Targeting Oxidative Stress and Inflammation with Vitis vinifera Leaf Extract: A Combined Experimental and Computational Pharmacological Study
Abstract
1. Introduction
2. Material and Methods
2.1. Ethical Consent
2.2. Chemicals
2.3. In Vitro Potential Assessment of V. vinifera Extract
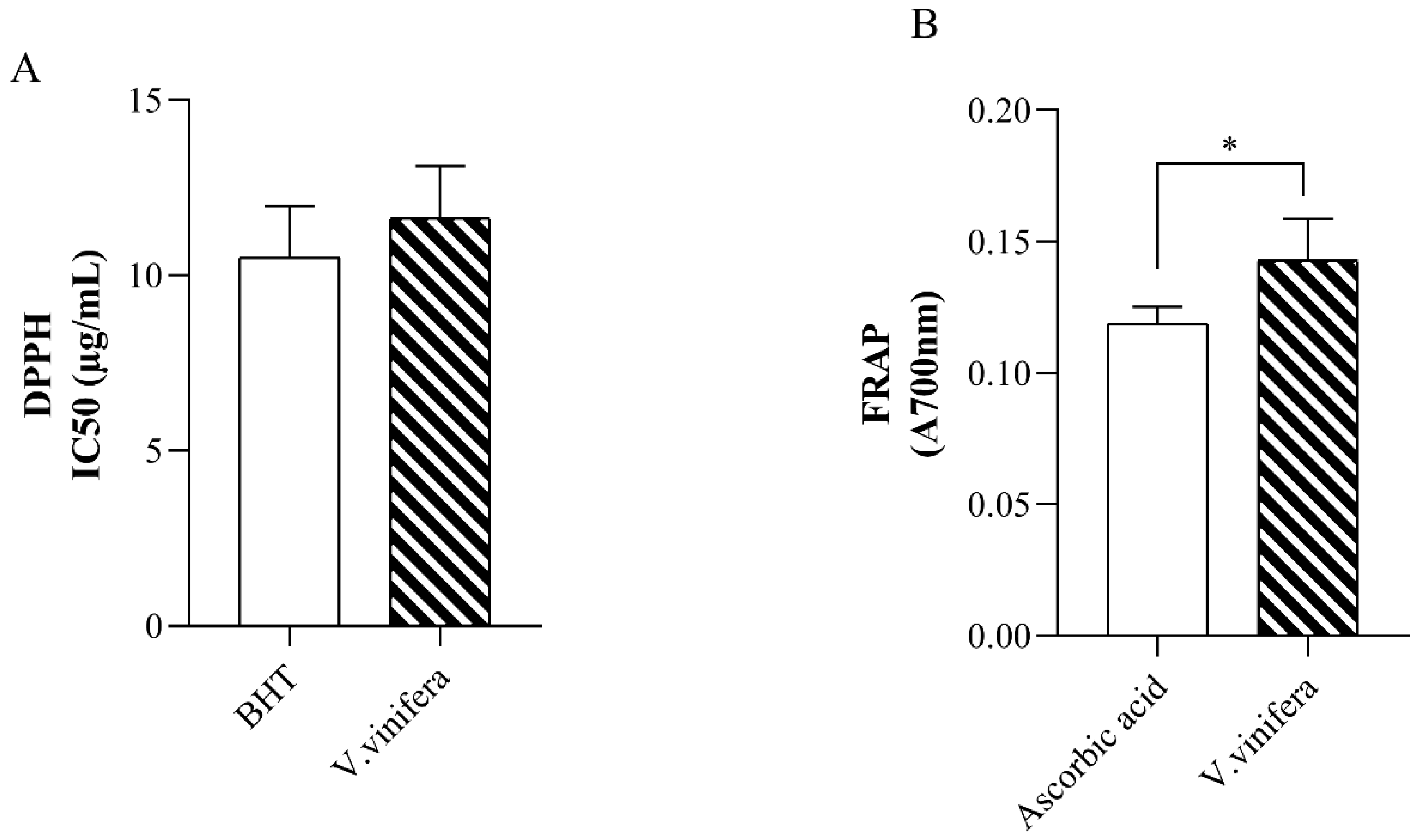
2.3.1. Assessment of DPPH Radical Scavenging Activity
2.3.2. Assessment of Ferric Reducing Antioxidant Power (FRAP) Assay
2.4. Study Design
- CTRL (control; n = 10)—healthy untreated rats;
- V. vinifera (n = 10) experimental group—rats who drank tap water containing 150 mg/kg V. vinifera water extract for 14 days.
2.5. Evaluation of Effect of V. vinifera Extract on Systemic Redox State
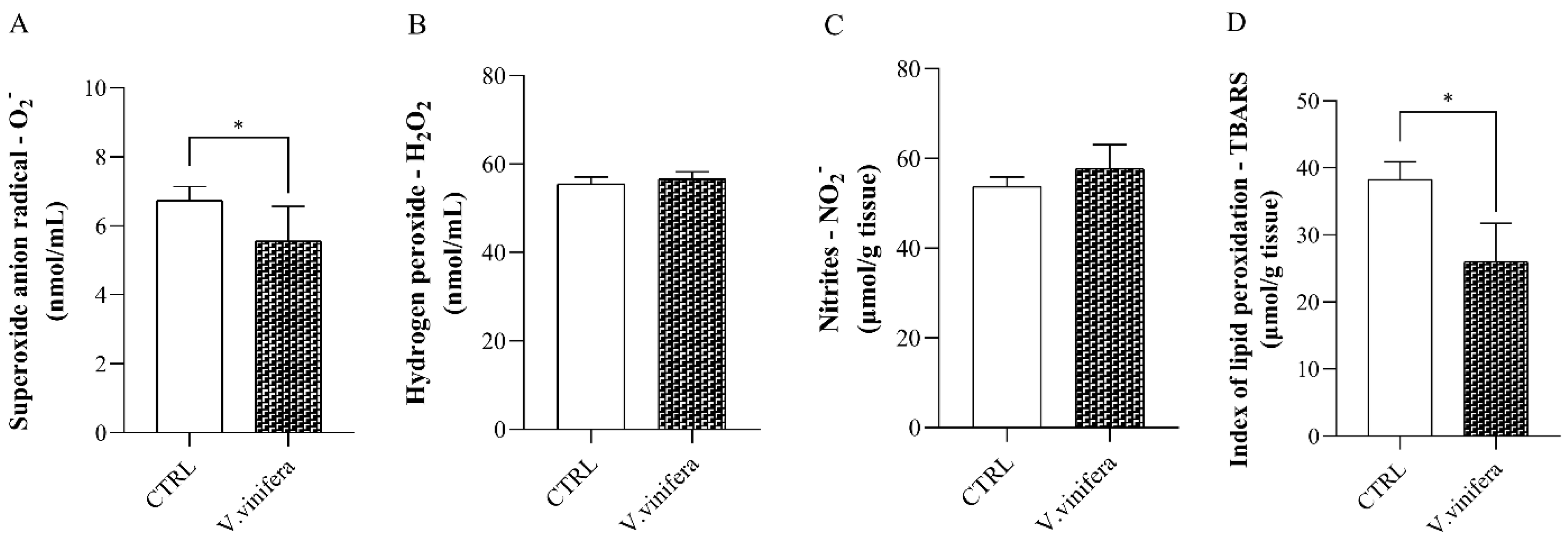
2.5.1. Determination of Pro-Oxidative Parameters
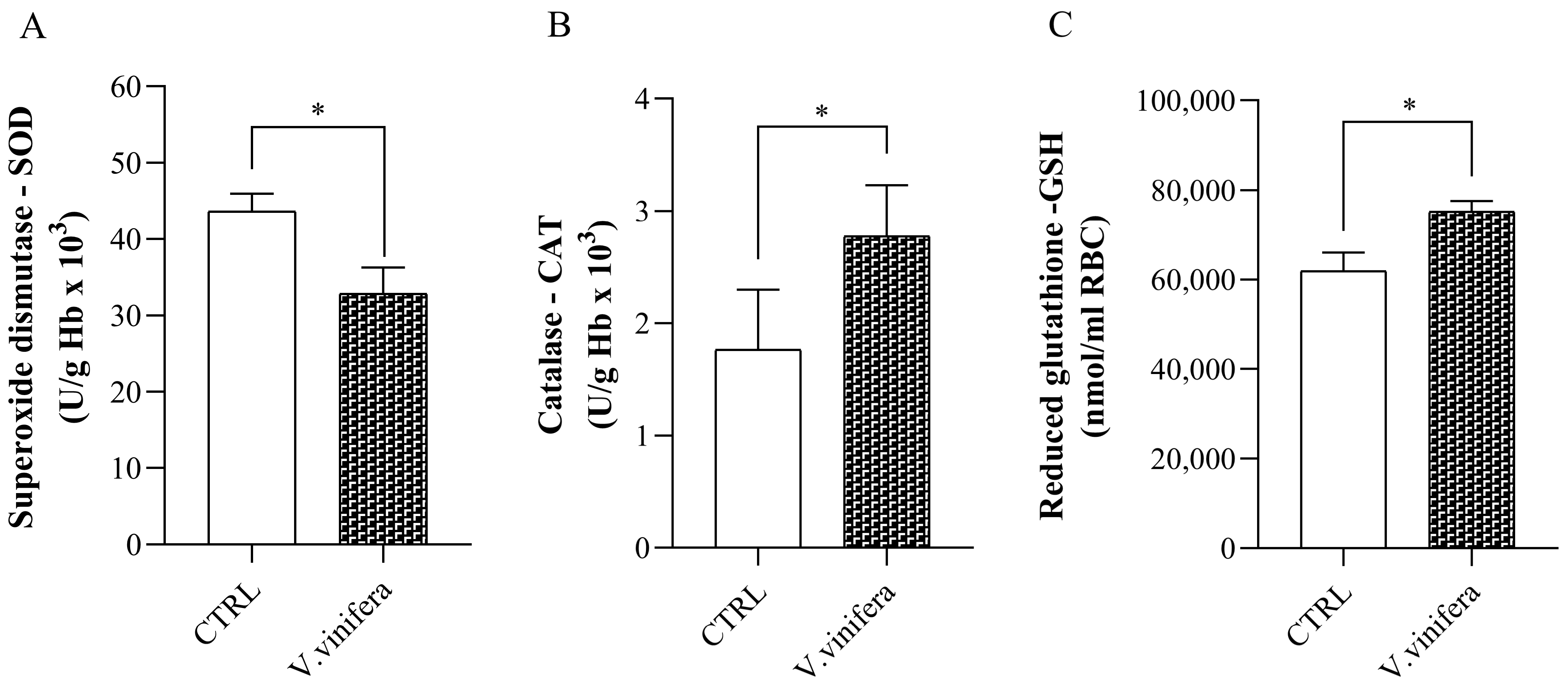
2.5.2. Determination of Antioxidative Parameters
2.6. Evaluation of Anti-Inflammatory Potential of V. vinifera Extract
2.7. In Silico Analysis
2.8. Statistical Analysis
3. Results
3.1. In Vitro Antioxidative Capacity of V. vinifera Extract
3.2. Effect of V. vinifera Extract on Pro-Oxidative Markers
3.3. Effect of V. vinifera Extract on Antioxidative Markers
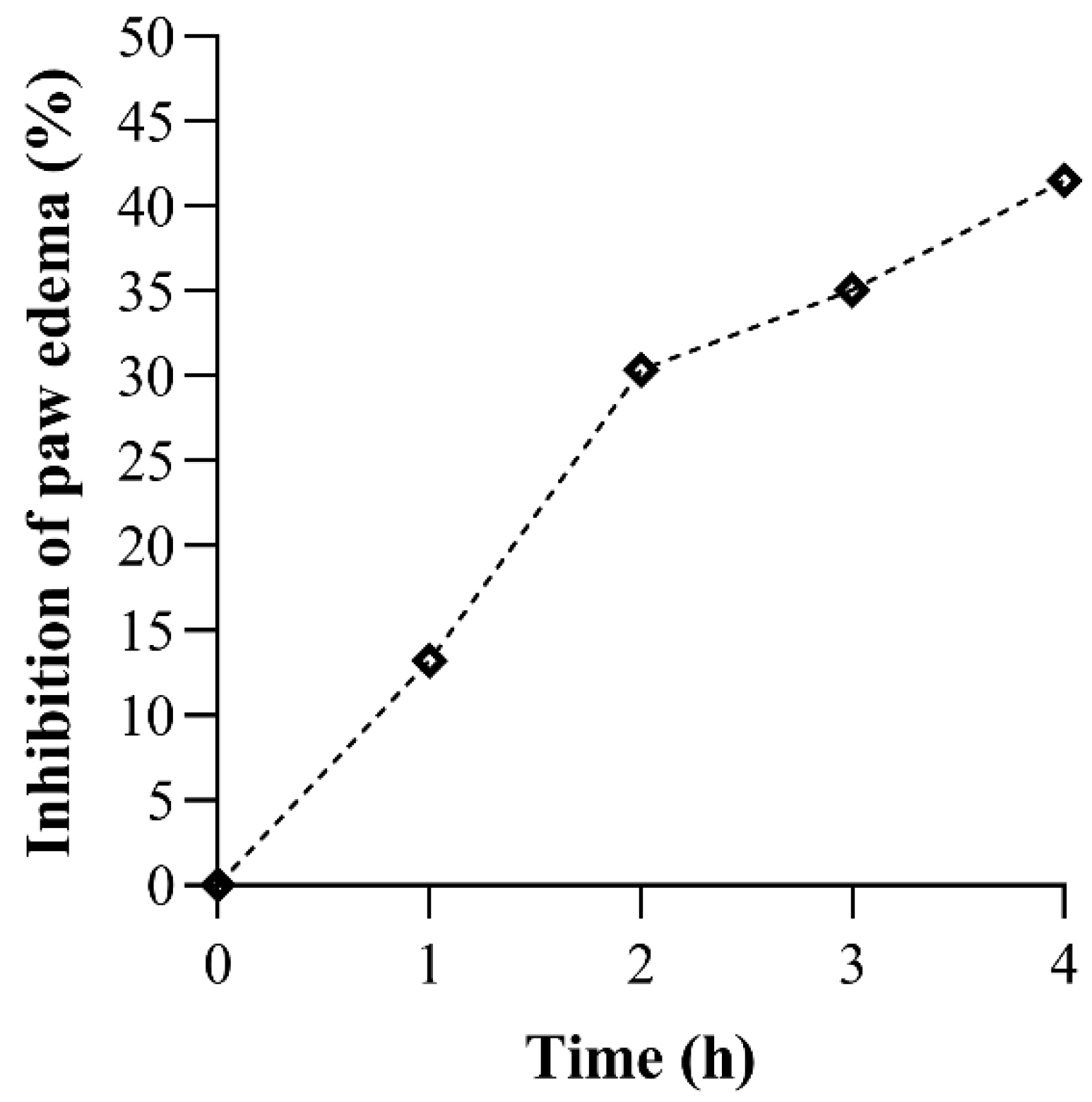
3.4. Anti-Inflammatory Activity of V. vinifera Extract
3.5. In Silico Simulations
3.5.1. Blind Molecular Docking Studies
3.5.2. Focused Molecular Docking Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. [Google Scholar] [CrossRef]
- Demirci-Çekiç, S.; Özkan, G.; Avan, A.N.; Uzunboy, S.; Çapanoğlu, E.; Apak, R. Biomarkers of Oxidative Stress and Antioxidant Defense. J. Pharm. Biomed. Anal. 2022, 209, 114477. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Zhang, H.; Dhalla, N.S. The Role of Pro-Inflammatory Cytokines in the Pathogenesis of Cardiovascular Disease. Int. J. Mol. Sci. 2024, 25, 1082. [Google Scholar] [CrossRef]
- Biswas, S.K. Does the Interdependence between Oxidative Stress and Inflammation Explain the Antioxidant Paradox? Oxidative Med. Cell. Longev. 2016, 2016, 5698931. [Google Scholar] [CrossRef]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the Pharmacological Effects of Vitis vinifera (Grape) and its Bioactive Constituents: An Update. Phytother. Res. 2016, 30, 1392–1403. [Google Scholar] [CrossRef]
