AI-Driven Image Analysis for Precision Screening Transposon-Mediated Transgenesis of NFκB eGFP Reporter System in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
2.1. AI and Imaging Systems
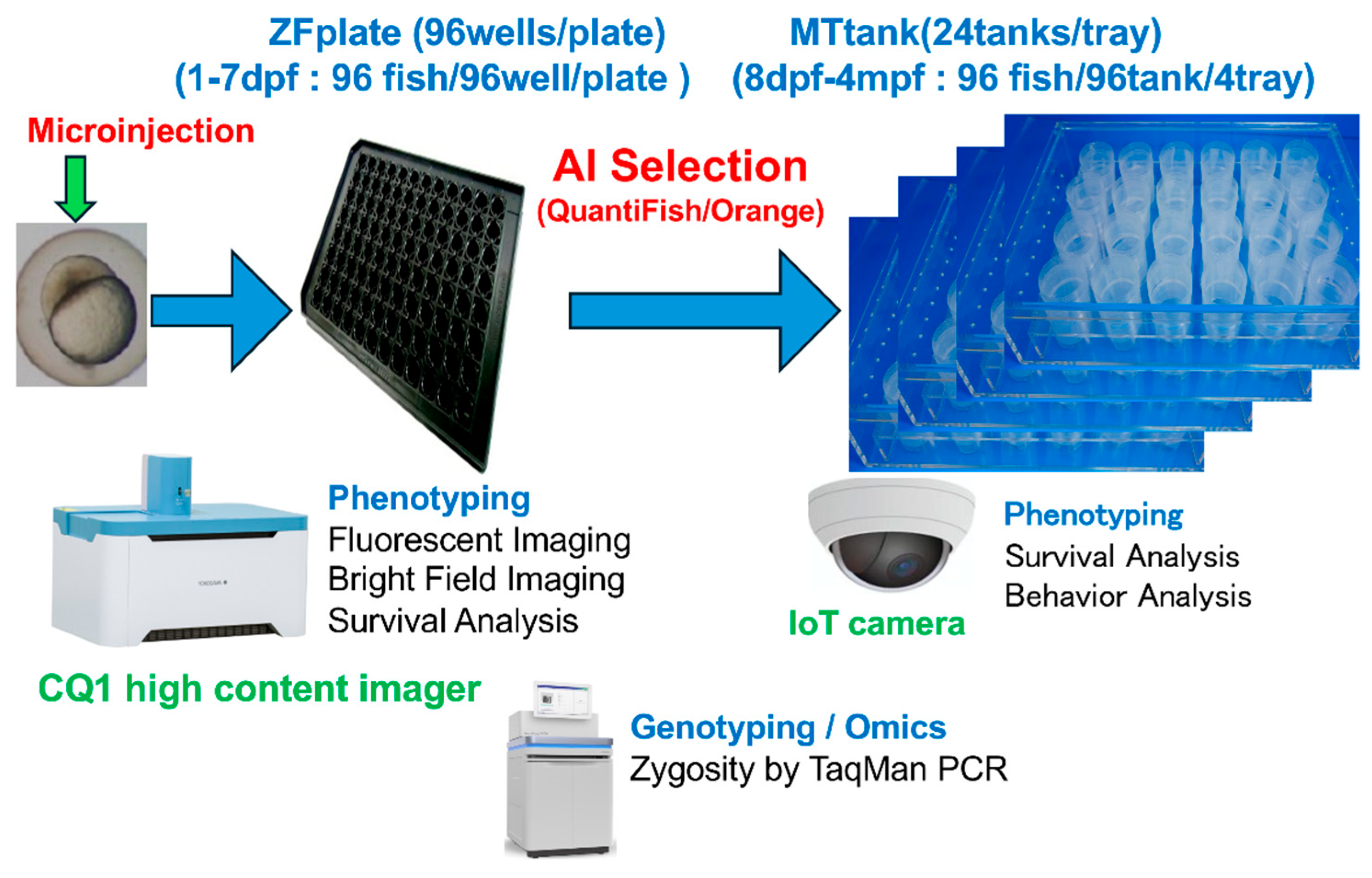
2.2. Key Components of the Zebrafish Precision System (Figure 1 and Figure 2)
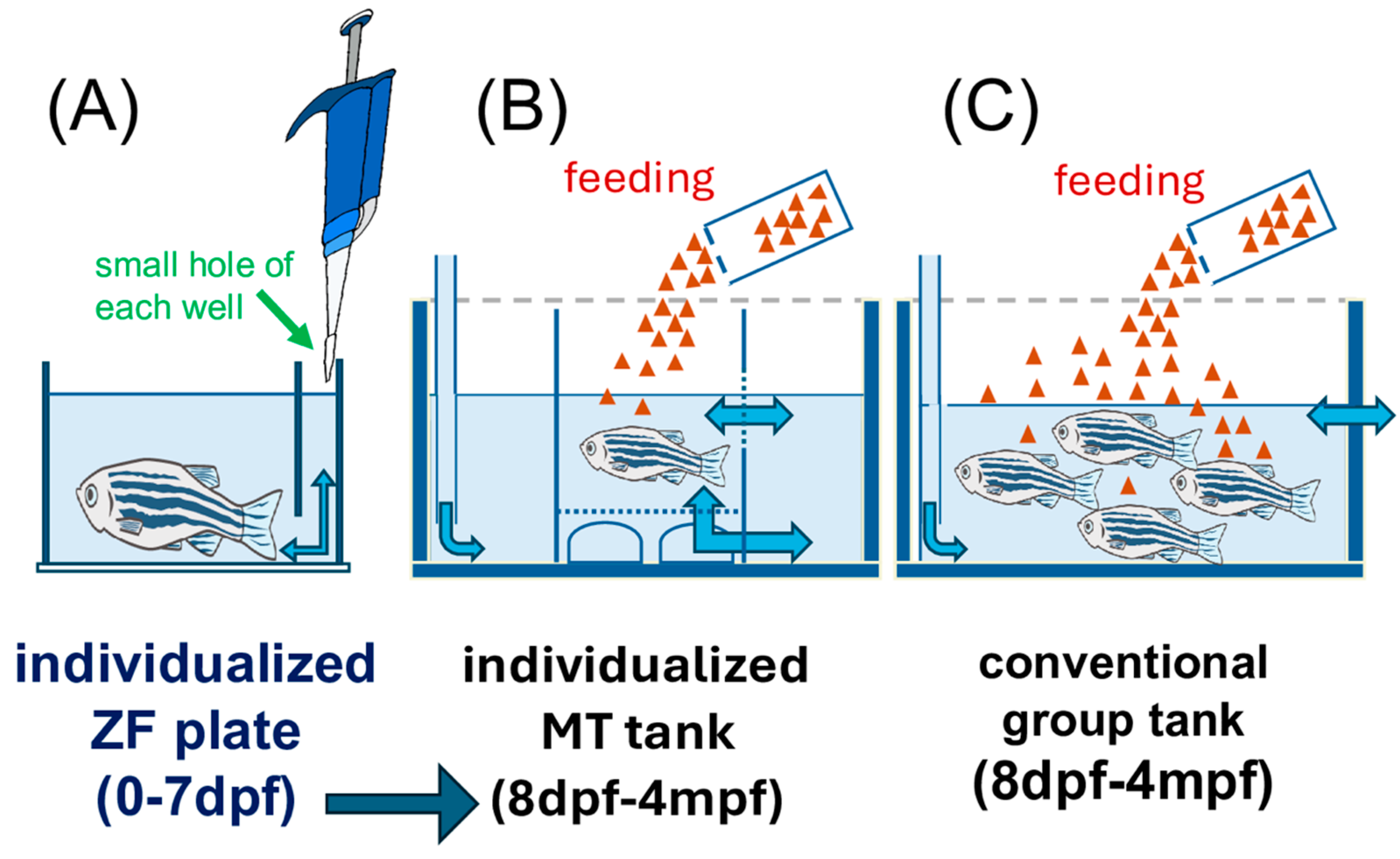
- ZF Plates (0–7 pdf) enable individualized embryo and larva handling and early fluorescence imaging screening, and using a fine-tipped pipette or aspiration tool, carefully access the small hole at the bottom of each well in the ZF plate. This allows for the removal of excess medium or debris and facilitates subsequent handling or imaging of individual zebrafish embryos in each well. Ensure that the pipette tip is inserted gently through the hole to avoid damaging the embryos. The small hole of each well in the ZF plate is used with an 8-channel pipette to avoid injury or stress in liquid handling, and the solution is removed (Figure 2). ZF plates were purchased from Funakoshi Co., Ltd., Tokyo, Japan [15].
- Individualized MT tanks (8 dpf–4 mpf): There were 24-tanks in the tray to provide precise environmental control for long-term phenotyping and breeding. This is compatible with the ZF plate, which is a 96-well plate for zebrafish fingerlings, and four MT trays are equivalent to one ZF plate (Figure 1 and Figure 2). Acclimate the rearing water in the MT tank by adjusting its temperature and chemical parameters to match those of the main system. Complete this acclimation at least one day prior to introducing zebrafish from the ZF plate. After acclimatization, suck one fish at a time with a p200 pipette with a wide-mouth tip from a ZF plate, and transfer it to a tank in the MT tank filled with rearing water from the receiving system. The zebrafish are fed paramecia (raw feed) from the day of entry. Feed only paramecia (raw feed) for the first month after the start of feeding twice daily using a syringe or pipette to dispense 2 mL per tank at a time. Feed powder food from 2mpf using a sprinkle container for feeding adult fish. The MT tanks were custom-made at the Systems Pharmacology Laboratory, Mie University Graduate School of Medicine, Mie, Japan. The individualized MT tanks were constructed using transparent polycarbonate or acrylic, with internal dimensions approximately 5.5 cm (diameter) × 8.0 cm (height), resulting in a water capacity of 100 mL per tank.
