1. Introduction
Neuroblastoma is recognized as a frequently occurring extracranial solid tumor in infants and children. It originates from the sympathetic nervous system [
1]. As a tumor of the sympathetic neural pathway, neuroblastoma can arise at any point along this pathway. The majority of neuroblastoma are primary tumors found in the abdomen, reflecting the distribution of the sympathetic nervous system. It accounts for approximately 8–10% of all childhood malignancies [
2]. Although it predominantly affects young children, the disease can also occur, albeit less frequently, in adolescents and young adults, where it is often associated with a poorer prognosis [
3].
Treatment strategies for the disease are determined by disease stage and risk group and include multimodal approaches such as surgery, chemotherapy, radiotherapy, stem cell transplantation, and, more recently, immunotherapy. Despite these multimodal treatments, high-risk neuroblastoma cases are characterized by high rates of disease recurrence, low long-term survival, and frequent development of treatment resistance. Consequently, intensive modal treatment strategies, including high-dose chemotherapy and immunotherapy combinations, are required, which substantially increase treatment-related toxicity and negatively affect patients’ quality of life [
4,
5]. Therefore, there remains a need for more effective therapeutic strategies that can reduce treatment-related side effects and improve quality of life in patients with high-risk neuroblastoma.
TMZ is an alkylating chemotherapeutic agent that has been utilized in the treatment of high-risk groups, particularly in the cases of glioblastoma and high-risk neuroblastoma [
6]. This drug methylates adenine and guanine bases at specific positions in DNA. This DNA methylation leads to single- and double-strand breaks, induces cell cycle arrest at the G2/M phase, and ultimately triggers apoptosis, resulting in cell death [
7]. Although it has these properties, its clinical efficacy remains limited due to intrinsic or acquired chemoresistance and dose-limiting toxicities. Furthermore, systemic cytotoxic therapy often brings along heavy toxicities such as myelosuppression, fatigue, neurotoxicity, and gastrointestinal toxicities. Among these, chemotherapy-related nausea and vomiting are particularly disturbing, resulting in severe functional impairment and also an impact on overall quality of life for the patients [
8].
To alleviate these side effects, antiemetic drugs are commonly given with chemotherapy. Granisetron (GRN) is a selective 5-hydroxytryptamine type 3 (5-HT
3) receptor antagonist, which has been frequently used for the prophylaxis and treatment of chemotherapy-induced nausea and vomiting [
9].
One of the primary neurotransmitters responsible for nausea and vomiting following chemotherapy or radiotherapy is 5-HT [
10]. Beyond its role in emesis, 5-HT plays a vital part in regulating cellular functions across the central and peripheral nervous systems, as well as in the hormonal and hematopoietic systems [
11]. In addition to its well-known physiological role, 5-HT has been shown recently that 5-HT also act as the mitogenic neurotransmitter that triggers the proliferation of cancer cells [
12]. Therefore, it has been linked to tumor progression, invasion, and metastasis, and thus represents a fundamental target in oncology [
13]. In contrast to the tumor-promoting activity of 5-HT, numerous reports have shown that 5-HT receptor antagonists, used alone or in combination with chemotherapeutics, can suppress tumor cell growth. Such discoveries have led to increased interest in molecular targets of serotonergic signaling as potential anticancer treatments [
12].
Some antiemetics used in cancer treatment exert their effects by blocking 5-HT receptors and by inhibiting 5-HT binding [
14]. Since both drugs interact with chemotherapeutics, drug–drug interaction analysis is fundamental to clinical practice in cancer patients [
15].
The role of 5-HT in the growth, proliferation, invasion, and migration of has been studied in several cancers, including prostate, bladder, lung, liver, and gliomas [
16,
17,
18,
19,
20,
21,
22,
23]. Numerous studies have also examined the effects of 5-HT receptor antagonists on cellular processes, particularly on cell viability. This reduction is achieved by inhibiting cell growth and proliferation [
16,
17,
19,
23,
24]. However, to date, no studies have investigated the interaction of 5-HT or 5-HT receptor antagonists with TMZ in SH-SY5Y or HMC-3 cells. Therefore, this study was conducted to compare the possible impacts of 5-HT and a 5-HT antagonist, as well as their potential synergistic or antagonistic interactions with an anticancer agent, using SH-SY5Y and HMC-3 cell lines, which may provide novel insights into improving therapeutic efficacy while minimizing toxicity.
2. Materials and Methods
2.1. Chemicals, Reagents, and Cell Lines
Serotonin hydrochloride (8367.2) was purchased from Carl Roth (Karlsruhe, Germany). A 380 mM stock solution was prepared in 100% dimethyl sulfoxide (DMSO). For experimental applications, this stock solution was further diluted using cell culture medium containing 10% fetal bovine serum (FBS).
Granisetron hydrochloride was purchased from Bostonchem (Boston, MA, USA). A 110 mM stock solution was prepared in 100% phosphate-buffered saline (PBS) and diluted with cell culture medium supplemented with 10% FBS during the experiments.
Temozolomide (T2744-25 mg) was purchased from TCI Chemicals. A 200 mM stock solution was prepared in 100% DMSO and diluted with cell culture medium containing 10% FBS for experimental use.
For cytotoxicity analysis, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was used, while crystal violet assays were performed to assess antiproliferative effects together with morphological alterations.
In this study, SH-SY5Y (ATCC CRL-2266) neuroblastoma cells and HMC-3 (ATCC CRL-3304) microglial cells were used, with the latter serving as the healthy control cell line. The SH-SY5Y neuroblastoma (ATCC CRL-2266) and HMC3 human microglial cell lines (ATCC CRL-3304) were obtained from the American Type Culture Collection (ATCC), Manassas, VA, USA.
2.2. Cell Culture and Cytotoxicity Assays
SH-SY5Y cells were maintained in the flasks using a growth medium composed of Dulbecco’s Modified Eagle’s Medium (DMEM) F12 (22.5 mL) and Eagle’s Minimum Essential Medium (EMEM) (22.5 mL), supplemented with 10% FBS and 1% penicillin/streptomycin solution (10 U/mL). The cells were incubated under controlled conditions at 37 °C with 90–95% humidity and 5% CO2 until sufficient proliferation was achieved.
HMC-3 microglial cells were cultured in EMEM (44.4 mL) supplemented with 10% FBS and 1% penicillin/streptomycin solution (10 U/mL) under identical conditions used for SH-SY5Y cells until adequate cell growth was obtained.
2.2.1. Determination of Cytotoxicity
The evaluation of cytotoxicity was investigated by MTT assay. One of the most popular techniques for determining cytotoxicity and cell viability is MTT. Based on variations in metabolic activity, this colorimetric assay is flexible, quick, and accurate in assessing cell viability.
SH-SY5Y cells were seeded at 1 × 10
4 cells/well and HMC3 cells at 8 × 10
3 cells/well into 96-well culture plates. After seeding, the cells were treated with single, dual, and triple combinations of 5-HT, GRN, and TMZ at the concentrations listed below for 48 h to evaluate cytotoxicity (
Table 1).
Hydrogen peroxide (250 µM, H2O2) was applied as a positive control, and no-treatment cells were used as a negative control. After exposure to 5-HT, GRN, and TMZ, culture medium (100 µL) was added with 20 µL of MTT solution (5 mg/mL), and incubated for a further 4 h at 37 °C. The formed formazan crystals were fully solubilized in DMSO. The absorbance of the resulting solubilized formazan solution, which correlates directly with the number of viable cells, was then measured at 570 nm using a microplate reader (Epoch, BioTek, Winooski, VT, USA).