- Singh, J.; Rasane, P.; Kaur, R.; Kaur, H.; Garg, R.; Kaur, S.; Ercisli, S.; Choudhary, R.; Skrovankova, S.; Mlcek, J. Valorization of grape (Vitis vinifera) leaves for bioactive compounds: Novel green extraction technologies and food-pharma applications. Front. Chem. 2023, 11, 1290619. [Google Scholar] [CrossRef]
- Pantelić, M.M.; Zagorac, D.Č.; Ćirić, I.Ž.; Pergal, M.V.; Relić, D.J.; Todić, S.R.; Natić, M.M. Phenolic profiles, antioxidant activity and minerals in leaves of different grapevine varieties grown in Serbia. J. Food Compos. Anal. 2017, 62, 76–83. [Google Scholar] [CrossRef]
- Kosar, M.; Küpeli, E.; Malyer, H.; Uylaser, V.; Türkben, C.; Baser, K.H.C. Effect of brining on biological activity of leaves of Vitis vinifera L. (cv. Sultani Cekirdeksiz) from Turkey. J. Agric. Food. Chem. 2007, 55, 4596–4603. [Google Scholar] [CrossRef]
- Dani, C.; Oliboni, L.S.; Agostini, F.; Funchal, C.; Serafini, L.; Henriques, J.A.; Salvador, M. Phenolic content of grapevine leaves (Vitis labrusca var. Bordo) and its neuroprotective effect against peroxide damage. Toxicol. Vitr. 2010, 24, 148–153. [Google Scholar] [CrossRef]
- Monagas, M.; Núñez, V.; Bartolomé, B.; Gómezcordoves, C. Anthocyanin derived pigments in Graciano, Tempramillo, and Carbernet Sauvignon wines 446 produced in Spain. Am. J. Enol. Vitic. 2003, 54, 163–169. [Google Scholar] [CrossRef]
- Orhan, D.D.; Orhan, N.; Ergun, F. Hepatoprotective effect of Vitis vinifera L. leaves on carbon tetrachloride-induced acute liver damage in rats. J. Ethnopharmacol. 2007, 112, 145–151. [Google Scholar] [CrossRef]
- Gheraibia, S.; Belattar, N.; Hassan, M.E.; El-Nekeety, A.A.; El-Sawy, E.R.; Abdel-Wahhab, M.A. Molecular docking of polyphenol compounds and exploring the anticoagulant activity of Costus speciosus extracts in vitro and in vivo. Toxicol. Rep. 2025, 14, 101961. [Google Scholar] [CrossRef]
- Ma, Y.; Tao, Y.; Qu, H.; Wang, C.; Yan, F.; Gao, X.; Zhang, M. Exploration of plant-derived natural polyphenols toward COVID-19 main protease inhibitors: DFT, molecular docking approach, and molecular dynamics simulations. RSC Adv. 2022, 12, 5357–5368. [Google Scholar] [CrossRef]
- Kiani, H.S.; Ahmad, W.; Nawaz, S.; Farah, M.A.; Ali, A. Optimized Extraction of Polyphenols from Unconventional Edible Plants: LC-MS/MS Profiling of Polyphenols, Biological Functions, Molecular Docking, and Pharmacokinetics Study. Molecules 2023, 28, 6703. [Google Scholar] [CrossRef] [PubMed]
- Dilkalal, A.; Annapurna, A.S.; Umesh, T.G. In vitro antioxidant, anticancer and in silico studies of polyphenol enriched leaf extract of Asystasia gangetica. Sci. Rep. 2024, 14, 28374. [Google Scholar] [CrossRef] [PubMed]
