In Vitro Antimicrobial and Antibiofilm Efficacy of an Aminochalcone-Loaded Hydrogel Against Candida spp.
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of AM-35
2.2. Preparation of Hydrogel (H+AM35)
2.3. Preparation of Specimens
2.4. Microorganism Strains and Culture Conditions
2.5. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Fungicidal Concentration (MFC)
2.6. Method for Time-Kill Assay
2.7. Effects of Hydrogel Containing AM-35 in Mono- and Mixed Biofilms of C. tropicalis and C. albicans in Acrylic Resin Specimens
2.8. Methodology for Evaluating Acute Systemic Toxicity in Galleria mellonella
2.9. Statistical Analysis
3. Results
3.1. Antifungal Activity of AM-35 Against Planktonic Cells
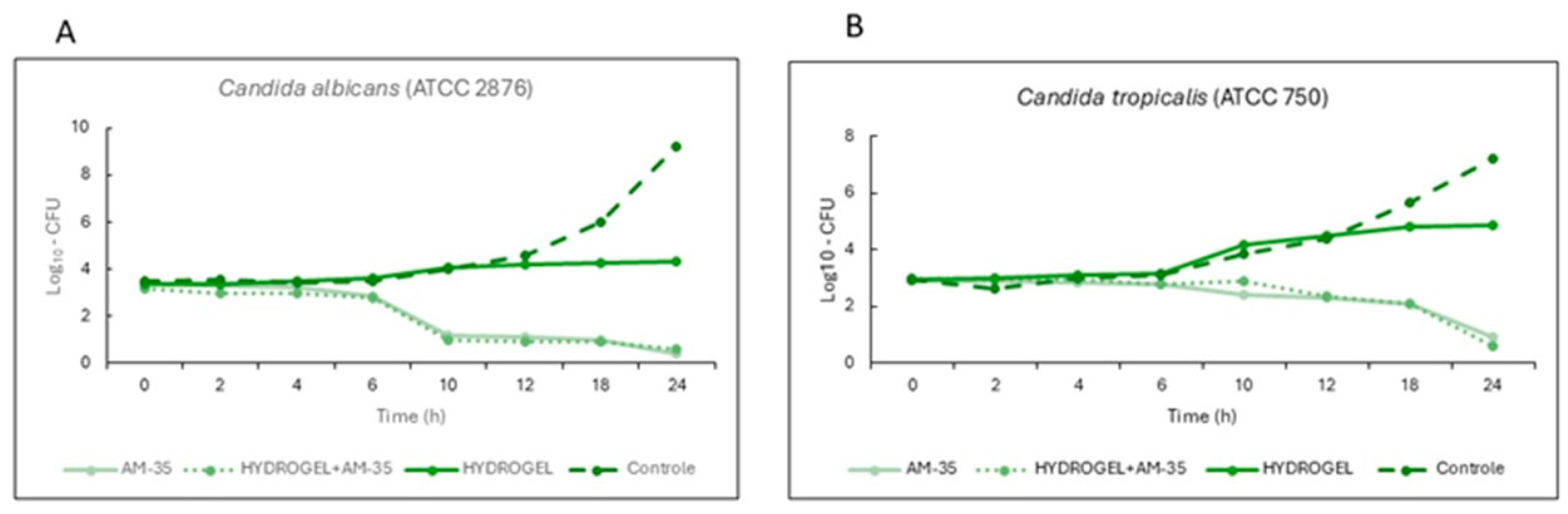
3.2. Time-Kill Assay Results
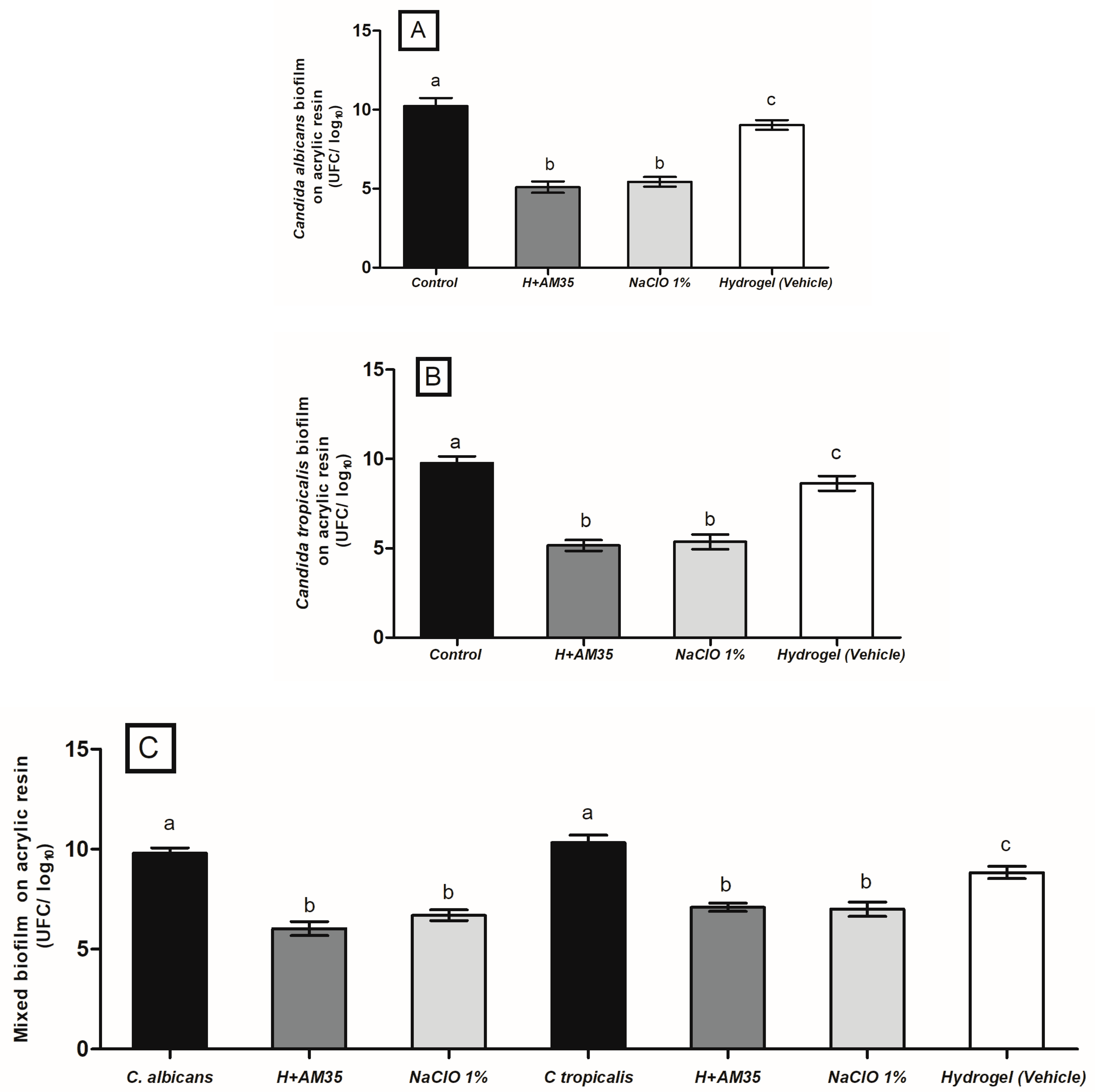
3.3. Hydrogel Containing AM-35 in Single and Mixed Biofilms
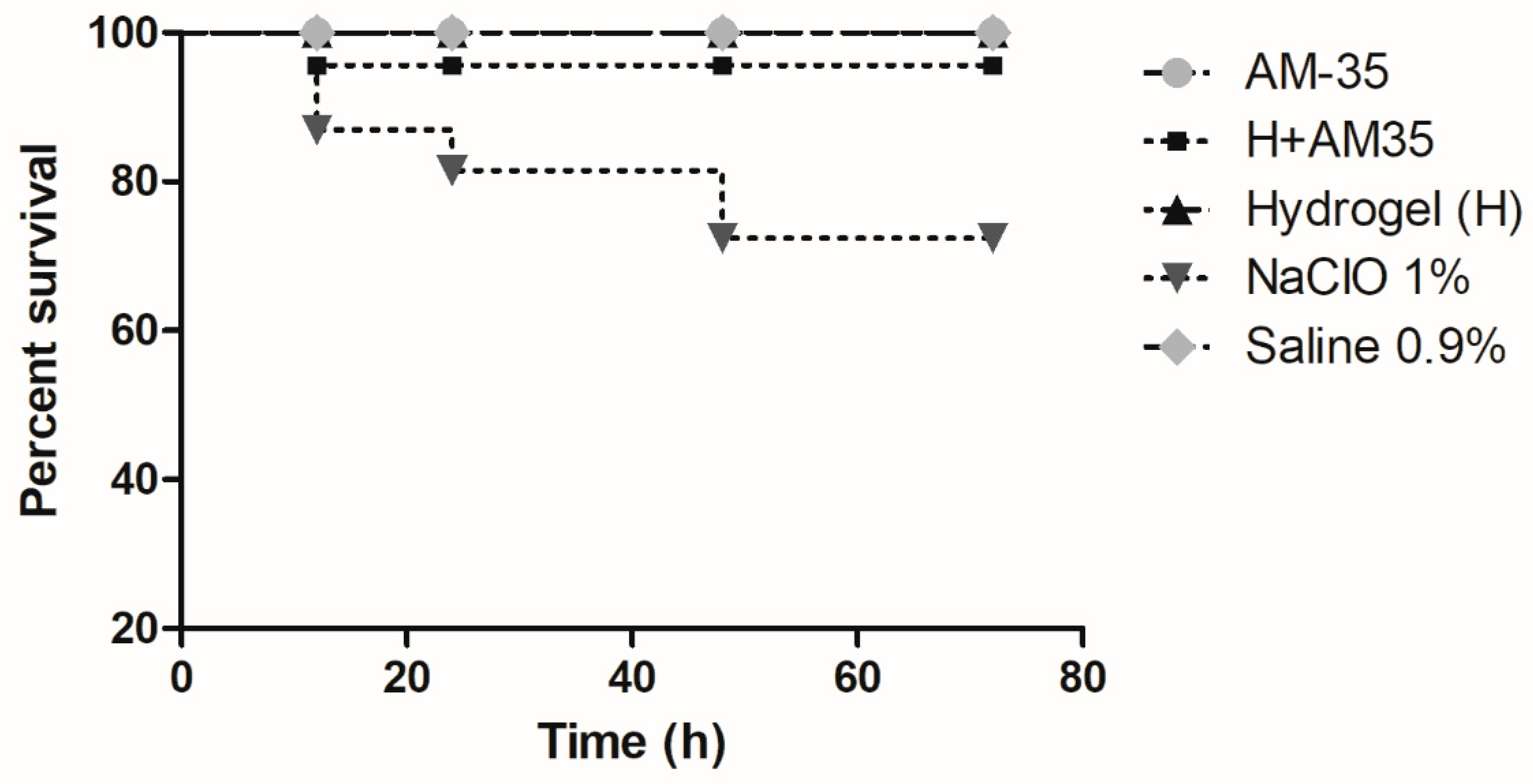
3.4. Results of Acute Systemic Toxicity in Galleria mellonella
4. Discussion
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Aguayo, S.; Marshall, H.; Pratten, J.; Bradshaw, D.; Brown, J.S.; Porter, S.R.; Spratt, D.; Bozec, L. Early Adhesion of Candida albicans onto Dental Acrylic Surfaces. J. Dent. Res. 2017, 96, 917–923. [Google Scholar] [CrossRef] [PubMed]
- McReynolds, D.E.; Moorthy, A.; Moneley, J.O.; Jabra-Rizk, M.A.; Sultan, A.S. Denture stomatitis—An interdisciplinary clinical review. J. Prosthodont. 2023, 32, 560–570. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, L.E.; Smith, K.; Williams, C.; Nile, C.J.; Lappin, D.F.; Bradshaw, D.; Lambert, M.; Robertson, D.P.; Bagg, J.; Hannah, V.; et al. Dentures are a reservoir for respiratory pathogens. J. Prosthodont. 2016, 25, 99–104. [Google Scholar] [CrossRef]
- Jackson, S.; Coulthwaite, L.; Loewy, Z.; Scallan, A.; Verran, J. Biofilm development by blastospores and hyphae of Candida albicans on abraded denture acrylic resin surfaces. J. Prosthet. Dent. 2014, 112, 988–993. [Google Scholar] [CrossRef] [PubMed]