- Quantifish (https://biii.eu/quantifish (accessed on 7 May 2025).) automated the imaging and quantification of eGFP expression [10].
- Orange (https://orangedatamining.com/ (accessed on 12 May 2025).) analyzes phenotypic data, predicts transgene stability, and optimizes experimental parameters [11].
- One-cell stage zebrafish embryos were microinjected with a mixture containing the NFκB: eGFP reporter construct and in vitro-transcribed Tol2 transposase mRNA. Following injection, embryos were individually transferred into the wells of a 96-well ZF plate and incubated at 28.5 °C. At 5 days post-fertilization, each larva was imaged using an automated confocal high-content imager (CQ1). Image analysis was performed using the Quantifish software, which provided automated detection, segmentation, and quantification of eGFP fluorescence within defined anatomical regions of interest. Quantitative data outputs were further analyzed in Orange to assess transgene expression patterns and efficiencies across experimental groups.
- Zebrafish strains. AB (wild-type fish) and albino (slc45a2b4) for retinal imaging were purchased from the Zebrafish International Resource Center (ZIRC), 5274 University of Oregon Eugene, OR 97403-5274, USA [18]. The zebrafish were incubated at 28 °C under a 14 h (7:00–21:00) light:10 h (21:00–7:00) dark cycle in environmental quality water, as previously described [19].