For every analysis, three independent experiments with six samples in each were performed. The cell viability was quantitatively determined as a percentage according to the following standardized Formula (1), with that of the control group considered as 100%.
2.2.2. Determination of Morphological Changes and Antiproliferative Effects
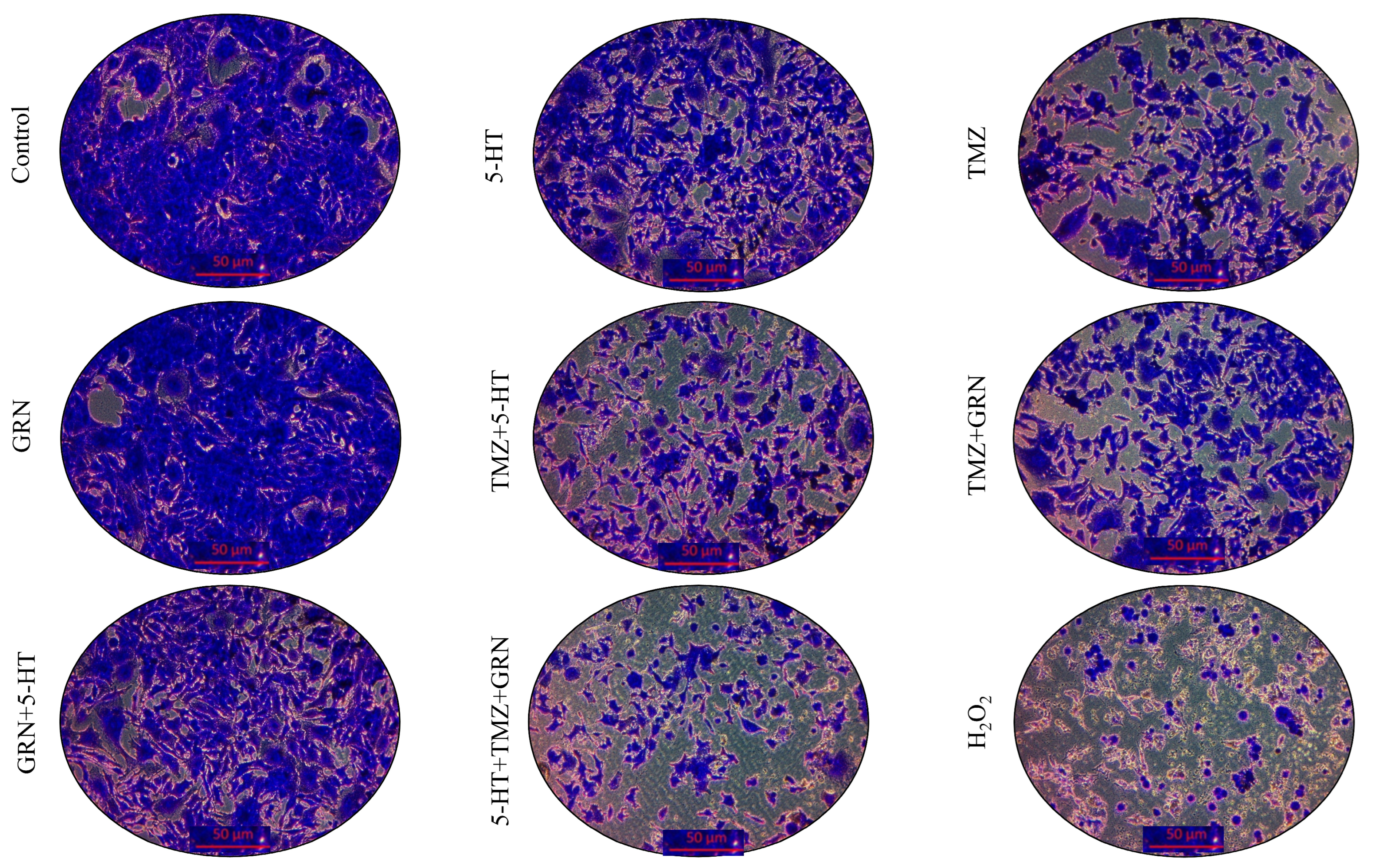
The crystal violet test is used to determine the effects of changes in cell proliferation on growth inhibition. It utilizes the dye’s binding to the proteins and DNA of viable, adherent cells. Dying cells lose their adherent properties, leading to their separation from the cell population and a decrease in color intensity in the culture. Crystal violet staining provides qualitative data on cell morphology. The decrease in staining intensity and morphological integrity of the cells directly correlates with the cytotoxic effect of the applied treatment, providing results that support toxicity.
For the crystal violet assay, cells were prepared as described for the MTT, and following treatment, the culture medium was aspirated after 48 h. Then, 20 µL of crystal violet solution was added to each well, and the plates were incubated at room temperature in the dark for 20 min. After the incubation period, the plates were cleaned and left to dry. Morphological changes were noted and captured with an inverted microscope. After that, 150 µL of 70% ethanol was added to each well to dissolve the crystal violet stain. A microplate reader (Epoch, BioTek, Winooski, VT, USA) was used to measure absorbance at 570 nm.
For each analysis, three independent experiments were carried out in six replicates. The viability of cells was calculated using the absorbance of the control group as 100%, and the proliferation values for the other groups were calculated as a percentage using Formula (1).
