Insights into the Migration Routes and Historical Dispersion of Species Surviving the Messinian Crisis: The Case of Patella ulyssiponensis and Epizoic Rhodolith Lithophyllum hibernicum
Abstract
1. Introduction
2. Material and Methods
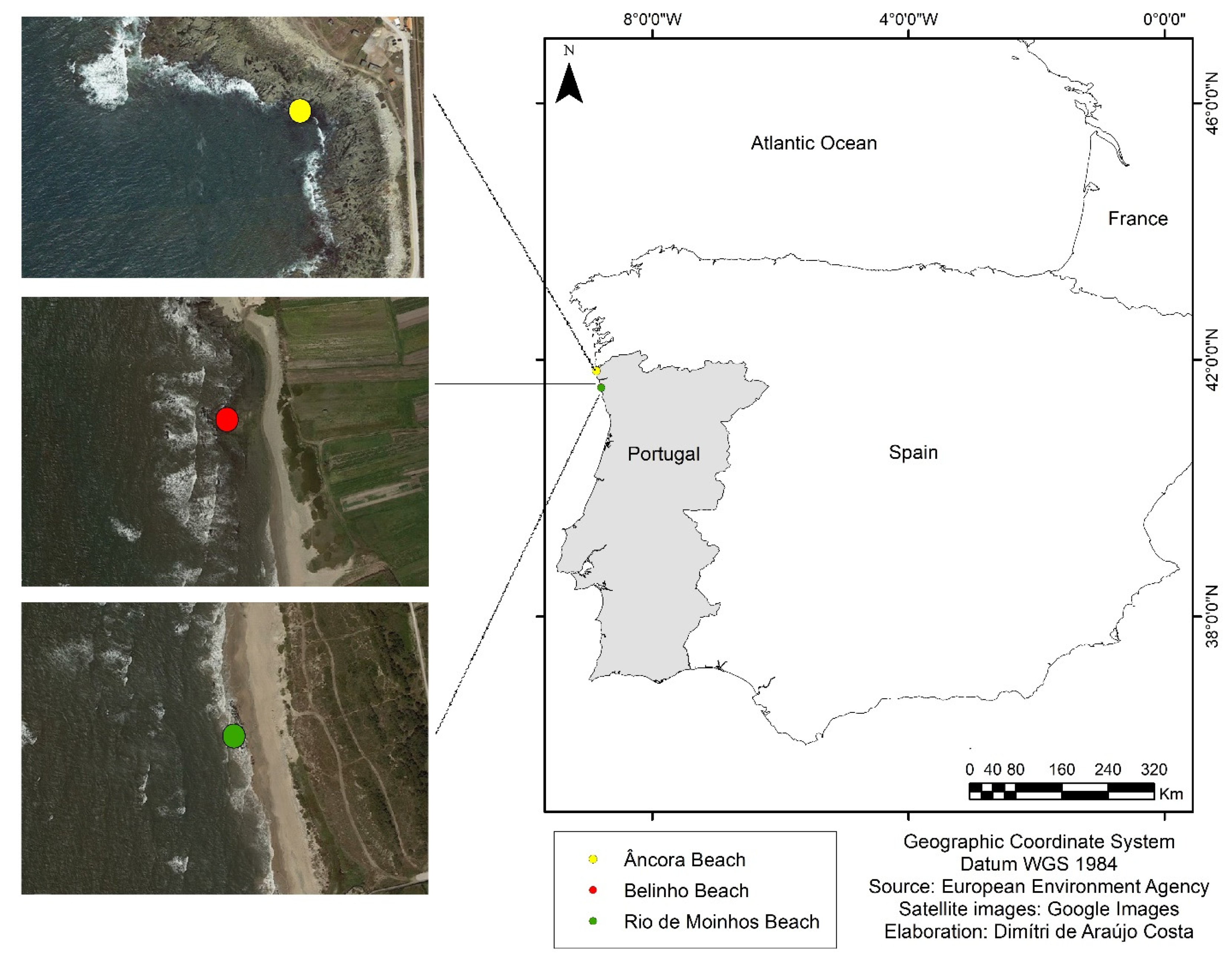
2.1. Study Area
2.2. Data Collection
2.3. Data Analyses
2.4. Geological Maps
3. Results
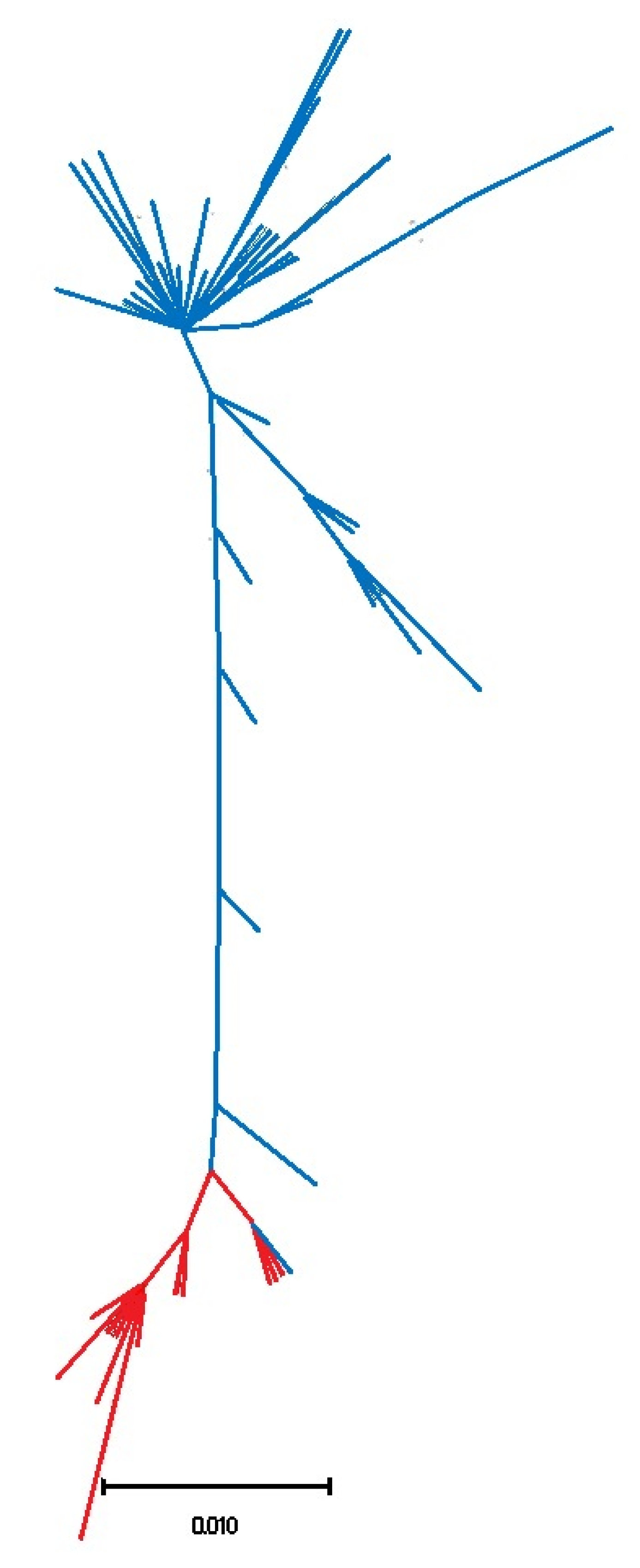
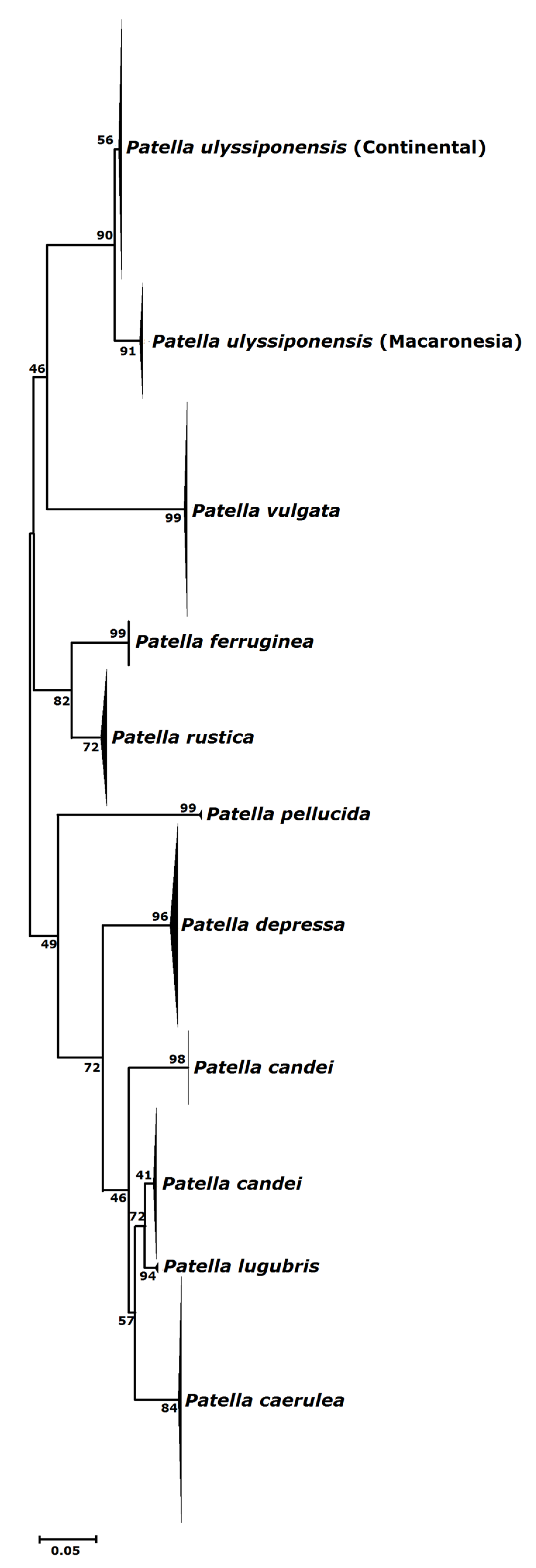
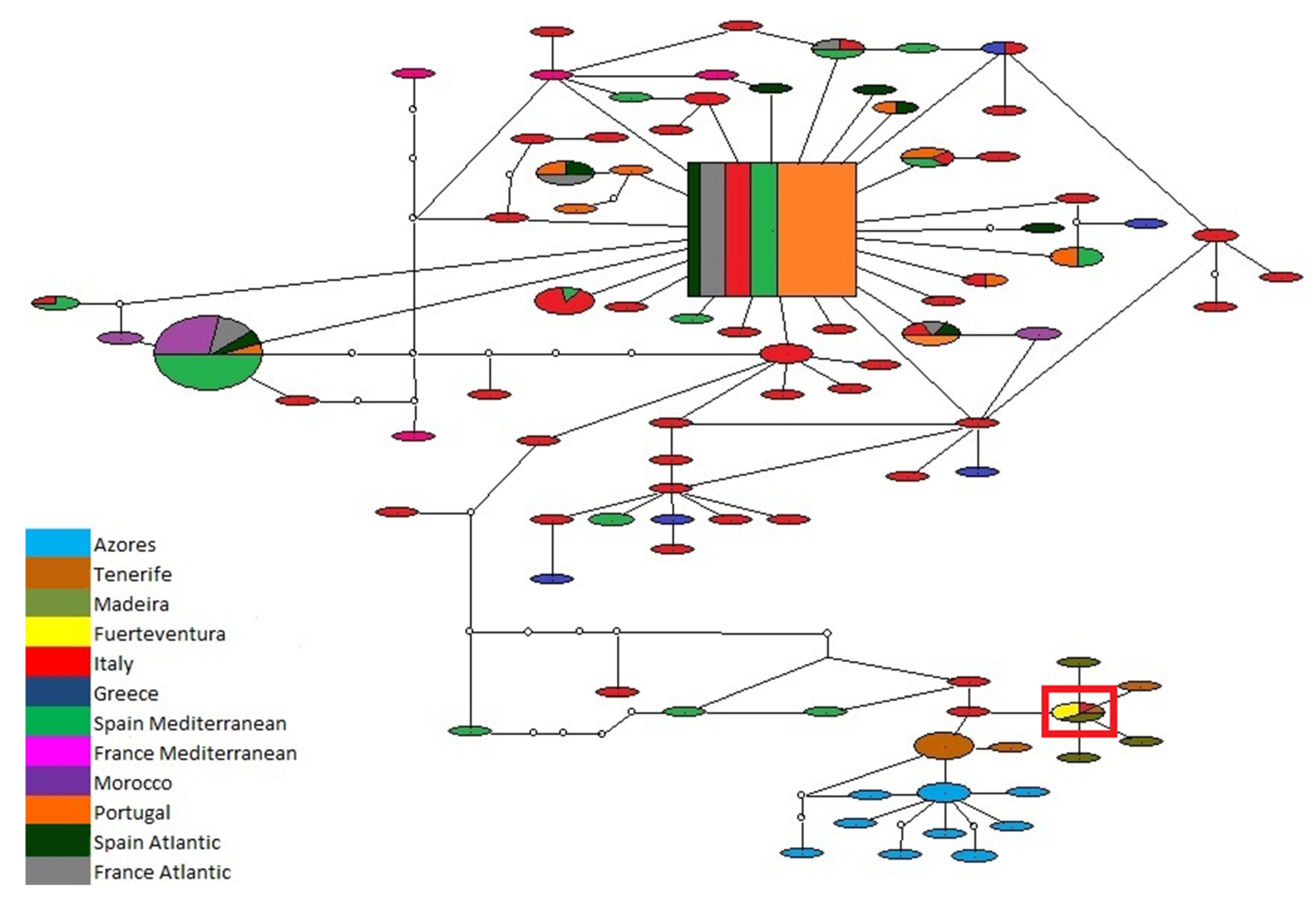
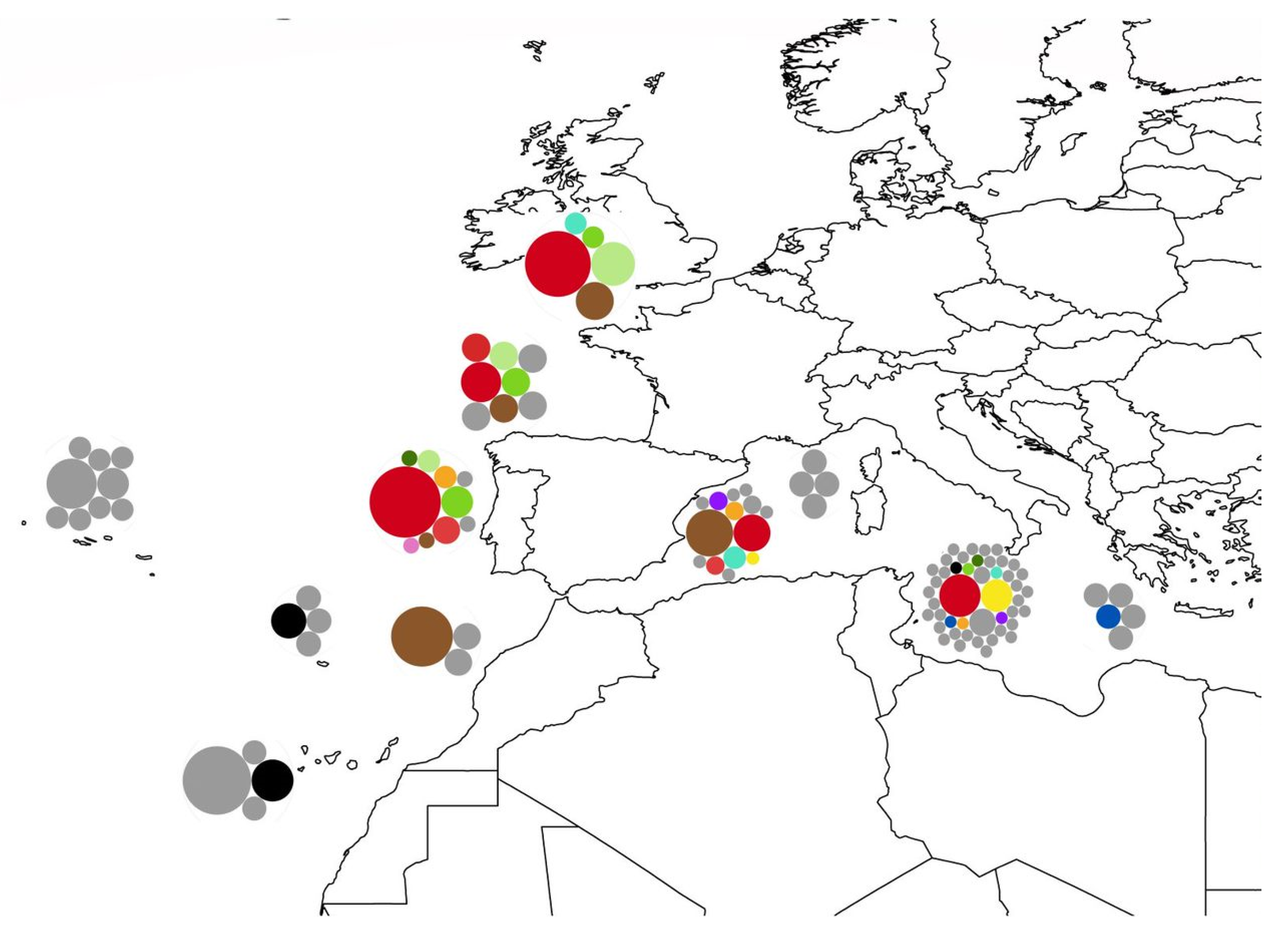
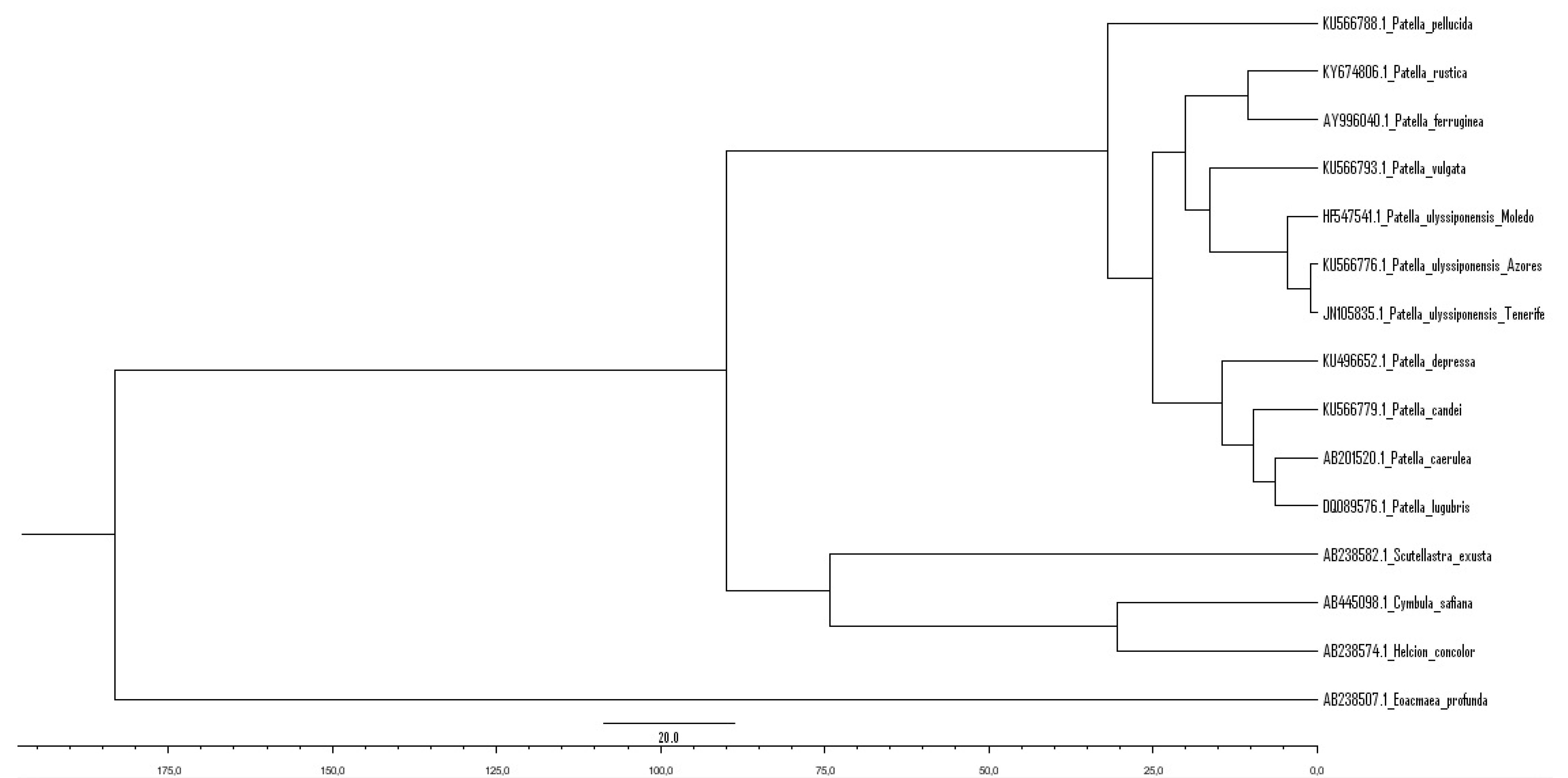
3.1. Biogeography Patterns and Genetic Analyses
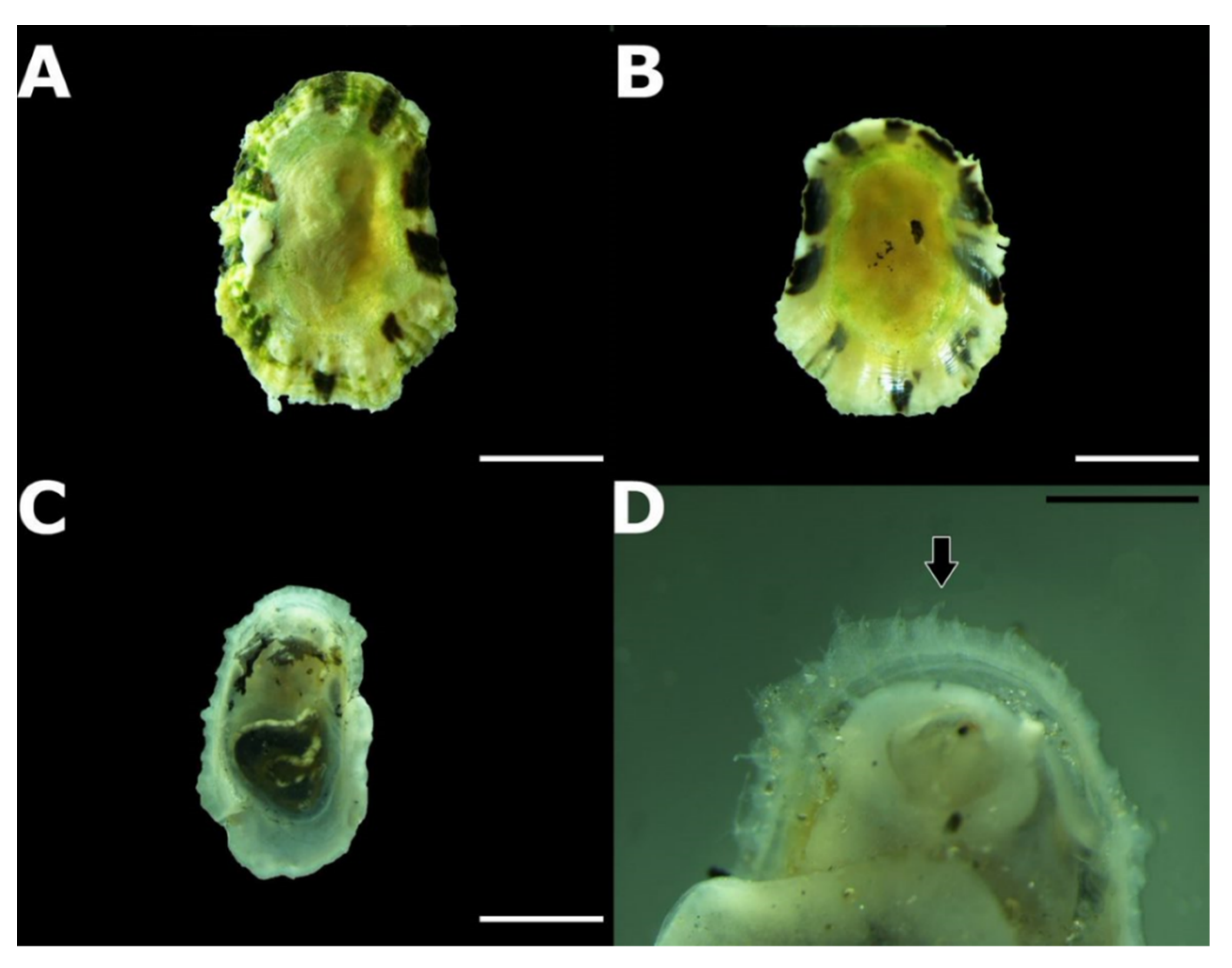
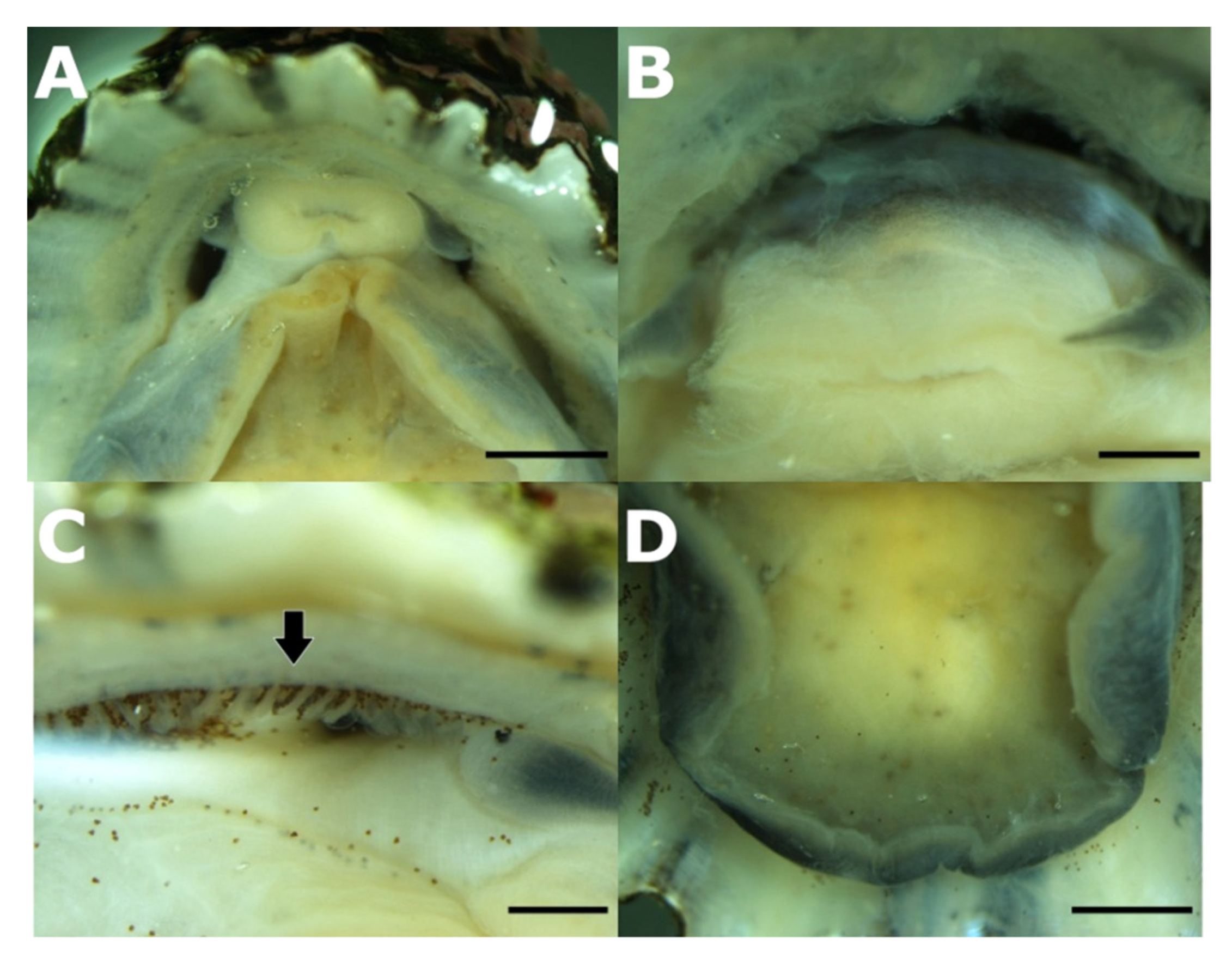
3.2. Taxonomic Account
- Kingdom Animalia Linnaeus, 1758
- Phylum Mollusca Cuvier, 1797
- Class Gastropoda Cuvier, 1795
- SubClass Patellogastropoda Lindberg, 1986
- SuperFamily Patelloidea Rafinesque, 1815
- Family Patellidae Rafinesque, 1815
- Genus Patella Linnaeus, 1758
- Patella ulyssiponensis Gmelin, 1791