2.3. F0 Generation Protocol (Figure 1 and Figure 2)
2.3.1. Preparation of Injection Mixture for Tg (6xNFκB:eGFP)
2.3.2. Microinjection
2.3.3. Screening in ZF Plates (Figure 1 and Figure 2)
2.3.4. Awakening from Anesthesia
2.3.5. Raising F0 Founders
2.4. F1 Generation Protocol (Figure 1 and Figure 2)
2.4.1. Breeding
2.4.2. Orange AI
- Importing CQ1-acquired image files into Quantifish.
- Automated identification and segmentation of each larva and specified regions of interest.
- Quantitative extraction of eGFP fluorescence intensity for each segmented region.
- Exporting the resulting quantitative data in .csv format.
- Data normalization and preprocessing.
- Application of machine learning algorithms (such as Random Forests and clustering) to classify expression patterns and identify trends.
- Visualization of results and prediction of optimal transgenic lines based on multivariate fluorescence data.
2.4.3. Validation
2.5. F2 Generation Protocol
2.5.1. Breeding in Individualized MT Tanks (Figure 2)
2.5.2. Phenotypic Validation Test
2.5.3. Statistical Analysis
3. Results
3.1. Accuracy, Speed, and Stability in This Precision Zebrafish System
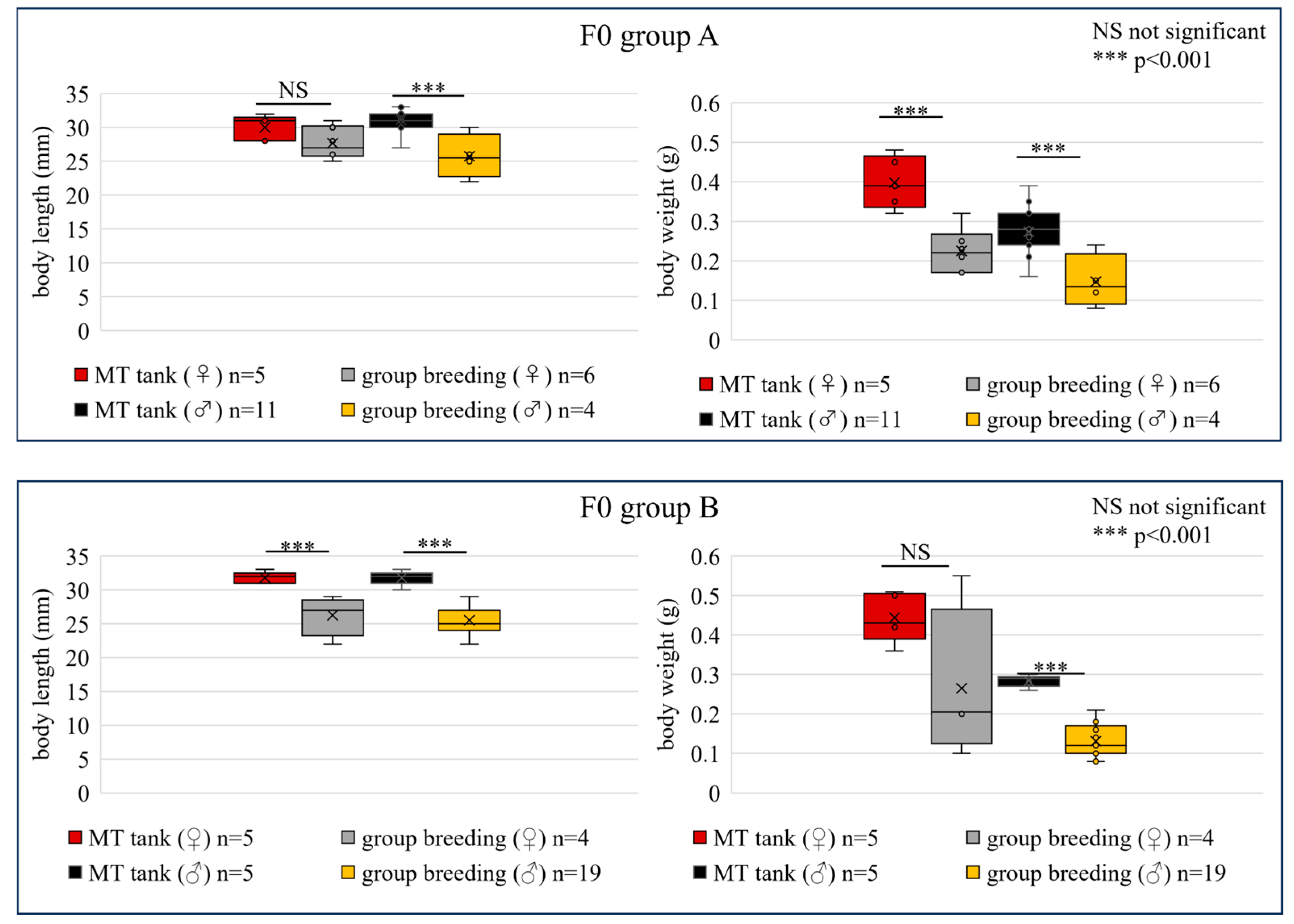
3.2. Longitudinal Stability in Individualized MT Tanks
3.3. AI-Driven CQ1 Image Analysis in the F0 and F1 Generations Accelerates Germline Transmission Efficiency
