Nanoparticle-Based Strategies to Enhance Catecholaminergic Drug Delivery for Neuropsychiatric Disorders: Advances, Challenges, and Therapeutic Opportunities
Abstract
1. Introduction
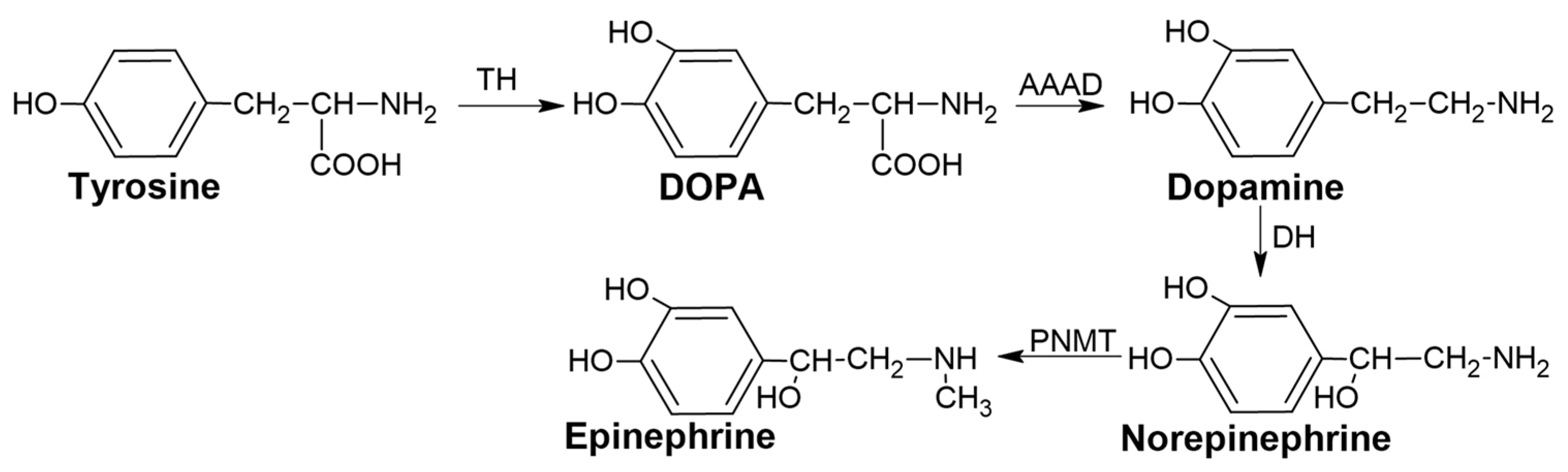
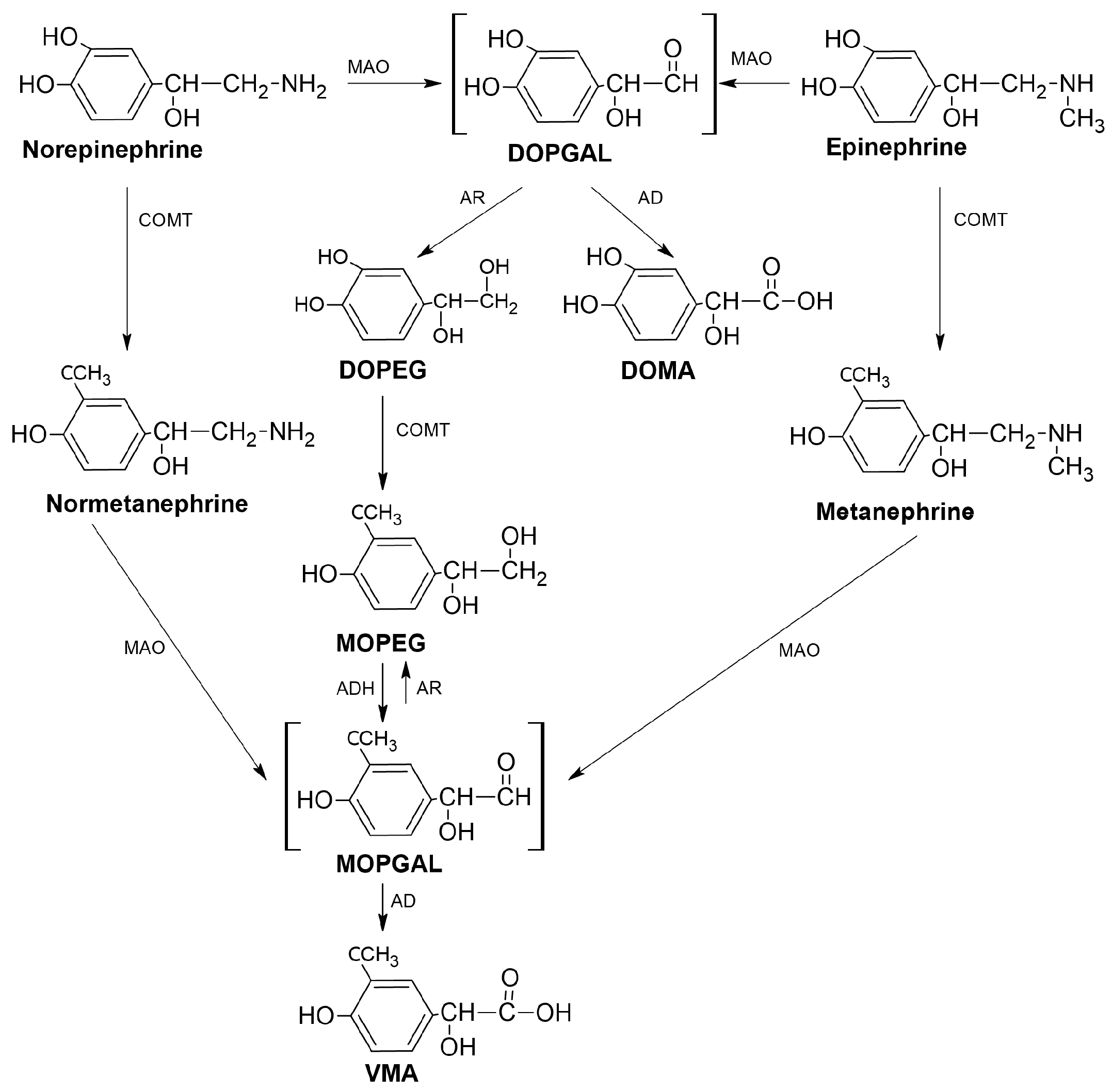
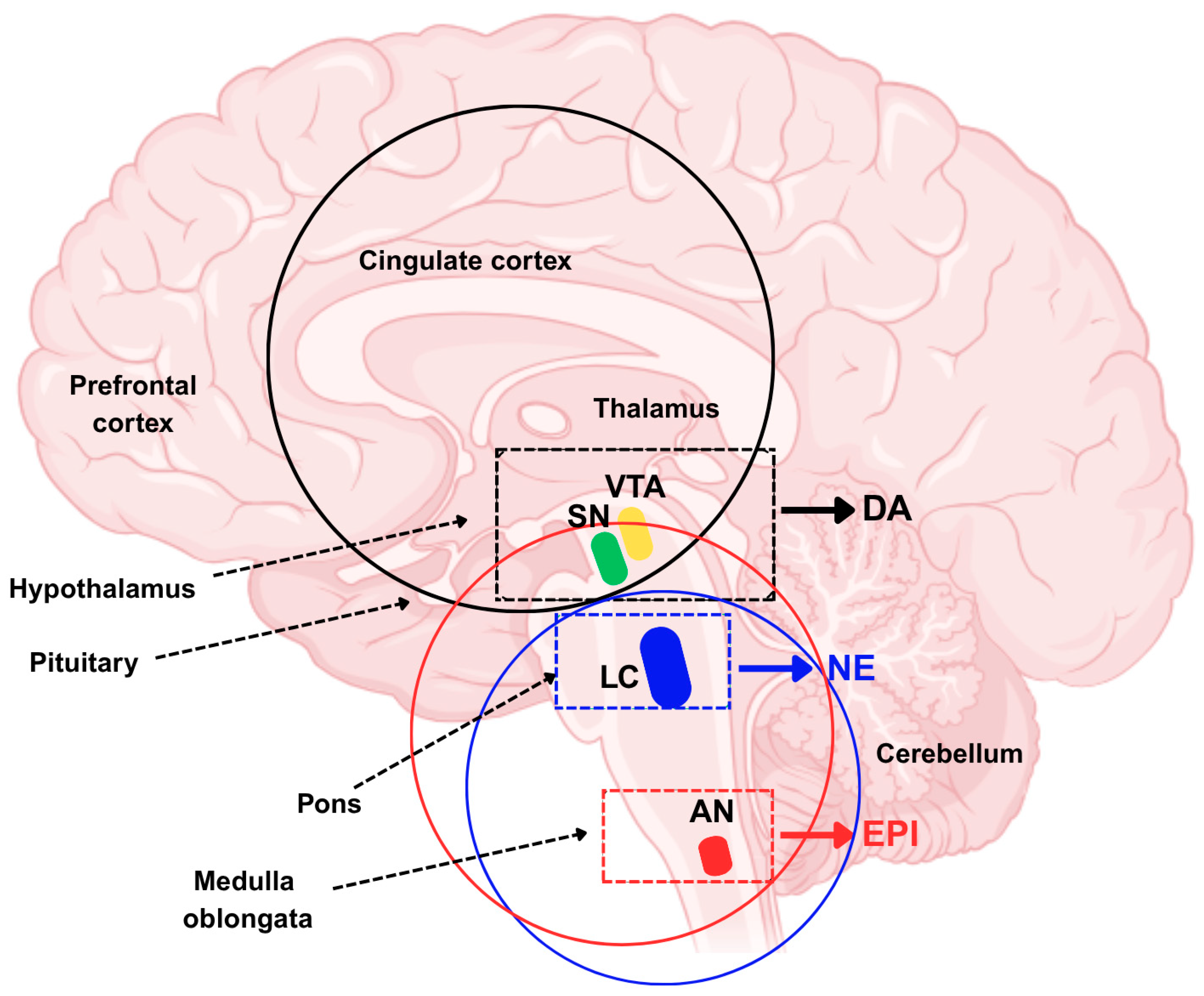
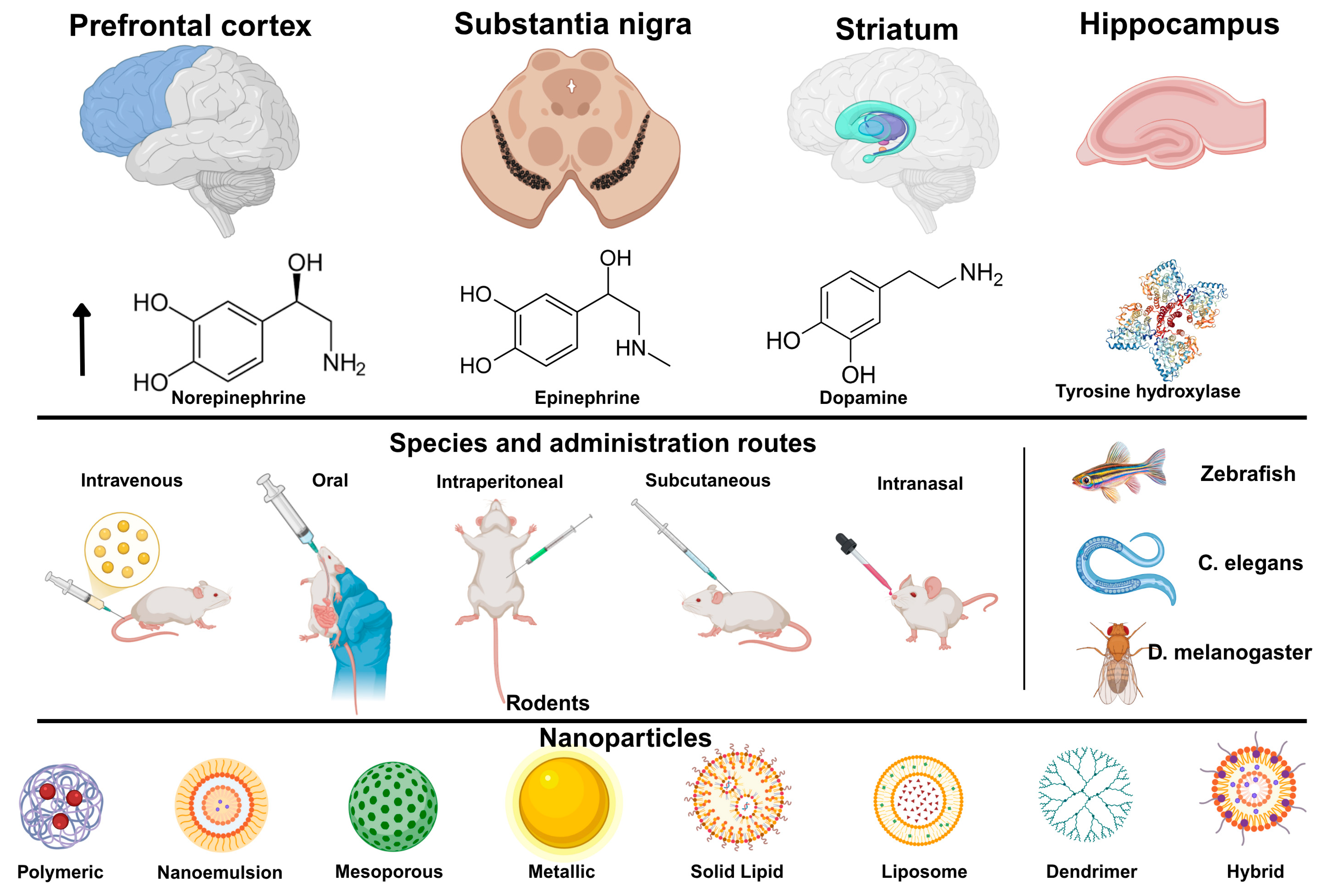
1.1. Catecholamines
1.2. Brain Diseases Related to Catecholamine Deficiencies
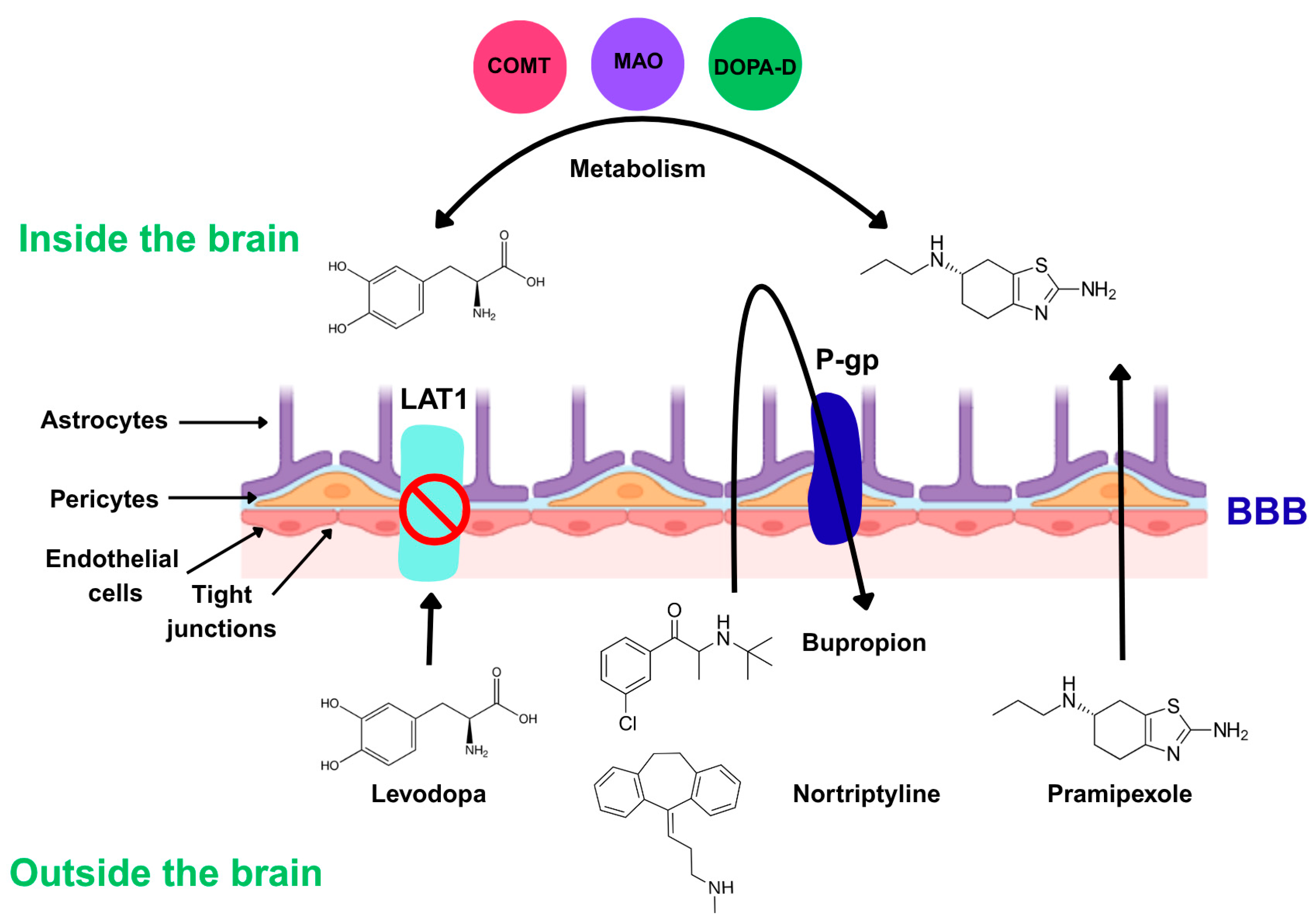
1.3. Challenges in the Treatment of Neuropsychiatric Diseases: The Blood–Brain Barrier (BBB)
1.4. Nanoparticles: New Therapeutic Strategies for Neuropsychiatric Diseases
2. Materials and Methods
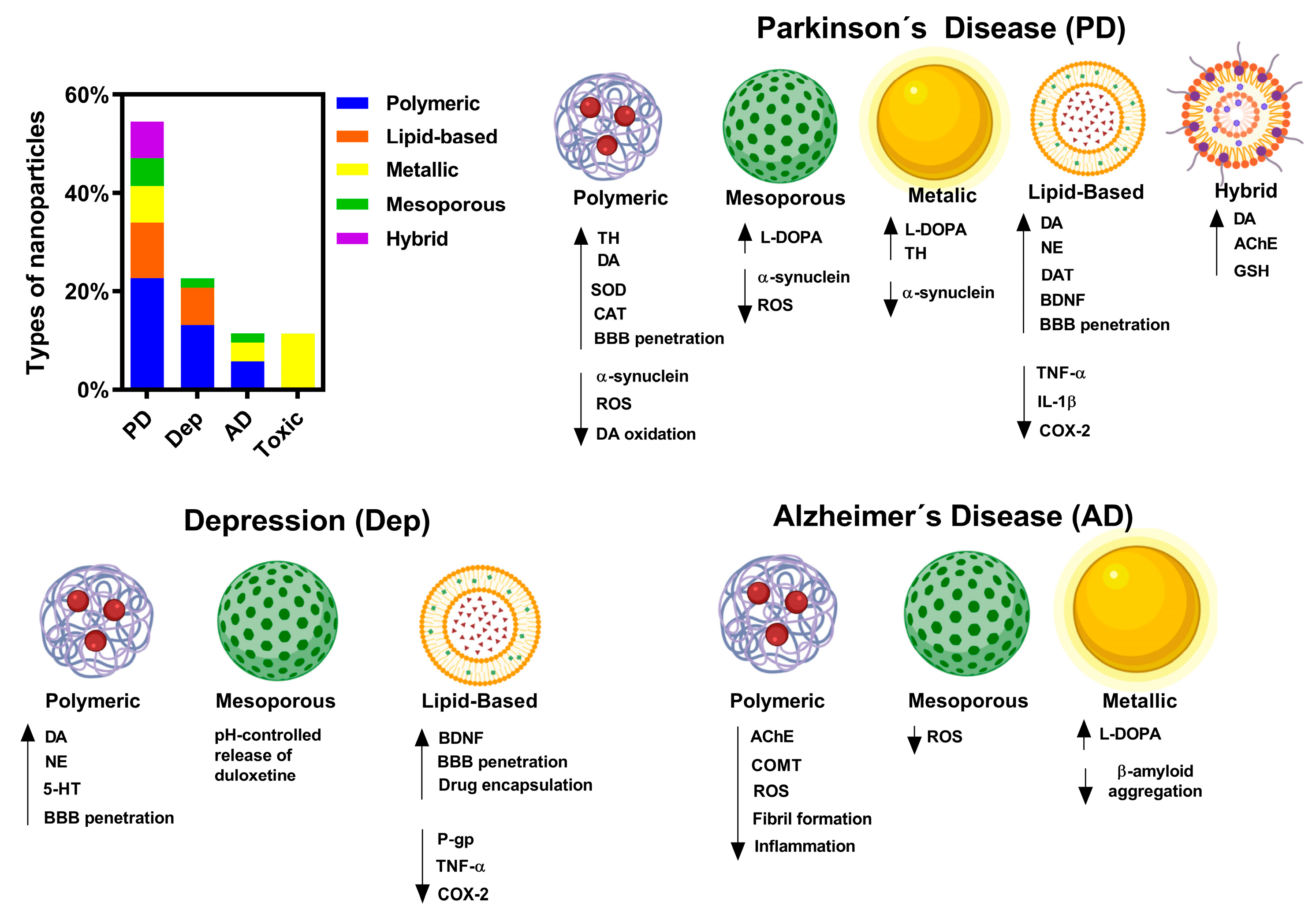
3. Results and Discussion
3.1. Influence of Particle Size on Catecholaminergic Restoration
3.2. Polymeric Nanoparticles
3.3. Lipid Nanoparticles
3.4. Levodopa and Dopaminergic Formulations
3.5. Blood–Brain Barrier Transport Mechanisms
3.6. Intranasal Administration
3.7. Controlled-Released and Smart Systems
3.8. Hybrid and Multifunctional Systems
3.9. Genetic Modulation of Therapeutic Targets
3.10. Safety Considerations
3.11. Clinical Translation of Catecholamine-Based Nanoparticles
3.12. Future Directions and Challenges
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Zou, D.; Li, Y.; Gu, S.; Dong, J.; Ma, X.; Xu, S.; Wang, F.; Huang, J.H. Monoamine Neurotransmitters Control Basic Emotions and Affect Major Depressive Disorders. Pharmaceuticals 2022, 15, 1203. [Google Scholar] [CrossRef]
- Moini, J.; Koenitzer, J.; LoGalbo, A. Chapter 2—Brain neurotransmitters. In Global Emergency of Mental Disorders; Moini, J., Koenitzer, J., LoGalbo, A., Eds.; Academic Press: Oxford, UK, 2021; pp. 31–40. [Google Scholar]
- Othumpangat, S. Catecholamines. In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 748–750. [Google Scholar]
- Dinter, E.; Saridaki, T.; Diederichs, L.; Reichmann, H.; Falkenburger, B.H. Parkinson’s disease and translational research. Transl. Neurodegener. 2020, 9, 43. [Google Scholar] [CrossRef]
- Saggu, S.; Bai, A.; Aida, M.; Rehman, H.; Pless, A.; Ware, D.; Deak, F.; Jiao, K.; Wang, Q. Monoamine alterations in Alzheimer’s disease and their implications in comorbid neuropsychiatric symptoms. Geroscience 2025, 47, 457–482. [Google Scholar] [CrossRef]
- Delva, N.C.; Stanwood, G.D. Dysregulation of brain dopamine systems in major depressive disorder. Exp. Biol. Med. 2021, 246, 1084–1093. [Google Scholar] [CrossRef]
- Godlewska, B.R.; Harmer, C.J. Cognitive neuropsychological theory of antidepressant action: A modern-day approach to depression and its treatment. Psychopharmacology 2021, 238, 1265–1278. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Pedrosa de Menezes, A.L.; Bloem, B.R.; Beckers, M.; Piat, C.; Benarroch, E.E.; Savica, R. Molecular Variability in Levodopa Absorption and Clinical Implications for the Management of Parkinson’s Disease. J. Parkinsons Dis. 2024, 14, 1353–1368. [Google Scholar] [CrossRef]
- Di Battista, V.; Hey-Hawkins, E. Development of Prodrugs for Treatment of Parkinson’s Disease: New Inorganic Scaffolds for Blood-Brain Barrier Permeation. J. Pharm. Sci. 2022, 111, 1262–1279. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Asimakidou, E.; Tan, J.K.S.; Zeng, J.; Lo, C.H. Blood-Brain Barrier-Targeting Nanoparticles: Biomaterial Properties and Biomedical Applications in Translational Neuroscience. Pharmaceuticals 2024, 17, 612. [Google Scholar] [CrossRef]
- Ding, S.; Khan, A.I.; Cai, X.; Song, Y.; Lyu, Z.; Du, D.; Dutta, P.; Lin, Y. Overcoming blood-brain barrier transport: Advances in nanoparticle-based drug delivery strategies. Mater. Today 2020, 37, 112–125. [Google Scholar] [CrossRef]
- Cai, H.; Liu, D.; Xue, W.W.; Ma, L.; Xie, H.T.; Ning, K. Lipid-based nanoparticles for drug delivery in Parkinson’s disease. Transl. Neurosci. 2024, 15, 20220359. [Google Scholar] [CrossRef]
- Sawicki, K.; Czajka, M.; Matysiak-Kucharek, M.; Fal, B.; Drop, B.; Męczyńska-Wielgosz, S.; Sikorska, K.; Kruszewski, M.; Kapka-Skrzypczak, L. Toxicity of metallic nanoparticles in the central nervous system. Nanotechnol. Rev. 2019, 8, 175–200. [Google Scholar] [CrossRef]
- Gong, J.Y.; Holt, M.G.; Hoet, P.H.M.; Ghosh, M. Neurotoxicity of four frequently used nanoparticles: A systematic review to reveal the missing data. Arch. Toxicol. 2022, 96, 1141–1212. [Google Scholar] [CrossRef] [PubMed]
- Alaei, M.; Koushki, K.; Taebi, K.; Yousefi Taba, M.; Keshavarz Hedayati, S.; Keshavarz Shahbaz, S. Metal nanoparticles in neuroinflammation: Impact on microglial dynamics and CNS function. RSC Adv. 2025, 15, 5426–5451. [Google Scholar] [CrossRef]
- Iversen, L.L.; Iversen, S.D.; Bloom, F.E.; Roth, R.H. (Eds.) Chapter 7—Catecholamines. In Introduction to Neuropsychopharmacology; Oxford University Press: Oxford, UK, 2009; pp. 150–213. [Google Scholar]