- Aljarba, N.H.; Hasnain, M.S.; Bin-Meferij, M.M.; Alkahtani, S. An in-silico investigation of potential natural polyphenols for the targeting of COVID main protease inhibitor. J. King Saud Univ. Sci. 2022, 34, 102214. [Google Scholar] [CrossRef]
- Bradic, J.; Petrovic, A.; Kocovic, A.; Mitrovic, S.; Jakovljevic, V.; Lazarevic, N.; Bolevich, S.; Simanic, I. Hypotensive and Cardioprotective Potential of Yellow Bedstraw Extract-Based Oral Liquid in Spontaneously Hypertensive Rats. Int. J. Mol. Sci. 2024, 25, 8346. [Google Scholar] [CrossRef] [PubMed]
- Bradic, J.; Petrovic, A.; Kocovic, A.; Ugrinovic, V.; Popovic, S.; Ciric, A.; Markovic, Z.; Avdovic, E. Development and Optimization of Grape Skin Extract-Loaded Gelatin-Alginate Hydrogels: Assessment of Antioxidant and Antimicrobial Properties. Pharmaceutics 2025, 17, 790. [Google Scholar] [CrossRef]
- Rankovic, M.; Krivokapic, M.; Bradic, J.; Petkovic, A.; Zivkovic, V.; Sretenovic, J.; Jeremic, N.; Bolevich, S.; Kartashova, M.; Jeremic, J.; et al. New Insight Into the Cardioprotective Effects of Allium ursinum L. Extract Against Myocardial Ischemia-Reperfusion Injury. Front. Physiol. 2021, 12, 690696. [Google Scholar] [CrossRef]
- Radonjic, T.; Rankovic, M.; Ravic, M.; Zivkovic, V.; Srejovic, I.; Jeremic, J.; Jeremic, N.; Sretenovic, J.; Matic, S.; Jakovljevic, V.; et al. The Effects of Thiamine Hydrochloride on Cardiac Function, Redox Status and Morphometric Alterations in Doxorubicin-Treated Rats. Cardiovasc. Toxicol. 2020, 20, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Nedeljković, N.; Dobričić, V.; Bošković, J.; Vesović, M.; Bradić, J.; Anđić, M.; Kočović, A.; Jeremić, N.; Novaković, J.; Jakovljević, V.; et al. Synthesis and Investigation of Anti-Inflammatory Activity of New Thiourea Derivatives of Naproxen. Pharmaceuticals 2023, 16, 666. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. AutoDockVina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Nzekoue, F.K.; Nguefang, M.L.K.; Alessandroni, L.; Mustafa, A.M.; Vittori, S.; Caprioli, G. Grapevine leaves (Vitis vinifera): Chemical characterization of bioactive compounds and antioxidant activity during leave development. Food Biosci. 2022, 50, 102120. [Google Scholar] [CrossRef]
- Schrödinger, L.; DeLano, W. PyMOL. Available online: http://www.pymol.org/pymol (accessed on 1 July 2025).