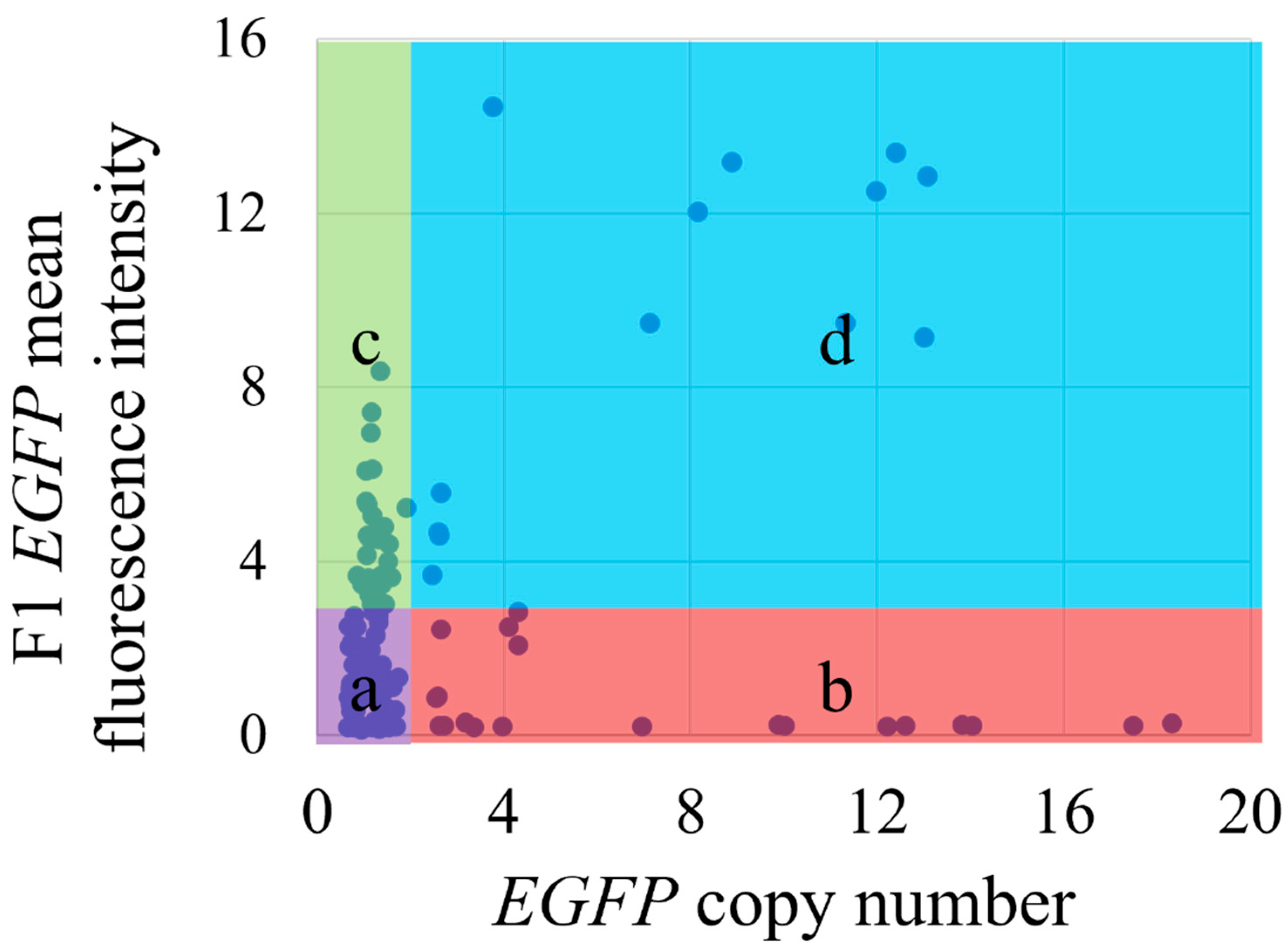
3.4. Cluster Analysis in F1 Generation of eGFP Fluorescence Intensity and Copy Number
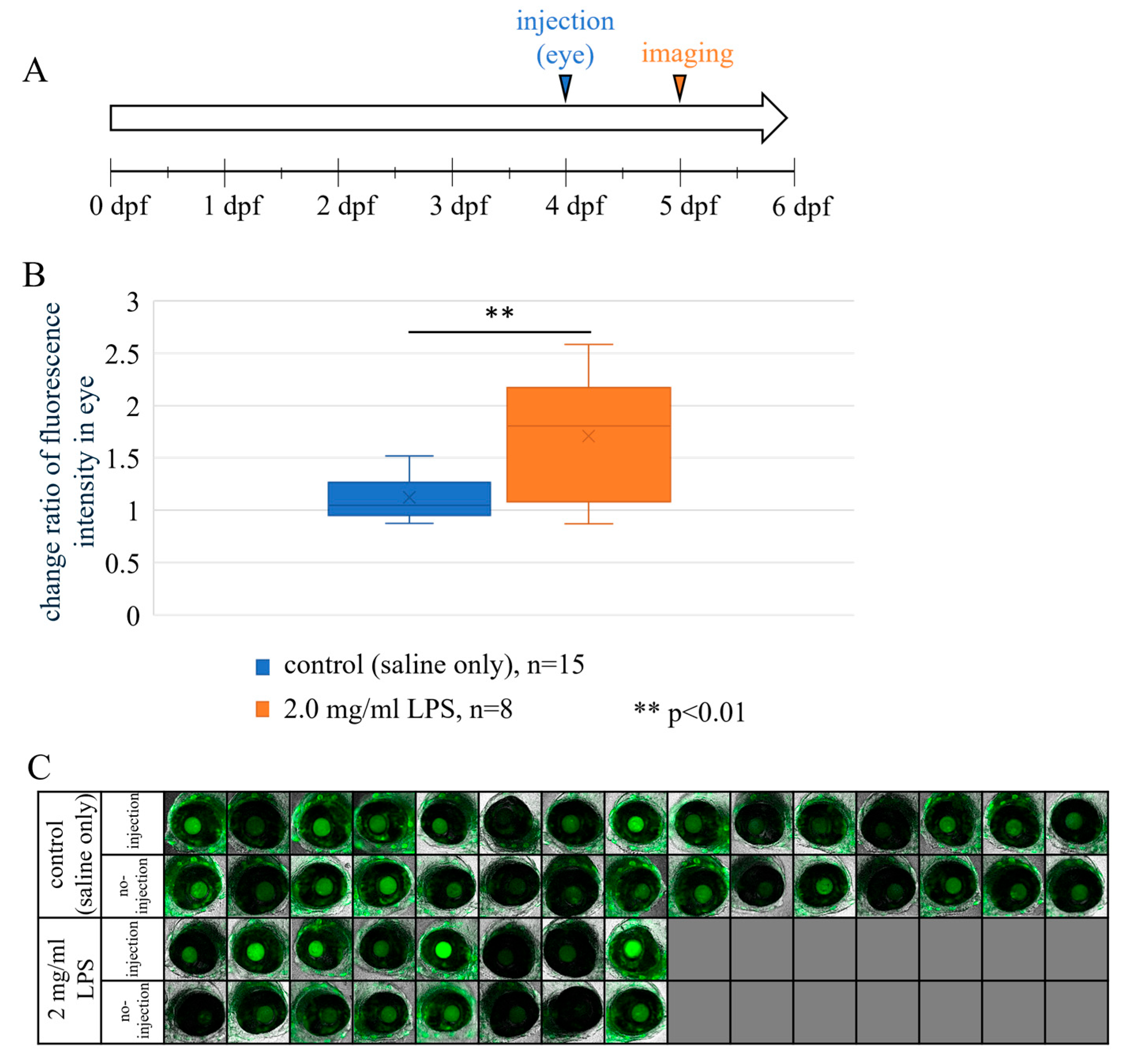
3.5. LPS-Dependent Zebrafish Retinal NFκB eGFP Reporter
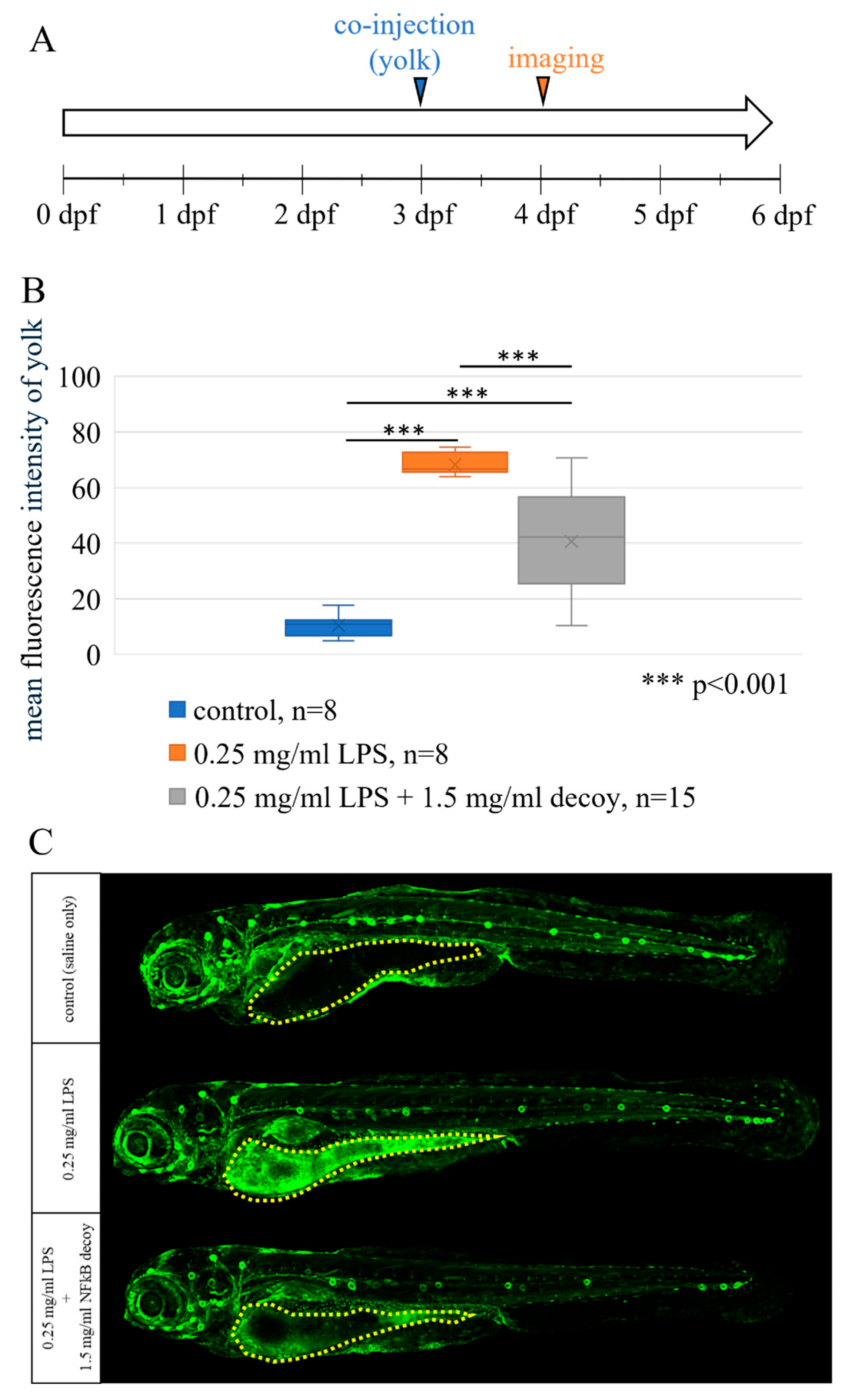
3.6. NFκB Decoy Pharmacology of NFκB eGFP Reporter Zebrafish Model System
4. Discussion
4.1. Advantages over Traditional Methods of This AI-Driven Analysis Protocol
4.1.1. Accuracy and Speed
4.1.2. Throughput
4.1.3. Tissue-Specific Analysis
4.2. Multimodalities in the Therapeutic Development of NFκB eGFP Reporter Zebrafish Model System
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zon, L.I.; Peterson, R.T. In vivo drug discovery in the zebrafish. Nat. Rev. Drug. Discov. 2005, 4, 35–44. [Google Scholar] [CrossRef]
- Rajpurohit, S.K.; Ouellette, L.; Sura, S.; Appiah, C.; O’Keefe, A.; McCarthy, K.; Kandepu, U.; Ye Mon, M.; Kimmerling, K.; Arora, V.; et al. Development of a Transparent Transgenic Zebrafish Cellular Phenotype Tg(6xNF-kB:EGFP); Casper(roy-/-, nacre-/-) to Study NF-kB Activity. Biomedicines 2023, 11, 1985. [Google Scholar] [CrossRef]
- Kawakami, K.; Shima, A.; Kawakami, N. Identification of a functional transposase of the Tol2 element, an Ac-like element from the Japanese medaka fish, and its transposition in the zebrafish germ lineage. Proc. Natl. Acad. Sci. USA 2000, 97, 11403–11408. [Google Scholar] [CrossRef]