- Gnegy, M.E. Chapter 14—Catecholamines. In Basic Neurochemistry, 8th ed.; Brady, S.T., Siegel, G.J., Albers, R.W., Price, D.L., Eds.; Academic Press: New York, NY, USA, 2012; pp. 283–299. [Google Scholar]
- Fernandez, C.J.; Hanna, F.W.F.; Pacak, K.; Nazari, M.A. Chapter 2—Catecholamines and blood pressure regulation. In Endocrine Hypertension; Pappachan, J.M., Fernandez, C.J., Eds.; Academic Press: New York, NY, USA, 2023; pp. 19–34. [Google Scholar]
- Finkielstain, G.; Jha, S.; Merke, D. Chapter 9—Adrenal disorders. In Biochemical and Molecular Basis of Pediatric Disease, 5th ed.; Dietzen, D., Bennett, M., Wong, E., Haymond, S., Eds.; Academic Press: New York, NY, USA, 2021; pp. 267–296. [Google Scholar]
- Breton-Provencher, V.; Drummond, G.T.; Sur, M. Locus Coeruleus Norepinephrine in Learned Behavior: Anatomical Modularity and Spatiotemporal Integration in Targets. Front. Neural Circuits 2021, 15, 638007. [Google Scholar] [CrossRef]
- Bucci, D.; Busceti, C.L.; Calierno, M.T.; Di Pietro, P.; Madonna, M.; Biagioni, F.; Ryskalin, L.; Limanaqi, F.; Nicoletti, F.; Fornai, F. Systematic Morphometry of Catecholamine Nuclei in the Brainstem. Front. Neuroanat. 2017, 11, 98. [Google Scholar] [CrossRef]
- Speranza, L.; di Porzio, U.; Viggiano, D.; de Donato, A.; Volpicelli, F. Dopamine: The Neuromodulator of Long-Term Synaptic Plasticity, Reward and Movement Control. Cells 2021, 10, 735. [Google Scholar] [CrossRef]
- Boku, S.; Nakagawa, S.; Toda, H.; Hishimoto, A. Neural basis of major depressive disorder: Beyond monoamine hypothesis. Psychiatry Clin. Neurosci. 2018, 72, 3–12. [Google Scholar] [CrossRef]
- Cui, L.; Li, S.; Wang, S.; Wu, X.; Liu, Y.; Yu, W.; Wang, Y.; Tang, Y.; Xia, M.; Li, B. Major depressive disorder: Hypothesis, mechanism, prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 30. [Google Scholar] [CrossRef]
- Sharma, M.; Pal, P.; Gupta, S.K. The neurotransmitter puzzle of Alzheimer’s: Dissecting mechanisms and exploring therapeutic horizons. Brain Res. 2024, 1829, 148797. [Google Scholar] [CrossRef]
- Gannon, M.; Che, P.; Chen, Y.; Jiao, K.; Roberson, E.D.; Wang, Q. Noradrenergic dysfunction in Alzheimer’s disease. Front. Neurosci. 2015, 9, 20. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Niculescu, A.-G.; Vladâcenco, O.; Grumezescu, A.M.; Teleanu, D.M. Neurotransmitters—Key Factors in Neurological and Neurodegenerative Disorders of the Central Nervous System. Int. J. Mol. Sci. 2022, 23, 5954. [Google Scholar] [CrossRef]
- Paredes-Rodriguez, E.; Vegas-Suarez, S.; Morera-Herreras, T.; De Deurwaerdere, P.; Miguelez, C. The Noradrenergic System in Parkinson’s Disease. Front. Pharmacol. 2020, 11, 435. [Google Scholar] [CrossRef]
- Radad, K.; Moldzio, R.; Krewenka, C.; Kranner, B.; Rausch, W.-D. Pathophysiology of non-motor signs in Parkinson’s disease: Some recent updating with brief presentation. Explor. Neuroprot. Ther. 2023, 3, 24–46. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef] [PubMed]
- Chai, A.B.; Callaghan, R.; Gelissen, I.C. Regulation of P-Glycoprotein in the Brain. Int. J. Mol. Sci. 2022, 23, 14667. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, L.; Cao, F.; Wang, H.; Gong, P.; Ma, C.; Ren, L.; Lin, Y.; Lin, X. The barrier and interface mechanisms of the brain barrier, and brain drug delivery. Brain Res. Bull. 2022, 190, 69–83. [Google Scholar] [CrossRef]
- Lau, K.; Kotzur, R.; Richter, F. Blood–brain barrier alterations and their impact on Parkinson’s disease pathogenesis and therapy. Transl. Neurodegener. 2024, 13, 37. [Google Scholar] [CrossRef]
- Najjar, S.; Pearlman, D.M.; Devinsky, O.; Najjar, A.; Zagzag, D. Neurovascular unit dysfunction with blood-brain barrier hyperpermeability contributes to major depressive disorder: A review of clinical and experimental evidence. J. Neuroinflamm. 2013, 10, 142. [Google Scholar] [CrossRef]
- O’Brien, F.E.; Dinan, T.G.; Griffin, B.T.; Cryan, J.F. Interactions between antidepressants and P-glycoprotein at the blood-brain barrier: Clinical significance of in vitro and in vivo findings. Br. J. Pharmacol. 2012, 165, 289–312. [Google Scholar] [CrossRef]
- Sweeney, M.D.; Sagare, A.P.; Zlokovic, B.V. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat. Rev. Neurol. 2018, 14, 133–150. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Kshirsagar, P.; Agrawal, P.; Murry, D.J. Crossing the Blood–Brain Barrier: Innovations in Receptor- and Transporter-Mediated Transcytosis Strategies. Pharmaceutics 2025, 17, 706. [Google Scholar] [CrossRef]
- Rust, R.; Sagare, A.P.; Mingzi, Z.; Zlokovic, B.V.; Kisler, K. The blood–brain barrier as a treatment target for neurodegenerative disorders. Expert Opin. Drug Deliv. 2025, 22, 673–692. [Google Scholar] [CrossRef]
