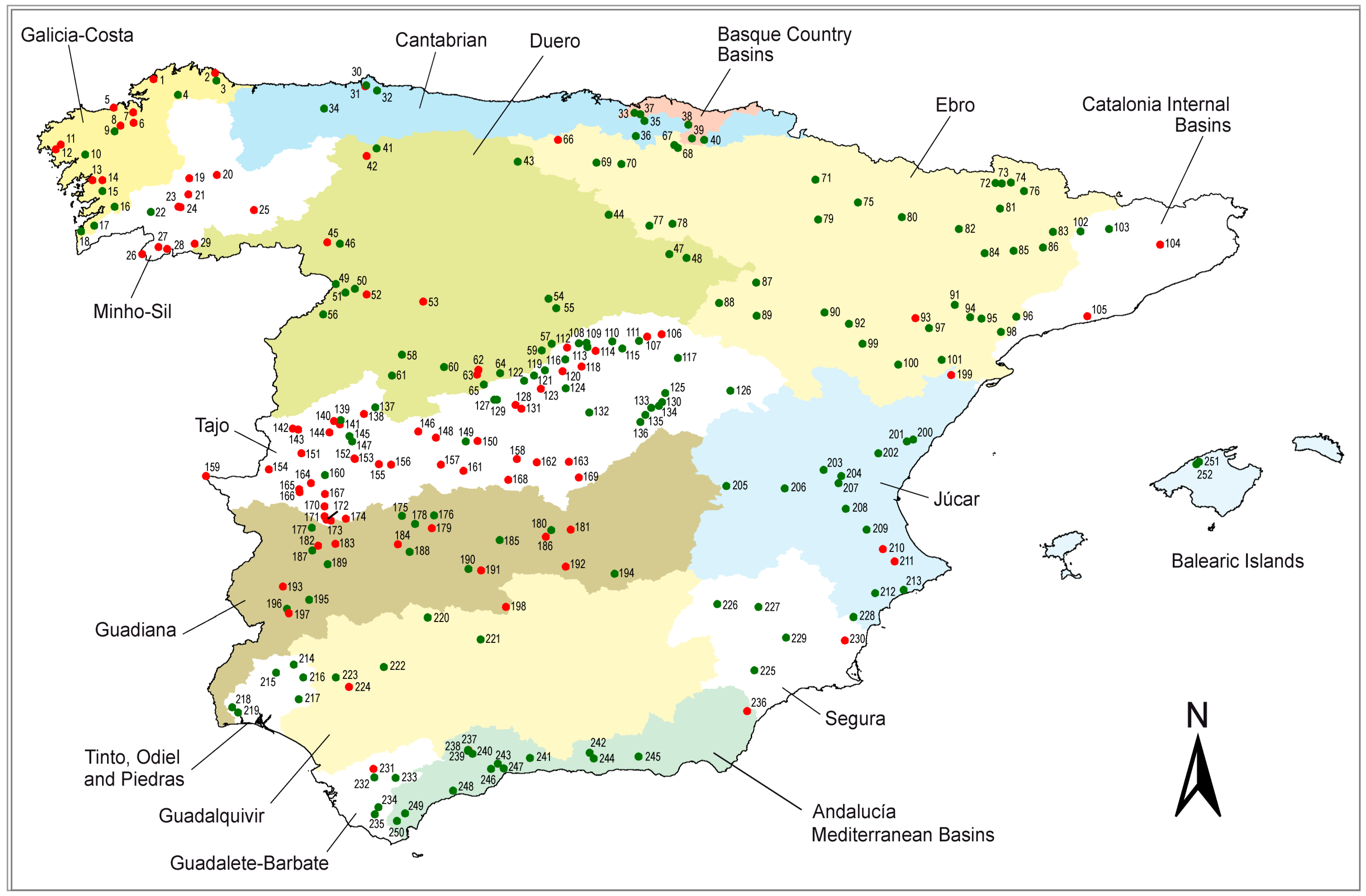

| RBD | Reservoirs in the RBD | With Cyanobateria | % | With Registered Blooms | % |
|---|---|---|---|---|---|
| G-C | 27 | 18 | 66.67 | 10 | 37.04 |
| M-S | 60 | 11 | 18.33 | 10 | 16.67 |
| C | 46 | 7 | 15.22 | 1 | 2.17 |
| BCB | 14 | 4 | 28.57 | 0 | 0.00 |
| D | 80 | 25 | 31.25 | 6 | 7.50 |
| E | 175 | 36 | 20.57 | 2 | 1.14 |
| CIB | 13 | 4 | 30.77 | 2 | 15.38 |
| T | 217 | 69 | 31.8 | 41 | 18.89 |
| Gd | 92 | 24 | 26.09 | 11 | 11.96 |
| J | 46 | 15 | 32.61 | 3 | 6.52 |
| TOP | 33 | 6 | 18.18 | 0 | 0.00 |
| Gq | 96 | 5 | 5.21 | 1 | 1.04 |
| S | 34 | 6 | 17.65 | 1 | 2.94 |
| G-B | 15 | 5 | 33.33 | 1 | 6.67 |
| AMB | 38 | 15 | 39.47 | 2 | 2.26 |
| BI | 2 | 2 | 100.00 | 0 | 0.00 |
| Total | 988 | 252 | 91 |
| Water Use | Total Reservoirs | Reservoirs with Cyanobacteria | Reservoirs with Registered Blooms | Number of Blooms Registered |
|---|---|---|---|---|
| Aqu | 2 | 0 | 0 | 0 |
| Fd | 36 | 6 | 1 | 1 |
| Fish | 4 | 0 | 0 | 0 |
| Hyd | 280 | 58 | 23 | 61 |
| Ind | 32 | 9 | 4 | 11 |
| Irr | 193 | 33 | 15 | 41 |
| Liv | 4 | 0 | 0 | 0 |
| Rec | 18 | 1 | 1 | 2 |
| Sto | 14 | 6 | 2 | 8 |
| Wd | 17 | 0 | 0 | 0 |
| Ws | 388 | 139 | 45 | 133 |
| Total | 988 | 252 | 91 | 257 |
| Genus | Distribution Range |
|---|---|
| Anabaena | 60.71 |
| Microcystis | 57.14 |
| Aphanizomenon | 50.00 |
| Aphanocapsa | 40.08 |
| Merismopedia | 38.89 |
| Pseudanabaena | 37.30 |
| Woronichinia | 35.32 |
| Oscillatoria | 30.95 |
| Chroococcus | 26.98 |
| Aphanothece | 22.22 |
| Planktothrix | 21.43 |
| Coelosphaerium | 9.92 |
| Anabaenopsis | 8.73 |
| Cylindrospermopsis | 7.94 |
| Phormidium | 7.54 |
