Antimicrobial Efficacy of Curcumin Nanoparticles Against Aquatic Bacterial Pathogens
Abstract
1. Introduction
2. Curcumin: Source, Biological Activity, and Bioavailability Challenges
3. Curcumin Nanoparticles: Synthesis and Properties
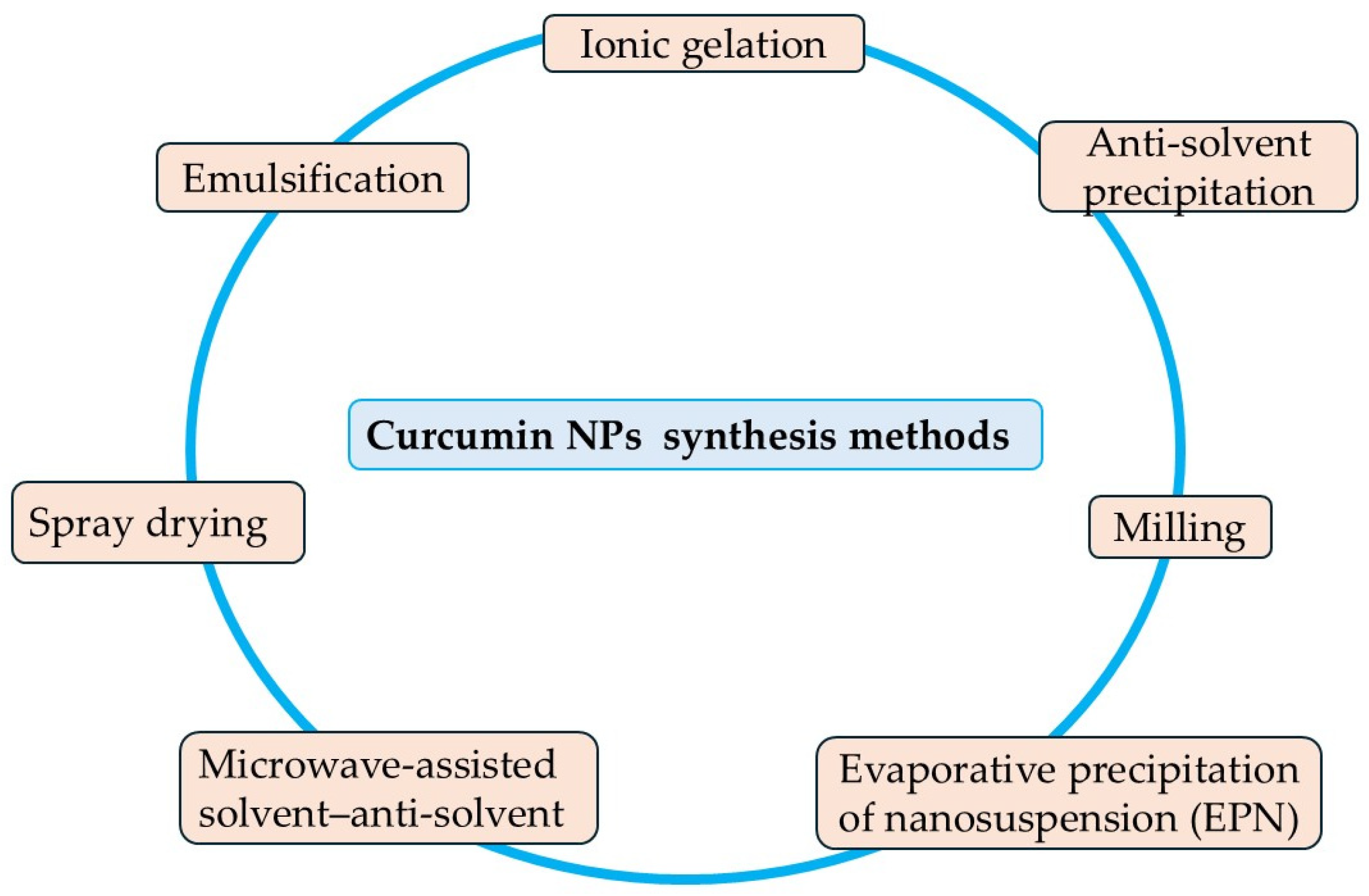
3.1. Nanoparticle Formulation Strategies
3.1.1. Milling Method
3.1.2. Anti-Solvent Precipitation
3.1.3. Evaporative Precipitation of Nanosuspension
3.1.4. Microwave-Assisted Solvent–Antisolvent Method
3.1.5. Emulsification Method
3.1.6. Ionic Gelation Method
3.1.7. Spray Drying Method
3.2. Physicochemical Properties of Curcumin Nanoparticles and Their Role in Antimicrobial Activity
3.2.1. Effect of Particle Size
3.2.2. Effect of Shape
3.2.3. Effect of Surface Charge (Zeta Potential) and Colloidal Stability
3.2.4. Effect of pH
3.3. Antimicrobial Efficacy of Curcumin Nanoparticles and Nanocomposites Against Aquatic Bacterial Pathogens
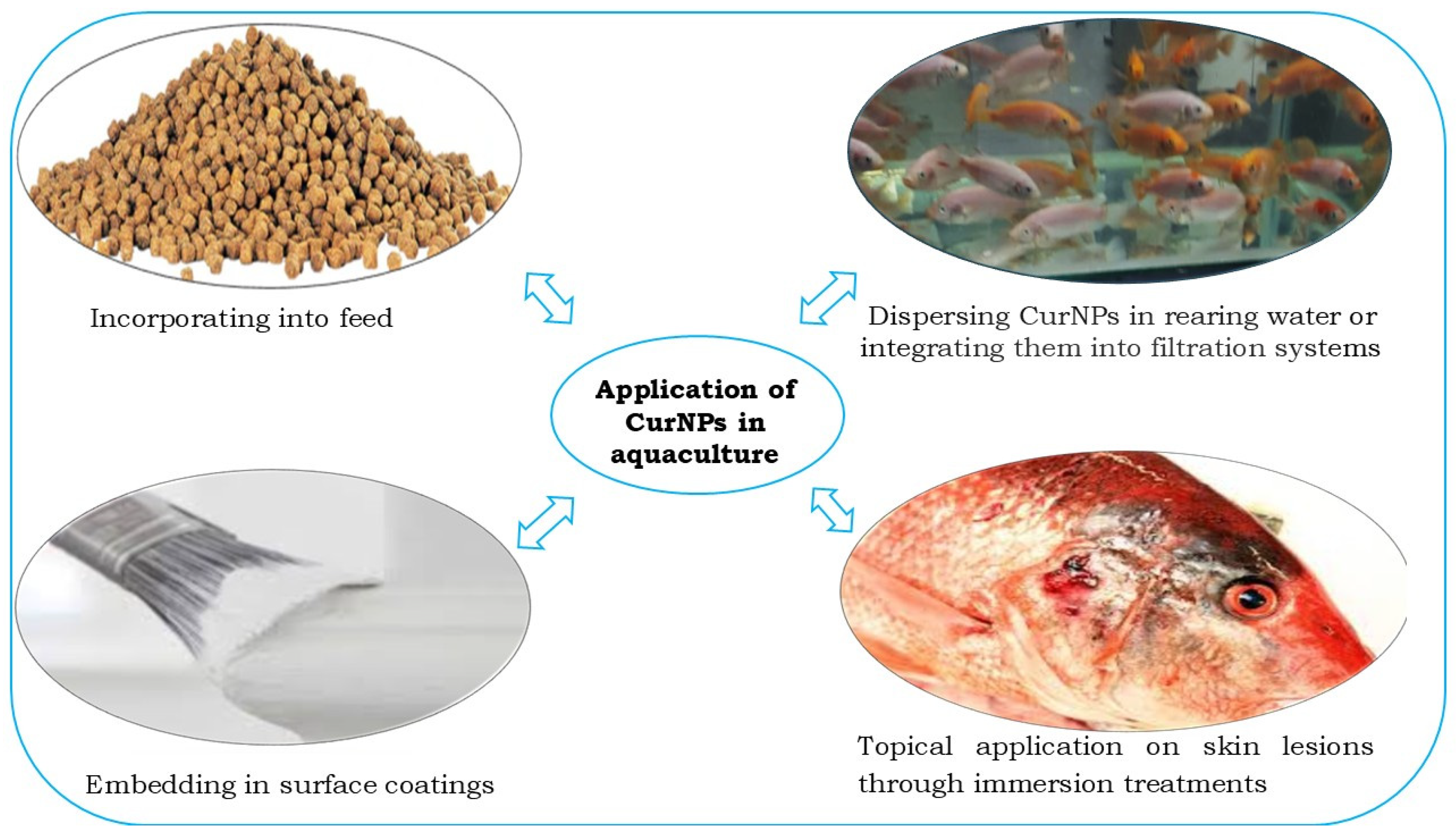
4. Applications in Aquaculture
4.1. Incorporating into Feed
4.2. Dispersing CurNPs in Rearing Water or Integrating Them into Filtration Systems
4.3. Topical Application Through Immersion Treatments
4.4. Embedding in Surface Coatings
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. In Brief to The State of World Fisheries and Aquaculture 2024; Blue Transformation Action; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Bouwmeester, M.M.; Goedknegt, M.A.; Poulin, R.; Thieltges, D.W. Collateral Diseases: Aquaculture Impacts on Wildlife Infections. J. Appl. Ecol. 2021, 58, 453–464. [Google Scholar] [CrossRef]
- Hegde, A.; Kabra, S.; Basawa, R.M.; Khile, D.A. Bacterial Diseases in Marine Fish Species: Current Trends and Future Prospects in Disease Management. World J. Microbiol. Biotechnol. 2023, 39, 317. [Google Scholar] [CrossRef]
- Yusoff, F.M.; Umi, W.A.D.; Ramli, N.M.; Harun, R. Water Quality Management in Aquaculture. Camb. Prism. Water 2024, 2, e8. [Google Scholar] [CrossRef]
- Mohammed, E.A.H.; Kovács, B.; Kuunya, R.; Mustafa, E.O.A.; Abbo, A.S.H.; Pál, K. Antibiotic Resistance in Aquaculture: Challenges, Trends Analysis, and Alternative Approaches. Antibiotics 2025, 14, 598. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.K.; Hussein, S.; Qurbani, K.; Ibrahim, R.H.; Fareeq, A.; Mahmood, K.A.; Mohamed, M.G. Antimicrobial Resistance: Impacts, Challenges, and Future Prospects. J. Med. Surg. Public Health 2024, 2, 100081. [Google Scholar] [CrossRef]
- Ripanda, A.; Rwiza, M.J.; Nyanza, E.C.; Hossein, M.; Alfred, M.S.; El Din Mahmoud, A.; Murthy, H.C.A.; Bakari, R.; Hamad Vuai, S.A.; Machunda, R.L. Ecological Consequences of Antibiotics Pollution in Sub-Saharan Africa: Understanding Sources, Pathways, and Potential Implications. Emerg. Contam. 2025, 11, 100475. [Google Scholar] [CrossRef]
- Chinemerem Nwobodo, D.; Ugwu, M.C.; Oliseloke Anie, C.; Al-Ouqaili, M.T.S.; Chinedu Ikem, J.; Victor Chigozie, U.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef]
- Ajanaku, C.O.; Ademosun, O.T.; Atohengbe, P.O.; Ajayi, S.O.; Obafemi, Y.D.; Owolabi, O.A.; Akinduti, P.A.; Ajanaku, K.O. Functional Bioactive Compounds in Ginger, Turmeric, and Garlic. Front. Nutr. 2022, 9, 1012023. [Google Scholar] [CrossRef]
- Cozmin, M.; Lungu, I.I.; Gutu, C.; Stefanache, A.; Duceac, L.D.; Șoltuzu, B.D.; Damir, D.; Calin, G.; Bogdan Goroftei, E.R.; Grierosu, C.; et al. Turmeric: From Spice to Cure. A Review of the Anti-Cancer, Radioprotective and Anti-Inflammatory Effects of Turmeric Sourced Compounds. Front. Nutr. 2024, 11, 1399888. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Y.; Alam, W.; Ullah, H.; Dacrema, M.; Daglia, M.; Khan, H.; Arciola, C.R. Antimicrobial Potential of Curcumin: Therapeutic Potential and Challenges to Clinical Applications. Antibiotics 2022, 11, 322. [Google Scholar] [CrossRef]
- Wahnou, H.; El Kebbaj, R.; Liagre, B.; Sol, V.; Limami, Y.; Duval, R.E. Curcumin-Based Nanoparticles: Advancements and Challenges in Tumor Therapy. Pharmaceutics 2025, 17, 114. [Google Scholar] [CrossRef]
- Karthikeyan, A.; Senthil, N.; Min, T. Nanocurcumin: A Promising Candidate for Therapeutic Applications. Front. Pharmacol. 2020, 11, 487. [Google Scholar] [CrossRef]
- Gera, M.; Sharma, N.; Ghosh, M.; Huynh, D.L.; Lee, S.J.; Min, T.; Kwon, T.; Jeong, D.K. Nanoformulations of Curcumin: An Emerging Paradigm for Improved Remedial Application. Oncotarget 2017, 8, 66680–66698. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Tagde, P.; Islam, F.; Tagde, S.; Shah, M.; Hussain, Z.D.; Rahman, M.H.; Najda, A.; Alanazi, I.S.; Germoush, M.O.; et al. The Multifaceted Role of Curcumin in Advanced Nanocurcumin Form in the Treatment and Management of Chronic Disorders. Molecules 2021, 26, 7109. [Google Scholar] [CrossRef]
- Moon, D.O. Curcumin in Cancer and Inflammation: An In-Depth Exploration of Molecular Interactions, Therapeutic Potentials, and the Role in Disease Management. Int. J. Mol. Sci. 2024, 25, 2911. [Google Scholar] [CrossRef] [PubMed]
- Sathyabhama, M.; Priya Dharshini, L.C.; Karthikeyan, A.; Kalaiselvi, S.; Min, T. The Credible Role of Curcumin in Oxidative Stress-Mediated Mitochondrial Dysfunction in Mammals. Biomolecules 2022, 12, 1405. [Google Scholar] [CrossRef] [PubMed]
