Abstract
Background/Objectives: Advances in understanding immune checkpoint pathways and tumor immune biology have enabled the development of immune checkpoint inhibitors (ICIs), particularly targeting the PD-1/PD-L1 axis, which has transformed cancer immunotherapy. While they have shown remarkable success in various cancer types, including melanoma, non-small cell lung cancer, and gastrointestinal malignancies, variability in patient response, immune-related adverse events (irAEs), and resistance mechanisms remain significant. This review aims to evaluate clinical pharmacology, mechanisms of action, resistance pathways, and pharmacogenomic influences shaping interindividual responses to ICIs. Methods: This comprehensive review synthesizes current literature on FDA-approved ICIs, exploring their clinical use, underlying biological mechanisms, and emerging pharmacogenomic data. It also assesses key biomarkers such as tumor mutational burden (TMB), microsatellite instability (MSI), HLA diversity, and epigenetic factors influencing ICI efficacy and safety. Results: We outline key mechanisms contributing to ICI resistance, including T cell dysfunction, altered antigen presentation, and immunosuppressive tumor microenvironment components. Furthermore, we highlight promising pharmacogenomic findings, including single-nucleotide polymorphisms (SNPs) in PD-1/PD-L1 and immune-regulatory genes, offering predictive and prognostic utility. Variability in PD-L1 expression and the role of epigenetic modifications are also addressed as challenges in treatment optimization. Conclusions: Interindividual variability in ICI response underscores the need for biomarker-driven strategies. By integrating pharmacogenomic insights with clinical pharmacology, future approaches may support more personalized and effective use of ICIs. Combination therapies and novel modalities hold promise for overcoming resistance, enhancing therapeutic efficacy, and enabling precision oncology.
1. Introduction
The understanding of tumor immune biology has led to the development of innovative immune-based treatments, known as cancer immunotherapy. This therapeutic approach involving novel agents has proven very promising in treating both hematological malignancies and solid tumors. In particular, these therapies are designed to boost the patient’s immune system to recognize and eliminate cancer cells more effectively [1]. This is achieved by the administration of monoclonal antibodies (mAbs) targeting immune checkpoint proteins known as immune checkpoint inhibitors (ICIs). The main protein targets are the programmed cell death protein-1 (PD1), programmed death ligand 1 and 2 (PD-L1 and PD-L2), and the cytotoxic T-lymphocyte-associated antigen-4 (CTLA4); the administration of these agents results in a derepression and/or reactivation of cytotoxic T cell function, enabling the patient’s immune system to attack cancer cells. That means that immune checkpoint blockade releases the brakes on the immune system, enhancing anticancer immune response. As a result, their use in various malignancies has the potential to induce a robust and long-lasting response.
Several ICIs have been approved by the United States Food and Drug Administration (FDA) since 2011 for the treatment of a wide range of advanced-stage tumors, and currently various ICIs are available as first- or second-line therapy for several malignancies. The first drugs that were approved are the anti-PD1 Pembrolizumab and Nivolumab, as well as the anti-CTLA4 Ipilimumab for the treatment of metastatic melanoma.
In this review, we focus on immune checkpoint inhibitors (ICIs), especially on agents acting against the programmed cell death protein 1 (PD-1)/programmed cell death protein 1 ligand (PD-L1) axis, which have drastically altered the therapy landscape of various malignancies. With sufficient background of the history and FDA approval of these treatments, the review examines their mechanisms of action and their current use in hematological and solid tumors. Further discussion ensues around the pharmacokinetic/pharmacodynamic properties of ICIs that affect exposure to the drug and response to therapeutics. Another focus is pharmacogenomics, including single-nucleotide polymorphisms (SNPs), HLA diversity, and other germline variations, which contribute to the interindividual variability in efficacy and immune-related adverse events. Also presented are analyses of the multiple resistance mechanisms to ICI treatment—primary and acquired-—with aspects varying from changes in tumor antigen presentation and immune microenvironment to epigenetic reprogramming and oncogenic signaling pathway dysregulation. Finally, this review underscores cutting-edge approaches driven by biomarkers and combination regimens to circumvent resistance and further targeted, cost-effective immunotherapies, consequently pushing precision medicine in oncology from theory into practice.