2.3. Cell Migration Analysis
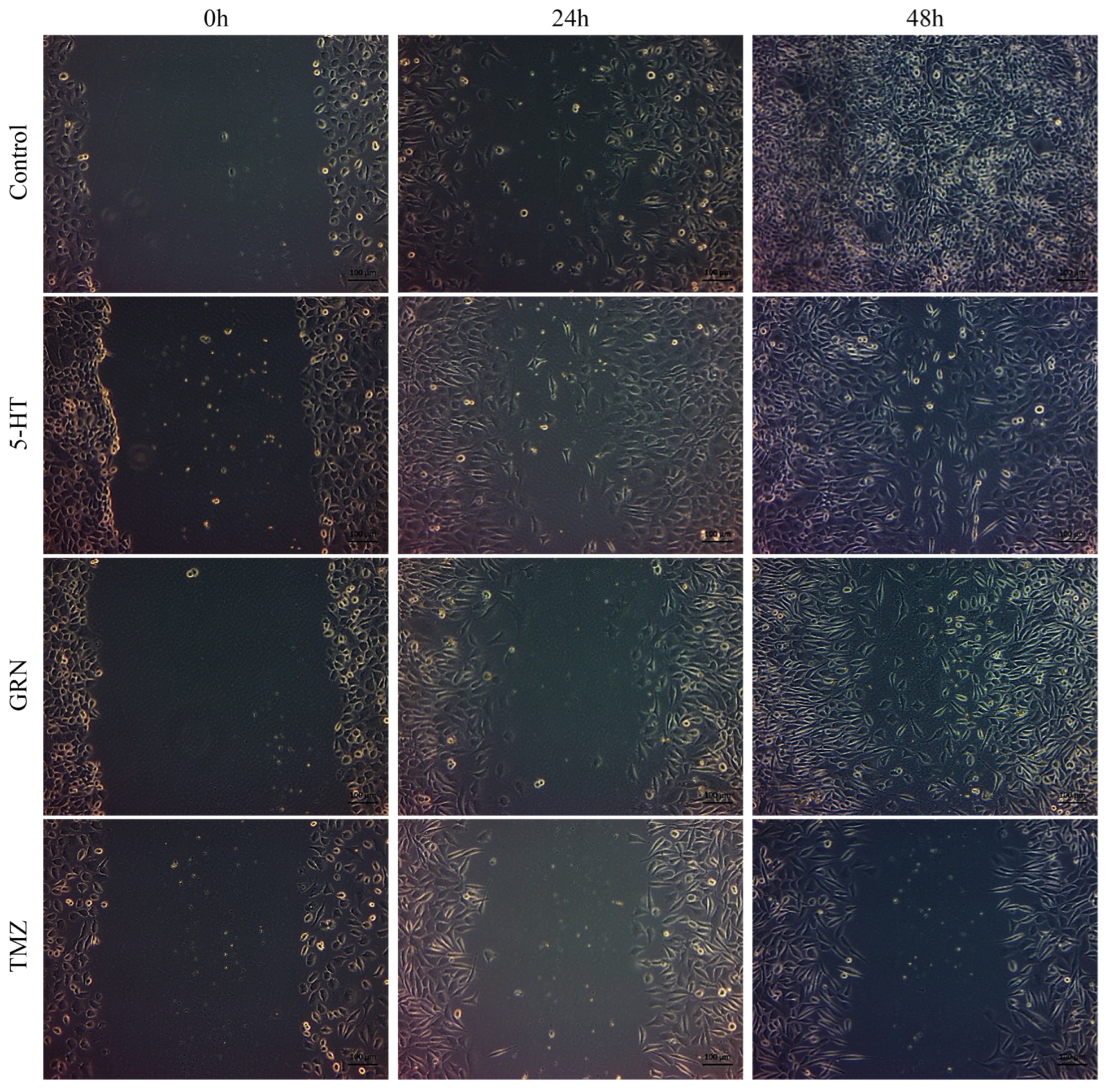
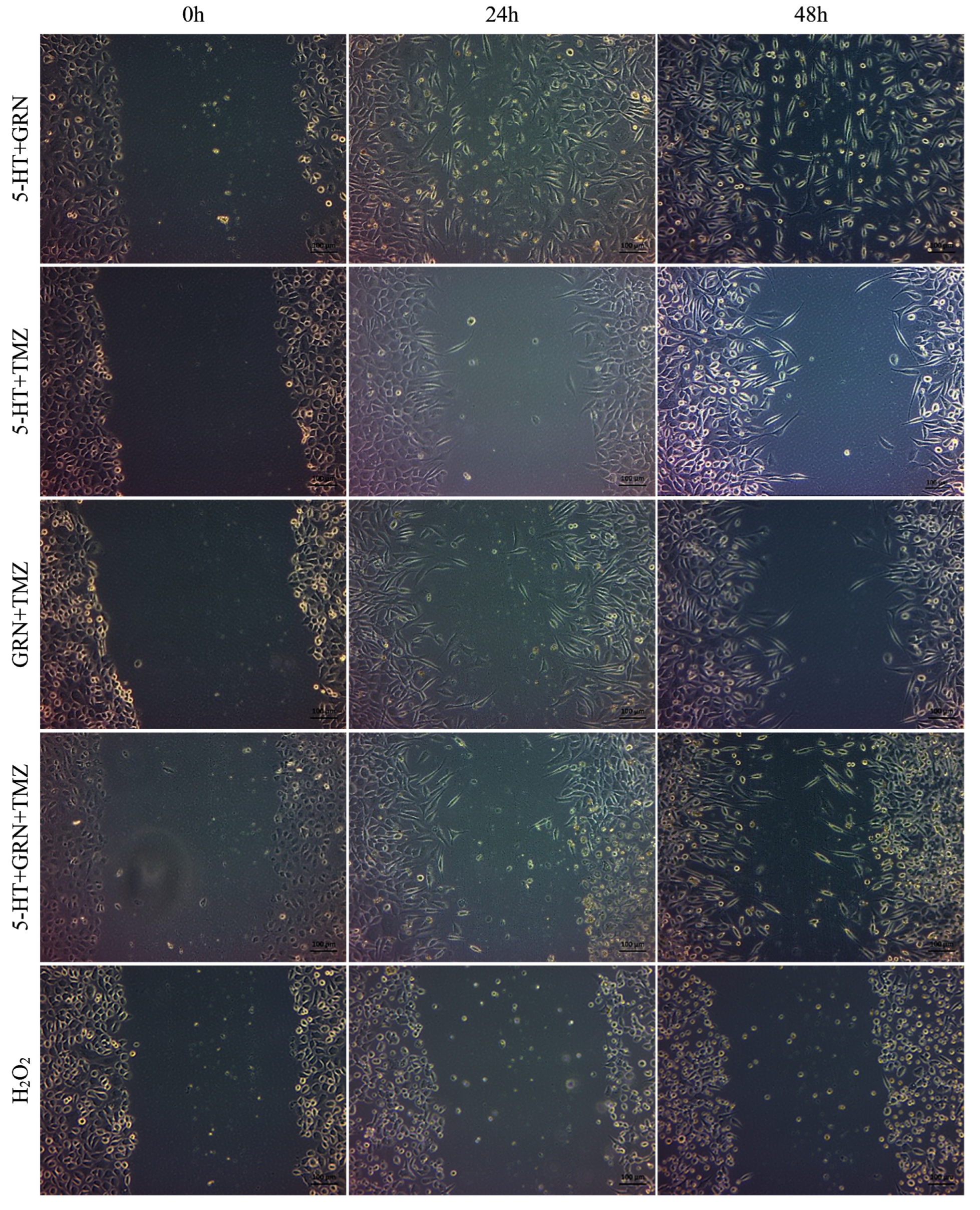
To determine how single, double, and triple combination treatments of 5-HT, GRN, and TMZ affected cell migration, SH-SY5Y cells were seeded at 1 × 104 cells/well and HMC3 cells at 8 × 103 cells/well in 24-well plates. The cells were placed in an incubator with set conditions (37 °C temperature and 5% CO2) for 48 h. They were examined under an inverted microscope. When cell density reached 70–80%, a line (wound) was drawn parallel to each other on the bottoms of the wells using a pipette tip. The medium in the wells was aspirated and washed with PBS. Based on IC50 values, 1.5 mL of single, double, and triple combinations of 5-HT, GRN, and TMZ were added to the wounded wells at selected concentrations. As a control, 1.5 mL of the cell-only medium was seeded in one well. Then, the image coordinates of each well were recorded, and images of the scratch distances created at the end of the 0th, 24th, and 48th h were taken.
2.4. Statistical Analysis
Standard deviation was obtained as ± SEM from three separate experiments done in six replicas. All the data were analyzed by GraphPad Prism 6.0. One-way analysis of variance (ANOVA) was conducted to assess the statistical significance, which was determined by a p-value less than 0.05, considered significant.