- Cox, F.F.; Misiou, A.; Vierkant, A.; Ale-Agha, N.; Grandoch, M.; Haendeler, J.; Altschmied, J. Protective Effects of Curcumin in Cardiovascular Diseases—Impact on Oxidative Stress and Mitochondria. Cells 2022, 11, 342. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Zhang, M. The Beneficial Effects of Curcumin on Aging and Age-Related Diseases: From Oxidative Stress to Antioxidant Mechanisms, Brain Health and Apoptosis. Front. Aging Neurosci. 2025, 17, 1533963. [Google Scholar] [CrossRef]
- Memarzia, A.; Khazdair, M.R.; Behrouz, S.; Gholamnezhad, Z.; Jafarnezhad, M.; Saadat, S.; Boskabady, M.H. Experimental and Clinical Reports on Anti-Inflammatory, Antioxidant, and Immunomodulatory Effects of Curcuma longa and Curcumin, an Updated and Comprehensive Review. BioFactors 2021, 47, 311–350. [Google Scholar] [CrossRef]
- Gabe, H.B.; Queiroga, F.R.; Taruhn, K.A.; Trevisan, R. Mitigating Oxidative Stress in Oyster Larvae: Curcumin Promotes Enhanced Redox Balance, Antioxidant Capacity, Development, and Resistance to Antifouling Compounds. Aquat. Toxicol. 2025, 279, 107231. [Google Scholar] [CrossRef]
- Wang, P.R.-X.; Zhou, M.; Ma, H.-L.; Qiao, P.Y.-B.; Li, P.Q.-S. The Role of Chronic Inflammation in Various Diseases and Anti-Inflammatory Therapies Containing Natural Products. ChemBioChem 2021, 16, 1576–1592. [Google Scholar] [CrossRef]
- Hasibuan, P.A.Z.; Simanjuntak, Y.; Hey-Hawkins, E.; Lubis, M.F.; Rohani, A.S.; Park, M.N.; Kim, B.; Syahputra, R.A. Unlocking the Potential of Flavonoids: Natural Solutions in the Fight against Colon Cancer. Biomed. Pharmacother. 2024, 176, 116827. [Google Scholar] [CrossRef]
- Hussain, Y.; Khan, H.; Alotaibi, G.; Khan, F.; Alam, W.; Aschner, M.; Jeandet, P.; Saso, L. How Curcumin Targets Inflammatory Mediators in Diabetes: Therapeutic Insights and Possible Solutions. Molecules 2022, 27, 4058. [Google Scholar] [CrossRef] [PubMed]
- Lan, Z.; Yang, R.; Wang, H.; Xue, X.; Sun, Y.; Wang, S.; Zhang, Y.; Meng, J. Rapid Identifying of COX-2 Inhibitors from Turmeric (Curcuma longa) by Bioaffinity Ultrafiltration Coupled with UPLC-Q Exactive-Orbitrap-MS and Zebrafish-Based in Vivo Validation. Bioorg. Chem. 2024, 147, 107357. [Google Scholar] [CrossRef] [PubMed]
- Dai, C.; Lin, J.; Li, H.; Shen, J.; Shen, Z.; Wang, Y.; Velkov, T. The Natural Product Curcumin as an Antibacterial Agent: Current Achievements and Problems. Antioxidants 2022, 11, 459. [Google Scholar] [CrossRef] [PubMed]
- Adamczak, A.; Ożarowski, M.; Karpiński, T.M. Curcumin, a Natural Antimicrobial Agent with Strain-Specific Activity. Pharmaceuticals 2020, 13, 153. [Google Scholar] [CrossRef]
- Dube, E.; Okuthe, G.E. Silver Nanoparticle-Based Antimicrobial Coatings: Sustainable Strategies for Microbial Contamination Control. Microbiol. Res. 2025, 16, 110. [Google Scholar] [CrossRef]
- Wang, W.; Li, M.; Wang, L.; Chen, L.; Goh, B.C. Curcumin in Cancer Therapy: Exploring Molecular Mechanisms and Overcoming Clinical Challenges. Cancer Lett. 2023, 570, 216332. [Google Scholar] [CrossRef]
- Sudhesh Dev, S.; Zainal Abidin, S.A.; Farghadani, R.; Othman, I.; Naidu, R. Receptor Tyrosine Kinases and Their Signaling Pathways as Therapeutic Targets of Curcumin in Cancer. Front. Pharmacol. 2021, 12, 772510. [Google Scholar] [CrossRef]
- Cao, Z.; He, S.; Peng, Y.; Liao, X.; Lu, H. Nanocurcumin Inhibits Angiogenesis via Down-Regulating Hif1a/VEGF-A Signaling in Zebrafish. Curr. Neurovasc. Res. 2020, 17, 147–154. [Google Scholar] [CrossRef]
- Baghcheghi, Y.; Razazpour, F.; Seyedi, F.; Arefinia, N.; Hedayati-Moghadam, M. Exploring the Molecular Mechanisms of PPARγ Agonists in Modulating Memory Impairment in Neurodegenerative Disorders. Mol. Biol. Rep. 2024, 52, 945. [Google Scholar] [CrossRef]
- Satpathy, L.; Parida, S.P. Neuroprotective Role of Curcumin Against Benzo[a]Pyrene-Induced Neurodegeneration in Zebrafish. Biointerface Res. Appl. Chem. 2022, 12, 7311–7320. [Google Scholar] [CrossRef]
- Cheng, M.; Ding, F.; Li, L.; Dai, C.; Sun, X.; Xu, J.; Chen, F.; Li, M.; Li, X. Exploring the Role of Curcumin in Mitigating Oxidative Stress to Alleviate Lipid Metabolism Disorders. Front. Pharmacol. 2025, 16, 1517174. [Google Scholar] [CrossRef] [PubMed]
- Racz, L.Z.; Racz, C.P.; Pop, L.; Tomoaia, G.; Mocanu, A.; Barbu, I.; Melinda, S.; Roman, I.; Avram, A.; Tomoaia-cotisel, M.; et al. Strategies for Improving Bioavailability, Bioactivity, and Physical-Chemical Behavior of Curcumin. Molecules 2022, 27, 6854. [Google Scholar] [CrossRef] [PubMed]
- Tabanelli, R.; Brogi, S.; Calderone, V. Improving Curcumin Bioavailability: Current Strategies and Future Perspectives. Pharmaceutics 2021, 13, 1715. [Google Scholar] [CrossRef]
- Rai, M.; Feitosa, C.M.; Ingle, A.P.; Golinska, P. Harnessing Bioactive Nanocurcumin and Curcumin Nanocomposites to Combat Microbial Pathogens: A Comprehensive Review. Crit. Rev. Biotechnol. 2025, 1–23. [Google Scholar] [CrossRef]
- No, D.S.; Algburi, A.; Huynh, P.; Moret, A.; Ringard, M.; Comito, N.; Drider, D.; Takhistov, P.; Chikindas, M.L. Antimicrobial Efficacy of Curcumin Nanoparticles against Listeria Monocytogenes Is Mediated by Surface Charge. J. Food Saf. 2017, 37, e12353. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Perera, Y.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, S.; Rajapakse, R.M.G. Comparison of Antibacterial Activity of Nanocurcumin with Bulk Curcumin. ACS Omega 2022, 7, 46494–46500. [Google Scholar] [CrossRef]