- Kingdom Plantae Haeckel, 1866
- SubKingdom Biliphyta Cavalier-Smith, 1981
- Phylum Rhodophyta Wettstein, 1901
- Subphylum Eurhodophytina Saunders & Hommersand, 2004
- Class Florideophyceae Cronquist, 1960
- Subclass Corallinophycidae Le Gall & Saunders, 2007
- Order Corallinales Silva & Johansen, 1986
- Family Lithophyllaceae Athanasiadis, 2016
- Genus Lithophyllum Philippi, 1837
- Lithophyllum hibernicum Foslie, 1906
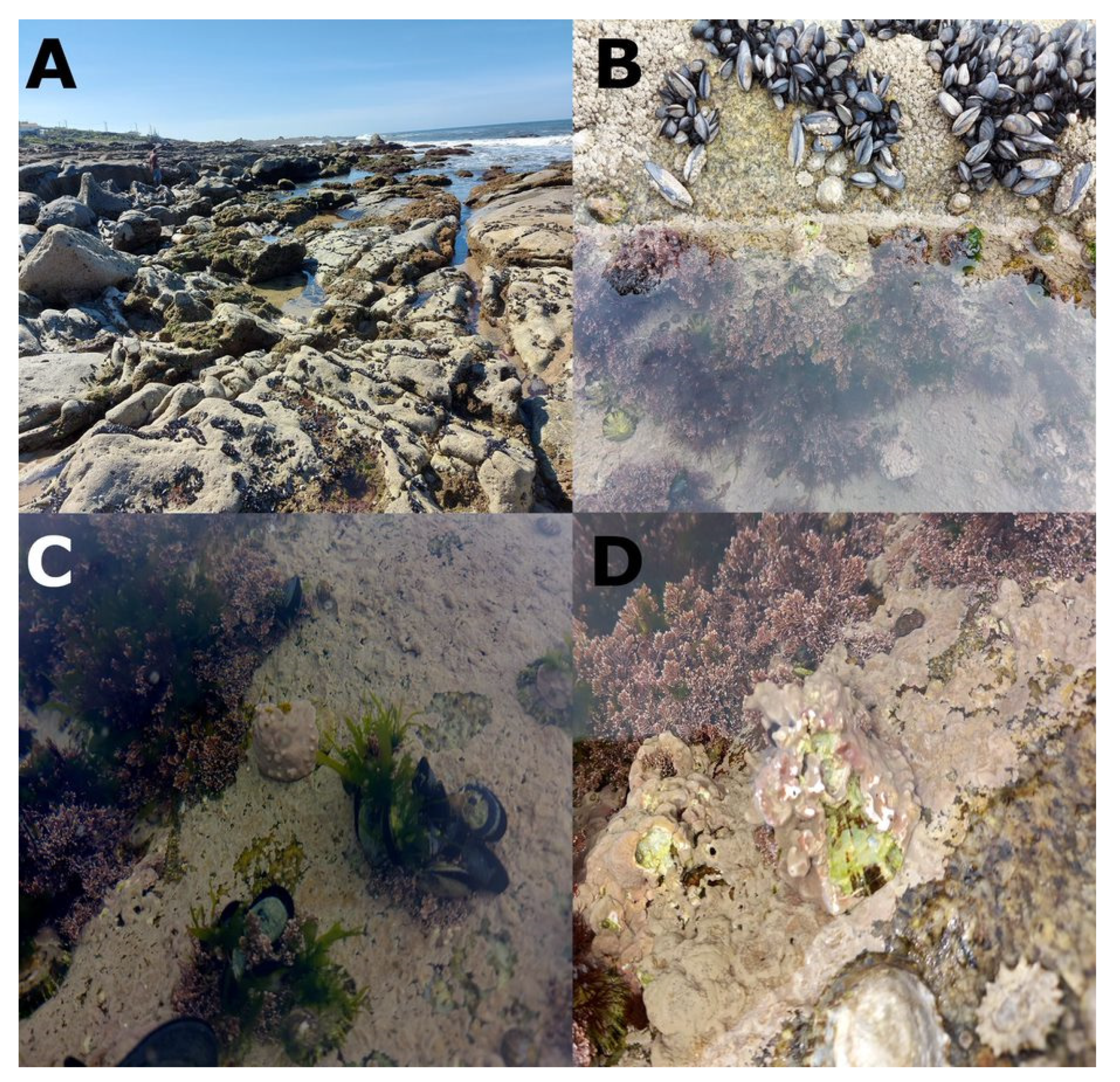
3.3. Association between P. ulyssiponensis and L. hibernicum
4. Discussion
4.1. Genetic Relationships of Two Patella ulyssiponensis Lineages
4.2. Biogeographic Patterns
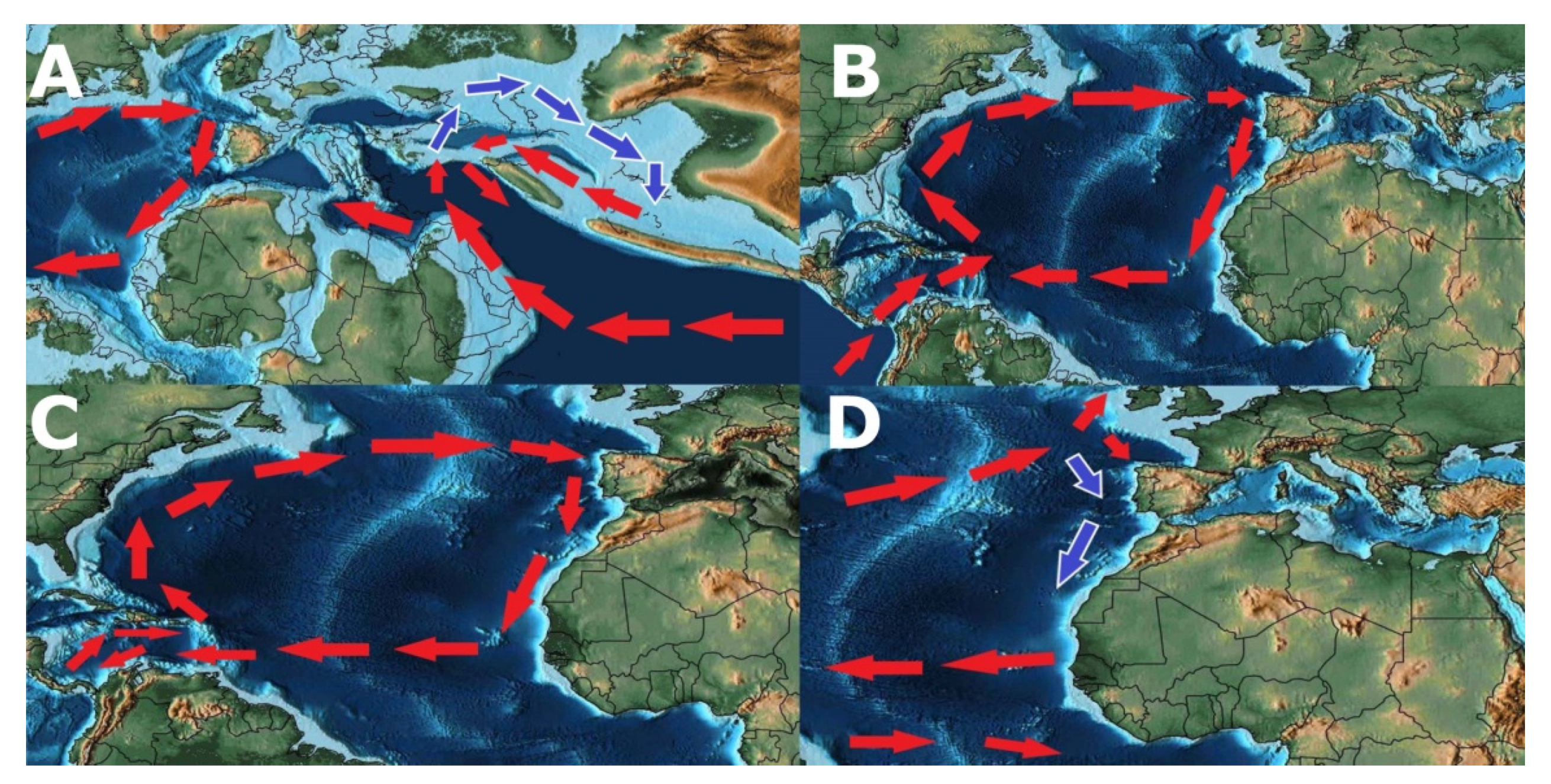
- Possible origin of Patellogastropoda in Tethys Ocean (Figure 13A).
- Vicariant speciation event during the breakup of the supercontinent of Pangea, originating two lineages that eventually led to the radiation of the genus Patella on the Northeast Atlantic Ocean and Scutellastra, Helcion and Cymbula on Southwest Africa (Figure 13B).
- Radiation of the recent species of the genus Patella followed by the closure of the Tethys Sea with Patella ulyssiponensis appearing somewhere between the Upper Oligocene and the Lower Miocene (6.3–33.2 Mya) (Figure 13B).
- Since from this event period, three hypotheses are proposed:
- Hypothesis A—Separation of the Atlantic Iberian and Mediterranean populations during the Messinian Salinity Crisis, with isolation in refuges during the desiccation of the Mediterranean Sea, followed by a colonization of the Macaronesian archipelagos after the Zanclean Flood (Figure 13C). Supported by the higher number of exclusive haplotypes for the Mediterranean, including the basal one, by the proximity of insular and Mediterranean lineages and the divergence date between continental and insular lineages occurring after the Zanclean Flood (5.35 Mya). Followed by a colonization of the Northern Atlantic promoted by the deglaciations after the Last Glacial Maximum.