2. Evolution of Immunotherapy in Cancer Treatment
The use of immune checkpoint inhibitors in clinical cancer care has dramatically changed our understanding of personalized medicine [2]. Since 2011, the US FDA has approved various agents that have significantly extended survival in patients with various cancer types, supported by preclinical and clinical data. The development of cancer immunotherapy was advanced by key discoveries, showing that T cell immune responses are regulated by immune checkpoints that act as on/off switches via the cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) pathway or the PD-1/PD-L1 pathway [3].
A critical first step in the development of ICIs was the elucidation of their biological components and mechanisms of action. The PD-1 gene was cloned as a new immunoglobulin gene superfamily from stimulated mouse T cell hybridoma by Tasuku Honjo in 1992 [4] through T cell activation. Subsequent studies by several groups identified the ligands of PD-1—namely programmed death-ligand 1 (PD-L1) and programmed death-ligand 2 (PD-L2)—were shown to physically bind to PD-1 and suppress T cell activity by delivering inhibitory signals. This led to the characterization of the physical interaction between PD-1 and its ligands (PD-L1 and PD-L2), contributing to the downregulation in T cell response [5,6]. In 2002, it was reported by Honjo and Lieping Chen groups that PD-L1, expressed by tumor cells, could inhibit tumor-reactive T cells as one of the immune evolution mechanisms. Thus, targeting the PD-1/PD-L1 axis could be a possible cancer treatment [7]. In 2006, Ono and Medarex developed antibodies targeting human PD-1 and human PD-L1, and started a phase I/II clinical study in cancer patients [8]. In 2018, the Nobel Prize in Physiology or Medicine 2018 was awarded to James P. Allison and Tasuku Honjo for their discovery in cancer immunotherapy through the inhibition of negative immune regulatory pathways [9].
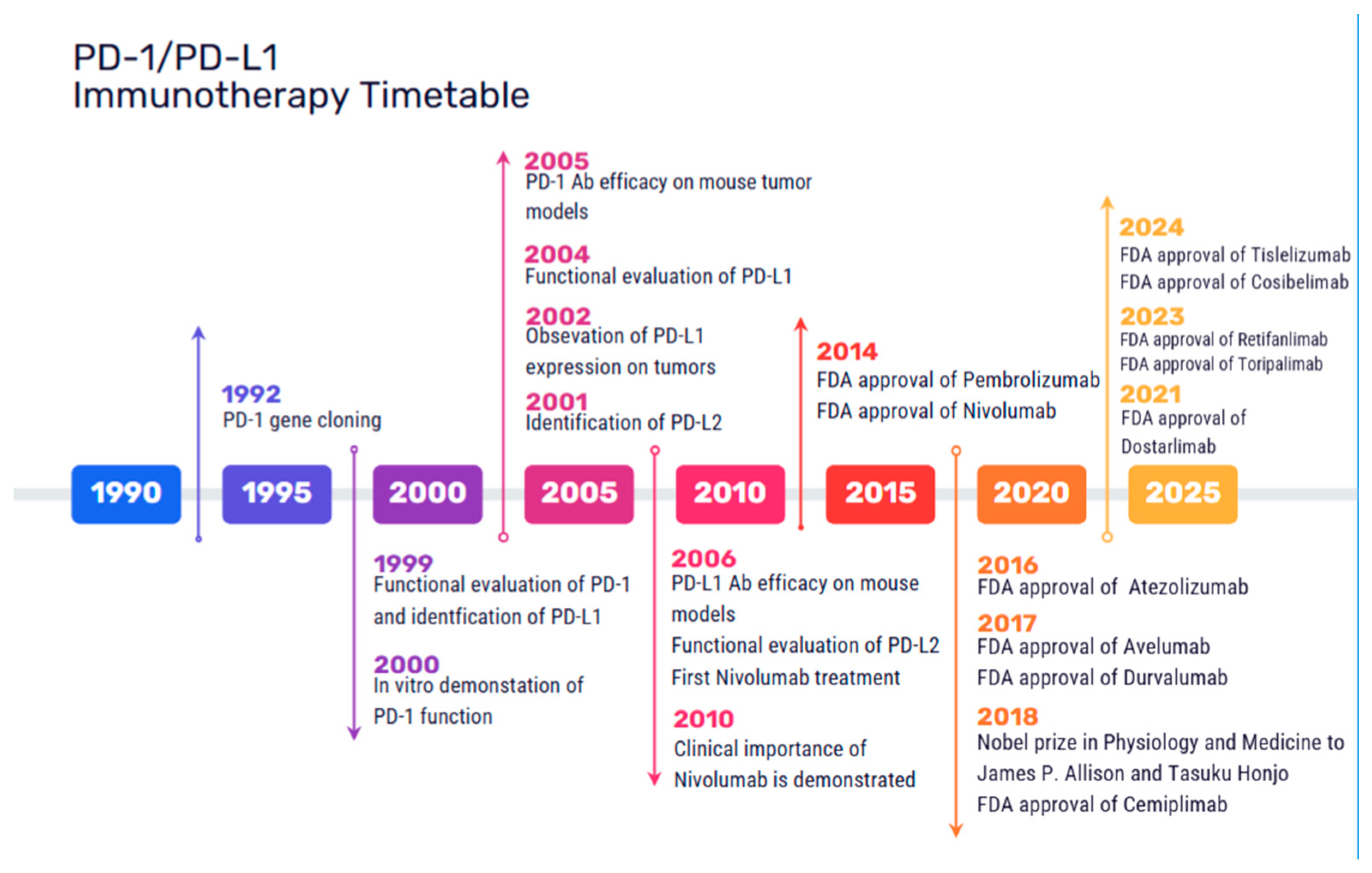