- Kumar, R.; Thakur, A.K.; Chaudhari, P.; Kumar, R.; Naresh, K.; Thapliyal, D.; Bedar, A.; Krishna, R.S. Nanoparticle Preparation of Pharmaceutical Compounds via Wet Milling: Current Status and Future Prospects. Powder Technol. 2024, 435, 119430. [Google Scholar] [CrossRef]
- Manikandan, S.; El Mabrouk, K.; Ballamurugan, A.M. Synthesis of Nanocurcumin and Evaluation of Its Properties for Biomedical Applications. Trends Biomater. Artif. Organs 2022, 36, 67–69. [Google Scholar]
- Lapcikova, B.; Valenta, T.; Lapcık, L.; Li, P. Curcuma Particle Size Evolution by Application of Bead Milling Process and Curcuminoids Content Determination. Int. J. Food Sci. Technol. 2023, 58, 5738–5744. [Google Scholar] [CrossRef]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical Nanocrystals: Production by Wet Milling and Applications. Drug Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef] [PubMed]
- Kuddushi, M.; Xu, B. Bin Recent Advances in Nanoprecipitation: From Mechanistic Insights to Applications in Nanomaterial Synthesis. Soft Matter 2025, 21, 2759–2781. [Google Scholar] [CrossRef] [PubMed]
- Kakran, M.; Sahoo, N.G.; Tan, I.-L.; Li, L. Preparation of Nanoparticles of Poorly Water-Soluble Antioxidant Curcumin by Antisolvent Precipitation Methods. J. Nanopart. Res. 2012, 14, 757. [Google Scholar] [CrossRef]
- Hettiarachchi, S.S.; Dunuweera, S.P.; Dunuweera, A.N.; Rajapakse, R.M.G. Synthesis of Curcumin Nanoparticles from Raw Turmeric Rhizome. ACS Omega 2021, 6, 8246–8252. [Google Scholar] [CrossRef]
- Yadav, D.; Kumar, N. Nanonization of Curcumin by Antisolvent Precipitation: Process Development, Characterization, Freeze Drying and Stability Performance. Int. J. Pharm. 2014, 477, 564–577. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, R.; Jain, V.K.; Nagpal, S. Preparation and Characterization of Nanocurcumin Based Hybrid Virosomes as a Drug Delivery Vehicle with Enhanced Anticancerous Activity and Reduced Toxicity. Sci. Rep. 2021, 11, 368. [Google Scholar] [CrossRef]
- Zamanidehyaghoubi, G.; Shahidi, F.; Edalatian Dovom, M.R.; Mohebbi, M.; Roshanak, S. Enhancing Curcumin Nanoparticle Synthesis through Wet-Milling: Comparative Analysis of Physico-Chemical and Antimicrobial Properties of Nano-Curcumin with Micro-Curcumin. LWT—Food Sci. Technol. 2024, 205, 116553. [Google Scholar] [CrossRef]
- Bhawana; Basniwal, R.K.; Buttar, H.S.; Jain, V.K.; Jain, N. Curcumin Nanoparticles: Preparation, Characterization, and Antimicrobial Study. J. Agric. Food Chem. 2011, 59, 2056–2061. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, M.U.K.; Sadiq, A.; Khan, S.; Faidah, H.S.; Naseemullah; Khurram, M.; Amin, M.U.; Haseeb, A. Fabrication, Characterization and in Vitro Evaluation of Silibinin Nanoparticles: An Attempt to Enhance Its Oral Bioavailability. Drug Des. Dev. Ther. 2017, 11, 1453–1464. [Google Scholar] [CrossRef]
- Kanwal, Q.; Ahmed, M.; Hamza, M.; Ahmad, M.; Atiq-ur-Rehman; Yousaf, N.; Javaid, A.; Anwar, A.; Khanf, I.H.; Muddassar, M. Curcumin Nanoparticles: Physicochemical Fabrication, Characterization, Antioxidant, Enzyme Inhibition, Molecular Docking and Simulation Studies. RSC Adv. 2023, 13, 22268–22280. [Google Scholar] [CrossRef]
- Aditya, N.P.; Hamilton, I.E.; Noon, J.; Norton, I.T. Microwave Assisted Nanonization of Poorly Water-Soluble Curcumin. ACS Sustain. Chem. Eng. 2019, 7, 9771–9781. [Google Scholar] [CrossRef]
- Shibatani, A.; Kan, H.; Matsumura, S.; Asakuma, Y.; Saptoro, A. Synergistic Effect of High Irradiation Power and Antisolvent Addition for Enhanced Microwave Assisted Nanoparticle Synthesis Process. J. Chem. Eng. Jpn. 2020, 53, 397–401. [Google Scholar] [CrossRef]
- Al Fatease, A.; Alqahtani, A.; Khan, B.A.; Mohamed, J.M.M.; Farhana, S.A. Preparation and Characterization of a Curcumin Nanoemulsion Gel for the Effective Treatment of Mycoses. Sci. Rep. 2023, 13, 22730. [Google Scholar] [CrossRef] [PubMed]
- Inal, A.; Yenipazar, H.; Şahin-Yeşilçubuk, N. Preparation and Characterization of Nanoemulsions of Curcumin and Echium Oil. Heliyon 2022, 8, e08974. [Google Scholar] [CrossRef]
- Moghaddasi, F.; Housaindokht, M.R.; Darroudi, M.; Bozorgmehr, M.R.; Sadeghi, A. Synthesis of Nano Curcumin Using Black Pepper Oil by O/W Nanoemulsion Technique and Investigation of Their Biological Activities. LWT—Food Sci. Technol. 2018, 92, 92–100. [Google Scholar] [CrossRef]
- Mogahed, N.; Gaafar, M.; Shalaby, T.; Sheta, E.; Arafa, F. Potential Efficacy of Curcumin and Curcumin Nanoemulsion against Experimental Cyclosporiasis. Parasitol. United J. 2023, 16, 197–207. [Google Scholar] [CrossRef]
- Aguilera, L.F.; Araujo, L.O.; Facchinatto, W.M.; Lima, R.G.; da Silva Pontes, M.; Pulcherio, J.H.V.; Caires, C.S.A.; de Oliveira, K.T.; de Oliveira, S.L.; Caires, A.R.L. Blue-Light Photoactivated Curcumin-Loaded Chitosan Nanoparticles Prepared by Nanoprecipitation and Ionic Gelation: A Promising Approach for Antimicrobial Photodynamic Inactivation. ACS Appl. Bio Mater. 2025, 8, 4055–4064. [Google Scholar] [CrossRef] [PubMed]