- Hypothesis B—Entrapment of Patella ulyssiponensis in the Mediterranean during the Messinian Salinity Crisis (Figure 13C), with the survival of some populations in refuges during the desiccation of the Sea and a posterior expansion in the Northeast Atlantic Ocean. Followed by a colonization of the Iberian and Northern Atlantic promoted by the deglaciations after the Last Glacial Maximum.
- Hypothesis C—Entrapment in the Iberian Atlantic with the almost extinction in the Mediterranean during the Messinian Salinity Crisis (Figure 13C) followed by a rapid population and genetic expansion in the Mediterranean after the Zanclean Flood. This hypothesis is mainly proposed due to the lack of fossil evidence and lack of haplotype sampling for some locations which could resolve the non-observed haplotypes in the haplotype network tree.
- Among the presented hypothesis we agree with hypothesis A, due to the presence of many exclusive haplotypes (including the most frequent one) in the Mediterranean, contrasting with the low haplotype diversity for the Northeast Atlantic, and the divergence date between continental and archipelago lineages occurring after the Zanclean Flood, according to TMRCA calculations
- Interruption of the gene flow between the populations from Azores and the populations from Madeira/Canary, possibly due to the global rise of sea level, consequence of the interglacial events during the Plio-Pleistocene (3.15–0.1 Mya), submerging the Proto-Madeira and Proto-Canary archipelagos. The disappearance of these islands may have cut the pathways of dispersal trough the entirety of the Macaronesian archipelagos (Figure 13D).
- Transport by association with rhodoliths—The southeastern coasts of the Macaronesian archipelagos show abundant tempestite deposits that incorporate fossil rhodoliths, belonging to the Mid Miocene to Lower Pliocene [153], transported from eastern locations by storms [154]. Contrasting with the relative scarcity of rhodoliths in Madeira and Azores in present day [153], the Plio-Pleistocene might have rebalanced the circulation patterns, interrupting the gene flow between islands. In the present day, rhodolith debris is still found abundantly in Fuerteventura and seldomly found in Selvagens [153], which may explain the haplotypes shared between Madeira, Fuerteventura, Tenerife and Bonifacio (France).
- Larval dispersal—Although no proof of long-range larval dispersal for Patella ulyssiponensis has been demonstrated, this pathway of colonization cannot be discarded. Contrary to other species of Patella, larvae of Patella ulyssiponensis show an optimal development at higher temperatures (8–24 °C) and a higher larval longevity combined with a higher longevity in substratum absence [155]. All these aspects, associated with the presence of pathways via the Proto-Macaronesian archipelagos, may have an impact on long range larval dispersal during the warmer Upper Miocene (Figure 13C).
4.3. Historical Context and Morphology: Relationships between Patella ulyssiponensis Gmelin, 1791 and Patella aspera Röding, 1798
4.4. Limpets and Rhodoliths
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WoRMS Patella Linnaeus. 1758. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=138312 (accessed on 5 May 2021).
- Ridgway, S.A.; Reid, D.G.; Taylor, J.D.; Branch, G.M.; Hodgson, A.N. A cladistic phylogeny of the family Patellidae (Mollusca: Gastropoda). Philos. Trans. R. Soc. Lond. Ser. B: Biol. Sci. 1998, 353, 1645–1671. [Google Scholar] [CrossRef]
- Christiaens, J. Révision du genre Patella (Mollusca, Gastropoda); Muséum National D’histoire Naturelle: Paris, France, 1973; Volume 121, pp. 1305–1392. [Google Scholar]
- Weber, L.I.; Hawkins, S.J. Patella aspera and P. ulyssiponensis: Genetic evidence of speciation in the North-east Atlantic. Mar. Biol. 2005, 147, 153–162. [Google Scholar] [CrossRef]
- Hayward, P.J.; Ryland, J.S. Handbook of the Marine Fauna of North-West Europe, 2nd ed.; Hayward, P.J., Ryland, J.S., Eds.; Oxford University Press: New York, NY, USA, 2017; ISBN 9780199549443. [Google Scholar]
- CICON Мoрскoе блюдечкo—Patella ulyssiponensis Gmelin. 1791. Available online: https://cicon.ru/morskoe-blyudechko-krym.html (accessed on 8 April 2021).
- Guerra, M.T.; Gaudencio, M.J. Aspects of the ecology of Patella spp. on the Portuguese coast. Hydrobiologia 1986, 142, 57–69. [Google Scholar] [CrossRef]
- Kooistra, W.H.C.F.; Joosten, A.M.T.; van den Hoek, C. Zonation Patterns in Intertidal Pools and their Possible Causes: A Multivariate Approach. Bot. Mar. 1989, 32, 9–26. [Google Scholar] [CrossRef]
- Firth, L.B.; Crowe, T.P. Large-scale coexistence and small-scale segregation of key species on rocky shores. Hydrobiologia 2008, 614, 233–241. [Google Scholar] [CrossRef]
- Spencer Davies, P. Physiological ecology of Patella I. The effect of body size and temperature on metabolic rate. J. Mar. Biol. Assoc. UK 1966, 46, 647–658. [Google Scholar] [CrossRef]
- Martins, H.R.; Santos, R.S.; Hawkins, S.J. Exploitation of limpets (Patella spp.) in the Azores with a preliminary analysis of the stocks. ICES Rep. 1987, 53, 1–18. [Google Scholar]
- Vasconcelos, P.; Umapathy, U.; Moura, P.; Pereira, F.; Carvalho, A.N.; Gaspar, M.B. Size at sex change and reproductive cycle of the limpets Patella vulgata and Patella ulyssiponensis (Mollusca: Patellogastropoda) from intertidal rocky shores of the Algarve coast (southern Portugal). Invertebr. Reprod. Dev. 2019, 63, 294–308. [Google Scholar] [CrossRef]
- Faria, J.; Pita, A.; Martins, G.M.; Ribeiro, P.A.; Hawkins, S.J.; Presa, P.; Neto, A.I. Inbreeding in the exploited limpet Patella aspera across the Macaronesia archipelagos (NE Atlantic): Implications for conservation. Fish. Res. 2018, 198, 180–188. [Google Scholar] [CrossRef]
- Hawkins, S.J.; Pannacciulli, F.G.; Weber, L.C.; Bishop, J.D.D. Thoughts on the ecology and evolution of the intertidal biota of the Azores and other Atlantic islands. Hydrobiologia 2000, 440, 3–17. [Google Scholar] [CrossRef]
- Sanna, D.; Dedola, G.L.; Lai, T.; Curini-Galletti, M.; Casu, M. PCR-RFLP: A practical method for the identification of specimens of Patella ulyssiponensis s.l. (Gastropoda: Patellidae). Ital. J. Zool. 2012, 79, 50–59. [Google Scholar] [CrossRef]
- Seabra, M.I.; Cruz, T.; Fernandes, J.N.; Silva, T.; Hawkins, S.J. Recruitment of the limpet Patella ulyssiponensis and its relationship with crustose coralline algae: Patterns of juvenile distribution and larval settlement. J. Mar. Biol. Assoc. UK 2019, 99, 1787–1796. [Google Scholar] [CrossRef]
- Bowman, R.S. The morphology of Patella spp. juveniles in Britain, and some phylogenetic inferences. J. Mar. Biol. Assoc. UK 1981, 61, 647–666. [Google Scholar] [CrossRef]
- Woelkerling, W.J.; Irvine, L.M.; Harvey, A.S. Growth-forms in non-geniculate coralline red algae (Corallinales, rhodophyta). Aust. Syst. Bot. 1993, 6, 277–293. [Google Scholar] [CrossRef]
- Sañé, E.; Chiocci, F.L.; Basso, D.; Martorelli, E. Environmental factors controlling the distribution of rhodoliths: An integrated study based on seafloor sampling, ROV and side scan sonar data, offshore the W-Pontine Archipelago. Cont. Shelf Res. 2016, 129, 10–22. [Google Scholar] [CrossRef]
- Johansen, H.W. Coralline Algae, A First Synthesis; CRC Press: Boca Raton, FL, USA, 1981. [Google Scholar]
- Riosmena-Rodríguez, R.; Nelson, W.; Aguirre, J. Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Coastal Research Library; Springer International Publishing: Cham, Switzerland, 2017; Volume 15, ISBN 978-3-319-29313-4. [Google Scholar]
- Barbera, C.; Bordehore, C.; Borg, J.A.; Glémarec, M.; Grall, J.; Hall-Spencer, J.M.; de la Huz, C.; Lanfranco, E.; Lastra, M.; Moore, P.G.; et al. Conservation and management of northeast Atlantic and Mediterranean maerl beds. Aquat. Conserv. Mar. Freshw. Ecosyst. 2003, 13, S65–S76. [Google Scholar] [CrossRef]
- Peña, V.; Bárbara, I. Non-coralline crustose algae associated with maerl beds in Portugal: A reappraisal of their diversity in the Atlantic Iberian beds. Bot. Mar. 2013, 56, 481–493. [Google Scholar] [CrossRef]
- Baweja, P.; Kumar, S.; Sahoo, D.; Levine, I. Biology of Seaweeds. In Seaweed in Health and Disease Prevention; Elsevier: Amsterdam, The Netherlands, 2016; pp. 41–106. ISBN 9780128027936. [Google Scholar]
- Adey, W.H. The effects of light and temperature on growth rates in boreal-subarctic crustose corallines. J. Phycol. 1970, 6, 269–276. [Google Scholar] [CrossRef]
- Lobo, J.; Costa, P.M.; Teixeira, M.A.L.; Ferreira, M.S.G.; Costa, M.H.; Costa, F.O. Enhanced primers for amplification of DNA barcodes from a broad range of marine metazoans. BMC Ecol. 2013, 13, 34. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Hebert, P.D.N.; Ratnasingham, S. The Barcode of Life Data System BOLD. Mol. Ecol. Notes 2007, 7, 355–364. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Benson, D.A. GenBank. Nucleic Acids Res. 2004, 33, D34–D38. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Huelsenbeck, J.P.; Ronquist, F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 2001, 17, 754–755. [Google Scholar] [CrossRef]
- Clement, M.; Posada, D.; Crandall, K.A. TCS: A computer program to estimate gene genealogies. Mol. Ecol. 2000, 9, 1657–1659. [Google Scholar] [CrossRef]
- Peakall, R.; Smouse, P.E. GenALEx 6.5: Genetic analysis in Excel. Population genetic software for teaching and research—An update. Bioinformatics 2012, 28, 2537–2539. [Google Scholar] [CrossRef]
- Excoffier, L.; Lischer, H.E.L. Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Mol. Ecol. Resour. 2010, 10, 564–567. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef]
- Marko, P.B. Fossil Calibration of Molecular Clocks and the Divergence Times of Geminate Species Pairs Separated by the Isthmus of Panama. Mol. Biol. Evol. 2002, 19, 2005–2021. [Google Scholar] [CrossRef]
- Rambaut, A.; Drummond, A.J.; Xie, D.; Baele, G.; Suchard, M.A. Posterior Summarization in Bayesian Phylogenetics Using Tracer 1.7. Syst. Biol. 2018, 67, 901–904. [Google Scholar] [CrossRef]
- Müller, R.D.; Cannon, J.; Qin, X.; Watson, R.J.; Gurnis, M.; Williams, S.; Pfaffelmoser, T.; Seton, M.; Russell, S.H.J.; Zahirovic, S. GPlates: Building a Virtual Earth Through Deep Time. Geochem. Geophys. Geosyst. 2018, 19, 2243–2261. [Google Scholar] [CrossRef]
- Encyclopædia Britannica Britannica Website. Available online: https://www.britannica.com/ (accessed on 6 October 2021).