CTLA-4 is an immune checkpoint molecule that negatively regulates the initiation of T cell activation. It competes with CD28 for binding to the ligands CD80 and CD86, resulting in reduced T cell activation [10]. The first approved ICI in 2011 was Ipilimumab, a CTLA-4 inhibitor for the treatment of metastatic and nonresectable melanoma. PD-1 mainly regulates T cell proliferation following activation. PD-1 is expressed on a variety of immune cell types, limiting the immune response and T cell activity in peripheral tissues. PD-L1 and PD-L2 are PD-1 receptor ligands, mediating immunosuppressive effects. PD-1/PD-L1 inhibitors have been developed since 2014 and they are currently the cornerstone of cancer immunotherapy, with over 2000 clinical trials exploring their efficacy as monotherapies or combination therapies across a broad spectrum of malignancies. The first agents that were approved include the PD-1 inhibitors Pembrolizumab and Nivolumab in 2014 for advanced melanoma; Nivolumab in 2015 for advanced non-small cell lung cancer (NSCLC) and renal cell carcinoma (RCC), as well as Pembrolizumab for advanced NSCLC; in 2016 the PD-L1 inhibitor Atezolizumab for the treatment of locally advanced or metastatic urothelial carcinoma [11]; and in 2017, Durvalumab for advanced urothelial bladder cancer [12] and Avelumab for refractory metastatic Merkel cell carcinoma [13]. Currently, approved immune checkpoint inhibitors mainly include agents targeting PD-1 (Nivolumab, Pembrolizumab, Cemiplimab, Dostarlimab, Tislelizumab, Retifanlimab, Toripalimab and Camrelizumab), PD-L1 (Atezolizumab, Avelumab, Durvalumab and Cosibelimab), CTLA-4 (Ipilimumab and Tremelimumab), and LAG3 (Relatlimab, Fianlimab and Favezelimab) [1]. A timetable of the FDA-approved ICIs is shown in Figure 1.
Figure 1.
Milestones in the development and FDA approval of immune checkpoint inhibitors targeting the PD-1/PD-L1 axis. The timeline presents key discoveries in checkpoint biology, preclinical tests, pivotal clinical trials, and the regulatory act from 1992 up to 2024.
Due to the different methods of regulation of T cell function through CTLA-4 and PD-1, the combination of anti-PD-1 antibody (which promotes the proliferation and recruitment of pre-existing anti-tumor T cells) and anti-CTLA-4 antibody (which induces new T cell clones) has been used for the simultaneous blocking of the immune escape of tumor cells, enhancing the anti-tumor activity of T cells at different stages. Thus, the ICI combination therapy is currently FDA-approved for several malignancies [14].