- Channer, B.; Matt, S.M.; Nickoloff-Bybel, E.A.; Pappa, V.; Agarwal, Y.; Wickman, J.; Gaskill, P.J. Dopamine, Immunity, and Disease. Pharmacol. Rev. 2023, 75, 62–158. [Google Scholar] [CrossRef]
- Katsoulaki, E.-E.; Dimopoulos, D.; Hadjipavlou-Litina, D. Multitarget Compounds Designed for Alzheimer, Parkinson, and Huntington Neurodegeneration Diseases. Pharmaceuticals 2025, 18, 831. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood-Brain Barrier Breakdown in Alzheimer’s Disease: Mechanisms and Targeted Strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef]
- Beltran-Velasco, A.I.; Clemente-Suárez, V.J. Impact of Peripheral Inflammation on Blood—Brain Barrier Dysfunction and Its Role in Neurodegenerative Diseases. Int. J. Mol. Sci. 2025, 26, 2440. [Google Scholar] [CrossRef]
- Ejsing, T.B.; Linnet, K. Influence of P-glycoprotein inhibition on the distribution of the tricyclic antidepressant nortriptyline over the blood–brain barrier. Hum. Psychopharmacol. 2005, 20, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Okura, T.; Ito, R.; Ishiguro, N.; Tamai, I.; Deguchi, Y. Blood-brain barrier transport of pramipexole, a dopamine D2 agonist. Life Sci. 2007, 80, 1564–1571. [Google Scholar] [CrossRef]
- Pryor, K.O.; Storer, K.P. Chapter 11—Drugs for Neuropsychiatric Disorders. In Pharmacology and Physiology for Anesthesia; Hemmings, H.C., Egan, T.D., Eds.; W.B. Saunders: Philadelphia, PA, USA, 2013; pp. 180–207. [Google Scholar]
- Barcia, E.; Boeva, L.; García-García, L.; Slowing, K.; Fernández-Carballido, A.; Casanova, Y.; Negro, S. Nanotechnology-based drug delivery of ropinirole for Parkinson’s disease. Drug Deliv. 2017, 24, 1112–1123. [Google Scholar] [CrossRef]
- Ahmed-Farid, O.A.; Taha, M.; Bakeer, R.M.; Radwan, O.K.; Hendawy, H.A.M.; Soliman, A.S.; Yousef, E. Effects of bee venom and dopamine-loaded nanoparticles on reserpine-induced Parkinson’s disease rat model. Sci. Rep. 2021, 11, 21141. [Google Scholar]
- Pahuja, R.; Seth, K.; Shukla, A.; Shukla, R.K.; Bhatnagar, P.; Chauhan, L.K.S.; Saxena, P.N.; Arun, J.; Chaudhari, B.P.; Patel, D.K.; et al. Trans-Blood Brain Barrier Delivery of Dopamine-Loaded Nanoparticles Reverses Functional Deficits in Parkinsonian Rats. ACS Nano 2015, 9, 4850–4871. [Google Scholar] [CrossRef]
- Liu, Z.; Xiang, S.; Chen, B.; Li, J.; Zhu, D.; Xu, H.; Hu, S. Parkinson Disease-Targeted Nanocapsules for Synergistic Treatment: Combining Dopamine Replacement and Neuroinflammation Mitigation. Adv. Sci. 2024, 11, e2404717. [Google Scholar] [CrossRef]
- Mogharbel, B.F.; Cardoso, M.A.; Irioda, A.C.; Stricker, P.E.F.; Slompo, R.C.; Appel, J.M.; de Oliveira, N.B.; Perussolo, M.C.; Saçaki, C.S.; da Rosa, N.N.; et al. Biodegradable Nanoparticles Loaded with Levodopa and Curcumin for Treatment of Parkinson’s Disease. Molecules 2022, 27, 2811. [Google Scholar] [CrossRef]
- Morales, V.; McConnell, J.; Pérez-Garnes, M.; Almendro, N.; Sanz, R.; García-Muñoz, R.A. L-Dopa release from mesoporous silica nanoparticles engineered through the concept of drug-structure-directing agents for Parkinson’s disease. J. Mater. Chem. B 2021, 9, 4178–4189. [Google Scholar] [CrossRef]
- Guo, M.; Lin, R.; Xu, W.; Xu, L.; Liu, M.; Huang, X.; Zhang, J.; Li, X.; Ma, Y.; Yuan, M.; et al. Replenishing Cation-π Interactions for the Fabrication of Mesoporous Levodopa Nanoformulations for Parkinson Remission. ACS Nano 2024, 18, 30605–30615. [Google Scholar] [CrossRef] [PubMed]
- Yeni, Y.; Genc, S.; Ertugrul, M.S.; Nadaroglu, H.; Gezer, A.; Mendil, A.S.; Hacımuftuoglu, A. Neuroprotective effects of L-Dopa-modified zinc oxide nanoparticles on the rat model of 6-OHDA-ınduced Parkinson’s disease. Sci. Rep. 2024, 14, 19077. [Google Scholar] [CrossRef]
- Ortega Martínez, E.; Morales Hernández, M.E.; Castillo-González, J.; González-Rey, E.; Ruiz Martínez, M.A. Dopamine-loaded chitosan-coated solid lipid nanoparticles as a promise nanocarriers to the CNS. Neuropharmacology 2024, 249, 109871. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Trapani, G.; Di Gioia, S.; Dazzi, L.; De Giglio, E.; Trapani, A. In vitro investigations on dopamine loaded Solid Lipid Nanoparticles. J. Pharm. Biomed. Anal. 2020, 185, 113257. [Google Scholar] [CrossRef] [PubMed]
- Monge-Fuentes, V.; Biolchi Mayer, A.; Lima, M.R.; Geraldes, L.R.; Zanotto, L.N.; Moreira, K.G.; Martins, O.P.; Piva, H.L.; Felipe, M.S.S.; Amaral, A.C.; et al. Dopamine-loaded nanoparticle systems circumvent the blood-brain barrier restoring motor function in mouse model for Parkinson’s Disease. Sci. Rep. 2021, 11, 15185. [Google Scholar] [CrossRef]
- Raj, R.; Wairkar, S.; Sridhar, V.; Gaud, R. Pramipexole dihydrochloride loaded chitosan nanoparticles for nose to brain delivery: Development, characterization and in vivo anti-Parkinson activity. Int. J. Biol. Macromol. 2018, 109, 27–35. [Google Scholar] [CrossRef]