- Gmelin, J.F. Vermes. In Caroli a Linné. Systema Naturae per Regna Tria Naturae; Gmelin, J.F., Ed.; G.E. Beer: Leipzig, Germany, 1791; pp. 3021–3910. [Google Scholar]
- Dillwyn, L.W. A Descriptive Catalogue of Recent Shells: Arranged According to the Linnæan Method; with Particular Attention to the Synonymy; Printed for John and Arthur Arch: London, UK, 1817. [Google Scholar]
- Blainville, H.M. Dictionnaire des Sciences Naturelles, Dans Lequel on Traite Méthodiquement des Différens Êtres de la Nature, Considérés Soit en Eux-Mêmes, D’après L’état Actuel de nos Connoissances, Soit Relativement à L’utilité Qu’en Peuvent Retirer la Médecine, L’agric; Levrault; Tome, 38; Le Normant: Paris, France, 1825. [Google Scholar]
- Christiaens, J. Additifs à la “Révision du genre Patella”. Inf. Soc. Belg. Malacol. 1974, 5, 63–68. [Google Scholar]
- Ortea, J.A. El género Patella Linné 1758 en Asturias. Boletín Cienc. Nat. Inst. Estud. Astur. 1980, 26, 57–72. [Google Scholar]
- Titselaar, F.F.L.M. A revision of the recent European Patellidae (Mollusca: Gastropoda). Vita Mar. 1998, 45, 21–62. [Google Scholar]
- Öztürk, B.; Ergen, Z. Patella species (Archaeogastropoda) distributed in Saros Bay (Northest Aegean Sea) [Saros körfezi’nde (Kuzey Ege Denizi) daǧilim gösteren Patella (Archaeogastropoda) türleri]. Turkish J. Zool. 1999, 23, 513–519. [Google Scholar]
- Callapez, P.; Soares, A.F. Late Quaternary warm marine mollusks from Santa Maria (Azores); paleoecologic and paleobiogeographic considerations. Ciências Terra 2000, 14, 313–322. [Google Scholar]
- Bouchet, P.; Le Renard, J.; Gofas, S. Mollusca. In European Register of Marine Species: A Check-List of the Marine Species in Europe and a Bibliography of Guides to their Identification; Costello, M.J., Emblow, C., White, R.J., Eds.; Muséum National d’Histoire Naturelle, Institute D’écologie et de Gestion de la Biodiversité Service du Patrimoine Naturel: Paris, France, 2001; pp. 180–213. [Google Scholar]
- Ávila, S.P.; Amen, R.; Azevedo, J.M.N.; Cachão, M.; García-Talavera, F. Checklist of the Pleistocene Marine Molluscs of Praínha and Lagoínhas (Santa Maria Island, Azores). Açoreana. Rev. Estud. Açoreanos 2002, 9, 343–370. [Google Scholar]
- Cabral, J.P. Characterization and multivariate analysis of Patella intermedia, Patella ulyssiponensis and Patella vulgata from Póvoa de Varzim (Northwest Portugal). Iberus Rev. la Soc. Española Malacol. 2003, 21, 1–17. [Google Scholar]
- Cabral, J.P.; Silva, A.C.F. Morphometric analysis of limpets from an Iron-Age shell midden found in northwest Portugal. J. Archaeol. Sci. 2003, 30, 817–829. [Google Scholar] [CrossRef]
- Cabral, J.P. Shape and growth in European Atlantic Patella limpets (Gastropoda, Mollusca). Ecological implications for survival. Web Ecol. 2007, 7, 11–21. [Google Scholar] [CrossRef]
- Hodgson, A.N.; Le Quesne, W.J.F.; Hawkins, S.J.; Bishop, J.D.D. Factors affecting fertilization success in two species of patellid limpet (Mollusca: Gastropoda) and development of fertilization kinetics models. Mar. Biol. 2007, 150, 415–426. [Google Scholar] [CrossRef]
- Dhora, D. Mollusks of Albania. Arch. Biol. Sci. 2009, 61, 537–553. [Google Scholar] [CrossRef]
- Ivanov, S.P.; Fateryga, A.V. Красная Книга Республики Крым: Живoтные [Red Book of the Republic of Crimea: Animals]; PP “ARIAL” LLC: Simferopol, Republic of Crimea, 2015; ISBN 9785906813886. [Google Scholar]
- Borges, L.M.S.; Hollatz, C.; Lobo, J.; Cunha, A.M.; Vilela, A.P.; Calado, G.; Coelho, R.; Costa, A.C.; Ferreira, M.S.G.; Costa, M.H.; et al. With a little help from DNA barcoding: Investigating the diversity of Gastropoda from the Portuguese coast. Sci. Rep. 2016, 6, 20226. [Google Scholar] [CrossRef] [PubMed]
- Titselaar, F.F.L.M. Notes on the nomenclature of the Macaronesian Patella candei d’Orbigny complex, with special reference to Patella ordinaria Mabile and Patella crenata Gmelin (Patellogastropoda, Patellida). Basteria 2019, 4–6, 158–165. [Google Scholar]
- Crocetta, F.; Bitar, G.; Zibrowius, H.; Oliverio, M. Increase in knowledge of the marine gastropod fauna of Lebanon since the 19th century. Bull. Mar. Sci. 2020, 96, 5343. [Google Scholar] [CrossRef]
- Röding, P.F. Museum Boltenianum, Sive, Catalogus Cimeliorum e Tribus Regnis Naturae Quae Olim Collegerat Joa. Fried. Bolten… : Pars Secunda Continens Conchylia Sive Testacea Univalvia, Bivalvia & Multivalvia; Typis Johan. Christi. Trappii: Hamburgi, Germany, 1798. [Google Scholar]
- De Lamarck, J.-B. Histoire Naturelle des Animaux Sans Vertèbres; Verdiere, Libraire: Paris, France, 1819. [Google Scholar]
- Reeve, L.A. Conchologia Iconica: Or, Illustrations of the Shells of Molluscous Animals; Lovell Reeve: London, UK, 1855; Volume VIII. [Google Scholar]
- Servain, G. Étude sur les Patellidae des Mers d’Europe; Germain & G. Grassin: Angers, France, 1886. [Google Scholar]
- Simroth, H. Zur Kenntniss der Azorenfauna. Arch. für Nat. 1888, 54, 179–234. [Google Scholar]
- Watson, R.B. On the Marine Mollusca of Madeira; with Descriptions of Thirty-five new Species, and an Index-List of all the known Sea-dwelling Species of that Island. J. Linn. Soc. Lond. Zool. 1897, 26, 233–329. [Google Scholar] [CrossRef][Green Version]
- Nobre, A. Materiais Para o Estudo da Fauna dos Açores; Instituto de Zoologia da Universidade do Porto: Porto, Portugal, 1930. [Google Scholar]
- Mermod, G. Les Types de la Collection Lamarck au Muséum de Genève. Mollusques vivants, I. Rev. Suisse Zool. 1950, 57, 687–756. [Google Scholar] [CrossRef]
- Evans, R.G. Studies on the biology of British limpets-the genus Patella on the south coast of England. Proc. Zool. Soc. Lond 1953, 123, 357–376. [Google Scholar] [CrossRef]
- Evans, R.G. The genus Patella on the West coast of France. J. Conchyliol. 1958, 98, 126–151. [Google Scholar]
- Fischer-Piette, E.; Gaillard, J.M. Les patelles, au long des cotes Atlantiques Ibériques et Nord-Marocaines. J. Conchyliol. 1959, 99, 135–200. [Google Scholar]
- Kolstad, K. Patella aspera Lamarck, New to Norway. Nature 1959, 184, 1886–1887. [Google Scholar] [CrossRef]
- Morton, B. Malacological Report. In Final Report Published by Chelsea College Azores Expedition (July–October 1965); Chelsea College of Science and Technology, University of London: London, UK, 1967; pp. 30–39. [Google Scholar]
- McMillan, N.F. British Shells; F. Warne: London, UK, 1968; ISBN 0723209650. [Google Scholar]
- Nordsieck, F. Die europäischen Meeres-Gehäuseschnecken (Prosobranchia). Vom Eismeer bis Kapverden und Mittelmeer; Gustav Fischer: Stuttgart, Germany, 1968. [Google Scholar]
- Powell, A.W.B. The patellid limpets of the world (Patellidae). Indo-Pac. Mollusca 1973, 3, 75–206. [Google Scholar]
- Cuerda, J.; Galiana, R. Nuevo yacimiento del Pleistoceno superior marino en la costa norte de Mallorca. Bolletí Soc. d’Història Nat. Balear. 1976, 21, 115–124. [Google Scholar]
- Nordsieck, F.; García-Talavera, F. Moluscos marinos de Canarias y Madera. (Gastropoda); Aula de Cultura de Tenerife: Madrid, Spain, 1979. [Google Scholar]
- Gaffney, P.M. On the number of Patella species in south-west England. J. Mar. Biol. Assoc. UK 1980, 60, 565–574. [Google Scholar] [CrossRef]
- Van Aartsen, J.J.; Menkhorst, H.P.M.G.; Gittenberger, E. The marine Mollusca of the Bay of Algeciras, Spain, with general notes on Mitrella, Marginellidae and Turridae. Basteria 1984, 2, 3–135. [Google Scholar]
- Campbell, A.C. Country Life Guides. The Seashore and Shallow Seas of Britain and Europe; Country Life Books: Feltham, UK, 1985. [Google Scholar]
- Backeljau, T. Lijst van de Recente Mariene Mollusken van België [A Checklist of the Recent Marine Molluscs of Belgium]; Documents; Koninklijk Belgisch Instituut voor Natuurwetenschappen: Brussels, Belgium, 1986. [Google Scholar]
- Bosch, M.; Moreno, I. Contribución al conocimiento del género Patella Linné 1758, en la isla de Mallorca. Bolletí Soc. d’Història Nat. Balear. 1986, 30, 127–135. [Google Scholar]
- Cervella, P.; Ramella, L.; Robotti, C.A.; Sella, G. Chromosome analysis of three species of Patella (Archaeogastropoda). Genetica 1988, 77, 97–103. [Google Scholar] [CrossRef]
- Graham, A. Molluscs: Prosobranch and Pyramidellid Gastropods. Keys and Notes for the Identification of the Species; Kermack, D.M., Barnes, R.S.K., Eds.; The Linnean Society of London & The Estuarine and Brackish-Water Sciences Association: Leiden, The Netherlands, 1988; ISBN 9004087710. [Google Scholar]
- Lindner, G. Guide des Coquillages Marins, 2nd ed.; Delachaux et Niestlé, Neuchâtel: Paris, France, 1989; ISBN 2603006894. [Google Scholar]
- Bellomo, E. Presenza di «Ospiti boreali» (Mollusca, Gastropoda) nel Pleistocene di Contrada Case Alte (Villa S. Giovanni). Boll. della Soc. Paleontol. Ital. 1993, 32, 393–399. [Google Scholar]
- Weber, M. Aguda, Entre as Marés: Fauna e Flora do Litoral da Praia da Aguda; Edições Af.; Rainho & Neves: Porto, Portugal, 1997; ISBN 9723604329. [Google Scholar]
- Nakano, T.; Ozawa, T. Worldwide phylogeography of limpets of the order Patellogastropoda: Molecular, morphological and palaeontological evidence. J. Molluscan Stud. 2007, 73, 79–99. [Google Scholar] [CrossRef]
- Santos, R.S.; Delgado, R.; Ferraz, R. Background Document for Azorean Limpet Patella aspera; OSPAR Commission: London, UK, 2010. [Google Scholar]
- Castejón, D.; Cañizares, J.M.; Nogueira, N.; Andrade, C.A.P. Artificial maturation and larval production of the limpet Patella aspera Röding, 1798 (Patellogastropoda, Mollusca): Enhancing fertilization success of oocytes using NaOH-alkalinized seawater. Aquac. Res. 2021, 52, 1904–1914. [Google Scholar] [CrossRef]
- Bean, W. A supplement of new species. In British Marine Conchology; Being a Descriptive Catalogue, Arranged According to the Lamarckian System, of the Salt Water Shells of Great Britain, by Charles Thorpe, Assisted by Several Distinguished Conchologists, and Illustrated with Numerous Delineation; Thorpe, C., Ed.; Edward Lumley: London, UK, 1844; pp. 263–267. [Google Scholar]
- Forbes, E.; Hanley, S. A history of British Mollusca, and their Shells. In Including the Remaining Families of Bivalves, the Pteropoda, and the Gasteropoda as far as Ianthinidae; John Van Voorst: London, UK, 1853; Volume 2. [Google Scholar]
- Dautzenberg, P. Une Excursion malacologique à Saint-Lunaire (Ille-et-Vilaine) et aux environs de cette localité. Bull. Société d’études Sci. Paris 1887, 9, 1–27. [Google Scholar]
- Nobre, A. Moluscos Testáceos Marinhos do Arquipélago da Madeira; Coimbra University Press: Coimbra, Portugal, 1937; pp. 1–101. [Google Scholar]