3. Results
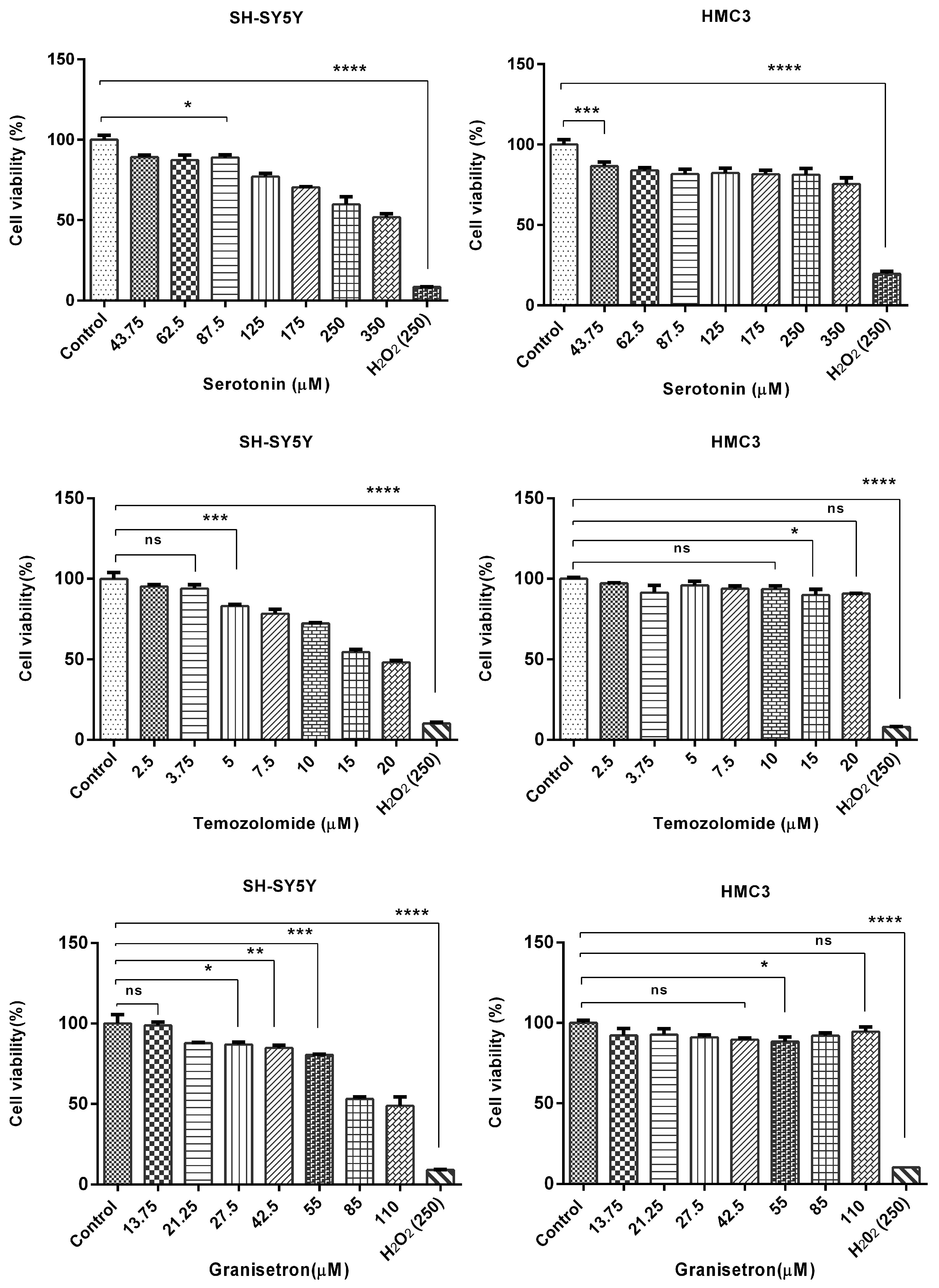
3.1. Serotonin, Temozolomide, and Granisetron Reduced Cell Viability in a Concentration-Dependent Manner
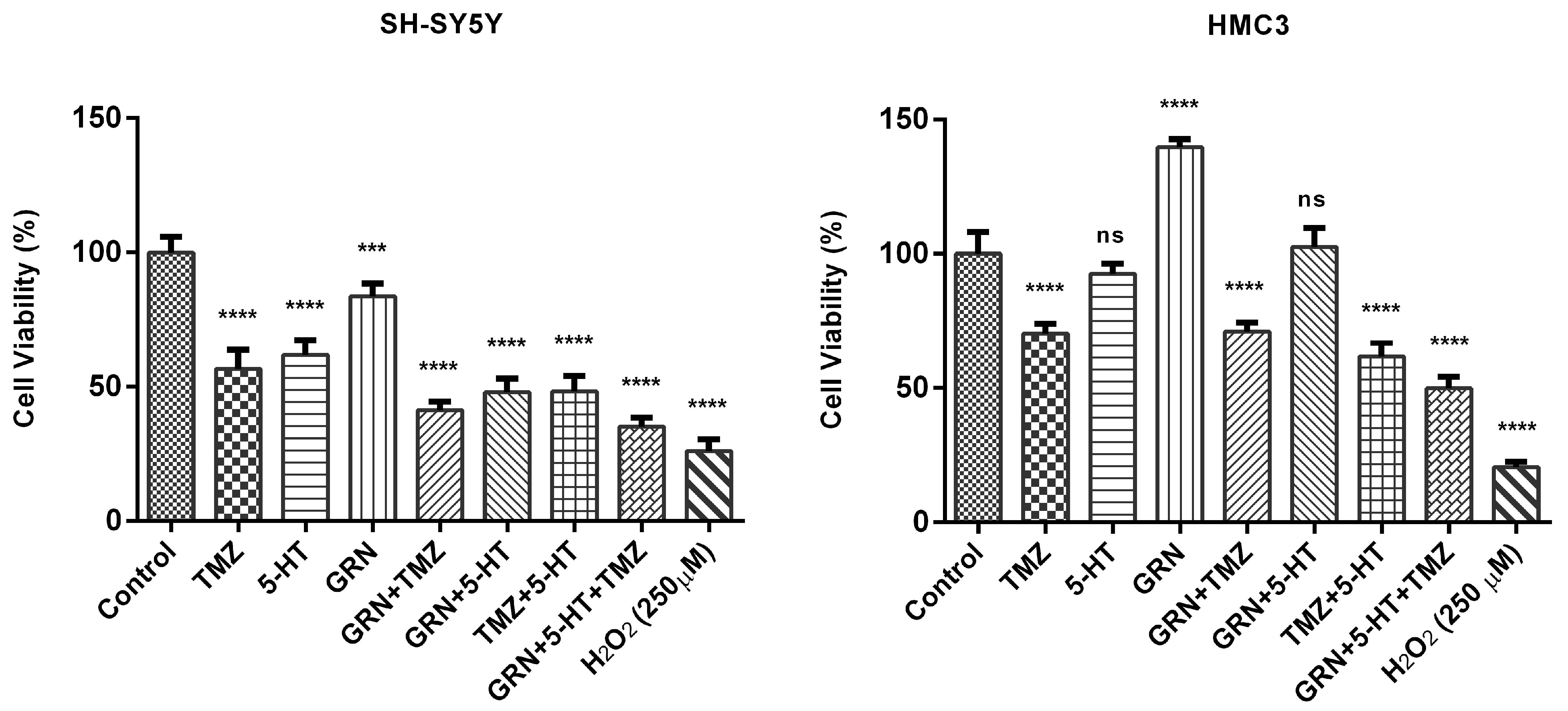
SH-SY5Y and HMC3 cells were treated with 5-HT, TMZ, and GRN for 48 h. Some cells received no treatment (control-untreated). After the incubation period, the MTT assay was implemented to analyze the effects of 5-HT, TMZ, and GRN applied to the cells at selected concentrations on cell viability.
Figure 1 shows the MTT results. MTT analysis results revealed that single applications of 5-HT, GRN, and TMZ in SH-SY5Y caused a progressive decline in cell viability as the concentration increased. A significant decrease in viability occurred as the concentration of 5-HT was increased. TMZ, on the other hand, caused significant cytotoxicity starting at low concentrations. Suppression of cell proliferation was also observed at higher concentrations of GRN.