- Tzankov, B.; Voycheva, C.; Yordanov, Y.; Aluani, D.; Spassova, I.; Kovacheva, D.; Lambov, N.; Tzankova, V. Development and in vitro safety evaluation of pramipexole-loaded hollow mesoporous silica (HMS) particles. Biotechnol. Biotechnol. Equip. 2019, 33, 1204–1215. [Google Scholar] [CrossRef]
- Gunay, M.S.; Ozer, A.Y.; Erdogan, S.; Bodard, S.; Baysal, I.; Gulhan, Z.; Guilloteau, D.; Chalon, S. Development of Nanosized, Pramipexole-Encapsulated Liposomes and Niosomes for the Treatment of Parkinson’s Disease. J. Nanosci. Nanotechnol. 2017, 17, 5155–5167. [Google Scholar] [CrossRef]
- Nie, T.; He, Z.; Zhu, J.; Chen, K.; Howard, G.P.; Pacheco-Torres, J.; Minn, I.; Zhao, P.; Bhujwalla, Z.M.; Mao, H.-Q.; et al. Non-invasive delivery of levodopa-loaded nanoparticles to the brain via lymphatic vasculature to enhance treatment of Parkinson’s disease. Nano Res. 2021, 14, 2749–2761. [Google Scholar] [CrossRef]
- Liu, L.; Li, M.; Xu, M.; Wang, Z.; Zeng, Z.; Li, Y.; Zhang, Y.; You, R.; Li, C.-H.; Guan, Y.-Q. Actively targeted gold nanoparticle composites improve behavior and cognitive impairment in Parkinson’s disease mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 114, 111028. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Ke, W.; Liu, Y.; Wu, D.; Feng, L.; Jiang, C.; Pei, Y. Gene therapy using lactoferrin-modified nanoparticles in a rotenone-induced chronic Parkinson model. J. Neurol. Sci. 2010, 290, 123–130. [Google Scholar] [CrossRef]
- Bezem, M.T.; Johannessen, F.G.; Jung-Kc, K.; Gundersen, E.T.; Jorge-Finnigan, A.; Ying, M.; Betbeder, D.; Herfindal, L.; Martinez, A. Stabilization of Human Tyrosine Hydroxylase in Maltodextrin Nanoparticles for Delivery to Neuronal Cells and Tissue. Bioconjug. Chem. 2018, 29, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Hanumanthappa, R.; Venugopal, D.M.; P C, N.; Shaikh, A.; B.M, S.; Heggannavar, G.B.; Patil, A.A.; Nanjaiah, H.; Suresh, D.; Kariduraganavar, M.Y.; et al. Polyvinylpyrrolidone-Capped Copper Oxide Nanoparticles-Anchored Pramipexole Attenuates the Rotenone-Induced Phenotypes in a Drosophila Parkinson’s Disease Model. ACS Omega 2023, 8, 47482–47495. [Google Scholar] [CrossRef]
- Rana, I.; Khan, N.; Ansari, M.M.; Shah, F.A.; Din, F.u.; Sarwar, S.; Imran, M.; Qureshi, O.S.; Choi, H.-I.; Lee, C.-H.; et al. Solid lipid nanoparticles-mediated enhanced antidepressant activity of duloxetine in lipopolysaccharide-induced depressive model. Colloids Surf. B Biointerfaces 2020, 194, 111209. [Google Scholar] [CrossRef]
- Saeedi, M.; Morteza-Semnani, K.; Akbari, J.; Siahposht-Khachaki, A.; Firouzi, M.; Goodarzi, A.; Abootorabi, S.; Babaei, A.; Hassan Hashemi, S.M.; Nokhodchi, A. Brain targeting of venlafaxine HCl as a hydrophilic agent prepared through green lipid nanotechnology. J. Drug Deliv. Sci. Technol. 2021, 66, 102813. [Google Scholar] [CrossRef]
- Salem, H.F.; Ali, A.A.; Rabea, Y.K.; Abo El-Ela, F.I.; Khallaf, R.A. Optimization and Appraisal of Chitosan-Grafted PLGA Nanoparticles for Boosting Pharmacokinetic and Pharmacodynamic Effect of Duloxetine HCl Using Box-Benkhen Design. J. Pharm. Sci. 2023, 112, 544–561. [Google Scholar] [CrossRef]
- Tong, G.-F.; Qin, N.; Sun, L.-W. Development and evaluation of Desvenlafaxine loaded PLGA-chitosan nanoparticles for brain delivery. Saudi Pharm. J. 2017, 25, 844–851. [Google Scholar] [CrossRef]
- Cayero-Otero, M.D.; Perez-Caballero, L.; Suarez-Pereira, I.; Hidalgo-Figueroa, M.; Delgado-Sequera, A.; Montesinos, J.M.; Berrocoso, E.; Martín-Banderas, L. Venlafaxine-PLGA nanoparticles provide a fast onset of action in an animal model of depression via nose-to-brain. Int. J. Pharm. 2025, 678, 125692. [Google Scholar] [CrossRef]
- Shoaib, M.; Arif, H.; Awan, A.N.; Khan, M.M.; Batool, S.; Ahmed, S. Synthesis and optimization of fluoxetine-loaded polymeric nanoparticles for dual therapeutic applications in cancer and depression. DARU J. Pharm. Sci. 2025, 33, 18. [Google Scholar] [CrossRef]
- Ganesh, M.; Ubaidulla, U.; Hemalatha, P.; Peng, M.M.; Jang, H.T. Development of duloxetine hydrochloride loaded mesoporous silica nanoparticles: Characterizations and in vitro evaluation. AAPS PharmSciTech 2015, 16, 944–951. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, G.; Rao, Z.; Yang, Y.; Zhou, Q.; Qin, H.; Wei, Y.; Wu, X. Increased brain uptake of venlafaxine loaded solid lipid nanoparticles by overcoming the efflux function and expression of P-gp. Arch. Pharm. Res. 2015, 38, 1325–1335. [Google Scholar] [CrossRef]
- Valadez-Lemus, R.E.; Góngora-Alfaro, J.L.; Jiménez-Vargas, J.M.; Alamilla, J.; Mendoza-Muñoz, N. Nanoencapsulation of amitriptyline enhances the potency of antidepressant-like effects and exhibits anxiolytic-like effects in Wistar rats. PLoS ONE 2025, 20, e0316389. [Google Scholar] [CrossRef]