- Jha, R.; Mayanovic, R.A. A Review of the Preparation, Characterization, and Applications of Chitosan Nanoparticles in Nanomedicine. Nanomaterials 2023, 13, 1302. [Google Scholar] [CrossRef]
- Wathoni, N.; Herdiana, Y.; Suhandi, C.; Mohammed, A.F.A.; El-Rayyes, A.; Narsa, A.C. Chitosan/Alginate-Based Nanoparticles for Antibacterial Agents Delivery. Int. J. Nanomed. 2024, 19, 5021–5044. [Google Scholar] [CrossRef] [PubMed]
- Shende, P.; Gupta, H. Formulation and Comparative Characterization of Nanoparticles of Curcumin Using Natural, Synthetic and Semi-Synthetic Polymers for Wound Healing. Life Sci. 2020, 253, 117588. [Google Scholar] [CrossRef] [PubMed]
- Bhoopathy, S.; Inbakandan, D.; Rajendran, T.; Chandrasekaran, K.; Prabha S, B.; Reddy, B.A.; Kasilingam, R.; RameshKumar, V.; Dharani, G. Dietary Supplementation of Curcumin-Loaded Chitosan Nanoparticles Stimulates Immune Response in the White Leg Shrimp Litopenaeus vannamei Challenged with Vibrio harveyi. Fish Shellfish Immunol. 2021, 117, 188–191. [Google Scholar] [CrossRef]
- Malamatari, M.; Charisi, A.; Malamataris, S.; Kachrimanis, K.; Nikolakakis, I. Spray Drying for the Preparation of Nanoparticle-Based Drug Formulations as Dry Powders for Inhalation. Processes 2020, 8, 788. [Google Scholar] [CrossRef]
- Attri, N.; Kaur, S.; Aggarwal, P. Spray Drying: A Promising Technique in Food Processing—Concept, Applications, and Latest Advancements. In Novel Drying Technologies in Food Science; Chandrapala, J., Ed.; IntechOpen: London, UK, 2025. [Google Scholar]
- Dube, E. Antibacterial Activity of Engineered Nanoparticles against Fish Pathogens. Aquac. Rep. 2024, 37, 102240. [Google Scholar] [CrossRef]
- Jastaniah, S.D.; Mansour, A.A.; Al-Tarawni, A.H.; El-Haroun, E.; Munir, M.B.; Saghir, S.A.M.; Abdul Kari, Z.; Téllez-Isaías, G.; Bottje, W.G.; AL-Farga, A.; et al. The Effects of Nano-Curcumin on Growth Performance, Feed Utilization, Blood Biochemistry, Disease Resistance, and Gene Expression in European Seabass (Dicentrarchus labrax) Fingerlings. Aquac. Rep. 2024, 36, 102034. [Google Scholar] [CrossRef]
- Gandapu, U.; Chaitanya, R.K.; Kishore, G.; Reddy, R.C.; Kondapi, A.K. Curcumin-Loaded Apotransferrin Nanoparticles Provide Efficient Cellular Uptake and Effectively Inhibit HIV-1 Replication In Vitro. PLoS ONE 2011, 6, e23388. [Google Scholar] [CrossRef]
- Unnikrishnan, G.; Joy, A.; Megha, M.; Kolanthai, E.; Senthilkumar, M. Exploration of Inorganic Nanoparticles for Revolutionary Drug Delivery Applications: A Critical Review; Springer: New York, NY, USA, 2023; Volume 18, ISBN 0123456789. [Google Scholar]
- Zhao, Y.; Wang, Y.; Ran, F.; Cui, Y.; Liu, C.; Zhao, Q.; Gao, Y.; Wang, D.; Wang, S. A Comparison between Sphere and Rod Nanoparticles Regarding Their in Vivo Biological Behavior and Pharmacokinetics. Sci. Rep. 2017, 7, 4131. [Google Scholar] [CrossRef]
- Xuan, Y.; Zhang, W.; Zhu, X.; Zhang, S. An Updated Overview of Some Factors That Influence the Biological Effects of Nanoparticles. Front. Bioeng. Biotechnol. 2023, 11, 1254861. [Google Scholar] [CrossRef]
- Pochapski, D.J.; Carvalho Dos Santos, C.; Leite, G.W.; Pulcinelli, S.H.; Santilli, C.V. Zeta Potential and Colloidal Stability Predictions for Inorganic Nanoparticle Dispersions: Effects of Experimental Conditions and Electrokinetic Models on the Interpretation of Results. Langmuir 2021, 37, 13379–13389. [Google Scholar] [CrossRef] [PubMed]
- Ly, P.D.; Ly, K.N.; Phan, H.L.; Nguyen, H.H.T.; Duong, V.A.; Nguyen, H.V. Recent Advances in Surface Decoration of Nanoparticles in Drug Delivery. Front. Nanotechnol. 2024, 6, 1456939. [Google Scholar] [CrossRef]
- Abbas, Q.; Yousaf, B.; Amina; Ali, M.U.; Munir, M.A.M.; El-Naggar, A.; Rinklebe, J.; Naushad, M. Transformation Pathways and Fate of Engineered Nanoparticles (ENPs) in Distinct Interactive Environmental Compartments: A Review. Environ. Int. 2020, 138, 105646. [Google Scholar] [CrossRef]
- Gad, H.A.; Alshubaily, F.A.; Alsieni, M.A.; Tayel, A.A.; Diab, A.M. Biosynthesis of Nano-Curcumin/Nano-Selenium Composite and Their Potentialities as Bactericides against Fish-Borne Pathogens. Green Process. Synth. 2022, 11, 1098–1107. [Google Scholar] [CrossRef]
- Sayyar, Z.; Jafarizadeh-Malmiri, H. Enhancing the Efficacy of Nano-Curcumin on Cancer Cells through Mixture Design Optimization of Three Emulsifiers. BMC Chem. 2024, 18, 62. [Google Scholar] [CrossRef]
- Singh, A.K.; Prakash, P.; Singh, R.; Nandy, N.; Firdaus, Z.; Bansal, M.; Singh, R.K.; Srivastava, A.; Roy, J.K.; Mishra, B.; et al. Curcumin Quantum Dots Mediated Degradation of Bacterial Biofilms. Front. Microbiol. 2017, 8, 1517. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Chen, L.; Zhang, W. Influence of Ionic Surfactants on the Properties of Nanoemulsions Emulsified by Nonionic Surfactants Span 80/Tween 80. J. Dispers. Sci. Technol. 2016, 37, 1511–1517. [Google Scholar] [CrossRef]
- Kumavat, S.D.; Chaudhari, Y.S.; Borole, P.; Mishra, P.; Shenghani, K.; Duvvuri, P. Degradation Studies of Curcurmin. Int. J. Pharm. Rev. Res. 2015, 3, 50–55. [Google Scholar]