- Putnam, C.D.; Arvai, A.S.; Bourne, Y.; Tainer, J.A. Active and inhibited human catalase structures: Ligand and NADPH binding and catalytic mechanism. J. Mol. Biol. 2000, 296, 295–309. [Google Scholar] [CrossRef]
- DiDonato, M.; Craig, L.; Huff, M.E.; Thayer, M.M.; Cardoso, R.M.; Kassmann, C.J.; Lo, T.P.; Bruns, C.K.; Powers, E.T.; Kelly, J.W.; et al. ALS mutants of human superoxide dismutase form fibrous aggregates via framework destabilization. J. Mol. Biol. 2003, 332, 601–615. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, R.S.; Lee, J.Y.; Yuan, C.; Smith, W.L. Comparison of cyclooxygenase-1 crystal structures: Cross-talk between monomers comprising cyclooxygenase-1 homodimers. Biochemistry 2010, 49, 7069–7079. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Miyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclooxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar] [CrossRef]
- Biovia, D.S.; Berman, H.M.; Westbrook, J.; Feng, Z.; Gilliland, G.; Bhat, T.N.; Richmond, T.J. Dassault Systèmes BIOVIA, Discovery Studio Visualizer, V. 17.2, San Diego: Dassault Systèmes. J. Chem. Phys. 2000, 10, 21–9991. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Wilkinson, F.L.; Sandhu, M.A.; Lightfoot, A.P. The interplay of oxidative stress and inflammation: Mechanistic insights and therapeutic potential of antioxidants. Oxidative Med. Cell. Longev. 2021, 2021, 9851914. [Google Scholar] [CrossRef]
- Lugrin, J.; Rosenblatt-Velin, N.; Parapanov, R.; Liaudet, L. The role of oxidative stress during inflammatory processes. Biol. Chem. 2014, 395, 203–230. [Google Scholar] [CrossRef]
- Hulsmans, M.; Holvoet, P. The vicious circle between oxidative stress and inflammation in atherosclerosis. J. Cell. Mol. Med. 2010, 14, 70–78. [Google Scholar] [CrossRef]
- Scarian, E.; Viola, C.; Dragoni, F.; Di Gerlando, R.; Rizzo, B.; Diamanti, L.; Gagliardi, S.; Bordoni, M.; Pansarasa, O. New Insights into Oxidative Stress and Inflammatory Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 2698. [Google Scholar] [CrossRef]
- Yang, X.; Li, Y.; Li, Y.; Ren, X.; Zhang, X.; Hu, D.; Gao, Y.; Xing, Y.; Shang, H. Oxidative Stress-Mediated Atherosclerosis: Mechanisms and Therapies. Front. Physiol. 2017, 8, 600. [Google Scholar] [CrossRef]
- Wang, Y.; Ji, D.; Chen, T.; Li, B.; Zhang, Z.; Qin, G.; Tian, S. Production, Signaling, and Scavenging Mechanisms of Reactive Oxygen Species in Fruit-Pathogen Interactions. Int. J. Mol. Sci. 2019, 20, 2994. [Google Scholar] [CrossRef]
- de Almeida Sousa Cruz, M.A.; de Barros Elias, M.; Calina, D.; Sharifi-Rad, J.; Junger Teodoro, A. Insights into grape-derived health benefits: A comprehensive overview. Food Prod. Process. Nutr. 2024, 6, 91. [Google Scholar] [CrossRef]
- Vinci, G.; Prencipe, S.A.; Abbafati, A.; Filippi, M. Environmental Impact Assessment of an Organic Wine Production in Central Italy: Case Study from Lazio. Sustainability 2022, 14, 15483. [Google Scholar] [CrossRef]
- Armari, M.; Zavattaro, E.; Trejo, C.F.; Galeazzi, A.; Grossetti, A.; Veronese, F.; Savoia, P.; Azzimonti, B. Vitis vinifera L. Leaf Extract, a Microbiota Green Ally against Infectious and Inflammatory Skin and Scalp Diseases: An In-Depth Update. Antibiotics 2024, 13, 697. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.E.; Grao-Cruces, E.; Millan-Linares, M.C.; Montserrat-de la Paz, S. Grape (Vitis vinifera L.) Seed Oil: A Functional Food from the Winemaking Industry. Foods 2020, 9, 1360. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Hemmati, A.A.; Naghizadeh, B.; Mard, S.A.; Rezaie, A.; Ghorbanzadeh, B. A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats. Indian J. Pharmacol. 2015, 47, 292–298. [Google Scholar]
- Aouey, B.; Samet, A.M.; Fetoui, H.; Simmonds, M.S.J.; Bouaziz, M. Anti-oxidant, anti-inflammatory, analgesic and antipyretic activities of grapevine leaf extract (Vitis vinifera) in mice and identification of its active constituents by LC-MS/MS analyses. Biomed. Pharmacother. 2016, 84, 1088–1098. [Google Scholar] [CrossRef]