Furthermore, over the last decade, other immunotherapies such as adoptive T cell therapy have been developed. Firstly, Chimeric antigen receptor T (CAR-T) cell therapy, a class of immunotherapy, combines the antigen-binding site of a monoclonal antibody with the signal-activated machinery of a T cell, enabling major histocompatibility complex (MHC)-independent antigen recognition by T cells [15]. CAR-T has been used in the treatment of hematological malignancies, including B-cell leukemia, lymphoma, and multiple myeloma, demonstrating significant improvements in patient outcomes and overall survival [16]. Another category of immune cells which are implicated in cancer immunology are the T cell receptor-engineered T cells (TCR-T). They act in a similar way as the natural T cells in the human body by recognizing MHC-presented antigens through affinity-optimized or purely natural TCRs. TCR-T cells recognize tumor antigens that are processed intracellularly (including nuclear antigens) and presented on the surface of tumor cells by MHC molecules [17]. Tumor-infiltrating lymphocyte (TIL) therapy represents a promising strategy, where lymphocytes extracted from the patient’s tumor are expanded outside the body and reintroduced to enhance the antitumor immune response [18]. An emerging therapy concerns the Bispecific T cell engager (BiTE), which is a bispecific antibody construct, designed to simultaneously bind an antigen on tumor cells and a T cell surface receptor, effectively promoting targeted tumor cell lysis [19]. Recently, these agents have been explored in the treatment of both solid tumors and hematopoietic malignancies, showing potential clinical benefits as promising emerging therapies [20]. Another innovative category for cancer treatment involves therapeutic cancer vaccines demonstrating potential by stimulating T cell responses that target tumor-specific antigens [21]. Finally, oncolytic virus (OV) therapy represents a novel class of immunotherapy that exerts antitumor effects through direct lysis of infected tumor cells specifically and also by activating the innate and adaptive immune response [22]. These therapies will not be included in this review.
3. Mechanisms of ICIs Action
The PD-1/PD-L1 axis is the most extensively studied negative regulatory immune checkpoint pathway in recent years and plays a key role in tumor immune evasion. PD-1, also known as PDCD1 and CD279, is a type I transmembrane protein and a member of the B7/CD28 receptor superfamily, consisting of 288 amino acids, PD-L1 (CD274) and PD-L2 (CD273), ligands of PD-1, are also type I transmembrane proteins belonging to the same superfamily. They consist of 290 and 273 amino acids, respectively, sharing 37% sequence homology [23,24].
The structure of PD-1 includes of four regions: an immunoglobulin variable region (IgV), a transmembrane region, immunoreceptor tyrosine-based inhibitory motifs (ITIMs), and immunoreceptor tyrosine-based switch motifs (ITSMs) [25]. Upon ligand binding to the receptor, these motifs become phosphorylated, recruiting tyrosine acid phosphatase Src homology phosphatase 1 (SHP-1) and Src homology phosphatase 2 (SHP-2) [26]. These phosphatases repress T cell receptor (TCR) signaling, dephosphorylating several key downstream proteins in signaling pathways, such as phosphoinositide 3-kinase (PI3K), protein kinase B (PKB/AKT), mammalian target of rapamycin (mTOR), rat sarcoma (RAS), mitogen-activated protein kinase (MAPK/MEK), extracellular regulated protein kinase (ERK), etc. This inhibition leads to suppression of related gene expression, blockade of T cell cycle progression, inhibition of cytokine production and proliferation, and differentiation of T cells, causing immune tolerance within the tumor microenvironment [27].