- Sedghi, R.; Ashrafzadeh, S.; Heidari, B. PH-Sensitive Molecularly Imprinted Polymer Based on Graphene Oxide for Stimuli Actuated Controlled Release of Curcumin. J. Alloys Compd. 2021, 857, 157603. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.; Xia, L.; Jiang, S.; Kong, Y.; Chen, X.; Wang, H. The Kinetics and Release Behaviour of Curcumin Loaded PH-Responsive PLGA/Chitosan Fibers with Antitumor Activity against HT-29 Cells. Carbohydr. Polym. 2021, 265, 118077. [Google Scholar] [CrossRef]
- Zhu, J.; Sanidad, K.Z.; Sukamtoha, E.; Zhang, G. Potential Roles of Chemical Degradation in the Biological Activities of Curcumin. Food Funct. 2017, 8, 907–914. [Google Scholar] [CrossRef]
- Morsy, M.K.; Al-Dalain, S.Y.; Haddad, M.A.; Diab, M.; Abd-Elaaty, E.M.; Abdeen, A.; Ibrahim, S.F.; Shukry, M.; Banatean-Dunea, I.; Fericean, L.; et al. Curcumin Nanoparticles as a Natural Antioxidant and Antimicrobial Preservative against Foodborne Pathogens in Processed Chicken Fingers. Front. Sustain. Food Syst. 2023, 7, 1267075. [Google Scholar] [CrossRef]
- Ring, L.C.; Yenn, T.W.; Wen-Nee, T.; Tumin, N.D.; Yusof, F.A.M.; Yacob, L.S.; Bin Rosli, M.I.H.; Taher, M.A. Synthesis of Curcumin Quantum Dots and Their Antimicrobial Activity on Necrotizing Fasciitis Causing Bacteria. Mater. Today Proc. 2020, 31, 31–35. [Google Scholar] [CrossRef]
- Hosseini, A.; Nejadsattari, T.; Zargar, M. In Vitro Anti-Biofilm Activity of Curcumin Nanoparticles in Acinetobacter Baumannii: A Culture-Based and Molecular Approach. Arch. Clin. Infect. Dis. 2019, 14, e83263. [Google Scholar] [CrossRef]
- Mansour, S.; Bakry, K.A.; Alwaleed, E.A.; Ahmed, H.; Al-Amgad, Z.; Mohammed, H.H.; Emeish, W.F.A. Dietary Nanocurcumin Impacts Blood Biochemical Parameters and Works Synergistically with Florfenicol in African Catfish Challenged with Aeromonas Veronii. Fishes 2023, 8, 298. [Google Scholar] [CrossRef]
- Panwar, D.; Sidhu, K.; Bhushan, J.; Kakkar, V.; Mehta, M.; Sharma, J. Evaluation of Antimicrobial Efficacy of Nanocurcumin-coated Gutta-percha against Enterococcus faecalis: An in Vitro Study. J. Conserv. Dent. 2023, 26, 160–164. [Google Scholar] [CrossRef]
- Khan, M.A.; Moghul, N.B.; Butt, M.A.; Kiyani, M.M.; Zafar, I.; Bukhari, A.I. Assessment of Antibacterial and Antifungal Potential of Curcuma longa and Synthesized Nanoparticles: A Comparative Study. J. Basic Microbiol. 2021, 61, 603–611. [Google Scholar] [CrossRef]
- Negahdari, R.; Sharifi, S.; Ghavimi, M.A.; Memar, M.Y. Curcumin Nanocrystals: Production, Physicochemical Assessment, and In Vitro Evaluation of the Antimicrobial Effects against Bacterial Loading of the Implant Fixture. Appl. Sci. 2020, 10, 8356. [Google Scholar] [CrossRef]
- Adahoun, M.A.; Al-Akhras, M.A.H.; Jaafar, M.S.; Bououdina, M. Enhanced Anti-Cancer and Antimicrobial Activities of Curcumin Nanoparticles. Artif. Cells Nanomed. Biotechnol. 2017, 45, 98–107. [Google Scholar] [CrossRef]
- Shariati, A.; Asadian, E.; Fallah, F.; Azimi, T.; Hashemi, A.; Sharahi, J.Y.; Moghadam, M.T. Evaluation of Nano-Curcumin Effects on Expression Levels of Virulence Genes and Biofilm Production of Multidrug-Resistant Pseudomonas aeruginosa Isolated from Burn Wound Infection in Tehran, Iran. Infect. Drug Resist. 2019, 12, 2223–2235. [Google Scholar] [CrossRef]
- Shome, S.; Das, A.; Nath, R.; Tewari, S. Curcumin-ZnO Nanocomposite Mediated Inhibition of Pseudomonas aeruginosa Biofilm and Its Mechanism of Action. J. Drug Deliv. Sci. Technol. 2023, 81, 104301. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Noroozian, M.; Ahmad Akhoundi, M.S.; Bahador, A. Antimicrobial Effects and Mechanical Properties of Poly(Methyl Methacrylate) as an Orthodontic Acrylic Resin Containing Curcumin-Nisin-Poly(l-Lactic Acid) Nanoparticle: An in Vitro Study. BMC Oral Health 2022, 22, 158. [Google Scholar] [CrossRef]
- Maher, C.; Hassan, K.A. The Gram-Negative Permeability Barrier: Tipping the Balance of the in and the Out. Antimicrob. Chemother. 2023, 14, e01205-23. [Google Scholar] [CrossRef]
- Modi, S.K.; Gaur, S.; Sengupta, M.; Singh, M.S. Mechanistic Insights into Nanoparticle Surface-Bacterial Membrane Interactions in Overcoming Antibiotic Resistance. Front. Microbiol. 2023, 14, 1135579. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Pourakbari, B.; Bahador, A. Contribution of Antimicrobial Photo-Sonodynamic Therapy in Wound Healing: An in Vivo Effect of Curcumin-Nisin-Based Poly (L-Lactic Acid) Nanoparticle on Acinetobacter Baumannii Biofilms. BMC Microbiol. 2022, 22, 28. [Google Scholar] [CrossRef]
- El-Kattan, N.; Ibrahim, M.A.; Emam, A.N.; Metwally, K.; Youssef, F.S.; Nassar, N.A.; Mansour, A.S. Evaluation of the Antimicrobial Activity of Chitosan- and Curcumin-Capped Copper Oxide Nanostructures against Multi-Drug-Resistant Microorganisms. Nanoscale Adv. 2025, 7, 2988–3007. [Google Scholar] [CrossRef]
- Pourasgar, S.; Ranji, N.; Asadpour, L.; Shahriarinour, M.; Nikpassand, M. Antibacterial and Anti-Cancer Properties of Curcumin-Functionalized Silica-Coated Fe3O4 Magnetic Nanoparticles. Arab. J. Sci. Eng. 2024, 50, 6231–6249. [Google Scholar] [CrossRef]