- Sterzenbach, T.; Helbig, R.; Hannig, C.; Hannig, M. Bioadhesion in the oral cavity and approaches for biofilm management by surface modifications. Clin. Oral. Investig. 2020, 24, 4237–4260. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Liu, Y.; Liu, Y.; Lv, R.; Sun, W.; Ding, W.; Cai, Y.; Li, W.; Liu, X.; Qu, W. Candida albicans biofilms: Antifungal resistance, immune evasion, and emerging therapeutic strategies. Int. J. Antimicrob. Agents 2022, 60, 106673. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, M.; Teixeira, M.C. Candida Biofilms: Threats, Challenges, and Promising Strategies. Front. Med. 2018, 5, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sasani, E.; Khodavaisy, S.; Rezaie, S.; Salehi, M.; Yadegari, M.H. The relationship between biofilm formation and mortality in patients with Candida tropicalis candidemia. Microb. Pathog. 2021, 155, 104889. [Google Scholar] [CrossRef] [PubMed]
- Abuhajar, E.; Ali, K.; Zulfiqar, G.; Al Ansari, K.; Raja, H.Z.; Bishti, S.; Anweigi, L. Management of Chronic Atrophic Candidiasis (Denture Stomatitis)—A Narrative Review. Int. J. Environ. Res. Public Health 2023, 20, 3029. [Google Scholar] [CrossRef]
- Felton, D.; Cooper, L.; Duqum, I.; Minsley, G.; Guckes, A.; Haug, S.; Meredith, P.; Solie, C.; Avery, D.; Chandler, N.D.; et al. Evidence-based guidelines for the care and maintenance of complete dentures: A publication of the American College of Prosthodontists. J. Am. Dent. Assoc. 2011, 142 (Suppl. S1), S1–S12. [Google Scholar] [CrossRef] [PubMed]
- da Costa, R.M.B.; Poluha, R.L.; De la Torre Canales, G.; Junior, J.F.S.; Conti, P.C.R.; Neppelenbroek, K.H.; Porto, V.C. The effectiveness of microwave disinfection in treating Candida-associated denture stomatitis: A systematic review and metaanalysis. Clin. Oral Investig. 2020, 24, 3821–3832. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.G.; Pavarina, A.C.; Dovigo, L.N.; Palomari Spolidorio, D.M.; Giampaolo, E.T.; Vergani, C.E. Denture disinfection by microwave irradiation: A randomized clinical study. J. Dent. 2009, 37, 666–672. [Google Scholar] [CrossRef] [PubMed]