- Kuri, P.; Ellwanger, K.; Kufer, T.A.; Leptin, M.; Bajoghli, B. A high-sensitivity bi-directional reporter to monitor NF-κB activity in cell culture and zebrafish in real time. J. Cell Sci. 2017, 130, 648–657. [Google Scholar] [CrossRef]
- Kanther, M.; Sun, X.; Mühlbauer, M.; Mackey, L.C.; Flynn, E.J.; Bagnat, M.; Jobin, C.; Rawls, J.F. Microbial colonization induces dynamic temporal and spatial patterns of NF-κB activation in the zebrafish digestive tract. Gastroenterology 2011, 141, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat Rev Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Leptin, M. Systemic response to ultraviolet radiation involves induction of leukocytic IL-1β and inflammation in zebrafish. J Immunol. 2014, 193, 1408–1415. [Google Scholar] [CrossRef]
- Icha, J.; Weber, M.; Waters, J.C.; Norden, C. Phototoxicity in live fluorescence microscopy, and how to avoid it. Bioessays 2017, 39, 1700003. [Google Scholar] [CrossRef]
- Stirling, D.R.; Suleyman, O.; Gil, E.; Elks, P.M.; Torraca, V.; Noursadeghi, M.; Tomlinson, G.S. Analysis tools to quantify dissemination of pathology in zebrafish larvae. Sci Rep. 2020, 10, 3149. [Google Scholar] [CrossRef]
- Demšar, J.; Curk, T.; Erjavec, A.; Gorup, Č.; Hočevar, T.; Milutinovič, M.; Možina, M.; Polajnar, M.; Toplak, M.; Starič, A.; et al. Orange: Data mining toolbox in Python. J. Mach. Learn. Res. 2013, 14, 2349–2353. [Google Scholar]