| Limnothrix | 7.54 |
| Synechococcus | 7.54 |
| Geitlerinema | 7.14 |
| Snowella | 5.95 |
| Planktolyngbya | 5.56 |
| Spirulina | 5.16 |
| Raphidiopsis | 4.37 |
| Synechocystis | 4.37 |
| Romeria | 3.97 |
| Lyngbya | 2.78 |
| Nostoc | 2.38 |
| Gomphosphaeria | 2.38 |
| Cyanogranis | 1.98 |
| Cylindrospermum | 1.59 |
| Radiocystis | 1.59 |
| Jaaginema | 1.59 |
| Tolypothrix | 0.79 |
| Arthrospira | 0.40 |
| Dermocarpella | 0.40 |
| Gloeocapsa | 0.40 |
| Schizothrix | 0.40 |
| Chamaesiphon | 0.40 |
| Coelomoron | 0.40 |
| Species | Distribution Range |
|---|---|
| Anabaena spp. | 38.49 |
| Microcystis aeruginosa | 34.13 |
| Woronichinia naegeliana | 32.54 |
| Aphanizomenon flos-aquae | 30.16 |
| Microcystis spp. | 26.19 |
| Pseudanabaena spp. | 23.41 |
| M. tenuissima | 23.41 |
| Aphanizomenon gracile | 18.25 |
| Anabaena flos-aquae | 17.46 |
| Microcystis flos-aquae | 17.46 |
| Aphanizomenon spp. | 17.06 |
| Aphanocapsa spp. | 16.67 |
| Oscillatoria spp. | 16.27 |
| Oscillatoria limnetica | 15.87 |
| Planktothrix agardhii | 15.08 |
| Aphanocapsa holsatica | 14.29 |
| Anabaena spiroides | 13.89 |
| Aphanocapsa incerta | 13.49 |
| Anabaena circinalis | 13.10 |
| Oscillatoria agardhii | 13.10 |
| Anabaena planctonica | 12.70 |
| Merismopedia spp. | 12.70 |
| Chroococcus spp. | 12.30 |
| Species | Frequency of Appearance |
|---|---|
| Microcystis aeruginosa | 17.9 |
| Aphanizomenon flos-aquae | 12.84 |
| Microcystis spp. | 11.28 |
| Woronichinia naegeliana | 9.34 |
| Oscillatoria limnetica | 5.06 |
| Merismopedia tenuissima | 4.67 |
| Aphanizomenon gracile | 4.67 |
| Anabaena spp. | 4.28 |
| Pseudanabaena spp. | 3.5 |
| Merismopedia spp. | 3.5 |