- Harini, K.; Girigoswami, K.; Vajagathali, M.; Bose, D.; Thirumalai, A.; Kiran, V.; Durgadevi, P.; Girigoswami, A. Enhanced behavioral impact of optimized bupropion-encapsulated bilosomes over traditional niosomes treating depression. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2025, 398, 4373–4392. [Google Scholar] [CrossRef] [PubMed]
- Harini, K.; Alomar, S.Y.; Vajagathali, M.; Manoharadas, S.; Thirumalai, A.; Girigoswami, K.; Girigoswami, A. Niosomal Bupropion: Exploring Therapeutic Frontiers through Behavioral Profiling. Pharmaceuticals 2024, 17, 366. [Google Scholar] [CrossRef] [PubMed]
- Alabsi, A.; Khoudary, A.C.; Abdelwahed, W. The Antidepressant Effect of L-Tyrosine-Loaded Nanoparticles: Behavioral Aspects. Ann. Neurosci. 2016, 23, 89–99. [Google Scholar] [CrossRef][Green Version]
- Qin, J.; Guan, Y.; Li, Z.; Guo, X.; Zhang, M.; Wang, D.; Tang, J. Aptamer conjugated polydopamine-coated gold nanoparticles as a dual-action nanoplatform targeting β-amyloid peptide for Alzheimer’s disease therapy. J. Mater. Chem. B 2022, 10, 8525–8534. [Google Scholar] [CrossRef]
- Panda, H.S.; Dhokne, M.D.; Thakur, S.; Singh, N.; Datusalia, A.K.; Panda, J.J. BDNF Loaded Amino Acid-Catecholamine Hybrid Nanoparticles as Curative Agents Against Cognitive Decline in Alzheimer’s Disease. Small 2025, e2411701. [Google Scholar] [CrossRef]
- Baysal, I.; Ucar, G.; Gultekinoglu, M.; Ulubayram, K.; Yabanoglu-Ciftci, S. Donepezil loaded PLGA-b-PEG nanoparticles: Their ability to induce destabilization of amyloid fibrils and to cross blood brain barrier in vitro. J. Neural Transm. 2017, 124, 33–45. [Google Scholar] [CrossRef]
- Çınar, E.; Mutluay, S.U.; Baysal, İ.; Gültekinoğlu, M.; Ulubayram, K.; Çiftçi, S.Y.; Tel, B.C.; Uçar, G. Donepezil-loaded PLGA-b-PEG Nanoparticles Enhance the Learning and Memory Function of Beta-Amyloid Rat Model of Alzheimer’s Disease. Noro Psikiyatr. Ars. 2022, 59, 281–289. [Google Scholar]
- Zhang, H.; Jiang, Y.; Zhao, S.-g.; Jiang, L.-q.; Meng, Y.; Liu, P.; Kim, M.O.; Li, S. Selective neuronal targeting, protection and signaling network analysis via dopamine-mediated mesoporous silica nanoparticles. MedChemComm 2015, 6, 1117–1129. [Google Scholar] [CrossRef]
- Chen, X.; Gao, W.; Sun, Y.; Dong, X. Multiple effects of polydopamine nanoparticles on Cu2+-mediated Alzheimer’s β-amyloid aggregation. Chin. J. Chem. Eng. 2023, 54, 144–152. [Google Scholar] [CrossRef]
- Molnár, Z.; Koplányi, G.; Farkas, R.; Péli, N.; Kenéz, B.; Decsi, B.; Katona, G.; Balogh, G.T.; Vértessy, B.G.; Balogh-Weiser, D. Immobilization of human tyrosine hydroxylase onto magnetic nanoparticles—A novel formulation of a therapeutic enzyme. Int. J. Biol. Macromol. 2024, 268, 131939. [Google Scholar] [CrossRef]
- Manickam, V.; Dhakshinamoorthy, V.; Perumal, E. Iron Oxide Nanoparticles Affects Behaviour and Monoamine Levels in Mice. Neurochem. Res. 2019, 44, 1533–1548. [Google Scholar] [CrossRef]
- Heidari, Z.; Mohammadipour, A.; Haeri, P.; Ebrahimzadeh-Bideskan, A. The effect of titanium dioxide nanoparticles on mice midbrain substantia nigra. Iran. J. Basic Med. Sci. 2019, 22, 745–751. [Google Scholar]
- Imam, S.Z.; Lantz-McPeak, S.M.; Cuevas, E.; Rosas-Hernandez, H.; Liachenko, S.; Zhang, Y.; Sarkar, S.; Ramu, J.; Robinson, B.L.; Jones, Y.; et al. Iron Oxide Nanoparticles Induce Dopaminergic Damage: In Vitro Pathways and In Vivo Imaging Reveals Mechanism of Neuronal Damage. Mol. Neurobiol. 2015, 52, 913–926. [Google Scholar] [CrossRef]
- Sadeghi, L.; Babadi, V.Y.; Tanwir, F. Manganese dioxide nanoparticle induces Parkinson like neurobehavioral abnormalities in rats. Bratisl. Lek. Listy 2018, 119, 379–384. [Google Scholar] [CrossRef]
- Ohta, S.; Kikuchi, E.; Ishijima, A.; Azuma, T.; Sakuma, I.; Ito, T. Investigating the optimum size of nanoparticles for their delivery into the brain assisted by focused ultrasound-induced blood-brain barrier opening. Sci. Rep. 2020, 10, 18220. [Google Scholar] [CrossRef]
- Poonkuzhali, K.; Seenivasagan, R.; Prabhakaran, J.; Karthika, A. Synthesis and characterization of chemical engineered PLGA nanosphere: Triggering mechanism of Catechol-O-methyltransferase inhibition on in vivo neurodegeneration. Bioorg. Chem. 2023, 139, 106673. [Google Scholar] [CrossRef]
- Wu, H.; Peng, B.; Mohammed, F.S.; Gao, X.; Qin, Z.; Sheth, K.N.; Zhou, J.; Jiang, Z. Brain Targeting, Antioxidant Polymeric Nanoparticles for Stroke Drug Delivery and Therapy. Small 2022, 18, e2107126. [Google Scholar] [CrossRef]
- Khongkow, M.; Yata, T.; Boonrungsiman, S.; Ruktanonchai, U.R.; Graham, D.; Namdee, K. Surface modification of gold nanoparticles with neuron-targeted exosome for enhanced blood—Brain barrier penetration. Sci. Rep. 2019, 9, 8278. [Google Scholar] [CrossRef] [PubMed]