- Evans, R.G. Studies on the Biology of British limpets. Proc. Zool. Soc. Lond. 1947, 117, 411–423. [Google Scholar] [CrossRef]
- Lucas, J.A.W. Het genus Patella in Nederland. Basteria 1954, 18, 36–40. [Google Scholar]
- Nordsieck, F. Die Europäischen Meeres-Gehäuseschnecken (Prosobranchia): Vom Eismeer bis Kapverden, Mittelmeer und Schwarzes Meer; 2. Völlig; Fischer: Stuttgart, Germany, 1982; ISBN 3437303600. [Google Scholar]
- Jay, J.C. A Catalogue of the Shells, Arranged According to the Lamarckian System: With Their Authorities, Synonymes, and References to Works Where Figured or Described: Together with a Supplement Containing Additional Species, Corrections, and Alterations/Contain; Craighead: New York, NY, USA, 1852. [Google Scholar]
- Drouët, H. Mollusques Marins des Iles Açores; Baillière: Paris, France, 1858. [Google Scholar]
- Payraudeau, B.C. Catalogue Descriptif et Méthodique des Annelides et des Mollusques de L’ile de Corse; Avec Huit Planches Représentant Quatre-Vingt-Huit Espéces, Dont Soixante-Huit Nouvelles; Béchet, Levrault, Paschoud, Treuttel et Wurtz: Paris, France, 1826. [Google Scholar]
- Weinkauff, H.C. Systematisches Conchylien-Cabinet; Küster, H.C., Ed.; Bauer und Raspe: Nürnberg, Germany, 1880. [Google Scholar]
- Anton, H.E. Verzeichniss der Conchylien: Welche sich in der Sammlung/von Hermann Eduard Anton befinden. Herausgegeben von dem Besitzer; E. Anton: Halle, Germany, 1838. [Google Scholar]
- Di Monterosato, T.A. Molluschi del Porto de Palermo. Specie e varietà. Bull. della Soc. Malacol. Ital. 1888, 13, 161–180. [Google Scholar]
- D’Orbigny, A. Mollusques, Echinodermes, Foraminifères et Polypiers recueillis aux Iles Canaries par MM. Webb et Berthelot. In Histoire naturelle des Iles Canaries; Barker-Webb, P., Berthelot, S., Eds.; Béthune: Paris, France, 1840; p. 117. [Google Scholar]
- Lecointre, G.; Tinkler, K.J.; Richards, H.G. The marine Quaternary of the Canary Islands. In Proceedings of The Academy of Natural Sciences of Philadelphia; The Academy of Natural Sciences of Drexel University: Philadelphia, PA, USA, 1967; Volume 119, pp. 325–344. [Google Scholar]
- Locard, A. Notices conchyliologiques. XXVIII. Description des deux coquilles marines nouvelles. L’Echange Rev. Linnéenne 1894, 10, 131–132. [Google Scholar]
- Milachewitch, K.O. Faune de la Russie et des Pays Limitrophes Fondée Principalement sur les Collections dur Musée Zoologique de l’Académie Impériale des Sciences de Petrograd. Les Mollusques de Mers Russes; Tome I. Le.: Petrograd, Russia, 1916. [Google Scholar]
- Dunker, G. Index Molluscorum, Quae in Itinere ad Guineam Inferiorem Collegit Georgius Tams, Med. dr.; Accedunt Novarum Specierum Diagnoses, Cirripedia Nonnulla et X. Tabulae Iconum; Typis et sumptibus Theodori Fischer, Cassellis Cattorum: Kreisfreie, Germany, 1853. [Google Scholar] [CrossRef]
- Mörch, O.A.L. Catalogus Conchyliorum Quae Reliquit D. Alphonso d’Aguirra & Gadea Comes de Yoldi: Regis Daniae Cubiculariorum Princeps, Ordinis Dannebrogici in Prima Classe & Ordinis Caroli Tertii Eques; Fasciculus; Ludovici Kleine: Hafniae, Denmark, 1852. [Google Scholar]
- Meuschen, F.C. Museum Geversianum Sive Index Rerum Naturalium: Continens Instructissimam Copiam Pretiosissimorum Omnis Generis ex Tribus Regnis Naturae Objectorum: Quam Dum in Vivis Erat Magna Diligentia Multaque Cura Comparavit vir Amplissimus Abrahamus Gevers; P. & J. Holsteyn Bibliopolas: Rotterdam, The Netherlands, 1787. [Google Scholar]
- Salis Marschlins, C.U. von Reisen in verschiedene provinzen des Königreichs Neapel; Geografia; Ziegler & Söhne: Zürich, Switzerland; Leipzig, Germany, 1793. [Google Scholar]
- Delessert, B. Recueil de Coquilles Décrites par Lamarck Dans son Histoire Naturelle des Animaux sans Vertèbres et non Encore Figurées; Chez Fortin, Masson et Cie, Libraires: Paris, France, 1841. [Google Scholar]
- Nordsieck, F. Conchiglie delle Isole Canarie. Parte 1: Patellae. La Conchiglia 1975, 73–74, 3–5. [Google Scholar]
- Mabille, J. De quelques coquilles nouvelles. Bull. Soc. Philomath. Paris 1888, 12, 73–82. [Google Scholar]
- Locard, A. Expéditions Scientifiques du Travailleur et du Talisman Pendant les Années 1880, 1881, 1882, 1883; Mollusques Testacés. Tome 2: Gastropoda; Milne-Edwards, A., Ed.; G. Masson: Paris, France, 1898. [Google Scholar]
- Poppe, G.T.; Goto, Y. European seashells. Vol. 1. Polyplacophora, Caudofoveata, Solenogastra, Gastropoda; Verlag Christa Hemmen: Wiesbaden, Germany, 1991; ISBN 9783925919077. [Google Scholar]
- Corte-Real, H.B.S.M.; Hawkins, S.J.; Thorpe, J.P. Genetic confirmation that intertidal and subtidal morphs of Patella ulyssiponensis aspera Röding (Mollusca: Gastropoda: Patellidae) are conspecific. Arquipélago-Life Earth Sci. 1992, 10, 55–66. [Google Scholar]
- De Gregorio, A. Studi su talune conchiglie mediterranee viventi e fossili con una rivista del genere Vulsella. Bull. della Soc. Malacol. Ital. 1884, 10, 36–128. [Google Scholar]
- Cabral, J.P. Concentrations of metals in Patella intermedia, Patella rustica, Patella ulyssiponensis and Patella vulgata shells along the Portuguese continental coast. Boll. Malacol. 2005, 41, 23–34. [Google Scholar]
- Foslie, M. Algologiske notiser II. Det K. Nor. Vidensk. Selsk. Skr. 1906, 1906, 1–28. [Google Scholar]
- Printz, H.M. Foslie: Contributions to a Monograph of the Lithothamnia; Kong. Norske Vidensk. Selsk. Mus.: Trondheim, Norway, 1929. [Google Scholar]
- Adey, W.H.; Lebednik, P.A. Catalog of the Foslie Herbarium; Det Kongelige Norske Videnskabers Selskab Museet: Trondheim, Norway, 1967. [Google Scholar]
- Woelkerling, W.J. Type collections of Corallinales (Rhodophyta) in the Foslie Herbarium (TRH). Gunneria 1993, 67, 65. [Google Scholar]
- Irvine, L.M.; Chamberlain, Y.M. Seaweeds of the British Isles, 1 (2B). Corallinales, Hildenbrandiales; HMSO: London, UK, 1994; ISBN 0113100167. [Google Scholar]
- Woelkerling, W.J.; Gustavsen, G.; Myklebost, H.E.; Prestø, T.; Såstad, S.M. The Coralline Red Algal Herbarium of Mikael Foslie: Revised Catalogue with Analyses; NTNU Vitenskapsmuseet: Trondheim, Norway, 2005; pp. 1–625. [Google Scholar]
- Hall-Spencer, J.M.; Kelly, J.; Maggs, C.A. Background Document for Maërl Beds; OSPAR Commission: Paris, France, 2010. [Google Scholar]
- Guiry, M.D. A Catalogue of Irish Seaweeds; A.R.G. Gantner Verlag K.G.: Ruggell, Liechtenstein, 2012; ISBN 9783905997101. [Google Scholar]
- Hernandez-Kantun, J.J.; Rindi, F.; Adey, W.H.; Heesch, S.; Peña, V.; Le Gall, L.; Gabrielson, P.W. Sequencing type material resolves the identity and distribution of the generitype Lithophyllum incrustans, and related European species L. hibernicum and L. bathyporum (Corallinales, Rhodophyta). J. Phycol. 2015, 51, 791–807. [Google Scholar] [CrossRef]
- Gallardo, T.; Bárbara, I.; Afonso-Carrillo, J.; Bermejo, R.; Altamirano, M.; Gómez Garreta, A.; Barceló Martí, M.C.; Rull Lluch, J.; Ballesteros, E.; De la Rosa, J. Nueva lista crítica de las algas bentónicas marinas de España [A new checklist of benthic marine algae of Spain]. Algas. Boletín Inf. la Soc. Española Ficología 2016, 51, 7–52. [Google Scholar]
- Lugilde, J.; Peña, V.; Bárbara, I. The order Corallinales sensu lato (Rhodophyta) in the Iberian Atlantic: Current state of knowledge. An. Jardín Botánico Madr. 2016, 73, e038. [Google Scholar] [CrossRef]
- Vázquez-Ferreira, R.; Peña, V. Type of sporangial division in Lithophyllum hibernicum (Corallinales, Rhodophyta) and implications on its life-history in Atlantic Europe [Tipo de división en esporocistes de Lithophyllum hibernicum (Corallinales, Rhodophyta) y su implicació. Nov. Acta Científica Compostel. 2016, 23, 99–106. [Google Scholar]
- Cormaci, M.; Furnari, G.; Alongi, G. Marine benthic flora of the Mediterranean Sea: Rhodophyta (excluding Rhodymeniophycida) [Flora marina bentonica del Mediterraneo: Rhodophyta (Rhodymeniophycidae escluse)]. Bull. Gioenia Acad. Catania 2017, 50, FP1–FP391. [Google Scholar]
- Hernandez-Kantun, J.J.; Hall-Spencer, J.M.; Grall, J.; Adey, W.; Rindi, F.; Maggs, C.A.; Bárbara, I.; Peña, V. North Atlantic rhodolith beds. In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 265–279. ISBN 9783319293158. [Google Scholar]
- Pardo, C.; Bárbara, I.; Barreiro, R.; Peña, V. Insights into species diversity of associated crustose coralline algae (Corallinophycidae, Rhodophyta) with Atlantic European maerl beds using DNA barcoding. An. Jardín Botánico Madr. 2017, 74, e059. [Google Scholar] [CrossRef]
- Burel, T.; Duff, M.L.; Gall, E.A. Updated check-list of the seaweeds of the French coasts, Channel and Atlantic Ocean. Les Cah. Nat. l’Observatoire Mar. 2019, 7, 1–38. [Google Scholar]
- Athanasiadis, A. A study of the type material of Lithophyllum hibernicum (Lithophyllaceae, Corallinales, Rhodophyta) with comments on L. bathyporum and L. incrustans. Mar. Biol. Res. 2020, 16, 68–76. [Google Scholar] [CrossRef]
- Foslie, M. On some Lithothamnia. Det K. Nor. Vidensk. Selsk. Skr. 1897, 1, 1–20. [Google Scholar]
- Foslie, M. New or critical calcareous algae. Det K. Nor. Vidensk. Selsk. Skr. 1900, 5, 1–34. [Google Scholar]
- Slatkin, M.; Hudson, R.R. Pairwise comparisons of mitochondrial DNA sequences in stable and exponentially growing populations. Genetics 1991, 129, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Sá-Pinto, A.; Branco, M.; Sayanda, D.; Alexandrino, P. Patterns of colonization, evolution and gene flow in species of the genus Patella in the Macaronesian Islands. Mol. Ecol. 2008, 17, 519–532. [Google Scholar] [CrossRef]
- Sá-Pinto, A.; Branco, M.S.; Alexandrino, P.B.; Fontaine, M.C.; Baird, S.J.E. Barriers to Gene Flow in the Marine Environment: Insights from Two Common Intertidal Limpet Species of the Atlantic and Mediterranean. PLoS ONE 2012, 7, e50330. [Google Scholar] [CrossRef]
- Sá-Pinto, A.; Branco, M.; Harris, D.J.; Alexandrino, P. Phylogeny and phylogeography of the genus Patella based on mitochondrial DNA sequence data. J. Exp. Mar. Bio. Ecol. 2005, 325, 95–110. [Google Scholar] [CrossRef]
- Hedegaard, C.; Lindberg, D.R.; Bandel, K. Shell microstructure of a Triassic patellogastropod limpet. Lethaia 2000, 30, 331–335. [Google Scholar] [CrossRef]
- Koufopanou, V.; Reid, D.G.; Ridgway, S.A.; Thomas, R.H. A Molecular Phylogeny of the Patellid Limpets (Gastropoda: Patellidae) and Its Implications for the Origins of Their Antitropical Distribution. Mol. Phylogenet. Evol. 1999, 11, 138–156. [Google Scholar] [CrossRef]
- Crame, J.A. Bipolar molluscs and their evolutionary implications. J. Biogeogr. 1993, 20, 145–161. [Google Scholar] [CrossRef]
- MolluscaBase (Ed.) MolluscaBase. Available online: https://www.molluscabase.org/ (accessed on 28 October 2021).