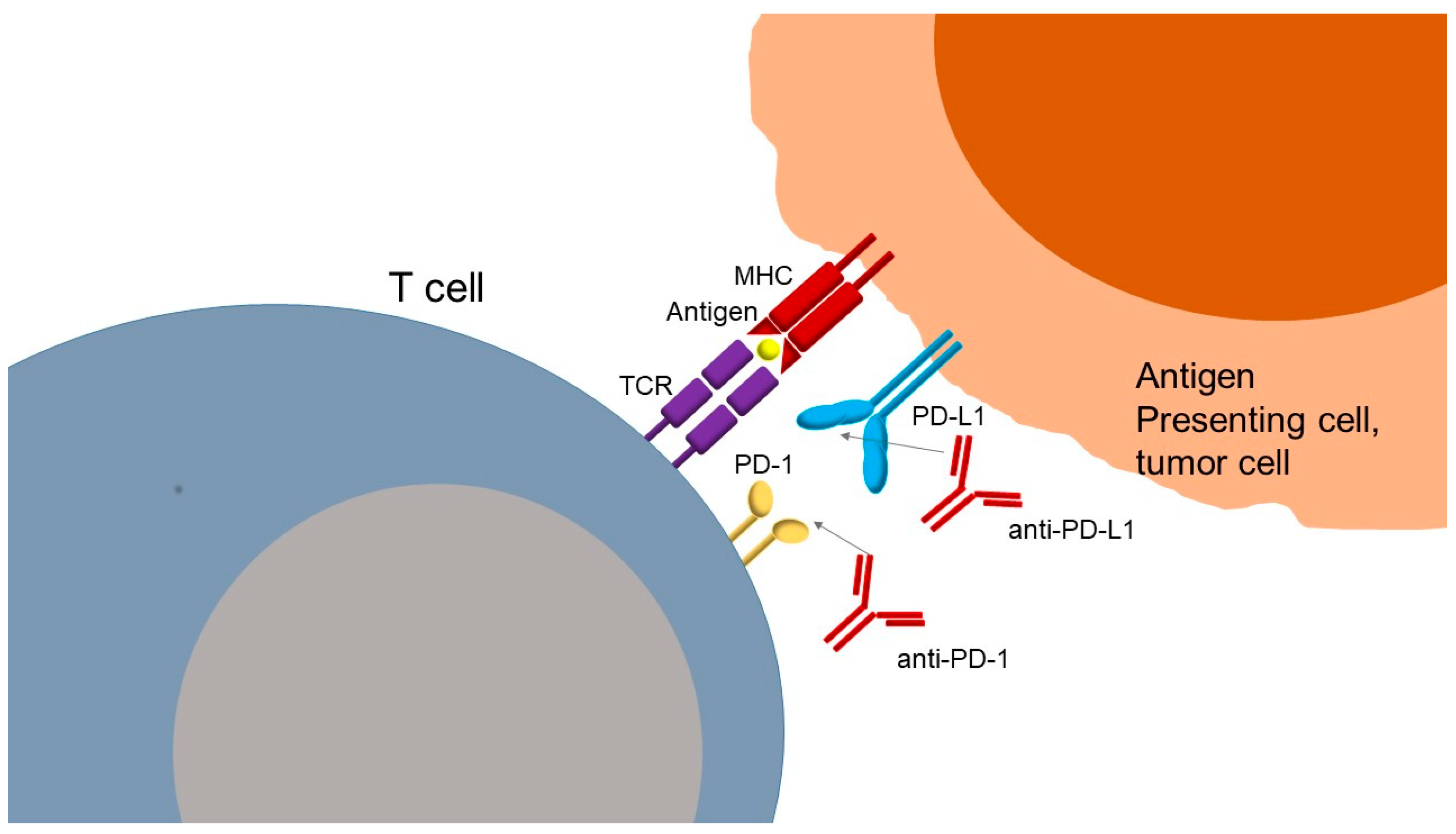
The mechanism of action of anti-PD-1 or anti-PD-L1 antibodies (Figure 2) is based on inhibition of the interaction of PD-1 with its ligands, PD-L1 and PD-L2, restoring T cell activity. PD-L1 is more widely expressed than PD-L2 but has a lower affinity for PD-1. Ligands are found at the surface of tumor cells, where their expressions can be induced by type I and II interferons, and at the surface of immune cells, such as macrophages and dendritic cells. PD-1 receptor is mainly expressed by lymphocytes secondarily to their activation. The interaction between PD-1 and its ligands negatively regulates lymphocyte function by suppressing signals by the TCR and the co-stimulation of molecules, as previously described. Thus, the PD-1/PD-L1 axis serves as a tumor immune escape mechanism. Blocking this pathway with anti-PD-1 or anti-PD-L1 antibodies can reactivate tumor-specific lymphocytes within the tumor, leading to effective tumor cell elimination. In addition to PD-1 and CTLA-4, other immune checkpoints have been identified that regulate T cell activation either positively or negatively. These include lymphocyte activation gene-3 (LAG-3), which binds to MHC class II molecules and lectins; T cell immunoglobulin mucin receptor 3 (TIM-3), which interacts with galectin-9; B and T lymphocyte attenuator (BTLA) and its ligand herpesvirus entry mediator (HVEM); TIGIT, which binds to CD155; and VISTA, a suppressor of T cell activation for which the ligand remains unclear [28,29]. In the past years, these molecules have been identified as checkpoints in immune regulators; however, current FDA-approved therapies still do not target them. Understanding their biology provides novel therapeutic opportunities, particularly in combination with PD-1/PD-L1 blockade to overcome resistance and improve clinical efficacy.