- Santos Sousa, T.M.; Rodrigues de Farias, O.; Dantas Batista, A.U.; Souto de Medeiros, E.; Santiago, B.M.; Cavalcanti, Y.W. Effectiveness of denture microwave disinfection for treatment of denture stomatitis: A systematic review and meta-analysis. Int. J. Dent. Hyg. 2021, 19, 62–77. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, L.A.; Daily, A.M.; Horava, S.D.; Peppas, N.A. Therapeutic applications of hydrogels in oral drug delivery. Expert Opin. Drug Deliv. 2014, 11, 901–915. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Samiraninezhad, N.; Asadi, K.; Rezazadeh, H.; Gholami, A. Using chitosan, hyaluronic acid, alginate, and gelatin-based smart biological hydrogels for drug delivery in oral mucosal lesions: A review. Int. J. Biol. Macromol. 2023, 252, 126573. [Google Scholar] [CrossRef] [PubMed]
- Chai, N.; Sun, A.; Zhu, X.; Li, Y.; Wang, R.; Zhang, Y.; Mao, Z. Antifungal evaluation of quinoline-chalcone derivatives combined with FLC against drug-resistant Candida albicans. Bioorg. Med. Chem. Lett. 2023, 86, 129242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.H.; Dong, H.H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.Y.; Jin, Y.S. The synthesis and synergistic antifungal effects of chalcones against drug resistant Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef] [PubMed]
- Graciani, J.; Rosalen, P.L.; de Oliveira Chaves Dos Santos, E.; Rocha, K.A.P.; Balen, B.R.T.; Garcia, M.A.R.; Lazarini, J.G.; da Silva, D.R.; Carvalho, S.G.; Regasini, L.O.; et al. Evaluation of efficacy of new chalcone-based endodontic irrigant against dual biofilm Enterococcus faecalis and Candida albicans: A study in vitro. Odontology 2023, 111, 573–579. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.A.R.; Theodoro, R.S.; Sardi, J.C.O.; Santos, M.B.; Ayusso, G.M.; Pavan, F.R.; Costa, A.R.; Santa Cruz, L.M.; Rosalen, P.L.; Regasini, L.O. Design, synthesis and antibacterial activity of chalcones against MSSA and MRSA planktonic cells and biofilms. Bioorg. Chem. 2021, 116, 105279. [Google Scholar] [CrossRef] [PubMed]