- Bocsan, I.C.; Măgureanu, D.C.; Pop, R.M.; Levai, A.M.; Macovei, Ș.O.; Pătrașca, I.M.; Chedea, V.S.; Buzoianu, A.D. Antioxidant and Anti-Inflammatory Actions of Polyphenols from Red and White Grape Pomace in Ischemic Heart Diseases. Biomedicines 2022, 10, 2337. [Google Scholar] [CrossRef]
- Liu, W.; Cui, X.; Zhong, Y.; Ma, R.; Liu, B.; Xia, Y. Phenolic metabolites as therapeutic in inflammation and neoplasms: Molecular pathways explaining their efficacy. Pharmacol. Res. 2023, 193, 106812. [Google Scholar] [CrossRef]
- Serreli, G.; Deiana, M. Role of Dietary Polyphenols in the Activity and Expression of Nitric Oxide Synthases: A Review. Antioxidants 2023, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Katalinic, V.; Mozina, S.; Generalic, I.; Skroza, D.; Ljubenkov, I.; Klancnik, A. Phenolic Profile, Antioxidant Capacity, and Antimicrobial Activity of Leaf Extracts from Six Vitis vinifera L. Varieties. Int. J. Food Prop. 2013, 16, 45–60. [Google Scholar] [CrossRef]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Palusiak, M.; Hoelm, M.; Zajdel, K.; Zajdel, R. In vitro evaluation and In Silico calculations of the antioxidant and anti-inflammatory properties of secondary metabolites from Leonurus sibiricus L. root extracts. Molecules 2023, 28, 6550. [Google Scholar] [CrossRef] [PubMed]
- Handoussa, H.; Hanafi, R.; Eddiasty, I.; El-Gendy, M.; El Khatib, A.; Linscheid, M. Anti-Inflammatory and Cytotoxic Activities of Dietary Phenolics Isolated from Corchorus olitorius and Vitis vinifera. J. Funct. Foods 2013, 5, 1204–1216. [Google Scholar] [CrossRef]
- Mandour, Y.; Handoussa, H.; Swilam, N.; Hanafi, R.; Mahran, L. Structural docking studies of COX-II inhibitory activity for metabolites derived from Corchorus olitorius and Vitis vinifera. Int. J. Food Prop. 2016, 19, 2377–2384. [Google Scholar] [CrossRef]
- Amen, Y.; Sherif, A.E.; Shawky, N.M.; Abdelrahman, R.S.; Wink, M.; Sobeh, M. Grape-leaf extract attenuates alcohol-induced liver injury via interference with NF-κBsignaling pathway. Biomolecules 2020, 10, 558. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Rutin, a bioflavonoid antioxidant protects rat pheochromocytoma (PC-12) cells against 6-hydroxydopamine (6-OHDA)-induced neurotoxicity. Int. J. Mol. Med. 2013, 32, 235–240. [Google Scholar] [CrossRef]
- Iqbal, A.; Hafeez Kamran, S.; Siddique, F.; Ishtiaq, S.; Hameed, M.; Manzoor, M. Modulatory effects of rutin and vitamin A on hyperglycemia induced glycation, oxidative stress and inflammation in high-fat-fructose diet animal model. PLoS ONE 2024, 19, e0303060. [Google Scholar] [CrossRef] [PubMed]
- Robles, A.; Fabjanowicz, M.; Chmiel, T.; Płotka-Wasylka, J. Determination and identification of organic acids in wine samples. Problems and challenges. TrAC Trends Anal. Chem. 2019, 120, 115630. [Google Scholar] [CrossRef]
- Al-Warhi, T.; Zahran, E.M.; Selim, S.; Al-Sanea, M.M.; Ghoneim, M.M.; Maher, S.A.; Mostafa, Y.A.; Alsenani, F.; Elrehany, M.A.; Almuhayawi, M.S.; et al. Antioxidant and Wound Healing Potential of Vitis vinifera Seeds Supported by Phytochemical Characterization and Docking Studies. Antioxidants 2022, 11, 881. [Google Scholar] [CrossRef]
- Pozzo, L.; Grande, T.; Raffaelli, A.; Longo, V.; Weidner, S.; Amarowicz, R.; Karamać, M. Characterization of Antioxidant and Antimicrobial Activity and Phenolic Compound Profile of Extracts from Seeds of Different Vitis Species. Molecules 2023, 28, 4924. [Google Scholar] [CrossRef]
- Shaban, N.Z.; El-Faham, A.A.; Abu-Serie, M.M.; Habashy, N.H. Targeting apoptosis in MCF-7 and Ehrlich ascites carcinoma cells by saponifiable fractions from green and black Vitis vinifera seed oil. Biomed. Pharmacother. 2023, 157, 114017. [Google Scholar] [CrossRef]
- Fernandes, F.; Ramalhosa, E.; Pires, P.; Verdial, J.; Valentão, P.; Andrade, P.; Bento, A.; Pereira, J.A. Vitis vinifera leaves towards bioactivity. Ind. Crops Prod. 2013, 43, 434–440. [Google Scholar] [CrossRef]