In HMC3 cells, after 48 h of 5-HT, TMZ, and GRN administration, linearity was observed in the concentration-dependent change trends in comparison with the control group in terms of relative viability percentages (
Figure 1). In other words, no significant decrease in viability rates was revealed in HMC3 cells after the same treatments, suggesting that these cells may be more resistant to toxic effects.
Moreover, based on MTT data, half-maximal inhibitory concentrations (IC50) of 5-HT, GRN, and TMZ were determined. The values for the IC50 were 368 ± 12.71 µM, 18.08 ± 0.89 µM, and 93.6 ± 4.2 µM for 5-HT, TMZ, and GRN, respectively.
The results suggest that 5-HT at high, peritumoral concentrations is cytotoxic rather than mitogenic. The findings are consistent with those of earlier studies showing that the effect of 5-HT on tumor cells is a function of increasing concentration and cell type.
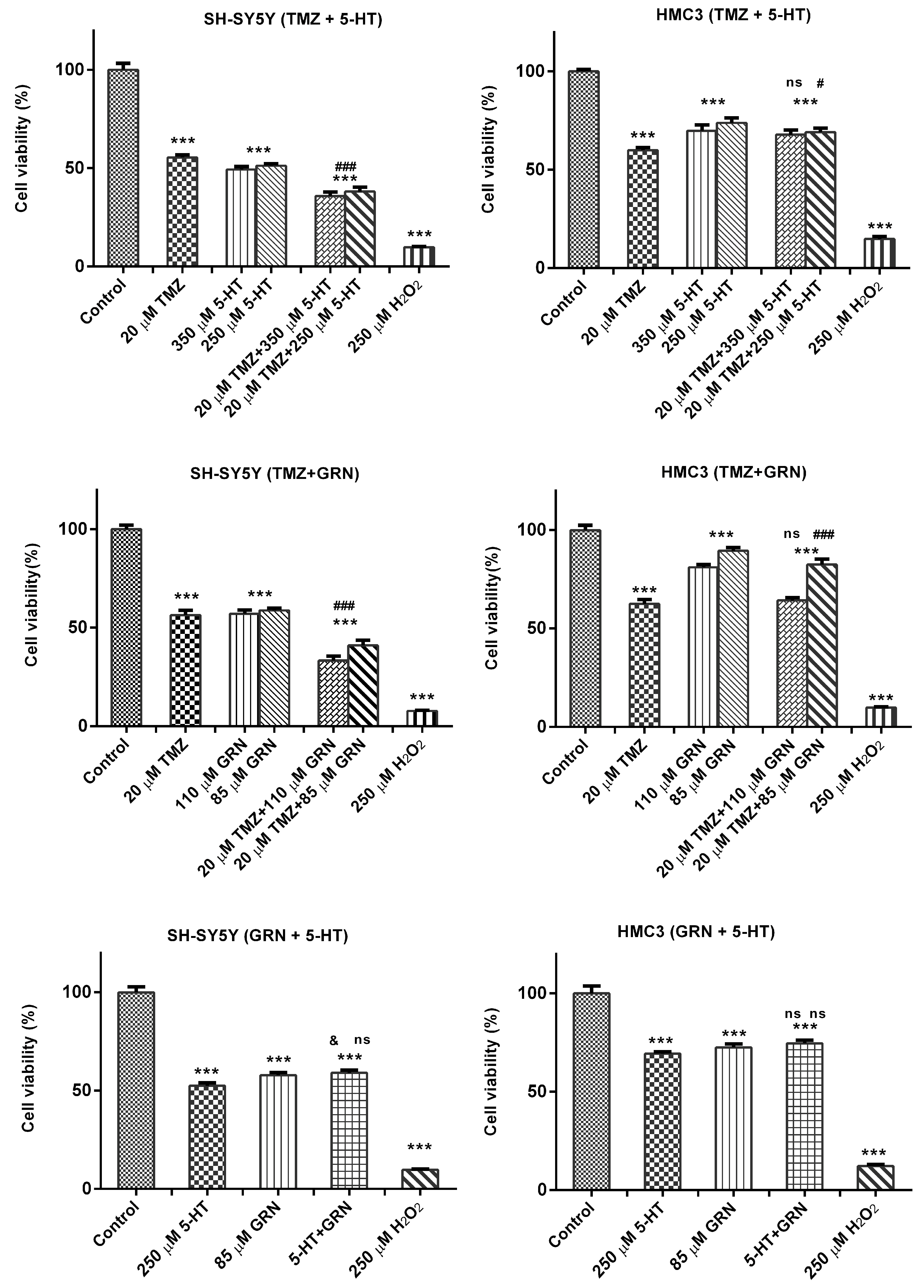
3.2. Dual Combinations of Serotonin, Temozolomide, and Granisetron Reduced Cell Viability in SH-SY5Y Cells via Synergistic-like Effects
SH-SY5Y and HMC3 cells were treated for 48 h with dual combinations of TMZ–5-HT, TMZ–GRN, and GRN–5-HT. A subset of cells received no treatment and served as the untreated control group. After the incubation period, the MTT test was performed to assess the effects of the various concentrations of these dual combinations on cell viability. In SH-SY5Y cells, 48 h exposure to the combinations of TMZ–5-HT, TMZ–GRN, and GRN–5-HT led to a significant reduction in cellular viability in comparison with the control group (
Figure 2). All three paired combinations produced a markedly greater loss of viability than the corresponding single-agent treatments (
p < 0.001). Notably, the TMZ–GRN combination produced a pronounced potential synergistic effect. This synergy suggests that blocking 5-HT receptors together with DNA-damaging chemotherapeutic agents may enhance cell death.
In HMC3 cells, however, no significant reduction in viability was observed after 48-h exposure to any of the dual combinations compared with the control group, suggesting that these combinations may exert a selective cytotoxic effect on cancer cells.