- Koca, F.D.; Demirezen Yilmaz, D.; Ertas Onmaz, N.; Ocsoy, I. Peroxidase-like Activity and Antimicrobial Properties of Curcumin-Inorganic Hybrid Nanostructure. Saudi J. Biol. Sci. 2020, 27, 2574–2579. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, S.; Mishra, P. Antimicrobial and Antibiofilm Activity of Curcumin-Silver Nanoparticles with Improved Stability and Selective Toxicity to Bacteria over Mammalian Cells. Med. Microbiol. Immunol. 2018, 207, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Shajari, M.; Zamani, M.; Ahmadi, N.; Rostamizadeh, K.; Shapouri, R. Improving the Antibacterial Activity of Curcumin Loaded Nanoparticles in Wastewater Treatment by Enhancing Permeability and Sustained Release. J. Polym. Environ. 2022, 30, 2658–2668. [Google Scholar] [CrossRef]
- Amresh, M.; Sagar, S.; Rethinam, S.; Jeyaraj, G. Antibacterial Curcumin Nanoparticles Loaded with Aloe Vera Gel for Topical Application. Afr. J. Biomed. Res. 2024, 27, 4246–4250. [Google Scholar] [CrossRef]
- Albratty, M.; Makeen, H.A. Eco-Friendly Fabrication of SnO2-Curcumin Nanoparticles via Pterocarpus marsupium Extract: Unveiling Potent Antimicrobial and Anticancer Applications. Indian J. Pharm. Educ. Res. 2025, 59, 719–726. [Google Scholar] [CrossRef]
- Singh, A.K.; Mishra, H.; Firdaus, Z.; Yadav, S.; Aditi, P.; Nandy, N.; Sharma, K.; Bose, P.; Pandey, A.K.; Chauhan, B.S.; et al. MoS2-Modified Curcumin Nanostructures: The Novel Theranostic Hybrid Having Potent Antibacterial and Antibiofilm Activities against Multidrug-Resistant Hypervirulent Klebsiella pneumoniae. Chem. Res. Toxicol. 2019, 32, 1599–1618. [Google Scholar] [CrossRef]
- Rahimi, M.; Piroozmand, A.; Shayestehpour, M.; Salamat, S.; Falak, F.P.; Shakerimoghaddam, A.; Moosavi, G.A.; Khaledi, A. Effect of Curcumin Nanoparticles and Alcoholic Extract of Falcaria vulgaris on the Growth Rate, Biofilm, and Gene Expression in Pseudomonas aeruginosa Isolated from Burn Wound Infection. Mol. Biol. Rep. 2023, 50, 6681–6690. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, G.; Kaviyil, J.E.; Paul, W.; Sasi, R.; Joseph, R. Gallium—Curcumin Nanoparticle Conjugates as an Antibacterial Agent against Pseudomonas aeruginosa: Synthesis and Characterization. ACS Omega 2022, 7, 6795–6809. [Google Scholar] [CrossRef]
- Di Salle, A.; Viscusi, G.; Di Cristo, F.; Valentino, A.; Gorrasi, G.; Lamberti, E.; Vittoria, V.; Calarco, A.; Peluso, G. Antimicrobial and Antibiofilm Activity of Curcumin-Loaded Electrospun Nanofibers for the Prevention of the Biofilm-Associated Infections. Molecules 2021, 26, 4866. [Google Scholar] [CrossRef]
- Gao, M.; Long, X.; Du, J.; Teng, M.; Zhang, W.; Wang, Y.; Wang, X.; Wang, Z.; Zhang, P.; Li, J. Enhanced Curcumin Solubility and Antibacterial Activity by Encapsulation in PLGA Oily Core Nanocapsules. Food Funct. 2020, 11, 448–455. [Google Scholar] [CrossRef]
- Ali, S.M.A.; Khan, J.; Shahid, R.; Shabbir, S.; Ayoob, M.F.; Imran, M. Chitosan-Carrageenan Microbeads Containing Nano-Encapsulated Curcumin: Nano-in-Micro Hydrogels as Alternative-Therapeutics for Resistant Pathogens Associated with Chronic Wounds. Int. J. Biol. Macromol. 2024, 278, 134841. [Google Scholar] [CrossRef]
- Targhi, A.A.; Moammeri, A.; Jamshidifar, E.; Abbaspour, K.; Sadeghi, S.; Lamakani, L.; Akbarzadeh, I. Synergistic Effect of Curcumin-Cu and Curcumin-Ag Nanoparticle Loaded Niosome: Enhanced Antibacterial and Anti-Biofilm Activities. Bioorg. Chem. 2021, 115, 105116. [Google Scholar] [CrossRef]
- Fan, Y.L.; Liu, H.J.; Wang, Z.L.; Wang, Z.L.; Zhu, L.L.; Wang, Y.Z.; Song, F. A One-Nano MOF-Two-Functions Strategy Toward Self-Healing, Anti-Inflammatory, and Antibacterial Hydrogels for Infected Wound Repair. Chem. Eng. J. 2024, 497, 155037. [Google Scholar] [CrossRef]
- Montoya-Hinojosa, E.I.; Salazar-Sesatty, H.A.; Alvizo-Baez, C.A.; Terrazas-Armendariz, L.D.; Luna-Cruz, I.E.; Alcocer-González, J.M.; Villarreal-Treviño, L.; Flores-Treviño, S. Antibiofilm and Antimicrobial Activity of Curcumin-Chitosan Nanocomplexes and Trimethoprim-Sulfamethoxazole on Achromobacter, Burkholderia, and Stenotrophomonas Isolates. Expert Rev. Anti. Infect. Ther. 2023, 21, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Parvin, N.; Joo, S.W.; Mandal, T.K. Nanomaterial-Based Strategies to Combat Antibiotic Resistance: Mechanisms and Applications. Antibiotics 2025, 14, 207. [Google Scholar] [CrossRef] [PubMed]
- Dube, E. Nanoparticle-Enhanced Fish Feed: Benefits and Challenges. Fishes 2024, 9, 322. [Google Scholar] [CrossRef]
- Eissa, E.S.H.; Khattab, M.S.; Elbahnaswy, S.; Elshopakey, G.E.; Alamoudi, M.O.; Aljàrari, R.M.; Munir, M.B.; Kari, Z.A.; Naiel, M.A.E. The Effects of Dietary Spirulina Platensis or Curcumin Nanoparticles on Performance, Body Chemical Composition, Blood Biochemical, Digestive Enzyme, Antioxidant and Immune Activities of Oreochromis Niloticus Fingerlings. BMC Vet. Res. 2024, 20, 215. [Google Scholar] [CrossRef] [PubMed]
- Dube, E.; Okuthe, G.E. Applications of Antimicrobial Photodynamic Therapy in Aquaculture: Effect on Fish Pathogenic Bacteria. Fishes 2024, 9, 99. [Google Scholar] [CrossRef]
- Chaubey, S.; Mehra, S.; Yadav, A.; Kumar, A.; Shahi, V.K. Investigation of Antifouling and Antibacterial Properties of Curcumin-Enriched Surfactant Nanoparticles Modified Polysulfone Nanocomposite Membranes. Mater. Today Chem. 2022, 26, 101130. [Google Scholar] [CrossRef]