- Sangiovanni, E.; Di Lorenzo, C.; Piazza, S.; Manzoni, Y.; Brunelli, C.; Fumagalli, M.; Magnavacca, A.; Martinelli, G.; Colombo, F.; Casiraghi, A.; et al. Vitis vinifera L. Leaf Extract Inhibits In Vitro Mediators of Inflammation and Oxidative Stress Involved in Inflammatory-Based Skin Diseases. Antioxidants 2019, 8, 134. [Google Scholar] [CrossRef]
- Dubois-Deruy, E.; Peugnet, V.; Turkieh, A.; Pinet, F. Oxidative Stress in Cardiovascular Diseases. Antioxidants 2020, 9, 864. [Google Scholar] [CrossRef]
- Nunes, M.A.; Pimentel, F.; Costa, A.S.G.; Alves, R.C.; Oliveira, M.B.P.P. Cardioprotective properties of grape seed proanthocyanidins: An update. Trends Food Sci. Technol. 2016, 57, 31–39. [Google Scholar] [CrossRef]
- Di Renzo, L.; Carraro, A.; Valente, R.; Iacopino, L.; Colica, C.; De Lorenzo, A. Intake of red wine in different meals modulates oxidized LDL level, oxidative and inflammatory gene expression in healthy people: A randomized crossover trial. Oxidative Med. Cell. Longev. 2014, 2014, 681318. [Google Scholar] [CrossRef] [PubMed]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Andrés Juan, C.; Plou, F.J.; Pérez-Lebeña, E. Superoxide Anion Chemistry-Its Role at the Core of the Innate Immunity. Int. J. Mol. Sci. 2023, 24, 1841. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, K.; Rao, L.; Hu, Y.; Yang, H.; Wang, Y.; Zhao, L.; Liao, X. Investigating the non-covalent interactions between rutin and superoxide dismutase: Focus on thermal stability, structure, and in vitro digestion. LWT 2024, 212, 116977. [Google Scholar] [CrossRef]

| Rat Paw Thickness (mm) (% of Inhibition) | |||||
|---|---|---|---|---|---|
| Groups | 0 h | 1 h | 2 h | 3 h | 4 h |
| CTRL | 4.30 ± 0.40 | 6.00 ± 0.30 | 6.50 ± 0.30 | 6.20 ± 0.40 | 5.90 ± 0.40 |
| V. vinifera | 4.37 ± 0.15 | 6.57 ± 1.16 (13.235%) | 6.07 ± 0.72 (30.337%) * | 5.77 ± 0.42 (35.065%) * | 5.30 ± 0.26 (41.538%) * |
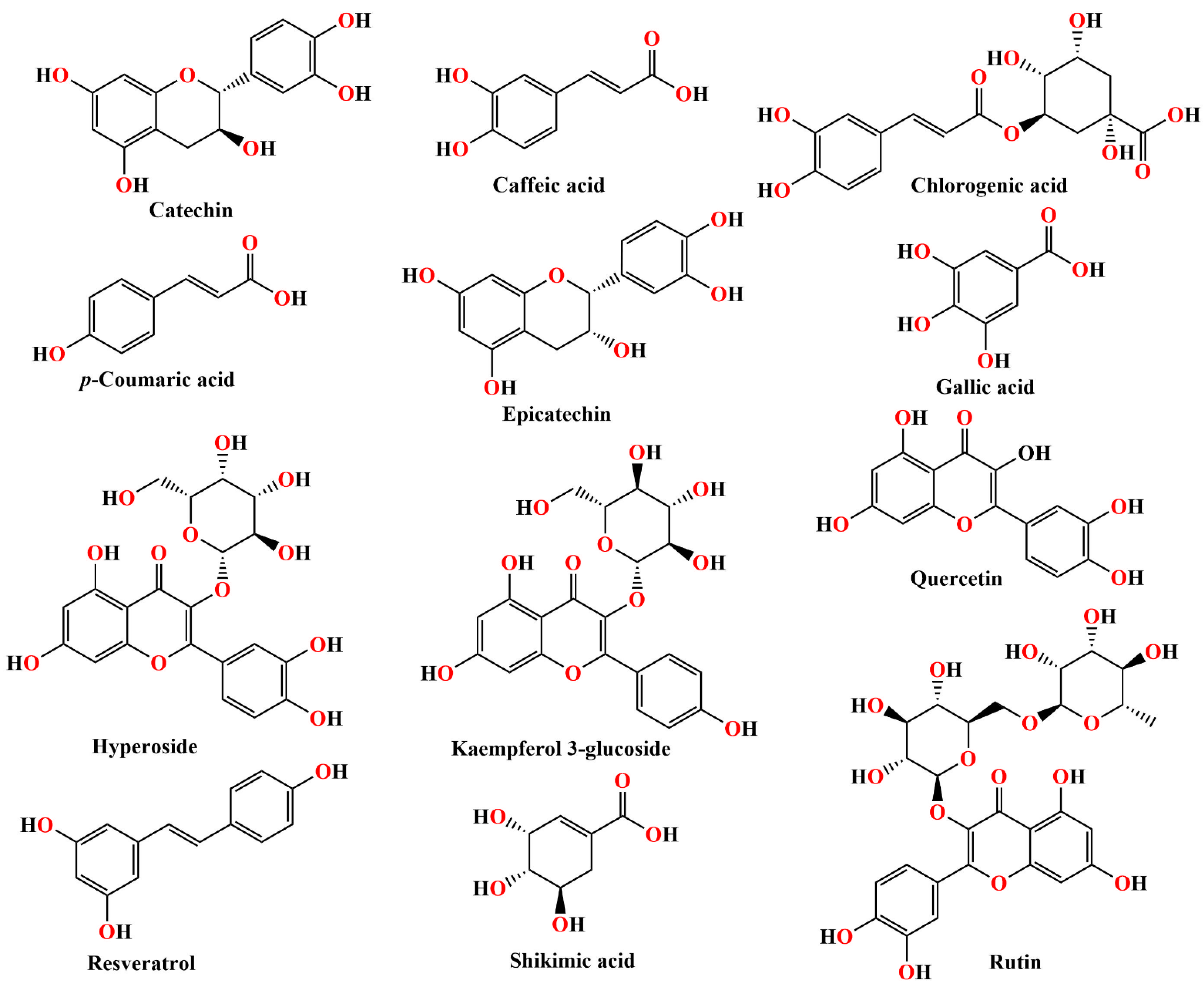
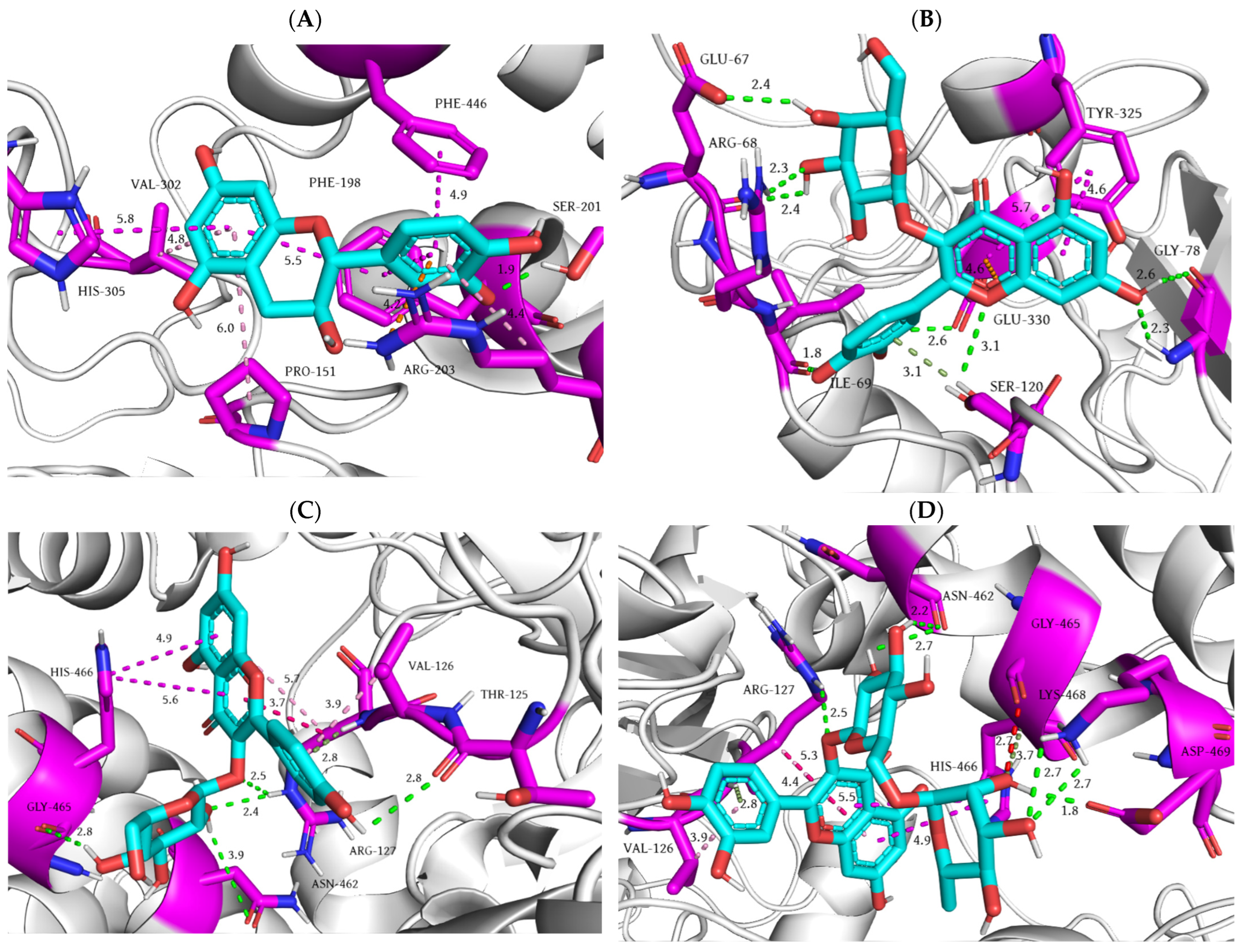
| Ligand | Docking Score (kcal/mol) | Kb (M−1) | Number of Favorable Binding Interaction | Interacting Residue * |
|---|---|---|---|---|
| Catechin | −8.3 | 1.22 × 106 | 9 | Pro151 (π-alkyl), Phe198 (π-π x 2), Ser201 (HBD), Arg203 (π-alkyl), Arg203 (π-cation), Val302 (π-alkyl), His305 (π-π), Phe446 (π-π) |
| Caffeic acid | −6.1 | 2.98 × 104 | 4 | Asn369 (HBD), Ile373 (π-alkyl), Met392 (π-alkyl), Met394 (π-alkyl) |
| Chlorogenic acid | −6.4 | 4.94 × 104 | 11 | Lys221 (HBD x 2), Ser337 (HBA x 2), Met339 (HBA), Met339 (π-σ), Ala345 (π-alkyl), His421 (HBA x 2), His421 (CHBA), Tyr425 (HBA) |
| p-coumaric acid | −5.1 | 5.5 × 103 | 6 | Pro7 (HBA), His14 (HBD), Phe266 (π-π), Ile269 (HBA), Ala270 (π-alkyl), Asn321 (HBD) |
| Epicatechin | −6.9 | 1.15 × 105 | 9 | Arg363 (HBD), Pro368 (HBA), Pro368 (π-alkyl), His372 (CHBD), His372 (π-cation x 2), His372 ((π-π), Gln387 (HBA), Gln387 (CHBA) |
| Gallic acid | −5.8 | 1.79 × 104 | 8 | Asn369 (HBA), His372 (π-cation x 2), His372 (π-π) Asn385 (HBD), Asp389 (HBA x 2), Gln398 (HBD), |
| Hyperoside | −7.7 | 7.37 × 105 | 12 | Glu67 (HBA x 2), Arg68 (HBD), Ile69 (HBA), Gly78 (HBD), Gly78 (HBA), Ser120 (HBD), Ser120 (π-donor HBD), Tyr325 (π-π x 2), Glu330 (HBA), Glu330 (π-anion) |
| Kaempferol 3-glucoside | −7.8 | 5.25 × 105 | 11 | Thr125 (HBA), Val126 (π-alkyl), Arg127 (HBD x 2), Arg127 (π-donor HBD), Arg127 (π-alkyl), Arg127 (π-σ), Asn462 (HBA), Gly465 (HBA), His466 (π-π x 2) |
| Quercetin | −7.4 | 2.67 × 105 | 12 | Arg127 (HBD), Arg127 (π-donor HBD), Arg127 (π-cation), Arg127 (π-alkyl x 3), Gln168 (HBD), Val247 (π-alkyl), Asn462 (HBA), His466 (HBD), His466 (π-cation), His466 (π-π) |
| Resveratrol | −6.4 | 4.94 × 104 | 6 | Glu67 (HBA), Arg68 (π-alkyl), Ile69 (HBA), Pro70 (HBA), Lys77 (CHBD), Glu330 (π-anion) |