- Dash, S.N.; Patnaik, L. Flight for fish in drug discovery: A review of zebrafish-based screening of molecules. Biol Lett. 2023, 19, 20220541. [Google Scholar] [CrossRef]
- Suster, M.L.; Kikuta, H.; Urasaki, A.; Asakawa, K.; Kawakami, K. Transgenesis in zebrafish with the Tol2 transposon system. Methods Mol. Biol. 2009, 561, 41–63. [Google Scholar] [PubMed]
- Mosimann, C.; Puller, A.C.; Lawson, K.L.; Tschopp, P.; Amsterdam, A.; Zon, L.I. Site-directed zebrafish transgenesis into single landing sites with the phiC31 integrase system. Dev. Dyn. 2013, 242, 949–963. [Google Scholar] [CrossRef] [PubMed]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little fish, big data: Zebrafish as a model for cardiovascular and metabolic disease. Physiol Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi Co. Ltd ZF Plate 96-Well Microplate Specifications. Product Code: ZF-96-BLK-G. Tokyo, Japan. 2022. Available online: https://www.funakoshi.co.jp/contents/63672 (accessed on 19 August 2025).
- Yokogawa Electric Corporation CQ1 High-Content Analysis System Technical Specifications. IMSOL Technical Documentation 2022; v3.1.2. Available online: https://www.yokogawa.co.jp/solutions/products-and-services/life-science/high-content-analysis/cellvoyager-cq1/#%E8%A9%B3%E7%B4%B0 (accessed on 19 August 2025).