- Lozouet, P.; Maestrati, P. Nouvelles espèces de mollusques de l’Oligocène (Stampen) pour les bassins de Paris et d’Aquitaine. Arch. für Molluskenkd. 1982, 112, 165–189. [Google Scholar]
- Lozouet, P. Nouvelles espèces de gastéropodes (Mollusca: Gastropoda) de l’Oligocène et du Miocène inférieur de l’Aquitaine (Sud-Ouest de la France). Cossmanniana 1999, 5, 61–102. [Google Scholar]
- Landau, B.; Van Dingenen, F.; Ceulemans, L. The upper Miocene gastropods of northwestern France, 1. Patellogastropoda and Vetigastropoda. Cainozoic Res. 2017, 17, 75–166. [Google Scholar]
- Martín-González, E.; Vera-Peláez, J.L.; Castillo, C.; Lozano-Francisco, M.C. New fossil gastropod species (Mollusca: Gastropoda) from the upper miocene of the canary islands (Spain). Zootaxa 2018, 4422, 191–218. [Google Scholar] [CrossRef] [PubMed]
- Forli, M.; Dell’Angelo, B.; Montagna, P.; Taviani, M. A new large Patella in the Pliocene of the Mediterranean Sea.: (Mollusca: Patellogastropoda). Boll. Malacol. 2004, 40, 49–78. [Google Scholar]
- Pacaud, J.M. Les espèces de mollusques du Danien décrites par Alcide d’Orbigny en 1850. In Stratotype Danien [Patrimoine Géologique Volume 9; Muséum National d’Histoire Naturelle: Paris, France, 2018; pp. 234–258. [Google Scholar]
- Johnson, M.E.; Ledesma-Vázquez, J.; Ramalho, R.S.; da Silva, C.M.; Rebelo, A.C.; Santos, A.; Baarli, B.G.; Mayoral, E.; Cachão, M. Taphonomic range and sedimentary dynamics of modern and fossil rhodolith beds: Macaronesian realm (North Atlantic Ocean). In Rhodolith/Maërl Beds: A Global Perspective; Riosmena-Rodríguez, R., Nelson, W., Aguirre, J., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-29313-4. [Google Scholar]
- Johnson, M.E.; da Silva, C.M.; Santos, A.; Baarli, B.G.; Cachão, M.; Mayoral, E.J.; Rebelo, A.C.; Ledesma-Vázquez, J. Rhodolith transport and immobilization on a volcanically active rocky shore: Middle Miocene at Cabeço das Laranjas on Ilhéu de Cima (Madeira Archipelago, Portugal). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2011, 300, 113–127. [Google Scholar] [CrossRef]
- Ribeiro, P.A. Dispersal and Connectivity of Northeastern Atlantic Patellid Limpets: A Multidisciplinary Approach. Ph.D. Thesis, University of Southampton, Southampton, UK, 2008. [Google Scholar]
- Frizon de Lamotte, D.; Fourdan, B.; Leleu, S.; Leparmentier, F.; de Clarens, P. Style of rifting and the stages of Pangea breakup. Tectonics 2015, 34, 1009–1029. [Google Scholar] [CrossRef]
- Müller, R.D.; Seton, M.; Zahirovic, S.; Williams, S.E.; Matthews, K.J.; Wright, N.M.; Shephard, G.E.; Maloney, K.T.; Barnett-Moore, N.; Hosseinpour, M.; et al. Ocean Basin Evolution and Global-Scale Plate Reorganization Events Since Pangea Breakup. Annu. Rev. Earth Planet. Sci. 2016, 44, 107–138. [Google Scholar] [CrossRef]
- Barnett-Moore, N.; Müller, D.R.; Williams, S.; Skogseid, J.; Seton, M. A reconstruction of the North Atlantic since the earliest Jurassic. Basin Res. 2018, 30, 160–185. [Google Scholar] [CrossRef]
- Torsvik, T.H.; Cocks, L.R.M. Earth History and Palaeogeography; Cambridge University Press: Cambridge, UK, 2017; ISBN 9781316225523. [Google Scholar]
- Fernández-Palacios, J.M.; de Nascimento, L.; Otto, R.; Delgado, J.D.; García-del-Rey, E.; Arévalo, J.R.; Whittaker, R.J. A reconstruction of Palaeo-Macaronesia, with particular reference to the long-term biogeography of the Atlantic island laurel forests. J. Biogeogr. 2011, 38, 226–246. [Google Scholar] [CrossRef]
- Mitchell-Thomé, R.C. Radiometric studies in Macaronesia. Bol. Mus. Munic. Funchal 1985, 37, 52–85. [Google Scholar]
- De Favanne de Montcervelle, M. La Conchyliologie, Ou, Histoire Naturelle des Coquilles de Mer, D’eau Douce, Terrestres et Fossiles: Avec un Traité de la Zoomorphose, ou, Représentation des Animaux qui les Habitent, Ouvrage Dans Lequel on Trouve Une nouvelle Méthode de les Diviser/Par; Desallier D’Argenville, M., Ed.; Chez Guillaume De Bure Fils Aîné: Paris, France, 1780. [Google Scholar]
- MolluscaBase Patella aspera Lamarck. 1819. Available online: http://www.marinespecies.org/aphia.php?p=taxdetails&id=146455 (accessed on 25 March 2021).
- Costa, D.A.; Christoffersen, M.L. New status for hesionid polychaetes (Annelida, Polychaeta). Gaia Sci. 2016, 10, 160–165. [Google Scholar] [CrossRef]
- International Commission on Zoological Nomenclature International Code of Zoological Nomenclature. Available online: https://code.iczn.org/criteria-of-publication/article-8-what-constitutes-published-work/?frame=1 (accessed on 25 March 2021).
- Smith, S.M.; Heppell, D. Checklist of British Marine Mollusca; Trustees of the National Museums of Scotland: Edinburgh, UK, 1991. [Google Scholar]
- Sella, G.; Robotti, C.A.; Biglione, V. Genetic divergence among three sympatric species of Mediterranean Patella (Archaeogastropoda). Mar. Biol. 1993, 115, 401–405. [Google Scholar] [CrossRef]
- Mauro, A.; Arculeo, M.; Parrinello, N. Morphological and molecular tools in identifying the Mediterranean limpets Patella caerulea, Patella aspera and Patella rustica. J. Exp. Mar. Bio. Ecol. 2003, 295, 131–143. [Google Scholar] [CrossRef]
- Arguelles, E.D.L.R. First taxonomic records of epizoic freshwater algae on golden apple snails (Pomacea canaliculata Lamarck) from rice paddies in Laguna (Philippines). Philipp. J. Sci. 2021, 150, 829–844. [Google Scholar]
- Harvey, A.; Johnson, M.E.; Harvey, R. Heterozoan carbonate-enriched beach sand and coastal dunes—with particular reference to rhodoliths, Dirk Hartog Island, Shark Bay, Western Australia. Facies 2018, 64, 23. [Google Scholar] [CrossRef]
- Farr, T.; Broom, J.; Hart, D.; Neill, K.; Nelson, W. Common Coralline Algae of Northern New Zealand: An. Identification Guide; NIWA: Auckland, New Zealand, 2009; ISBN 1174-264X. [Google Scholar]
- Harvey, A.; William, W.; Far, T.; Neill, K.; Nelson, W. Coralline Algae of Central New Zealand An Identification Guide to Common ‘Crustose’ Species Coralline Algae of Central New Zealand; NIWA: Auckland, New Zealand, 2005. [Google Scholar]
- Woelkerling, W.J. A taxonomic reassessment of Lithophyllum (Corallinaceae, Rhodophyta) based on studies of R. A. Philippi’s original collections. Br. Phycol. J. 1983, 18, 299–327. [Google Scholar] [CrossRef]
- Barrientos, S.; Barreiro, R.; Olmedo, M.; Piñeiro-Corbeira, C. Can patch size and patch distance improve the recolonization of mussel-seed beds exploited for aquaculture? Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 1897–1908. [Google Scholar] [CrossRef]