| Planktothrix rubescens | 3.11 |
| Aphanocapsa spp. | 3.11 |
| Aphanizomenon spp. | 3.11 |
| Planktothrix agardhii | 2.72 |
| Microcystis flos-aquae | 2.72 |
| Anabaena planctonica | 2.72 |
| Anabaena flos-aquae | 2.33 |
| Pseudanabaena cfr. limnetica | 1.95 |
| Aphanocapsa holsatica | 1.95 |
| Anabaena aphanizomenoides | 1.95 |
| Oscillatoria agardhii | 1.56 |
| Geitlerinema spp. | 1.56 |
| Cyanogranis ferruginea | 1.56 |
| Woronichinia spp. | 1.17 |
| Pseudanabaena catenata | 1.17 |
| Limnothrix spp. | 1.17 |
| Cylindrospermopsis raciborskii | 1.17 |
| Anabaena crassa | 1.17 |
| Anabaena circinalis | 1.17 |
| Anabaena cfr. spiroides | 1.17 |
| Synechococcus cfr. nidulans | 0.78 |
| Microcystis smithii | 0.78 |
| Microcystis novacekii | 0.78 |
| Aphanocapsa cfr. grevillei | 0.78 |
| Aphanizomenon aphanizomenoides | 0.78 |
| Anabaena bergii | 0.78 |
| Anabaena affinis | 0.78 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira-Lanero, R.; Barca, S.; Cobo, M.C.; Cobo, F. Occurrence of Freshwater Cyanobacteria and Bloom Records in Spanish Reservoirs (1981–2017). Hydrobiology 2022, 1, 122-136. https://doi.org/10.3390/hydrobiology1010009
Vieira-Lanero R, Barca S, Cobo MC, Cobo F. Occurrence of Freshwater Cyanobacteria and Bloom Records in Spanish Reservoirs (1981–2017). Hydrobiology. 2022; 1(1):122-136. https://doi.org/10.3390/hydrobiology1010009
Chicago/Turabian StyleVieira-Lanero, Rufino, Sandra Barca, M. Carmen Cobo, and Fernando Cobo. 2022. "Occurrence of Freshwater Cyanobacteria and Bloom Records in Spanish Reservoirs (1981–2017)" Hydrobiology 1, no. 1: 122-136. https://doi.org/10.3390/hydrobiology1010009