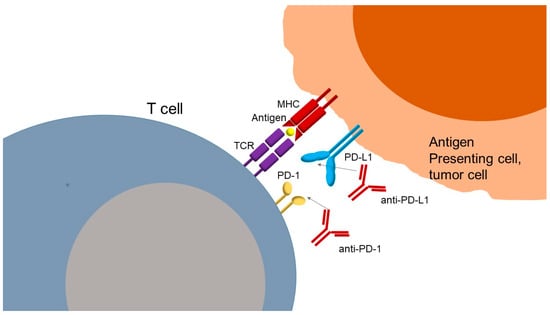
Figure 2.
Mechanism of action of PD-1 PD-L1 inhibitors against cancer cells. TCR: T cell receptor; MHC: major histocompatibility complex; PD-1: programmed cell death protein 1; PD-L1: programmed death-ligand 1; anti-PD-1: monoclonal antibody against PD-1; anti-PD-L1: monoclonal antibody against PD-L1.
Although the primary mechanism of action of PD-1/PD-L1 inhibitors involves reactivation of exhausted cytotoxic T lymphocytes by disrupting PD-1/PD-L1 signaling, a growing body of evidence has revealed additional effects that contribute to their efficacy. Notably, PD-1 is also expressed on tumor-associated macrophages (TAMs), where it suppresses phagocytic activity and antigen presentation [30]. The inhibition of PD-1 leads to restoration of innate immune functions and promotion of antitumor responses mediated by myeloid cells [31]. Furthermore, PD-L1 is not merely a passive ligand but can also function as a bidirectional signaling molecule. Beyond its interaction with PD-1, PD-L1 can transmit reverse signals within tumor cells, activating oncogenic pathways such as STAT3 and AKT, promoting epithelial–mesenchymal transition, and enhancing resistance to apoptosis and therapies [32,33,34]. These tumor-intrinsic functions suggest that PD-L1 blockade may exert direct antitumor effects.
Furthermore, PD-L1 is expressed on non-immune stromal cells and immunosuppressive myeloid populations, such as myeloid-derived suppressor cells (MDSCs), where it contributes to local immune evasion. Targeting PD-L1 in these cells may disrupt immune tolerance-promoting signaling pathways within the tumor, enhancing overall immune activation [33,35]. PD-1/PD-L1 blockade has also been shown to reshape immune cell metabolism and transcriptional programs. Transcriptomic and epigenetic profiling studies reveal that PD-1 inhibition can reprogram progenitor-exhausted CD8+ T cells, preserving their self-renewal capacity and enabling durable antitumor immunity. These effects extend beyond transient functional restoration and may underpin the long-term responses observed in some patients [35,36].
In addition to these mechanisms, PD-1/PD-L1 inhibitors have also been shown to modulate other components of the immune system. PD-1 is expressed on natural killer (NK) cells and dendritic cells (DCs), where it impairs cytotoxicity and antigen presentation, respectively. Immune checkpoint blockade in this setting enhances both innate cytotoxic responses and the initiation of adaptive immunity [33,35]. Moreover, immune checkpoint inhibition promotes the development of tertiary lymphoid structures (TLSs) within the tumor microenvironment. TLSs serve as ectopic lymphoid aggregates that facilitate local antigen presentation and T/B cell crosstalk. The presence of mature TLSs has been associated with improved immunotherapy outcomes in multiple tumor types [34]. Finally, PD-L1 blockade may disrupt intrinsic survival signaling in tumor cells, leading to direct cytotoxic effects independently of T cell activity. This mechanism may be particularly relevant in tumors with low immune infiltration or acquired resistance to immune attack [33,37]. Collectively, these emerging insights highlight the broader immunomodulatory potential of PD-1/PD-L1 inhibitors and support their use in combination therapies and biomarker-based strategies that extend beyond the classical T cell axis [38].
4. The Role of ICIs in the Treatment of Solid Tumors
Immunotherapy has been used in different types of malignancies, as mentioned bellow. The PD-1 and PD-L1 inhibitors that have been already approved by the FDA are shown in Table 1.