| Rutin | −8.6 | 2.03 × 106 | 13 | Val126 (π-alkyl), Arg127 (HBD), Arg127 (π-donor HBD), Arg127 (π-σ), Arg127 (π-alkyl), Asn462 (HBA x 2), His466 (CHBD), Gly465 (steric bump), His466 (π-π x 2), Lys468 (HBD x 2), Asp469 (HBA) |
| Shikimic acid | −5.2 | 6.51 × 103 | 7 | Ala76 (HBA), Gly78 (HBD), Ser120 (HBA x 3), Gly121 (HBA), Tyr325 (HBD), Glu330 (steric bump) |
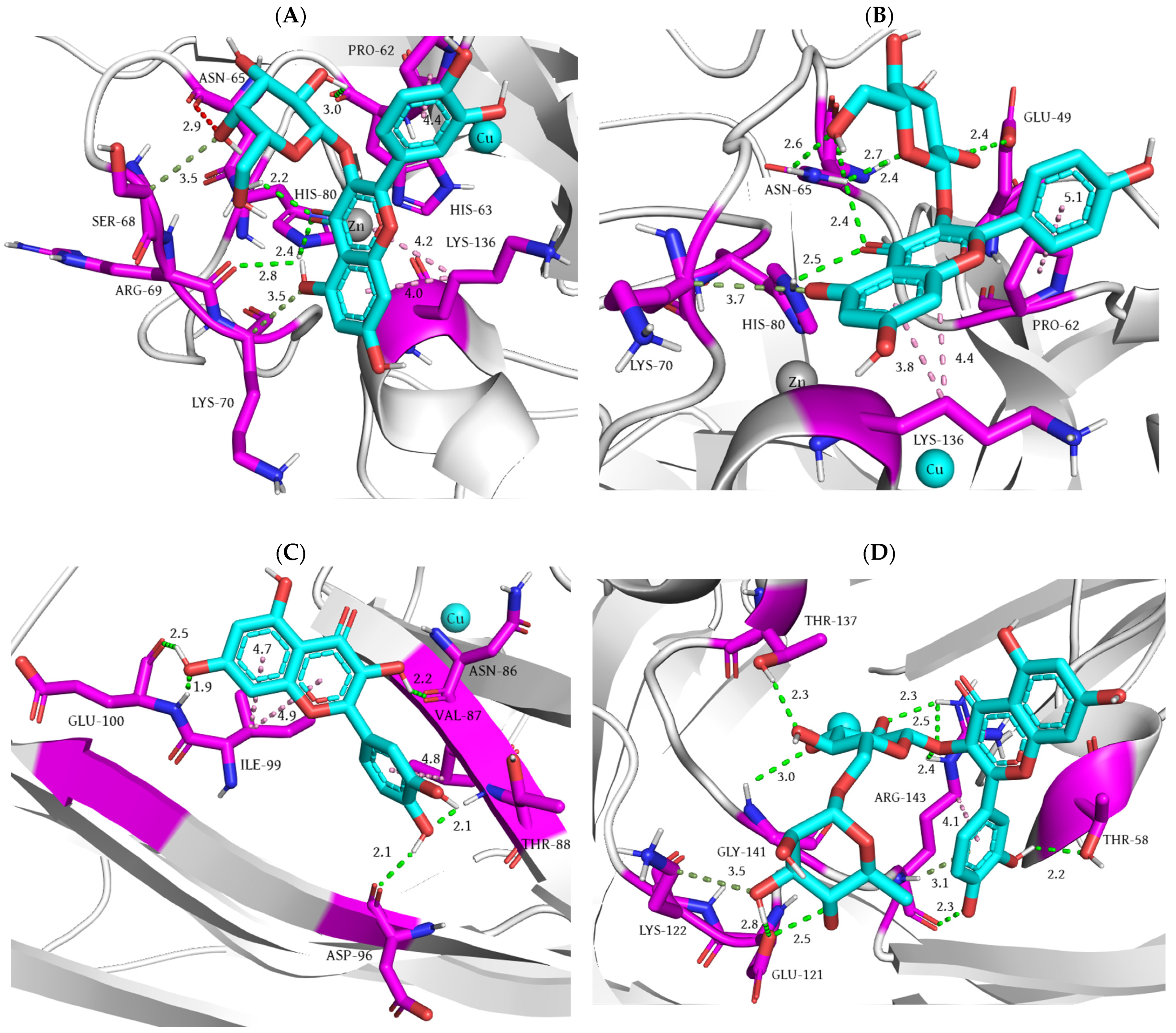
| Ligand | Docking Score (kcal/mol) | Kb (M−1) | Number of Favorable Binding Interaction | Interacting Residue * |
|---|---|---|---|---|
| Catechin | −5.9 | 2.12 × 104 | 9 | Arg69 (HBD), Ser102 (HBA), Ser107 (HBA), His110 (HBD x 2), His110 (π-σ), Val103 (CHBA), Val103 (π-σ x 2) |
| Caffeic acid | −4.5 | 2.00 × 103 | 6 | Thr39 (HBD), His43 (CHBD), His43 (π-π), Glu121 (HBA), Lys122 (π-alkyl), Ala123 (π-alkyl) |
| Chlorogenic acid | −5.7 | 1.51 × 104 | 7 | Glu133 (HBA x 2), Ala140 (HBD), Gly141 (HBD), Gly141 (CHBA), Arg143 (HBA), Arg143 (π-alkyl) |
| p-coumaric acid | −4.6 | 2.36 × 103 | 7 | Asp11 (HBD), Gly12 (HBD), Pro13 (π-alkyl), Val14 (π-alkyl), Gly37 (HBA), Leu144 (HBA), Leu144 (π-σ) |
| Epicatechin | −6.0 | 2.51 × 104 | 7 | Lys30 (π-alkyl x 2), Trp32 (π-π x 2), Ser98 (HBD), Glu100 (HBD), Glu100 (π-anion) |
| Gallic acid | −4.7 | 2.80 × 103 | 6 | Ala140 (HBD), Gly141 (HBD), Glu133 (HBA), Thr137 (π-σ), Arg143 (HBD x 2) |
| Hyperoside | −6.1 | 2.98 × 104 | 9 | Asn65 (steric bump), Pro62 (π-alkyl), His63 (HBA), Asn65 (HBD), Ser68 (CHBD), Arg69 (HBA), Lys70 (CHBD), His80 (HBD), Lys136 (π-alkyl x 2) |
| Kaempferol 3-glucoside | −6.2 | 3.52 × 104 | 10 | Glu49 (HBA), Pro62 (π-alkyl), Asn65 (HBD x 4), Lys70 (CHBD), His80 (HBD), Lys136 (π-alkyl x 2) |
| Quercetin | −6.3 | 4.17 × 104 | 8 | Asn86 (HBA), Val87 (π-alkyl), Thr88 (HBD), Asp96 (HBA), Ile99 (π-alkyl x 2), Glu100 (HBD), Glu100 (HBA) |
| Resveratrol | −5.5 | 1.08 × 104 | 7 | Val7 (π-alkyl), Lys9 (π-alkyl x 2), Asp11 (HBA), Cys146 (π-S x 2), Val148 (HBD) |
| Rutin | −7.4 | 2.67 × 105 | 12 | Thr58 (HBA), Glu121 (HBA x 2), Lys122 (CHBD), Thr137 (HBD), Gly141 (HBD), Arg143 (HBD x 3), Arg143 (π-donor HBD), Arg143 (HBA), Arg143 (π-alkyl) |
| Shikimic acid | −4.2 | 1.20 × 103 | 4 | His80 (HBD), Glu132 (HBA x 2), Thr135 (HBA) |
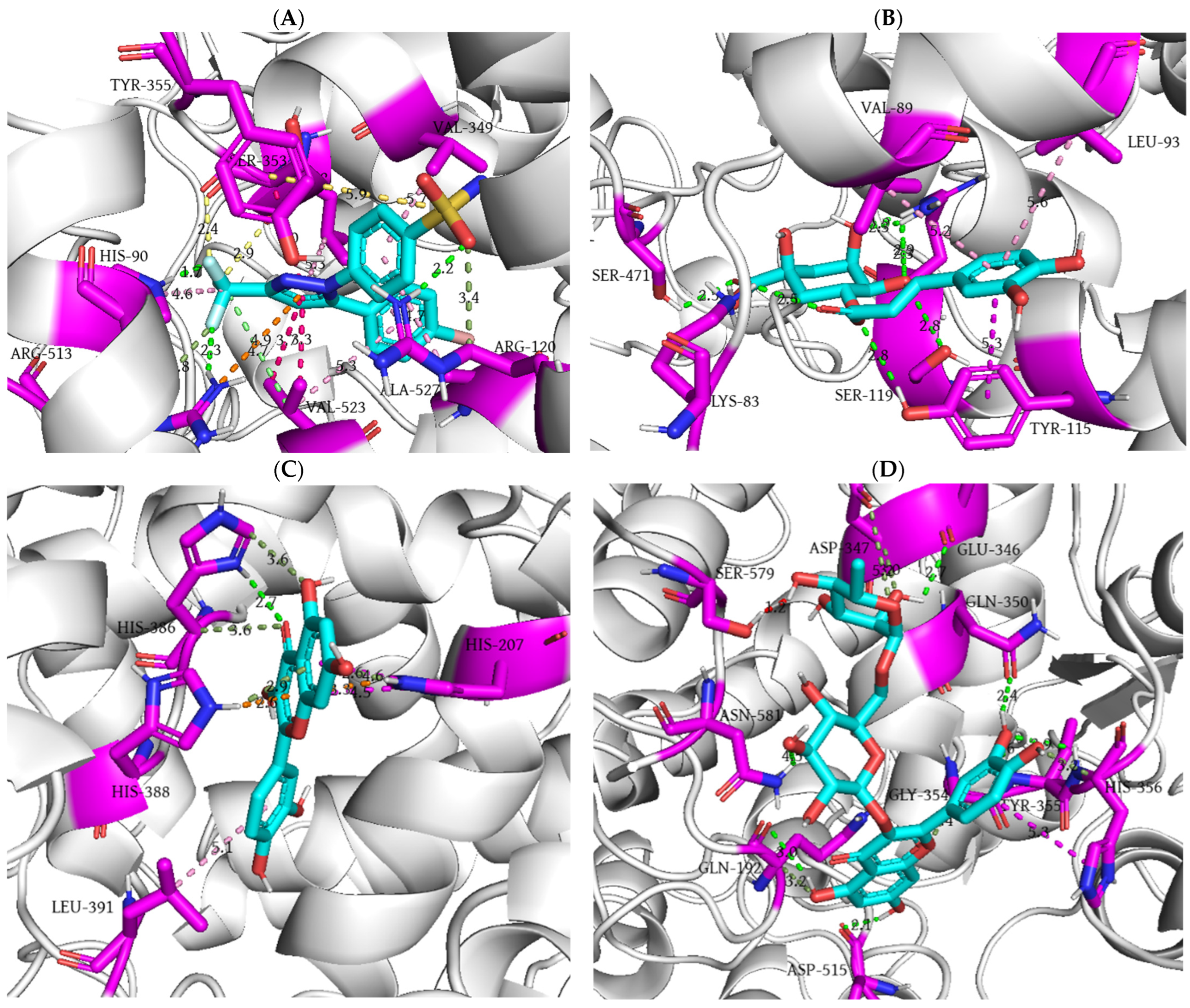
| Ligand | Docking Score (kcal/mol) | Ki (M) | Number of Favorable Binding Interaction | Interacting Residue * |
|---|---|---|---|---|
| Flurbiprofen | −9.3 | 1.50 × 10−7 | 7 | Arg120 (HBD), Arg120 (salt bridge), Val349 (π-σ), Leu 352 (π-alkyl), Ala527 (π-σ), Ser530 (HBD), Leu531 (π-alkyl) |
| Catechin | −7.7 | 2.24 × 10−6 | 8 | Ala199 (HBA), Ala202 (amide-π), Gln203 (amide-π), Thr206 (HBD), Tyr385 (CHBA), His386 (π-cation), His386 (π-π), Met391 (π-S) |
| Caffeic acid | −5.5 | 9.20 × 10−5 | 5 | Asn122 (HBA x 2), Pro125 (π-alkyl), Thr129 (steric bump), Tyr130 (HBD), Arg469 (HBD) |
| Chlorogenic acid | −7.5 | 3.14 × 10−6 | 5 | Thr94 (π-σ), Arg97 (HBD), Gln192 (HBD), Gln351 (HBA), Tyr355 (HBA) |
| p-coumaric acid | −6.5 | 1.70 × 10−5 | 3 | Ala202 (π-alkyl), Trp387 (HBA), Trp387 (π-donor HBD) |
| Epicatechin | −7.6 | 2.65 × 10−6 | 6 | Asn122 (HBA x 3), Pro125 (π-alkyl), Arg469 (HBD x 2) |
| Gallic acid | −6.1 | 3.34 × 10−5 | 5 | Ala199 (HBA), Ala202 (π-alkyl), Gln203 (CHBD), Thr206 (HBD), His207 (HBD) |
| Hyperoside | −8.1 | 1.14 × 10−6 | 4 | Gln192 (HBD), Gln192 (HBA), Glu347 (HBA), Phe356 (CHBD) |
| Kaempferol 3-glucoside | −7.5 | 3.14 × 10−6 | 6 | Arg97 (HBD), Gln192 (HBD), Gln350 (CHBA), Gly354 (CHBD), Tyr355 (HBA), Asn515 (HBA) |
| Quercetin | −7.4 | 3.71 × 10−6 | 6 | Asn122 (HBA x 2), Pro125 (π-alkyl), Pro125 (π-σ), Ser126 (HBD), Ser126 (π-donor HBD), Gln372 (steric bump) |
| Resveratrol | −7.9 | 1.60 × 10−6 | 8 | Ala202 (amide-π), Ala202 (π-alkyl), Gln203 (amide-π), Phe210 (π-π), Phe210 (π-donor HBA), Asn382 (steric bump), His386 (π-cation), His386 (π-π), Trp387 (HBA) |
| Rutin | −8.2 | 9.61 × 10−7 | 9 | Gln192 (HBA), Gln351 (HBD), Gln351 (HBA), Pro514 (HBA x 2), Asn515 (HBA x 2), His581 (HBD), His581 (π-alkyl) |