- Othman, A.S.; Shamekh, I.M.; Abdalla, M.; Eltayb, W.A.; Ahmed, N.A. Molecular Modeling Study of Micro and Nanocurcumin with in Vitro and in Vivo Antibacterial Validation. Sci. Rep. 2023, 13, 12224. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Bodón, J.; Moreno-Benitez, I.; Laza, J.M.; Larrea-Sebal, A.; Martin, C.; Irastorza, I.; Silvan, U.; Vilas-Vilela, J.L. Multifunctional Curcumin-Based Polymer Coating: A Promising Platform against Bacteria, Inflammation and Coagulation. Colloids Surf. B Biointerfaces 2024, 241, 114048. [Google Scholar] [CrossRef]

| Bacteria (Gram Reaction) | Size | Zeta Potential | Antibacterial Activity | Ref. |
|---|---|---|---|---|
| Acinetobacter baumannii (-) | 2 to 40 nm | - | NPs show antibacterial and anti-biofilm activity. | [85] |
| Aeromonas hydrophila (-) | - | −21.7 mV | NPs exhibited antibacterial activity with a ZOI of 17.6 ± 1.7 mm. | [75] |
| A. veronii (-) | 4.257 ± 0.897 nm | - | Dietary CurNPs reduced A. veronii-induced mortality in African catfish to 41.66%, compared to 58.33% in controls. | [86] |
| Bacillus cereus (+) | 80 ± 2 nm | 4.5 mV | NPs showed antibacterial activity with ZOI of 13 mm at 10 µg/mL). | [83] |
| Enterococcus faecalis (+) | - | - | Positive inhibitory action observed with a ZOI of 12.5 mm. | [87] |
| E. coli (-) | 50 to 100 nm | −24.7 mV | Antibacterial activity demonstrated by a ZOI of 31 mm. | [76] |
| 80 ± 2 nm | 4.5 mV | NPs exhibited both antimicrobial and antioxidant activities with ZOI of 15 ± 0.14 mm at 10 µg/g. | [83] | |
| - | −21.7 mV | NPs exhibited antibacterial activity with a ZOI of 15.9 ± 1.2 mm. | [75] | |
| 87 ± 8 nm | - | Better antibacterial activity than bulk curcumin with ZOI of 24.58 ± 1.12 mm. | [39] | |
| 42.64 nm | - | Antibacterial activity was concentration-dependent, with ZOIs of 15.7 ± 0.03 mm (5 mg/mL), 19.6 ± 0.02 mm (10 mg/mL), and 32.7 ± 0.02 mm (20 mg/mL). | [88] | |
| 95 nm | - | CurNPs inhibits the growth and proliferation of E. coli. | [89] | |
| 34.0 to 359.4 nm | - | Potent antimicrobial activities compared to bulk curcumin. | [90] | |
| Flavobacterium columnare (-) | 34.0 to 359.4 nm | - | Potent antimicrobial activities compared to bulk curcumin. | [90] |
| K. pneumoniae (-) | 42.64 nm | - | Antibacterial activity was concentration-dependent, with ZOIs of 12 ± 0.03 mm (5 mg/mL), 18 ± 0.02 mm (10 mg/mL), and 31.6 ± 0.03 mm (20 mg/mL). | [88] |
| 0.5–4.5 nm | −26 mV | Curcumin quantum dots exhibited excellent antibacterial activity and strong biofilm-degrading ability. | [77] | |
| 13.7 ± 4.9 nm | −13.3 mV | The antibacterial activity of curcumin quantum dots (1 mg/mL) was evidenced by a ZOI of 11.2 ± 0.4 mm. | [84] | |
| P. aeruginosa (-) | 34.0 to 359.4 nm | - | Potent antimicrobial activities compared to bulk curcumin. | [90] |
| 42.64 nm | - | Antibacterial activity was concentration-dependent, with ZOIs of 11.5 ± 0.03 mm (5 mg/mL), 17 ± 0.02 mm (10 mg/mL), and 30.8 ± 0.04 mm (20 mg/mL). | [88] | |
| 0.5–4.5 nm | −26 mV | A bactericidal effect was observed | [77] | |
| 20 to 40 nm | - | CurNPs effectively reduces bacterial growth, inhibits biofilm formation, and downregulates virulence gene. | [91] | |
| 35.96 ± 0.08 nm | - | Potent antimicrobial activities compared to bulk curcumin. | [49] | |
| 13.7 ± 4.9 nm | −13.3 mV | The antibacterial activity of curcumin quantum dots (1 mg/mL) was evidenced by a ZOI of 9.4 ± 0.5 mm. | [84] | |
| 154.2 nm | −19.9 mV | Cur-NPs inhibited biofilm formation up to 96 h, with significant efficacy at 300–700 μg/mL concentrations. | [92] | |
| Vibrio parahemolyticus (-) | 82.7 ± 11.1 nm. | - | Fish (Dicentrarchus labrax) fed 60 or 70 mg/kg nano-curcumin showed higher survival (75%, 60%) than controls (30%). | [67] |
| S. aureus (+) | 50 to 100 nm | −24.7 mV | Antibacterial activity was demonstrated by a 35 mm zone of inhibition. | [76] |
| 80 ± 2 nm | 4.5 mV | NPs exhibited antibacterial activity with a ZOI of 18 mm at 10 µg/mL. | [83] | |
| - | −21.7 mV | NPs exhibited antibacterial activity with a ZOI of 12.2 ± 1.0 mm. | [75] | |
| 42.64 nm | - | Antibacterial activity was concentration-dependent, with ZOIs of 18 ± 0.02 mm (5 mg/mL), 24 ± 0.02 mm (10 mg/mL), and 32.5 ± 0.02 mm (20 mg/mL). | [88] | |
| 87 ± 8 nm | - | Better antibacterial activity than bulk curcumin with ZOI of 29.91 ± 0.53 mm. | [39] | |
| 34.0 to 359.4 nm | - | Potent antimicrobial activities compared to bulk curcumin. | [90] | |
| 13.7 ± 4.9 nm | −13.3 mV | The antibacterial activity of curcumin quantum dots (1 mg/mL) was evidenced by a zone of inhibition measuring 11.3 ± 0.7 mm. | [84] | |
| Streptococcus sp. (+) | 13.7 ± 4.9 nm | −13.3 mV | The antibacterial activity of curcumin quantum dots (1 mg/mL) was evidenced by a zone of inhibition measuring 14.1 ± 0.6 mm. | [84] |
| Mycobacterium marinum (+) | 57.8 ± 17.9 nm | −30.7 ± 4.84 mV | 5% CurNisNps effectively restrained the growth of S. mutans. | [93] |
| Bacteria | Curcumin Nanocomposite (NC) | Size | Zeta Potential | Antibacterial Activity | Ref. |
|---|---|---|---|---|---|
| A. baumannii | Cur-Nisin-based poly (L-lactic acid) NC | 78.6 ± 17.9 nm | −30.7 ± 4.84 mV | Significant cell viability reduction occurred | [96] |
| Cur-Copper Oxide NC | 25 ± 10 nm | −1.07 mV | Concentration-dependent antibacterial activity observed. | [97] | |
| Cur-functionalized silica-coated Fe3O4 magnetic nanocomposite | 40 to 80 nm | −29.9 mV | Enhanced antibacterial activity. | [98] | |