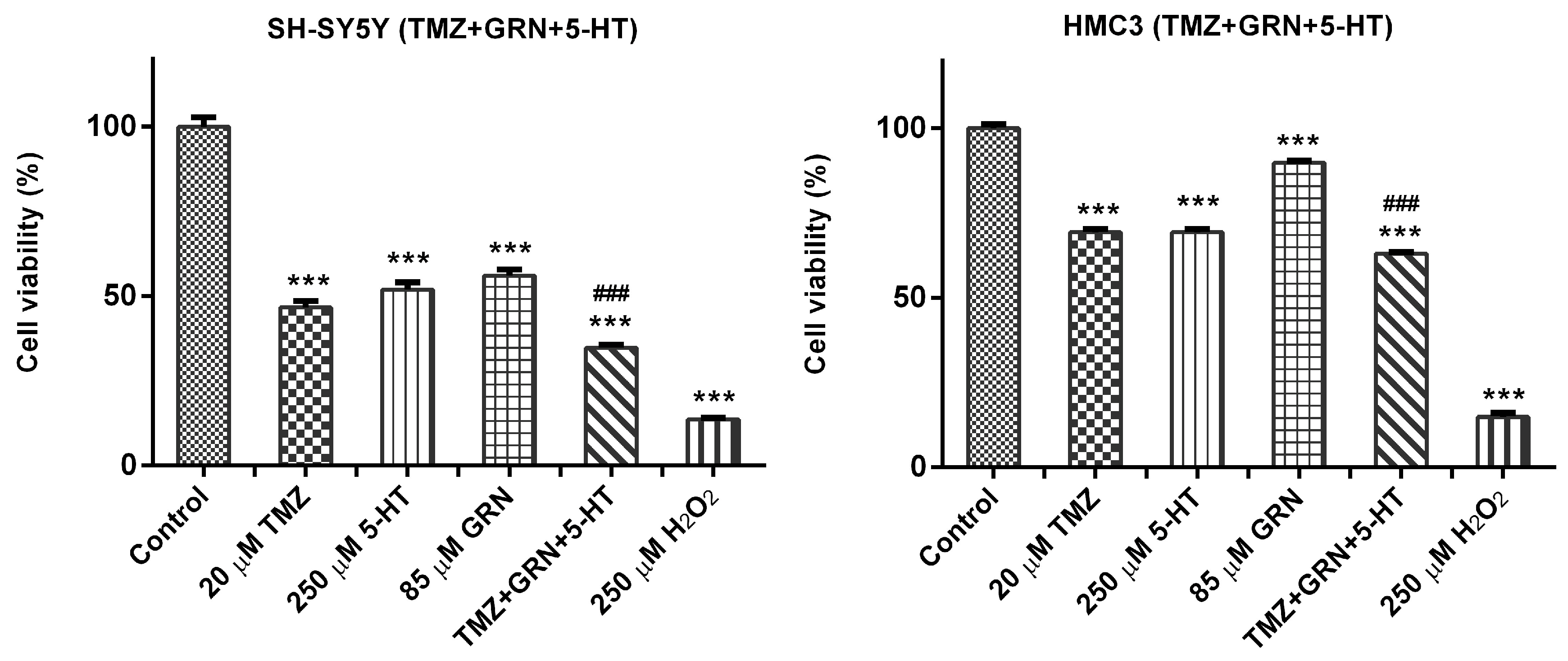
3.3. Triple Combinations of Serotonin, Temozolomide, and Granisetron Reduced Cell Viability in SH-SY5Y Cells via Synergistic-like Effects
SH-SY5Y and HMC3 cells were exposed to a triple combination of 5-HT–TMZ–GRN for 48 h. A subset of cells received no treatment and served as the untreated control group.
After the incubation period, MTT analyses were performed to assess the effects of various concentrations of the 5-HT–TMZ–GRN triple combination on cell viability. The results showed that 48 h exposure to the triple combination markedly reduced cell viability in SH-SY5Y cells in comparison with the control group (p < 0.001).
In HMC3 cells, however, a significant drop in viability was not observed following 48 h treatment with the 5-HT–TMZ–GRN combination compared with the control (
Figure 3).
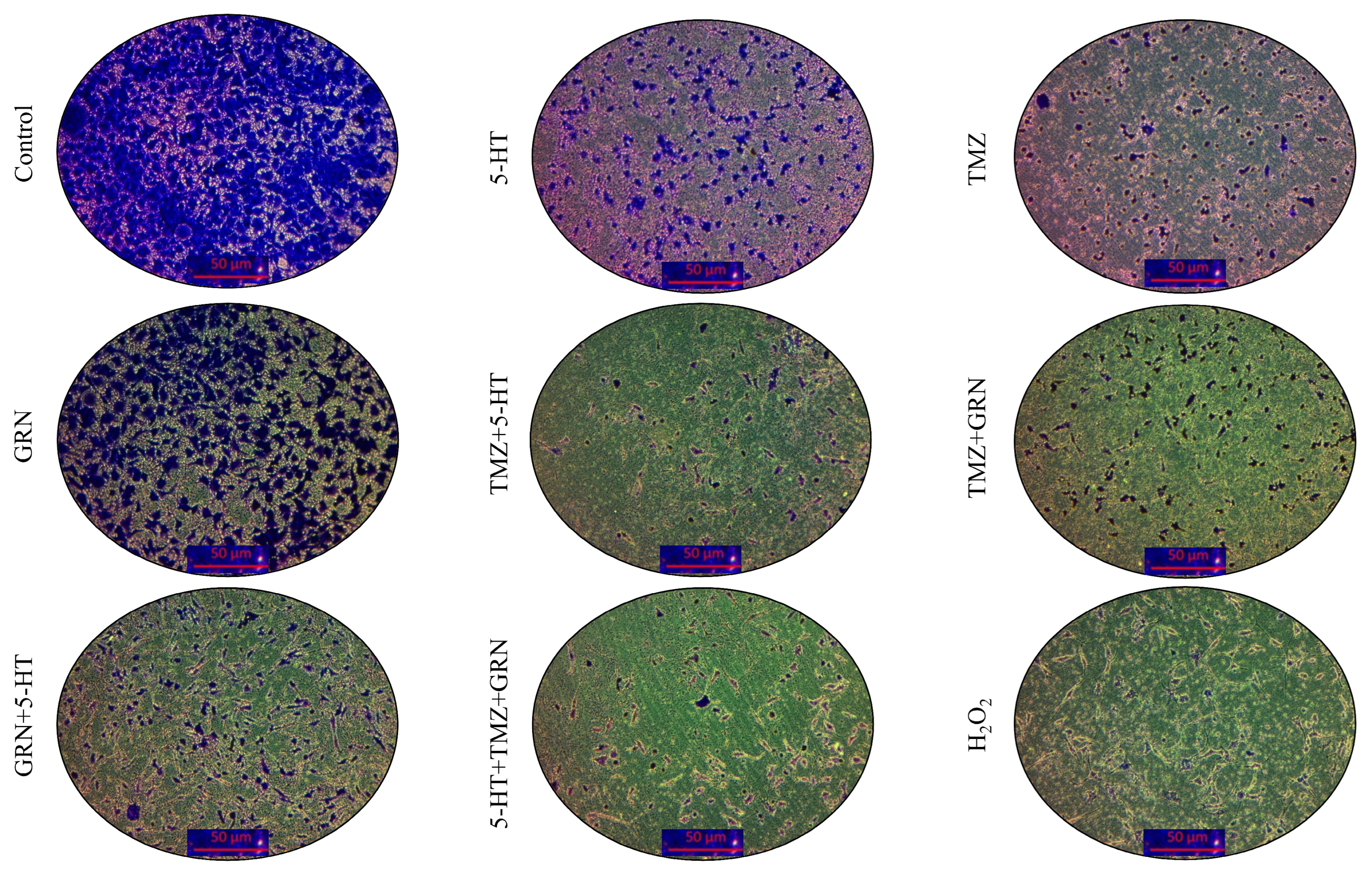
3.4. Serotonin, Temozolomide, and Granisetron Exhibited Antiproliferative Effects in SH-SY5Y Cells
SH-SY5Y and HMC3 cells were treated for 48 h with 5-HT, TMZ, GRN, and their dual and triple combinations. A subset of cells received no treatment and served as the untreated control group.
After the incubation period, crystal violet staining was performed to evaluate morphological changes and cell proliferation. The results showed that all combinations (GRN + TMZ, GRN + 5-HT, 5-HT + TMZ, GRN + 5-HT + TMZ) exerted strong cytotoxic effects on SH-SY5Y cells. This indicates that the combined treatments suppressed cell proliferation and reduced the number of adherent cells. Among all treatments, the triple combination of 5-HT, GRN, and TMZ produced the most pronounced antiproliferative effect in SH-SY5Y cells. In comparison with the control group, cell viability decreased significantly (p < 0.001), and morphological examination revealed disrupted cell integrity, cell shrinkage, and rounding with detachment from the surface. These findings suggest that TMZ may act in a synergistic-like manner with the high-concentration antiproliferative effects of GRN and 5-HT, thereby enhancing cell death.