| Shikimic acid | −5.8 | 5.54 × 10−5 | 5 | Ala199 (HBA), His207 (HBD), Tyr385 (HBA), Trp387 (HBD), His388 (HBD) |
| Ligand | Docking Score (kcal/mol) | Ki (M) | Number of Favorable Binding Interaction | Interacting Residue * |
|---|---|---|---|---|
| SC-558 | −8.5 | 5.79 × 10−7 | 19 | His90 (HBD), His90 (π-alkyl), Arg120 (HBD), Arg120 (CHBD), Val349 (π-alkyl), Leu352 (halogen interaction), Leu352 (π-alkyl x 2), Ser353 (halogen interaction), Ser353 (π-σ), Tyr355 (π-S), Arg513 (HBD), Arg513 (CHBD), Arg513 (π-cation), Val523 (π-σ x 2), Val523 (alkyl), Val523 (π-alkyl), Ala527 (π-alkyl) |
| Catechin | −8.2 | 9.61 × 10−7 | 7 | Ala202 (amide-π), Gln203 (amide-π), Thr206 (steric bump), His207 (π-cation), His207 (π-π), Asn382 (HBD), His386 (π-cation), His388 (HBD) |
| Caffeic acid | −7.0 | 7.30 × 10−6 | 3 | Ala202 (HBA), His207 (CHBD), Tyr385 (steric bump), His388 (π-π) |
| Chlorogenic acid | −7.8 | 1.89 × 10−6 | 11 | Lys83 (HBD), Val89 (π-alkyl), Leu93 (π-alkyl), Tyr115 (HBD), Tyr115 (π-π), Ser119 (HBA), Arg120 (HBD x 4), Ser471 (HBD) |
| p-coumaric acid | −7.1 | 6.16 × 10−6 | 8 | Ile124 (π-alkyl), Asp125 (HBD), Thr149 (HBA), Asn375 (HBD), Ala378 (π-alkyl), Phe381 (π-π), Phe529 (HBA), Phe529 (π-π) |
| Epicatechin | −7.7 | 2.24 × 10−6 | 8 | Gln203 (CHBD), Thr206 (HBD), His207 (π-cation), His207 (π-π), Asn382 (HBA), His386 (π-cation), His388 (HBD), Leu390 (π-alkyl) |
| Gallic acid | −6.5 | 1.70 × 10−5 | 5 | Ala202 (amide-π), Gln203 (amide-π), Thr206 (HBD), Tyr385 (HBA), His388 (π-π) |
| Hyperoside | −7.7 | 2.24 × 10−6 | 5 | Lys342 (CHBD), Asp347 (π-anion), Thr578 (HBA x 2), Phe580 (HBD) |
| Kaempferol 3-glucoside | −7.4 | 3.71 × 10−6 | 7 | Asp347 (HBA), Gln350 (HBA), Gly354 (CHBD), His356 (HBD), Asp515 (HBA), Asn581 (HBD x 2) |
| Quercetin | −8.6 | 4.89 × 10−7 | 10 | His207 (π-cation), His207 (π-donor HBD), His207 (π-π x 2), His386 (HBD), His386 (CHBD x 2), His388 (π-donor HBD), His388 (π-cation), Leu391 (π-alkyl) |
| Resveratrol | −7.6 | 2.65 × 10−6 | 9 | Arg120 (π-donor HBD), Val349 (π-alkyl), Leu352 (π-alkyl), Leu359 (π-alkyl), Gly526 (amide-π), Ala527 (amide-π), Ala527 (π-alkyl x 2), Leu531 (π-alkyl) |
| Rutin | −8.3 | 8.12 × 10−7 | 13 | Gln192 (HBA), Gln192 (CHBD), Glu346 (HBA), Asp347 (CHBA), Asp347 (CHBD), Gln350 (HBA), Gly354 (CHBD), Tyr355 (HBD), His356 (HBD), His356 (CHBD), His356 (π-π), Asp515 (HBA), Asn581(HBD), Ser579 (steric bump) |
| Shikimic acid | −5.7 | 6.56 × 10−5 | 4 | Ala199 (HBA), Gln203 (HBA), Thr206 (HBD), Tyr385 (HBA) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djakovic, S.; Nikolic, M.; Srejovic, I.; Nedeljkovic, N.; Karovic, M.; Bradic, J.; Andjic, M.; Jakovljevic, V.; Nikolic, M. Targeting Oxidative Stress and Inflammation with Vitis vinifera Leaf Extract: A Combined Experimental and Computational Pharmacological Study. Future Pharmacol. 2025, 5, 52. https://doi.org/10.3390/futurepharmacol5030052
Djakovic S, Nikolic M, Srejovic I, Nedeljkovic N, Karovic M, Bradic J, Andjic M, Jakovljevic V, Nikolic M. Targeting Oxidative Stress and Inflammation with Vitis vinifera Leaf Extract: A Combined Experimental and Computational Pharmacological Study. Future Pharmacology. 2025; 5(3):52. https://doi.org/10.3390/futurepharmacol5030052
Chicago/Turabian StyleDjakovic, Sanja, Marina Nikolic, Ivan Srejovic, Nikola Nedeljkovic, Marko Karovic, Jovana Bradic, Marijana Andjic, Vladimir Jakovljevic, and Milos Nikolic. 2025. "Targeting Oxidative Stress and Inflammation with Vitis vinifera Leaf Extract: A Combined Experimental and Computational Pharmacological Study" Future Pharmacology 5, no. 3: 52. https://doi.org/10.3390/futurepharmacol5030052
APA StyleDjakovic, S., Nikolic, M., Srejovic, I., Nedeljkovic, N., Karovic, M., Bradic, J., Andjic, M., Jakovljevic, V., & Nikolic, M. (2025). Targeting Oxidative Stress and Inflammation with Vitis vinifera Leaf Extract: A Combined Experimental and Computational Pharmacological Study. Future Pharmacology, 5(3), 52. https://doi.org/10.3390/futurepharmacol5030052