| A. hydrophila | Cur-copper hybrid nanostructures | 19 and 36 mm | - | Enhanced antibacterial activity. | [99] |
| Cur-selenium NC | 50.7 nm | −42.5 mV | A. hydrophila exhibited the greatest sensitivity to Cur-Se NPs compared to CurNPs alone. | [75] | |
| E. coli | Cur–silver (Ag) NC | 25 ± 5 nm | −35 mV | Enhanced antibacterial activity. | [100] |
| Cur-Methoxy-poly ethylene glycol-poly caprolac- tone (mPEG-PCL) NC | 105.62 ± 2.81 nm | −5.67 ± 5.26 mV | 0.125 µM mPEG-PCL/curcumin effectively reduced total microbial count. | [101] | |
| Cur-Copper Oxide NC | 25 ± 10 nm | −1.07 mV | Concentration-dependent antibacterial activity observed. | [97] | |
| Cur-Aloe vera gel NC | - | - | Antibacterial activity as evidenced by a ZOI of 37.16 ± 0.661 mm. | [102] | |
| K. pneumoniae | Cur-SnO2 NC | 50 nm | - | Antibacterial activity as evidenced by a ZOI of 10–12 mm at 2 mg/mL. | [103] |
| Cur-Copper Oxide NC | 25 ± 10 nm | −1.07 mV | Concentration-dependent antibacterial activity observed. | [97] | |
| Molybdenum disulfide–curcumin nanostructures | 13 nm | - | Enhanced antibacterial activity. | [104] | |
| P. aeruginosa | Cur–AgNC | 25 ± 5 nm | −35 mV | Enhanced antibacterial activity. | [100] |
| Cur-Copper Oxide NC | 25 ± 10 nm | −1.07 mV | Concentration-dependent antibacterial activity observed. | [97] | |
| Cur-Falcaria vulgaris extract NC | 10 nm | - | Enhanced antibacterial activity. | [105] | |
| Cur-Gallium NC | 25 to 35 nm | +22.1 mV | Enhanced antibacterial activity. | [106] | |
| Cur-ZnO NC | 253.2 nm | −22.3 mV | Cur-ZnO nanocomposites inhibited biofilm formation up to 96 h, with significant efficacy at 300–700 μg/mL concentrations. | [92] | |
| Cur-poly (ε-caprolactone) (PCL) and poly (lactic acid) (PLA) polymer nanofiber composite | 344 nm fiber diameter | - | The PCL-Cur/PLA membrane exhibited antibiofilm activity, reducing P. aeruginosa biofilm formation by 38 ± 3%. | [107] | |
| Cur loaded poly-(lactic-co-glycolic acid) (PLGA) nanocapsule | 158 nm | −29.1 mV | Encapsulation of curcumin in PLGA nanocomposites significantly enhanced its antibacterial activity. | [108] | |
| Nano-in-micro hydrogels (microbeads) of chitosan and κ-carrageenan (CCMBs) containing Cur-loaded rhamnosomes | 116 ± 7 nm | −24.5 ± 9.4 mV | Significant antibacterial activity, reducing P. aeruginosa counts in vitro and infected mice models. | [109] | |
| Cur-Methoxy-polyethylene glycol-poly caprolactone (mPEG-PCL) NC | 105.62 ± 2.81 nm | −5.67 ± 5.26 mV | 0.125 µM mPEG-PCL/curcumin effectively reduced total microbial count. | [101] | |
| Cur-AgNC-Niosomes | 188.1 ± 10.21 nm | - | Antibacterial activity as evidenced by a ZOI of 24.00 ± 1.67 mm. | [110] | |
| Cur-Copper Oxide NCs | 25 ± 10 nm | −1.07 mV | Concentration-dependent antibacterial activity observed. | [97] | |
| Cur-Copper (Cu) NC-Niosomes | 197.5 ± 6.42 nm | - | Antibacterial activity as evidenced by a ZOI of 27.00 ± 1.40 mm. | [110] | |
| Vibrio parahemolyticus | Cur-copper hybrid nanostructures | 19 and 36 nm | - | Enhanced antibacterial activity. | [99] |
| Lactococcus garvieae | Cur-copper hybrid nanostructures | 19 and 36 nm | - | Enhanced antibacterial activity. | [99] |
| S. aureus | Cur-Aloe vera gel NC | - | - | Antibacterial activity as evidenced by a ZOI of 38.75 ± 0.19 mm. | [102] |
| Cur–AgNC | 25 ± 5 nm | −35 mV | Enhanced antibacterial activity. | [100] | |
| Cur-AgNC-Niosomes | 188.1 ± 10.21 nm | - | Antibacterial activity as evidenced by a ZOI of 26.00 ± 1.50 mm. | [110] | |
| Nano-in-micro hydrogels (microbeads) of chitosan and κ-carrageenan (CCMBs) containing Cur-loaded rhamnosomes | 116 ± 7 nm | −24.5 ± 9.4 mV | Significant antibacterial activity, reducing S. aureus counts in vitro and infected mice models. | [109] | |
| Cur-loaded nano metal-organic framework (MOF) (Cur@Cu-UIO-66-NH2) | 200 nm | 19.6 ± 1.7 mV | The Cur@Cu-MOF/CMCS-OSA hydrogel markedly enhanced antibacterial activity and exhibited a strong inhibitory effect on biofilm formation. | [111] | |
| Cur-Copper (Cu) NC-Niosomes | 197.5 ± 6.42 nm | - | Antibacterial activity as evidenced by a ZOI of 29.00 ± 1.75 mm. | [110] | |
| S. maltophilia | Cur-chitosan and sodium tripolyphosphate magnetic NCs in combination with trimethoprim-sulfamethoxazole | ~100 nm | - | Biofilm inhibition activity at concentrations ranging from 4.69 to 18.75 µg/mL. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dube, E.; Okuthe, G.E. Antimicrobial Efficacy of Curcumin Nanoparticles Against Aquatic Bacterial Pathogens. Future Pharmacol. 2025, 5, 44. https://doi.org/10.3390/futurepharmacol5030044
Dube E, Okuthe GE. Antimicrobial Efficacy of Curcumin Nanoparticles Against Aquatic Bacterial Pathogens. Future Pharmacology. 2025; 5(3):44. https://doi.org/10.3390/futurepharmacol5030044
Chicago/Turabian StyleDube, Edith, and Grace Emily Okuthe. 2025. "Antimicrobial Efficacy of Curcumin Nanoparticles Against Aquatic Bacterial Pathogens" Future Pharmacology 5, no. 3: 44. https://doi.org/10.3390/futurepharmacol5030044
APA StyleDube, E., & Okuthe, G. E. (2025). Antimicrobial Efficacy of Curcumin Nanoparticles Against Aquatic Bacterial Pathogens. Future Pharmacology, 5(3), 44. https://doi.org/10.3390/futurepharmacol5030044