In contrast, HMC3 cells maintained or even increased their viability. This indicates that 5-HT, TMZ, and GRN may exert a proliferative or non-toxic effect on HMC3 cells.
As seen in
Figure 4, cell density in SH-SY5Y cells was significantly reduced in comparison with the control group, except for GRN. Furthermore, compared to the control group, intercellular junctions were significantly disrupted, and cells exhibited shrinkage and deformation. Conversely, in HMC3 cells, these changes were minimal, and overall, the cells preserved their normal morphology (
Figure 5).
According to the crystal violet staining results, the reduction in staining intensity in the combination treatments indicates a loss of cell adhesion and a pronounced suppression of cell proliferation (
Figure 6).
3.5. Migration Analysis Results
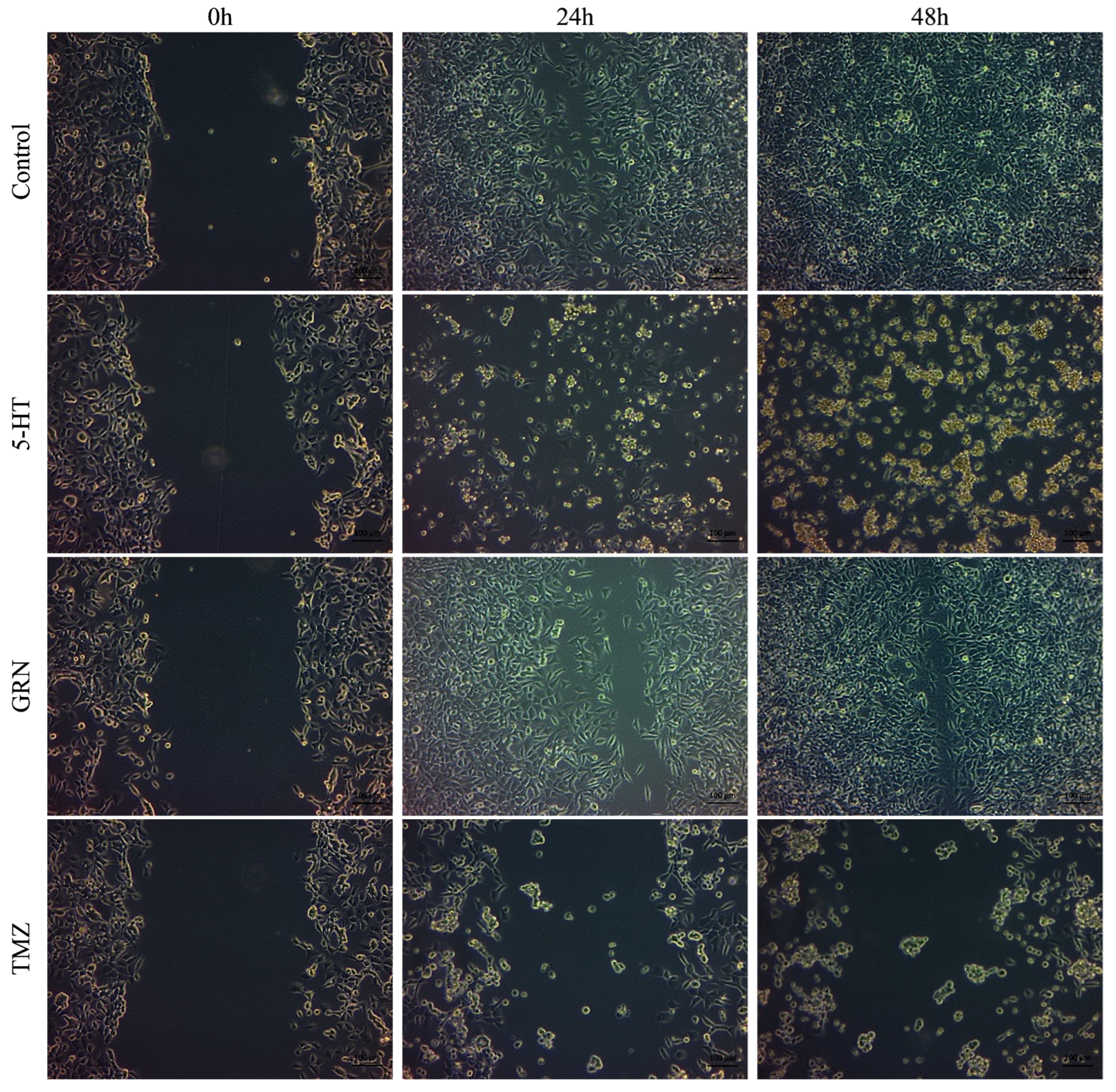
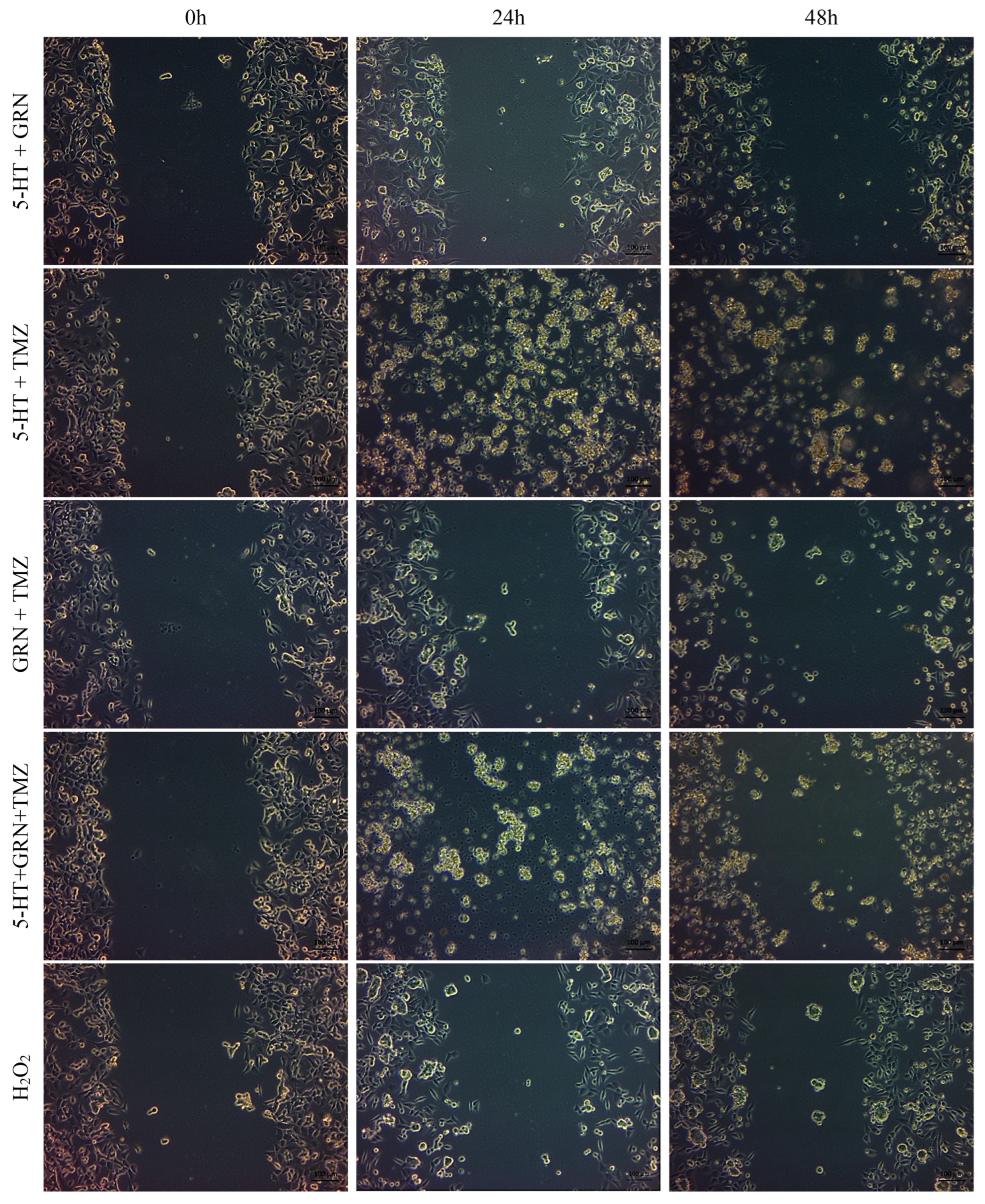
After seeding the 24-well plates, the cells were incubated for 48 h. Scratch marks were created in the wells containing SH-SY5Y and HMC3 cells. These cells were treated with single, double, and triple combinations of 5-HT, GRN, and TMZ at various concentrations. A culture medium containing no 5-HT, GRN, or TMZ was added to the control group. Images of the scratch areas in the wells were taken at three different times: 0, 24, and 48 h.