- Nomoto, T.; Mori, A.; Yamada, K.; Terami, F.; Shimizu, A.; Tanaka, T. In vivo assessment of individual and total proteinuria in zebrafish larvae using the solvatochromic compound ZMB741. Chem. Biomed. Imaging 2024, 2, 755–764. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio), 5th ed.; University of Oregon Press: Eugene, Oregon, 2007. [Google Scholar]
- Liu, W.; Saint, D.A. Validation of a quantitative method for real-time PCR kinetics. Anal Biochem. 2002, 294, 347–353. [Google Scholar] [CrossRef]
- TOYOBO CO., LTD. THUNDERBIRD® SYBR™ qPCR Mix (QPS-201)—Technical Manual; TOYOBO CO., LTD.: Osaka, Japan, 2014. [Google Scholar]
- Bludau, O.; Weber, A.; Bosak, V.; Kuscha, V.; Dietrich, K.; Hans, S.; Brand, M. Inflammation is a critical factor for successful regeneration of the adult zebrafish retina in response to diffuse light lesion. Front. Cell Dev. Biol. 2024, 12, 1332347. [Google Scholar] [CrossRef]
- Johnston, H.J.; Gillies, S.L.J.; Verdon, R.; Stone, V.; Henry, T.; Tran, L.; Tucker, C.; Rossi, A.G.; Tyler, C.R. Application of transgenic zebrafish for investigating inflammatory responses to nanomaterials: Recommendations for new users. F1000Research 2025, 12, 51. [Google Scholar] [CrossRef]
- Stirling, D.R.; Swain-Bowden, M.J.; Lucas, A.M.; Carpenter, A.E.; Cimini, B.A. CellProfiler 4: Improvements in speed, utility and usability. BMC Bioinf. 2020, 21, 433. [Google Scholar] [CrossRef]
- Nemoto, H.; Sakai, D.; Watson, D.; Masuda, K. Nuclear factor-κB decoy oligodeoxynucleotide attenuates cartilage resorption in vitro. Bioengineering 2024, 11, 46. [Google Scholar] [CrossRef]
- Walker, S.L.; Ariga, J.; Mathias, J.R.; Coothankandaswamy, V.; Xie, X.; Distel, M.; Köster, R.W.; Parsons, M.J.; Bhalla, K.N.; Saxena, M.T.; et al. Automated reporter quantification in vivo: High-throughput screening method for reporter-based assays in Zebrafish. PLoS ONE 2012, 7, e29916. [Google Scholar] [CrossRef]
- Wang, G.; Rajpurohit, S.K.; Delaspre, F.; Walker, S.L.; White, D.T.; Ceasrine, A.; Kuruvilla, R.; Li, R.-J.; Shim, J.S.; Liu, J.O.; et al. First quantitative high-throughput screen in zebrafish identifies novel pathways for increasing pancreatic β-cell mass. eLife 2015, 4, e08261. [Google Scholar] [CrossRef]
- Wong, R.L.; Choi, M.Y.; Wang, H.-Y.; Kipps, T.J. Mutation in Bruton Tyrosine Kinase (BTK) A428D confers resistance To BTK-degrader therapy in chronic lymphocytic leukemia. Leukemia 2024, 38, 1818–1821. [Google Scholar] [CrossRef]
- Mikami, I.; Takahashi, Y.; Koiwa, J.; Okamura, M.; Tanaka, T. Zebrafish yolk sac microinjection of thalidomide for assessment of developmental toxicology. Congenit Anom 2020, 60, 71–72. [Google Scholar] [CrossRef]
- Zanandrea, R.; Bonan, C.D.; Campos, M.M. Zebrafish as a model for inflammation and drug discovery. Drug Discov. Today 2020, 25, 2201–2211. [Google Scholar] [CrossRef]
| Primer Name | Sequence (5′→3′) | Amplicon Length |
|---|---|---|
| real-time_PCR_control-F | TCCAGTGTAAACAAGTCTGGAAAACT | 76 bp |
| real-time_PCR_control-R | GAGTAGCCATGCGGTCCAA | |
| real-time_PCR_eGFP-F | AGAACGGCATCAAGGTGAAC | 135 bp |
| real-time_PCR_eGFP-R | TGCTCAGGTAGTGGTTGTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iwata, Y.; Mori, A.; Shinogi, K.; Nishino, K.; Matsuoka, S.; Kushida, Y.; Satoda, Y.; Shimizu, A.; Terami, F.; Nonomura, T.; et al. AI-Driven Image Analysis for Precision Screening Transposon-Mediated Transgenesis of NFκB eGFP Reporter System in Zebrafish. Future Pharmacol. 2025, 5, 50. https://doi.org/10.3390/futurepharmacol5030050
Iwata Y, Mori A, Shinogi K, Nishino K, Matsuoka S, Kushida Y, Satoda Y, Shimizu A, Terami F, Nonomura T, et al. AI-Driven Image Analysis for Precision Screening Transposon-Mediated Transgenesis of NFκB eGFP Reporter System in Zebrafish. Future Pharmacology. 2025; 5(3):50. https://doi.org/10.3390/futurepharmacol5030050
Chicago/Turabian StyleIwata, Yui, Aoi Mori, Kana Shinogi, Kanako Nishino, Saori Matsuoka, Yuki Kushida, Yuki Satoda, Akiyoshi Shimizu, Fumihiro Terami, Toru Nonomura, and et al. 2025. "AI-Driven Image Analysis for Precision Screening Transposon-Mediated Transgenesis of NFκB eGFP Reporter System in Zebrafish" Future Pharmacology 5, no. 3: 50. https://doi.org/10.3390/futurepharmacol5030050
APA StyleIwata, Y., Mori, A., Shinogi, K., Nishino, K., Matsuoka, S., Kushida, Y., Satoda, Y., Shimizu, A., Terami, F., Nonomura, T., Kitajima, S., & Tanaka, T. (2025). AI-Driven Image Analysis for Precision Screening Transposon-Mediated Transgenesis of NFκB eGFP Reporter System in Zebrafish. Future Pharmacology, 5(3), 50. https://doi.org/10.3390/futurepharmacol5030050