- Khafajah, Y.; Shaheen, M.; Natour, D.E.; Merheb, M.; Matar, R.; Borjac, J. Neuroprotective Effects of Zinc Oxide Nanoparticles in a Rotenone-Induced Mouse Model of Parkinson’s Disease. Nanotheranostics 2024, 8, 497–505. [Google Scholar] [CrossRef]
- Evans, C.W.; Fitzgerald, M.; Clemons, T.D.; House, M.J.; Padman, B.S.; Shaw, J.A.; Saunders, M.; Harvey, A.R.; Zdyrko, B.; Luzinov, I.; et al. Multimodal Analysis of PEI-Mediated Endocytosis of Nanoparticles in Neural Cells. ACS Nano 2011, 5, 8640–8648. [Google Scholar] [CrossRef] [PubMed]
- Ozcicek, I.; Aysit, N.; Cakici, C.; Ayturk, N.U.; Aydeger, A.; Erim, U.C. The Effects of Various Surface Coatings of Gold Nanorods on Toxicity, Neuronal Localization, Microstructural Alterations, and In vitro/In vivo Biodistribution. Adv. Mater. Interfaces 2022, 9, 2101369. [Google Scholar] [CrossRef]
- Razzak, A.A.; Al-Garawi, Z.S.; Haider, A.J.; Hassan, F.A.A.N.B. New nanomanufacturing strategy through bioinspired design, for promising treatment of Parkinson’s disease. Sci. Rep. 2025, 15, 6904. [Google Scholar] [CrossRef]
- Lipsman, N.; Meng, Y.; Bethune, A.J.; Huang, Y.; Lam, B.; Masellis, M.; Herrmann, N.; Heyn, C.; Aubert, I.; Boutet, A.; et al. Blood-brain barrier opening in Alzheimer’s disease using MR-guided focused ultrasound. Nat. Commun. 2018, 9, 2336. [Google Scholar] [CrossRef]
- Niu, J.; Xie, J.; Guo, K.; Zhang, X.; Xia, F.; Zhao, X.; Song, L.; Zhuge, D.; Li, X.; Zhao, Y.; et al. Efficient treatment of Parkinson’s disease using ultrasonography-guided rhFGF20 proteoliposomes. Drug Deliv. 2018, 25, 1560–1569. [Google Scholar] [CrossRef]
- Zhang, N.; Yan, F.; Liang, X.; Wu, M.; Shen, Y.; Chen, M.; Xu, Y.; Zou, G.; Jiang, P.; Tang, C.; et al. Localized delivery of curcumin into brain with polysorbate 80-modified cerasomes by ultrasound-targeted microbubble destruction for improved Parkinson’s disease therapy. Theranostics 2018, 8, 2264–2277. [Google Scholar] [CrossRef]
- Lahiri, A.; V, B.; Pr, H.V.; Paul, K. A Novel and Green UFLC-MS/MS Method for Quantification of Amantadine and Levodopa in Polymeric Nanoparticles: Application to determine Drug loading (%DL), Drug entrapment (%DEE) and Drug release profile. Talanta Open 2025, 12, 100476. [Google Scholar] [CrossRef]
- Trivedi, R.; Minglani, V.V.; El-Gazzar, A.M.; Batiha, G.E.; Mahmoud, M.H.; Patel, M.; Patel, M. Optimization of Pramipexole-Loaded In Situ Thermosensitive Intranasal Gel for Parkinson’s Disease. Pharmaceuticals 2024, 17, 172. [Google Scholar] [CrossRef]
- Shang, M.; Niu, S.; Chang, X.; Li, J.; Zhang, W.; Guo, M.; Wu, T.; Zhang, T.; Tang, M.; Xue, Y. Silver nanoparticle-induced impaired autophagic flux and lysosomal dysfunction contribute to the microglia inflammation polarization. Food Chem. Toxicol. 2022, 170, 113469. [Google Scholar] [CrossRef]
- Siddiqi, N.J.; Abdelhalim, M.A.K.; El-Ansary, A.K.; Alhomida, A.S.; Ong, W.Y. Identification of potential biomarkers of gold nanoparticle toxicity in rat brains. J. Neuroinflamm. 2012, 9, 123. [Google Scholar] [CrossRef]
- Singh, G.; Sarwal, A.; Sharma, S.; Prasad, P.; Kuhad, A.; Ali, W. Polymer-based prolonged-release nanoformulation of duloxetine: Fabrication, characterization and neuropharmacological assessments. Drug Dev. Ind. Pharm. 2021, 47, 12–21. [Google Scholar] [CrossRef]
- National Library of Medicine. 31P-MRS Imaging to Assess the Effects of CNM-Au8 on Impaired Neuronal Redox State in Parkinson’s Disease (REPAIR-PD). Available online: https://clinicaltrials.gov/study/NCT03815916 (accessed on 2 September 2025).
- Wang, Z.; Henriques, A.; Rouvière, L.; Callizot, N.; Tan, L.; Hotchkin, M.T.; Rossignol, R.; Mortenson, M.G.; Dorfman, A.R.; Ho, K.S.; et al. A Mechanism Underpinning the Bioenergetic Metabolism-Regulating Function of Gold Nanocatalysts. Small 2024, 20, 2304082. [Google Scholar] [CrossRef]
- National Library of Medicine. Study of APH-1105 in Patients with Mild to Moderate Alzheimer’s Disease. Available online: https://clinicaltrials.gov/study/NCT03806478 (accessed on 2 September 2025).
- Vallorz, E.L.; Encinas-Basurto, D.; Schnellmann, R.G.; Mansour, H.M. Design, Development, Physicochemical Characterization, and In Vitro Drug Release of Formoterol PEGylated PLGA Polymeric Nanoparticles. Pharmaceutics 2022, 14, 638. [Google Scholar] [CrossRef]
- Hemmrich, E.; McNeil, S. Active ingredient vs excipient debate for nanomedicines. Nat. Nanotechnol. 2023, 18, 692–695. [Google Scholar] [CrossRef] [PubMed]
- Farasati Far, B.; Omrani, M.; Naimi Jamal, M.R.; Javanshir, S. Multi-responsive chitosan-based hydrogels for controlled release of vincristine. Commun. Chem. 2023, 6, 28. [Google Scholar] [CrossRef] [PubMed]