- Gratieri, T.; Gelfuso, G.M.; Rocha, E.M.; Sarmento, V.H.; de Freitas, O.; Lopez, R.F. A poloxamer/chitosan in situ forming gel with prolonged retention time for ocular delivery. Eur. J. Pharm. Biopharm. 2010, 75, 186–193. [Google Scholar] [CrossRef] [PubMed]
- CLSI. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts: Approved Standard, 3rd ed.; CLSI document M27-A3; Clinical and Laboratory Standards Institute: Wayne, MI, USA, 2008; ISBN 1-56238-666-2. [Google Scholar]
- Kiraz, N.; Dag, I.; Yamac, M.; Kiremitci, A.; Kasifoglu, N.; Akgun, Y. Antifungal activity of caspofungin in combination with amphotericin B against Candida glabrata: Comparison of disk diffusion, Etest, and time-kill methods. Antimicrob. Agents Chemother. 2009, 53, 788–790. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bombarda, G.F.; Rosalen, P.L.; Paganini, E.R.; Garcia, M.A.; Silva, D.R.; Lazarini, J.G.; Freires, I.A.; Regasini, L.O.; Sardi, J.C. Bioactive molecule optimized for biofilm reduction related to childhood caries. Future Microbiol. 2019, 14, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Badaró, M.M.; Salles, M.M.; Leite, V.M.F.; Arruda, C.N.F.; Oliveira, V.C.; Nascimento, C.D.; Souza, R.F.; Paranhos, H.F.O.; Silva-Lovato, C.H. Clinical trial for evaluation of Ricinus communis and sodium hypochlorite as denture cleanser. J. Appl. Oral Sci. 2017, 25, 324–334. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Okolo, E.N.; Ugwu, D.I.; Ezema, B.E.; Ndefo, J.C.; Eze, F.U.; Ezema, C.G.; Ezugwu, J.A.; Ujam, O.T. New chalcone derivatives as potential antimicrobial and antioxidant agent. Sci. Rep. 2021, 11, 21781. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Megaw, J.; Thompson, T.P.; Lafferty, R.A.; Gilmore, B.F. Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chemosphere 2015, 139, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral. Sci. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Senel, S.; Ikinci, G.; Kaş, S.; Yousefi-Rad, A.; Sargon, M.F.; Hincal, A.A. Chitosan films and hydrogels of chlorhexidine gluconate for oral mucosal delivery. Int. J. Pharm. 2000, 193, 197–203. [Google Scholar] [CrossRef] [PubMed]
- Harish, N.M.; Prabhu, P.; Charyulu, R.N.; Gulzar, M.A.; Subrahmanyam, E.V. Formulation and Evaluation of in situ Gels Containing Clotrimazole for Oral Candidiasis. Indian J. Pharm. Sci. 2009, 71, 421–427. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lo, W.H.; Deng, F.S.; Chang, C.J.; Lin, C.H. Synergistic Antifungal Activity of Chitosan with Fluconazole against Candida albicans, Candida tropicalis, and Fluconazole-Resistant Strains. Molecules 2020, 25, 5114. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nikawa, H.; Hamada, T.; Yamashiro, H.; Kumagai, H. A review of in vitro and in vivo methods to evaluate the efficacy of denture cleansers. Int. J. Prosthodont. 1999, 12, 153–159. [Google Scholar] [PubMed]
- Bila, N.M.; Costa-Orlandi, C.B.; Vaso, C.O.; Bonatti, J.L.C.; de Assis, L.R.; Regasini, L.O.; Fontana, C.R.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. 2-Hydroxychalcone as a potent compound and photosensitizer against dermatophyte biofilms. Front. Cell Infect. Microbiol. 2021, 13, 679470. [Google Scholar] [CrossRef] [PubMed]
- Emeri, F.T.A.S.; Rosalen, P.L.; Paganini, É.R.; Garcia, M.A.R.; Nazaré, A.C.; Lazarini, J.G.; Alencar, S.M.; Regasini, L.O.; Sardi, J.C.O. Antimicrobial activity of nitrochalcone and pentyl caffeate against hospital pathogens results in decreased microbial adhesion and biofilm formation. Biofouling 2019, 35, 129–142. [Google Scholar] [CrossRef] [PubMed]
| Compounds | C. tropicalis (ATCC 750 (µg/mL) | C. albicans MYA2876 (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| MIC | MFC | MFC/MIC | SI | MIC | MFC | MFC/MIC | SI | |
| Original Chalcone | >250 | >250 | - | - | >250 | >250 | - | - |