In SH-SY5Y cells, treatments with TMZ, 5-HT, and GRN markedly inhibited cell migration. In the triple combination group, migration was almost completely stopped. This finding indicates disruption of cytoskeletal dynamics and a substantial decrease in proliferative capacity (
Figure 7 and
Figure 8). The deterioration in cell morphology and the reduction in the migration rate also suggest activation of apoptotic processes.
In HMC3 cells, the migration rate was only slowed, and cell viability was preserved (
Figure 9 and
Figure 10). The migration patterns of control cells and those treated with 5-HT, GRN, and TMZ at different time intervals are presented in
Figure 7,
Figure 8,
Figure 9 and
Figure 10.
4. Discussion
5-Hydroxytryptamine (5-HT), which is a multi-effect neurotransmitter, contributes to the regulatory activity of gastrointestinal motility, vascular tone, platelet aggregation, and central nervous system activity. It is also important in the pathophysiology of several diseases, such as mood disorders and emesis [
25]. While 5-HT itself is not used directly as a therapeutic agent, many serotonergic drugs (agonists and antagonists at various 5-HT receptors; selective serotonin reuptake inhibitors [SSRIs] attenuating the uptake of 5-HT; and drugs affecting the release of 5-HT) are in routine clinical use [
26]. In oncology, these drugs are frequently used to treat chemotherapy-induced nausea and vomiting, which is related to raised levels of 5-HT, and psychiatric symptoms occurring in cancer patients [
27,
28,
29].
There is now accumulating evidence that 5-HT functions as a mitogenic factor and can stimulate proliferative signal pathways in both normal and cancer cells. In contrast, 5-HT at low concentrations was described to inhibit tumor growth through induction of vasoconstriction by inhibiting blood flow to the tumor due to vasoconstrictor actions via a subset of 5-HT receptors [
12,
30]. These data suggest a concentration-dependent effect of 5-HT on tumor biological features. Some lines of evidence come to support this idea: it has been demonstrated that 5-HT receptor antagonists, serotonin transporter inhibitors, and inhibitors of 5-HT biosynthesis can interfere with cancer cell proliferation [
12].
There are few studies on neuroblastoma regarding serotonergic signaling in cancer, although there is ample literature in the field of cancer-related research. The clarification of the influence of 5-HT and 5-HT receptor antagonists in SH-SY5Y neuroblastoma cells, especially when combined with classical anticancer drugs, is therefore a matter of great interest. In the current study, we examined 5-HT and its receptor antagonist granisetron (GRN), as well as their effects on temozolomide (TMZ)-induced responses in neuroblastoma (SH-SY5Y) and microglial cells (HMC3).
Our results showed that 5-HT, GRN, and TMZ alone or in single–double–triple combination could decrease the viability of SH-SY5Y cells in the MTT assay. Single–double–triple incorporation inhibited the proliferation and migration ability of SH-SY5Y markedly. In addition, these treatments enhanced the rate of cell death in a concentration-dependent manner. Of note, combined treatments applied more robust cytotoxic activities than when used as single agents, suggesting that 5-HT, GRN, and TMZ potentially had an interacting (possibly synergistic) action on each other.
Heterogeneous 5-HT effects on cancer cells have been described in previous studies. Although lower amounts of 5-HT would stimulate proliferation, higher concentrations were reported to cause cytotoxicity as well as induce apoptosis [
16,
30]. 5-HT has been demonstrated to induce proliferation in cells of SCLC and PRC, whereas 5-HT receptor antagonists suppress proliferation and activate apoptotic routes [
16,
20,
31,
32]. Similar proliferative effects of 5-HT and inhibition of its antagonists have also been shown in breast and bladder cancer models [
23,
33].
5-HT has exhibited anti-inflammatory, antioxidant, and anti-apoptotic properties on SH-SY5Y cells [
34]. In contrast, 5-HT receptor antagonists, including GRN, have been shown to exhibit antiproliferative effects in various cancer cells, such as prostate cancer, bladder cancer, and glioma [
17,
19,
21]. Our results were also consistent with those of researchers using GRN-like antagonists such as tropisetron and ketanserin, which demonstrated significant concentration-dependent cell viability and proliferation loss in a lung carcinoma cell line. Moreover, cotreatment with TMZ led to a potential synergistic cytotoxic effect compared with individual treatment [
35].
TMZ is a well-established chemotherapy drug with known anticancer effects as an alkylating agent. TMZ has been reported in several studies to time- and concentration-dependently suppress cell viability and proliferation of SH-SY5Y cells, with significant effects evident after exposure to 48 h [
36,
37]. Furthermore, glioblastoma cell lines were described to be more resistant towards TMZ than SH-SY5Y cells, indicating a tumor-type specific divergence of the drug sensitivity [
38]. These results are consistent with our findings and further demonstrate the superior efficacy of combination regimens.
In summary, our results demonstrate that the combination of dual serotonergic modulation (5-HT/GRN) with temozolomide (TMZ) effectively decreases cell viability, proliferation, and migration and stimulates cell death in SH-SY5Y neuroblastoma cells. These combinations were superior to TMZ alone, and they could be evidence for serotonergic pathways potentiating the antitumor effects of TMZ. However, accurate determination of drug synergy could not be quantitatively confirmed, since no CI analysis was performed in the context of this study. Future drug synergistic studies are required to test the true nature of such drug–drug interactions by designing fixed ratios of drug combinations and CI values. These novel combination strategies are of considerable interest from a translational research perspective for creating integrated formulation strategies. For example, in a single delivery system, it may be possible to co-encapsulate TMZ with cytotoxic agents that influence 5-HT such as granisetron. This encapsulation provides a unique platform for enhancing drug stability, allowing synchronized release, and minimizing systemic toxicity, an important consideration in pediatric oncology.
Subsequent studies should involve apoptosis, cell cycle regulation, and DNA damage response-associated mechanistic assays, as well as serotonin receptor subtypes and signaling pathway assessment. In vivo neuroblastoma models would be essential to establish the therapeutic efficacy, toxicity, and drug synergy, as well as the potential clinical application of serotonergic modulation-based treatment.