- Foond and Drug Administration. Drug Products, Including Biological Products, that Contain Nanomaterials Guidance for Industry. Available online: https://www.fda.gov/files/drugs/published/Drug-Products--Including-Biological-Products--that-Contain-Nanomaterials---Guidance-for-Industry.pdf (accessed on 2 September 2025).
- European Medicines Agency. Nanotechnology-Based Medicinal Products for Human Use. Available online: https://www.ema.europa.eu/en/documents/report/nanotechnology-based-medicinal-products-human-use-eu-horizon-scanning-report_en.pdf (accessed on 2 September 2025).
| Nanoparticle Type (NPs)/Size | Material | Drug(s) | Administration Route/Specie | Target Disease(s) | Mechanism/Key Features | Main Therapeutic Outcomes | Ref. |
|---|---|---|---|---|---|---|---|
| Polymeric/≈150 nm | PLGA | Ropinirole | Intraperitoneal (1 mg/kg/day)/Male rat | Parkinson’s disease | ↑ TH in substantia nigra | Reversal of motor symptoms | [48] |
| Nanoemulsion/≈10 nm | Lecithin/Peanut oil | DA | Oral (25 mg/kg)/Male rat | Parkinson’s disease | Restores DA & NE in hippocampus/striatum, ↓ TNF-α, IL-1β | ↓ oxidative stress & neuroinflammation | [49] |
| Polymeric/≈120 nm | PLGA | DA | Intravenous (4.5 mg/kg)/Rat | Parkinson’s disease | ↑ DA in striatum, avoids DA oxidation | Reverses neurochemical & behavioral deficits | [50] |
| Nanocapsule/≈92 nm | Polymeric core functionalized with Angiopep-2 and cRGD | DA, CAT | Intravenous (600 µg/kg)/Male mouse | Parkinson’s disease | ↑ DA in striatum/Substantia nigra, ↑ TH, ↓ α-synuclein | ↓ oxidative stress & inflammation | [51] |
| Polymeric/≈100 nm | Poly(ethylene oxide)/poly(ε-caprolactone) copolymer/GSH | L-DOPA, curcumin | - | Parkinson’s disease | GSH functionalization to cross BBB | Biocompatible with neuroendocrine cells | [52] |
| Mesoporous NPs/≈100 nm | Tetraethyl orthosilicate | L-DOPA | - | Parkinson’s disease | Sustained release at pH 7.4, minimal gastric release | pH-dependent release | [53] |
| Mesoporous NPs/≈200 nm | Na+ cation–π | L-DOPA | - | Parkinson’s disease | Sustained release, ↓ α-synuclein aggregation | High neuronal biocompatibility | [54] |
| Metallic/≈20 nm | Zinc oxide | L-DOPA | Oral (30–60 mg/kg)/Male rat | Parkinson’s disease | Protects neurons in the substantia nigra, ↓ α-synuclein | improved motor function | [55] |
| Solid Lipid NPs/≈260 nm | Glycerol tripalmitin, polysorbate 80, chitosan | DA | - | Parkinson’s disease | Nanocarrier for DA | Efficient BBB transit in vitro | [56] |
| Solid Lipid NPs/≈150 nm | Gelucire®, Tween 85, Glycol Chitosan | DA | - | Parkinson’s disease | Sustained release of DA | Potential intranasal route | [57] |
| Polymeric/≈350 nm | PLGA | DA, Albumin | Intraperitoneal (10 and 20 mg)/Male mouse | Parkinson’s disease | ↑ DA in striatum and substantia nigra | ↑ Motor coordination | [58] |
| Polymeric/≈300–500 nm | Chitosan, sodium tripolyphosphate | Pramipexole | Intranasal (0.3 mg/kg)/Male rat | Parkinson’s disease | ↑ DA, SOD, and CAT | ↓ catalepsy | [59] |
| Hollow mesoporous NPs/≈550 nm | Chitosan, alginate | Pramipexole | SH-SY5Y cells (200 µg/mL) | Parkinson’s disease | ↓ Oxidative stress | Neuroprotection | [60] |
| Liposome/≈120 nm | PEG, Dipalmitoylphosphatidylcholine | Pramipexole | Intraperitoneal (0.5 mg/mL)/Male rat | Parkinson’s disease | ↑ DAT | ↓ Dose | [61] |
| Niosome/≈100 nm | PEG, polyglyceryl-3 cetyl ether | Pramipexole | Intraperitoneal (0.5 mg/mL)/Male rat | Parkinson’s disease | ↓ DAT | ↓ Dose | |
| Polymeric/≈50 nm | Tannic acid, polyvinyl alcohol | L-DOPA | Subcutaneous (2 mg)/Rat | Parkinson’s disease | ↓ ROS, ↑ DA, TH, and SOD in striatum | ↓ Movement disorders and cerebral oxidative stress | [62] |
| Metallic/≈200 nm | Chloroauric acid, cholesterol, lecithin | α-synuclein DNA plasmid, docosahexaenoic acid, nerve growth factor | Intraperitoneal (2 mg/kg)/Male mouse | Parkinson’s disease | ↓ α-synuclein, ↑ TH in substantia nigra | ↑ Spatial memory ↓ motor dysfunction | [63] |
| Dendrimer | PAMAM, PEG | Lactoferrin vector | Intravenous/Male rat | Parkinson’s disease | ↑ TH in substantia nigra, ↑ DA in striatum | ↑ Locomotor activity | [64] |
| Porous polysaccharides/≈100 nm | Maltodextrin, lipid core | TH | Intracranial (3 µL)/Male mouse | Parkinson’s disease | ↑ TH in caudate putamen | ↑ TH activity | [65] |
| Hybrid (Metallic/Polymeric)/≈110 nm | Copper oxide, polyvinylpyrrolidone | Pramipexole | Drosophila melanogaster (46.5 µg via diet) | Parkinson’s disease | ↑ DA, AChE and GSH | ↓ locomotor defects | [66] |
| Solid Lipid NPs/≈150 nm | Stearyl alcohol, poloxamer 188, Tween 80 | Duloxetine | Intraperitoneal (30 mg/kg)/Male rat | Depression | 80% encapsulation, 52% sustained release | ↑ BDNF, ↓ TNF-α, COX-2 in prefrontal cortex | [67] |