| AM-35 | 3.9 | 7.8 | 2 | 32 | 7.8 | 7.8 | 1 | 16 |
| Hydrogel+AM-35 | 1.95 | 3.9 | 2 | 32 | 3.9 | 3.9 | 1 | 16 |
| Hydrogel (pure) | 125 | 125 | - | - | 125 | 125 | - | - |
| Sodium Hypochlorite (control) | 1% | 1% | - | - | 1% | 1% | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Chaves dos Santos, E.; Rosalen, P.L.; Graciani, J.; Lazarini, J.G.; Macedo, M.L.R.; Romário-Silva, D.; Garcia, M.A.R.; Carvalho, S.G.; da Mata Siqueira Mesut, P.; Prates, A.C.C.N.; et al. In Vitro Antimicrobial and Antibiofilm Efficacy of an Aminochalcone-Loaded Hydrogel Against Candida spp. Future Pharmacol. 2025, 5, 47. https://doi.org/10.3390/futurepharmacol5030047
de Oliveira Chaves dos Santos E, Rosalen PL, Graciani J, Lazarini JG, Macedo MLR, Romário-Silva D, Garcia MAR, Carvalho SG, da Mata Siqueira Mesut P, Prates ACCN, et al. In Vitro Antimicrobial and Antibiofilm Efficacy of an Aminochalcone-Loaded Hydrogel Against Candida spp. Future Pharmacology. 2025; 5(3):47. https://doi.org/10.3390/futurepharmacol5030047
Chicago/Turabian Stylede Oliveira Chaves dos Santos, Emmanuely, Pedro Luiz Rosalen, Joice Graciani, Josy Goldoni Lazarini, Maria Ligia Rodrigues Macedo, Diego Romário-Silva, Mayara Aparecida Rocha Garcia, Suzana Gonçalves Carvalho, Paola da Mata Siqueira Mesut, Ana Claudia Castelã Nascimento Prates, and et al. 2025. "In Vitro Antimicrobial and Antibiofilm Efficacy of an Aminochalcone-Loaded Hydrogel Against Candida spp." Future Pharmacology 5, no. 3: 47. https://doi.org/10.3390/futurepharmacol5030047
APA Stylede Oliveira Chaves dos Santos, E., Rosalen, P. L., Graciani, J., Lazarini, J. G., Macedo, M. L. R., Romário-Silva, D., Garcia, M. A. R., Carvalho, S. G., da Mata Siqueira Mesut, P., Prates, A. C. C. N., Regasini, L. O., Chorilli, M., Consani, R. L. X., & Sardi, J. d. C. O. (2025). In Vitro Antimicrobial and Antibiofilm Efficacy of an Aminochalcone-Loaded Hydrogel Against Candida spp. Future Pharmacology, 5(3), 47. https://doi.org/10.3390/futurepharmacol5030047