- Johnson, M.E.; Baarli, B.G.; Cachão, M.; da Silva, C.M.; Ledesma-Vázquez, J.; Mayoral, E.J.; Ramalho, R.S.; Santos, A. Rhodoliths, uniformitarianism, and Darwin: Pleistocene and Recent carbonate deposits in the Cape Verde and Canary archipelagos. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2012, 329–330, 83–100. [Google Scholar] [CrossRef]
- Darwin, C. Geological Observations on the Volcanic Islands and Parts of South America Visited during the Voyage of H.M.S. “Beagle”, 3rd ed.; Appleton and Company: New York, NY, USA, 1891. [Google Scholar]
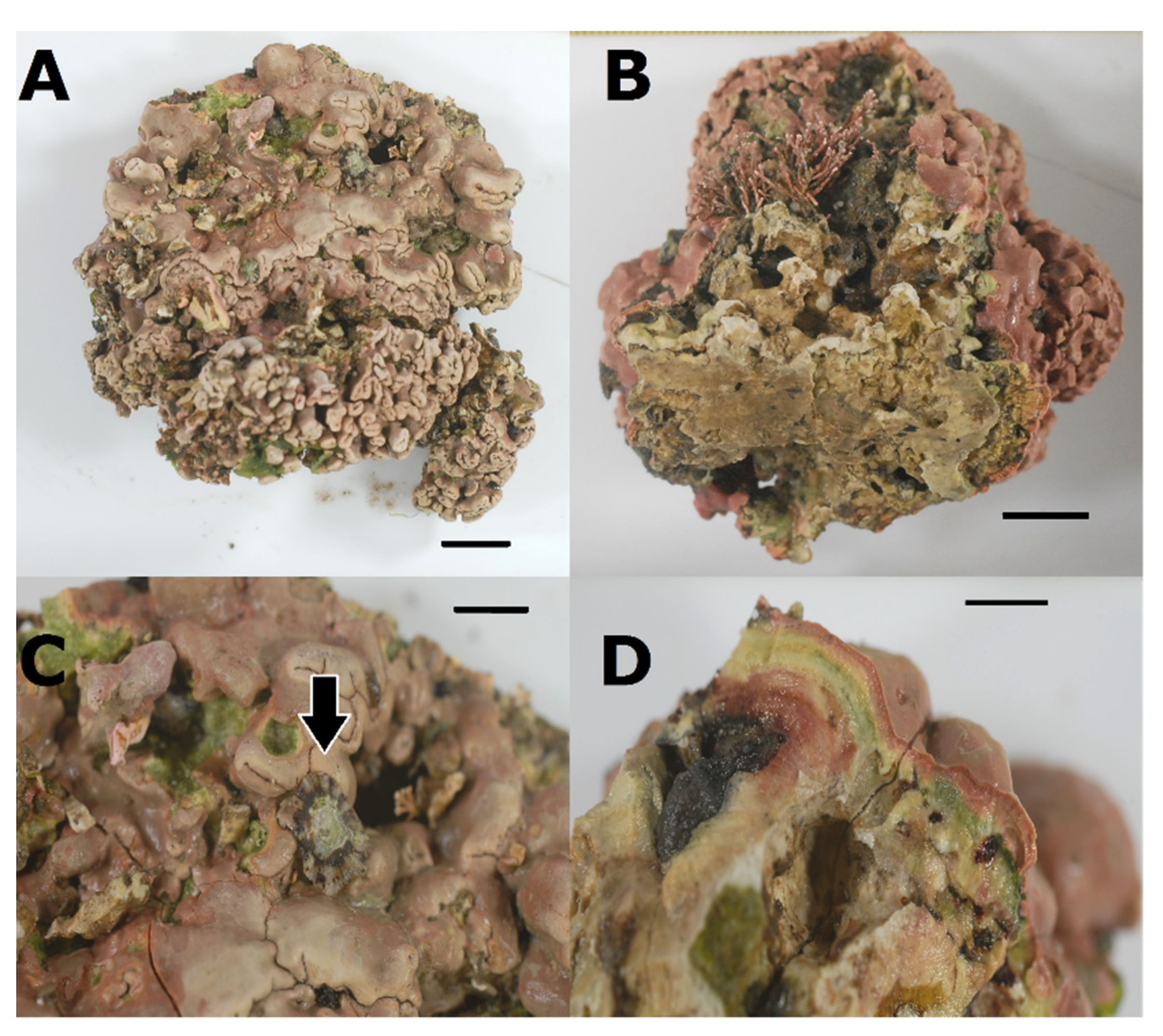
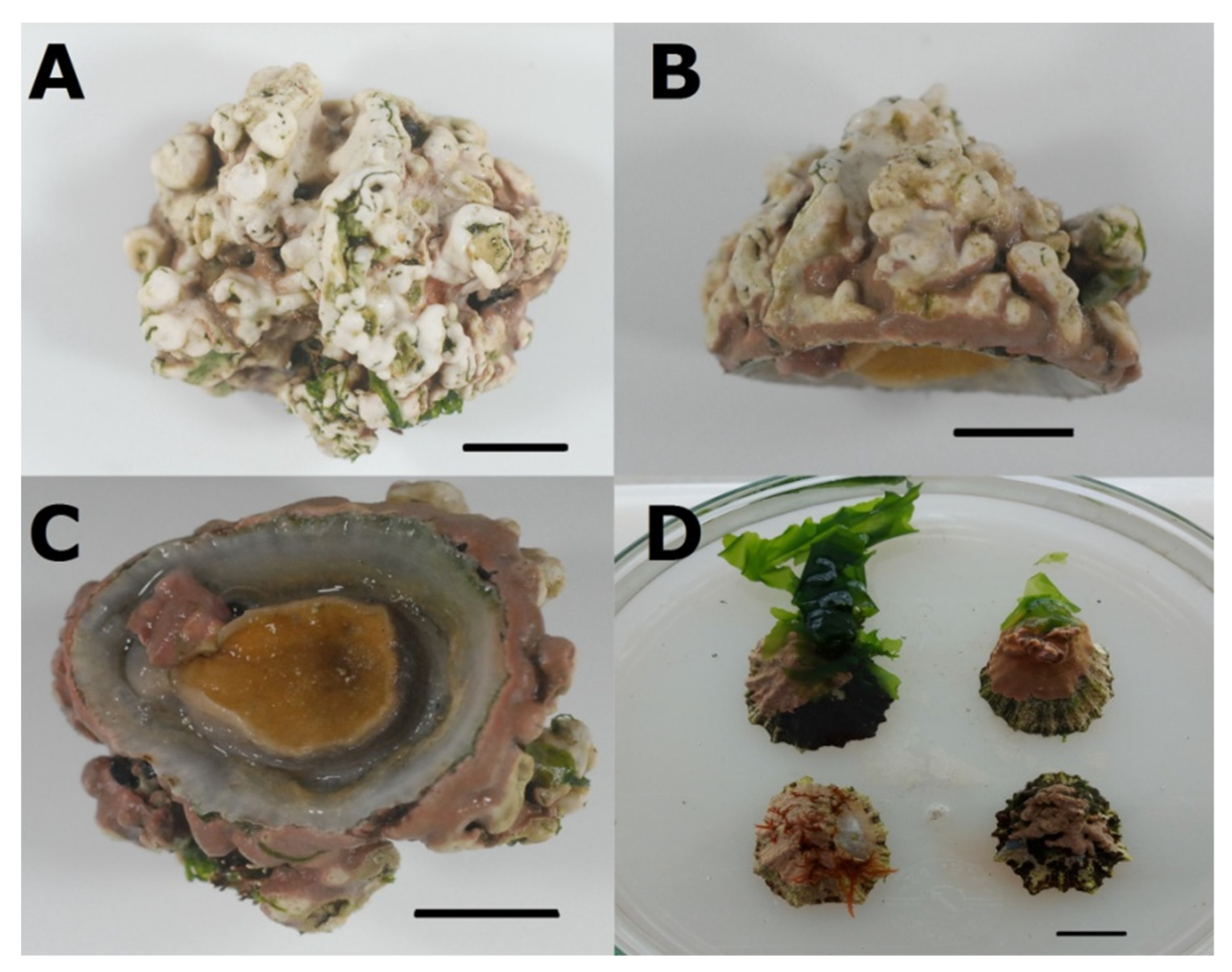
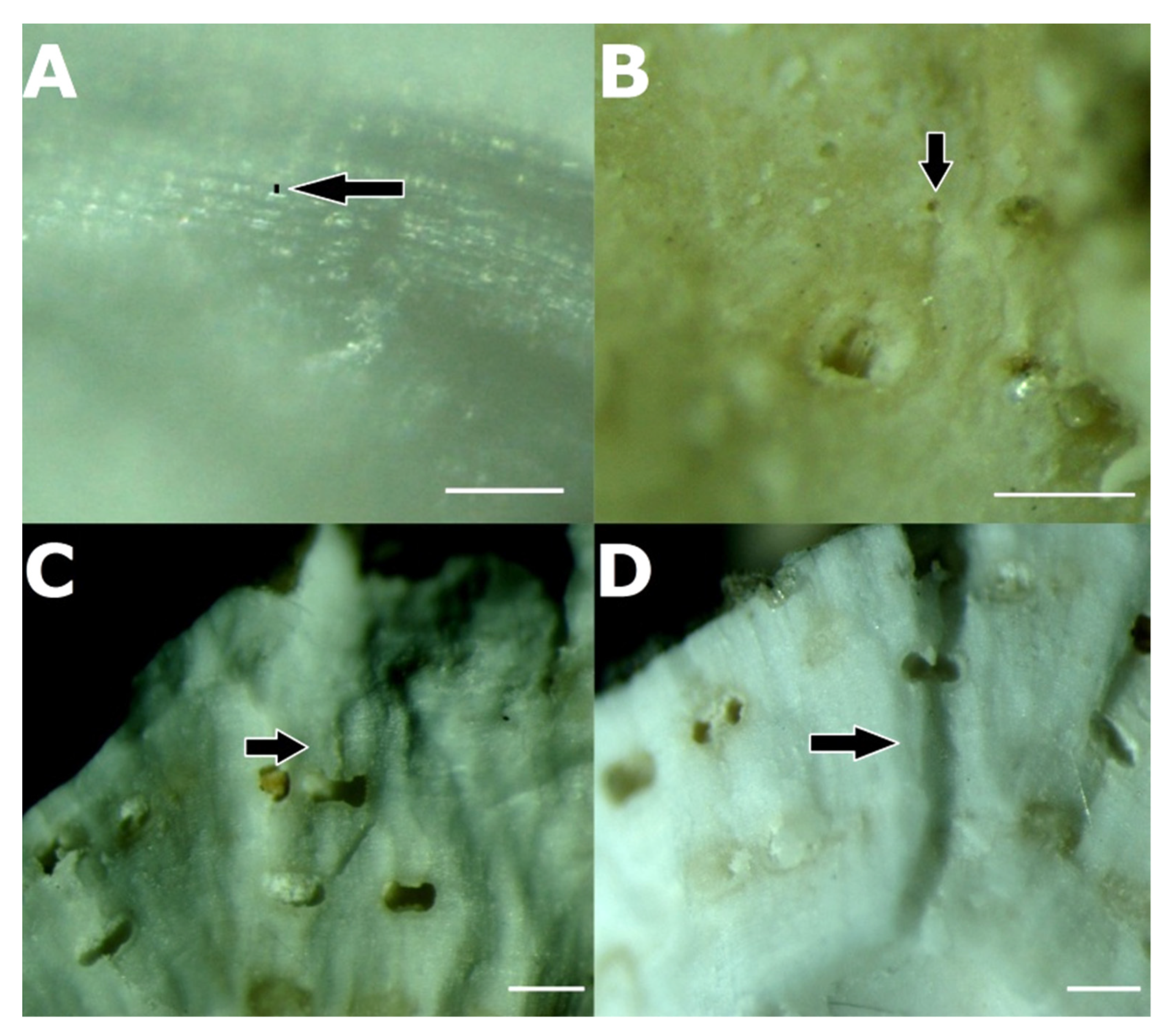
| Population | N° Seq. | Hap. | Hd | Π | Tajima’s D | Fu’s FS |
|---|---|---|---|---|---|---|
| Atlantic Continental | 94 | 17 | 0.7868 ± 0.0356 | 0.003691 ± 0.002537 | −1.9445 ** | −10.5684 ** |
| Mediterranean | 117 | 63 | 0.9524 ± 0.0117 | 0.009806 ± 0.005530 | −1.8685 ** | −26.0188 ** |
| Morocco | 14 | 3 | 0.4835 ± 0.1425 | 0.002806 ± 0.002217 | −0.5313 | 0.9689 |
| Azores | 14 | 9 | 0.8791 ± 0.0788 | 0.005465 ± 0.003655 | −1.5999 * | −4.7175 ** |
| Madeira/Canarias | 17 | 7 | 0.7500 ± 0.0924 | 0.004380 ± 0.003035 | −0.7066 | −2.1779 * |
| Population | N° Seq. | Hap. | Hd | Π | Tajima’s D | Fu’s FS |
|---|---|---|---|---|---|---|
| Alboran | 15 | 4 | 0.4667 ± 0.1478 | 0.002128 ± 0.001822 | −0.39 | −0.82 |
| Balearic | 23 | 12 | 0.8933 ± 0.0495 | 0.007148 ± 0.004401 | −1.75 ** | −5.01 ** |
| Tyrrhenian | 54 | 34 | 0.9399 ± 0.0214 | 0.009477 ± 0.005427 | −1.73 ** | −26.09 ** |
| Ionian | 15 | 15 | 1.0000 ± 0.0243 | 0.010790 ± 0.006393 | −1.24 | −13.69 ** |
| Aegean | 5 | 5 | 1.0000 ±0.1265 | 0.012766 ± 0.00876 | −0.65 | −1.41 |
| Atlantic | Mediterranean | Morroco | Azores | Canary + Madeira | |
|---|---|---|---|---|---|
| Atlantic | |||||
| Mediterranean | 0.04 ** | ||||
| Morocco | 0.25 ** | 0.12 * | |||
| Azores | 0.88 ** | 0.88 ** | 0.73 ** | ||
| Canary+Madeira | 0.89 ** | 0.90 ** | 0.74 ** | 0.40 ** |
| Alboran | Balearic | Tyrrhenian | Ionian | Aegean | |
|---|---|---|---|---|---|
| Alboran | |||||
| Balearic | 0.21 ** | ||||
| Tyrrhenian | 0.17 ** | 0.05 ** | |||
| Ionian | 0.23 ** | 0.03 | 0.06 | ||
| Aegean | 0.40 ** | 0.09 ** | 0.08 | −0.02 |
| Clade | TMRCA | CI |
|---|---|---|
| Patellidae | 96.6 | 64.7–143 |
| Patella | 36.1 | 13.6–67.6 |
| P. vulgata + P. ulyssiponensis | 18.0 | 6.3–33.2 |
| P. ulyssiponensis (continental and insular lineages) | 5.3 | 1.2–12 |
| P. ulyssiponensis (Azores and Tenerife) | 1.2 | 0.1–3.15 |
| Species | Period | Locality | Reference |
|---|---|---|---|
| Patella alternicostata Sandberger, 1859 † | Oligocene | Paris Basin, France | [147] |
| Patella estotiensis Lozouet, 1999 † | Upper Oligocene | Saint-Paul-lès-Dax, France | [148] |
| Patella protea Doderlein, 1862 † | Upper Miocene (Tortonian) | St-Clément-de-la-Place, France | [149] |
| Patella mahamensis Martín-González & Vera-Peláez, 2018 † | Upper Miocene (Tortonian) | Fuerteventura, Spain | [150] |
| Patella maxoratensis Martín-González & Vera-Peláez, 2018 † | Upper Miocene (Tortonian) | Fuerteventura; Lanzarote; Gran Canaria, Spain | [150] |
| Patella tintina Martín-González & Vera-Peláez, 2018 † | Upper Miocene (Tortonian) | Fuerteventura; Lanzarote; Gran Canaria, Spain | [150] |
| Patella ambroggii Lecointre, 1952 † | Pliocene | Mogador, Atlantic Morocco | [151] |
| Patella alessiae Forli, Dell’Angelo, Montagna & Taviani, 2004 † | Pliocene | Tuscany, Italy | [151] |
| Patella hebertiana (d’Orbigny, 1850) † | - | Vigny, France | [152] |
| Event | Date | Geographical Outcome | Ocean Current Pattern | References |
|---|---|---|---|---|
| Pangea breakup | Lower Jurassic (145 Mya) to Lower Cretaceous (110 Mya) | PaleoAfrica separation; Atlantic Ocean formation | Influx of the warm current from the Tethys Sea to the newly formed Atlantic Ocean | [156,157,158] |
| Tethys closure | Oligocene-Miocene 35 to 20 Mya | Collision of Arabian and Eurasia plates; Atlantic and Indian Oceans definitive separation | Influx of the warm current from the Pacific to all the Atlantic up to the United Kingdom; Total interruption of the current exchange between the Indic and Atlantic Oceans | [159] |
| Emergence and submersal of Proto-Madeira, Proto-Canary and Proto-Azores archipelagos | 60 to 8 Mya | Emergence of Gettysburg/Ormonde and Lars (60 Mya); Emergence of Great Meteor (16 Mya); Emergence of Porto Santo and Gran Canaria (10 Mya); Emergence of Santa Maria (8 Mya) | Great influx of the warm current from the Pacific to all the Proto-Macaronesian archipelagos | [160,161] |
| Messinian Salinity Crisis | 5.9 to 5.3 Mya | Closure of the Gibraltar Strait and subsequent desiccation of the Mediterranean Sea; Reopening of the Atlantic connection (Zanclean Flood) | Warm tides cycling on the North Atlantic Sea flows | [159] |
| Isthmus of Panama passage closure | 3.2 to 2.6 Mya | Formation of the land connection between North and South America | Apearence of the cold Canarian current (Canarian climate change from Tropical to Mediterranean) | [159,160] |
| Plio-Pleistocene glaciations | 2.7 Mya to 18 kyr | Multiple interglacial periods | Loss of land mass on Macaronesian archipelagos, with each island becoming more isolated | [159,160] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes, N.; Antunes, C.; Costa, D.d.A. Insights into the Migration Routes and Historical Dispersion of Species Surviving the Messinian Crisis: The Case of Patella ulyssiponensis and Epizoic Rhodolith Lithophyllum hibernicum. Hydrobiology 2022, 1, 10-38. https://doi.org/10.3390/hydrobiology1010003
Gomes N, Antunes C, Costa DdA. Insights into the Migration Routes and Historical Dispersion of Species Surviving the Messinian Crisis: The Case of Patella ulyssiponensis and Epizoic Rhodolith Lithophyllum hibernicum. Hydrobiology. 2022; 1(1):10-38. https://doi.org/10.3390/hydrobiology1010003
Chicago/Turabian StyleGomes, Nuno, Carlos Antunes, and Dimítri de Araújo Costa. 2022. "Insights into the Migration Routes and Historical Dispersion of Species Surviving the Messinian Crisis: The Case of Patella ulyssiponensis and Epizoic Rhodolith Lithophyllum hibernicum" Hydrobiology 1, no. 1: 10-38. https://doi.org/10.3390/hydrobiology1010003
APA StyleGomes, N., Antunes, C., & Costa, D. d. A. (2022). Insights into the Migration Routes and Historical Dispersion of Species Surviving the Messinian Crisis: The Case of Patella ulyssiponensis and Epizoic Rhodolith Lithophyllum hibernicum. Hydrobiology, 1(1), 10-38. https://doi.org/10.3390/hydrobiology1010003