| Solid Lipid NPs/≈300 nm | Glyceryl monostearate, Tween 80, Span 80 | Venlafaxine | Oral (22 mg/kg)/Male mouse | Depression | Surfactants ↑ BBB penetration | ↑ Brain bioavailability | [68] |
| Polymeric/≈120 nm | PLGA, chitosan | Duloxetine | Intranasal (20 mg/kg)/Male rat | Depression | ↑ NE in brain via trigeminal/olfactory transport | ↑ Antidepressant activity | [69] |
| Polymeric/≈170 nm | PLGA, chitosan | Desvenlafaxine | Intranasal (5 mg/kg)/Male rat | Depression | ↑ NE in brain | Reversed the signs of depression in rats | [70] |
| Polymeric/≈200 nm | PLGA | Venlafaxine | Intranasal (10 µL/day)/Male mouse | Depression | Direct brain transport, rapid onset | Reversed anhedonia, reduced immobility in 7 days | [71] |
| Polymeric/≈50 nm | PLGA, dextran | Fluoxetine | Oral (30 mg/kg/day)/Rat | Depression | ↑ 5-HT & DA, improved PK | ↑ Antidepressant activity | [72] |
| Mesoporous NPs | Tetraethyl orthosilicate | Duloxetine | - | Depression | pH-dependent release | Controlled release in intestinal pH | [73] |
| Solid Lipid NPs/≈190 nm | Poloxamer 188 | Venlafaxine | Intravenous (11 mg/kg)/Male mouse | Depression | ↓ P-gp activity | ↑ Brain concentration | [74] |
| Polymeric/≈200 nm | copolymer `poly (methyl vinil ether/Maleic acid) | Amitriptyline | Intranasal (10 mg/kg)/Male and female rat | Depression | ↑ BBB penetration | ↑ Antidepressant activity | [75] |
| Bilosome/Niosome/≈250–350 nm | Bile salt, span 20, cholesterol | Bupropion | Zebrafish | Depression | ↑ Encapsulation | ↓ Depressive behavior | [76,77] |
| Polymeric/≈140 nm | Polysorbate 20, poloxamer 420, polycaprolactone | L-tyrosine | Intraperitoneal (5–10 mg/kg)/Male rat | Depression | ↑ NE | ↑ Locomotor activity ↓ Depressive behavior | [78] |
| Metallic | Chloroauric acid | Polydopamine | - | Alzheimer’ s disease | ↓ β-amyloid aggregation and cytotoxicity. | Neuroprotector | [79] |
| Nanocomposite/≈25 nm | - | DA, tryptophan, EGCG, BDNF | - | Alzheimer’ s disease | ↓ β-amyloid fibrillation, ↓ neuronal damage, ↓ brain inflammation | ↑ Cognitive function | [80] |
| Polymeric/≈170–240 nm | PLGA, PEG | Donepezil | Intravenous (15 µg/kg)/Male rat | Alzheimer’ s disease | ↓ AChE activity, ↓ fibril formation, ↓ inflammatory markers | ↑ Memory | [81,82] |
| Mesoporous NPs/≈120 nm | Tetraethyl orthosilicate | DA, GSH | - | Alzheimer’ s disease | ↓ ROS | Neuroprotection | [83] |
| Polymeric/≈100 nm | NaOH | Polydopamine | Caenorhabditis elegans (100 µg/mL) | Alzheimer’ s disease | ↓ fibril formation, ↓ ROS | ↓ deposition of β-amyloid plaque | [84] |
| Magnetic/≈600 nm | Iron chloride, PEG, 3-aminopropyl-trimethoxysilane | TH | - | Therapeutic potential for treating diseases caused by catecholamine deficiencies. | ↑ L-DOPA synthesis in vitro | - | [85] |
| Metallic/≈45 nm | Iron oxide | - | Oral (25–50 µg/kg)/Male mouse | Safety studies | ↓ DA & EPI in prefrontal cortex and cerebellum, ↑ NE in hippocampus | ↓ Motor coordination and memory | [86] |
| Metallic/≈10 nm | Titanium dioxide | - | Oral (10–50 mg/kg)/Male mouse | Safety studies | ↓ TH in substantia nigra | Parkinson’s-like symptoms | [87] |
| Metallic/≈10 nm | Iron oxide | - | Intravenous (50 mg/kg)/Male rat | Safety studies | ↓ DA in striatum, ↑ ROS, ↑ α-synuclein | Neurotoxicity | [88] |
| Metallic/≈30–60 nm | Manganese dioxide | - | Intraperitoneal (50–100 µg/kg)/Male rat | Safety studies | ↓ DA & NE in hippocampus, ROS production, lipid peroxidation | Depression-like behaviors | [89] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cobos-Puc, L.E.; Rodríguez-Salazar, M.d.C.; Silva-Belmares, S.Y.; Aguayo-Morales, H. Nanoparticle-Based Strategies to Enhance Catecholaminergic Drug Delivery for Neuropsychiatric Disorders: Advances, Challenges, and Therapeutic Opportunities. Future Pharmacol. 2025, 5, 51. https://doi.org/10.3390/futurepharmacol5030051
Cobos-Puc LE, Rodríguez-Salazar MdC, Silva-Belmares SY, Aguayo-Morales H. Nanoparticle-Based Strategies to Enhance Catecholaminergic Drug Delivery for Neuropsychiatric Disorders: Advances, Challenges, and Therapeutic Opportunities. Future Pharmacology. 2025; 5(3):51. https://doi.org/10.3390/futurepharmacol5030051
Chicago/Turabian StyleCobos-Puc, Luis E., María del C. Rodríguez-Salazar, Sonia Y. Silva-Belmares, and Hilda Aguayo-Morales. 2025. "Nanoparticle-Based Strategies to Enhance Catecholaminergic Drug Delivery for Neuropsychiatric Disorders: Advances, Challenges, and Therapeutic Opportunities" Future Pharmacology 5, no. 3: 51. https://doi.org/10.3390/futurepharmacol5030051
APA StyleCobos-Puc, L. E., Rodríguez-Salazar, M. d. C., Silva-Belmares, S. Y., & Aguayo-Morales, H. (2025). Nanoparticle-Based Strategies to Enhance Catecholaminergic Drug Delivery for Neuropsychiatric Disorders: Advances, Challenges, and Therapeutic Opportunities. Future Pharmacology, 5(3), 51. https://doi.org/10.3390/futurepharmacol5030051