Table 1.
The PD-1 and PD-L1 inhibitors that have been approved by the FDA for the treatment of multiple cancer types.
4.1. Skin Cancers
Melanoma is a skin cancer that develops as the consequence of malignant transformation and proliferation of melanocytes. The main therapeutic procedure is surgery. In several cases, metastatic disease occurs and the survival outcome is poor (5-year overall survival (OS) 29.8%). The development of ICIs has revolutionized the treatment of advanced and metastatic cancers [39]. The approval of Ipilimumab by the FDA as a first-line treatment for unresectable stage III/IV melanoma marked the initiation of broader application of ICIs across multiple cancers types and ultimately led to an important transformation in the oncologic therapeutic strategy.
In 2015, the FDA approved the first combination of PD-1 (Nivolumab) and CTLA-4 (Ipilimumab) for immune checkpoint blockade for patients with BRAFV600 wild-type unresectable/metastatic melanoma [40], as this combination demonstrated advantage in progression free survival (PFS) and OS compared to monotherapy with Ipilimumab [41]. Very recently, for patients with resectable stage III melanoma, neoadjuvant Ipilimumab plus Nivolumab followed by surgery and response-driven adjuvant therapy resulted in longer event-free survival than surgery followed by adjuvant Nivolumab [42]. Another study showed that Nivolumab is a proven adjuvant treatment for resected melanoma at high risk of recurrence, with sustained, long-term improvement in recurrence-free survival with Ipilimumab and high OS rates. Identification of additional biomarkers is needed to better predict treatment outcome [43]. Furthermore, Pembrolizumab continued to prolong recurrence-free survival within >4 years follow-up [44].
For advanced/metastatic non-melanoma skin cancers (such as Merkel cell carcinoma and basal cell carcinoma), the ICIs Avelumab, Pembrolizumab, Cemiplimab, and Retifanlimab have also received FDA approval [13,45,46,47]. Finally, another anti PD-L1 inhibitor, Cosibelimab, was very recently approved by the FDA for metastatic or locally advanced cutaneous squamous cell carcinoma [48].
4.2. Lung Cancer
Lung cancer, the leading cause of cancer-related mortality worldwide, is frequently diagnosed at an advanced stage. Immunotherapy has provided increases in overall survival during the past decade in patients with non-small cell lung cancer (NSCLC) [49]. Nivolumab, Atezolizumab, and Pembrolizumab have been evaluated as potential first-line therapies, especially in cases with PD-L1 expression (determined by Tumor Proportion Score, TPS); it has been shown that patients with TPS ≥ 50% had a tumor response rate of 50% [50]. Atezolizumab and Cemiplimab have also been approved as first-line monotherapy agents for advanced NSCLC patients with PD-L1 tumor expression, as they demonstrated improvement in OS compared to platinum-based chemotherapy. Thus, anti-PD-L1 is considered as a standard first-line monotherapy for PD-L1 ≥ 50% advanced NSCLC [51,52]. Furthermore, a recently published meta-analysis of three phase III clinical trials in 559 advanced non-squamous NSCLC patients reported that patients with KRAS mutations showed improved response, though wildtype KRAS status alone is insufficient to predict lack of benefit [53,54]. Perioperative treatment with Nivolumab resulted in significantly longer event-free survival than chemotherapy in patients with resectable NSCLC. No new serious adverse events were observed in this study [55]. The FDA has also approved Pembrolizumab followed by resection in early-stage NSLC, as this treatment has benefits in survival [56].
Combination therapies with or without classical chemotherapy have also been approved [56,57,58,59,60]. A significant recent advancement in immunotherapy is the application of ICIs in treating early-stage NSCLC. Atezolizumab has been approved as adjuvant treatment following resection and platinum-based chemotherapy in adult patients with stage II-IIIA NSCLC whose tumors express PD-L1 on ≥1% of tumor cells [61]. Similar approvals have taken place for Pembrolizumab and Durvalumab as neoadjuvant treatment for resectable NSCLC as well [56,60].
Furthermore, for small cell lung cancer, a cancer with one of the poorest survival rates of all solid tumors (5-year survival rates less than 5%), ICIs have been evaluated as treatment options; however, no significant response was recorded. Atezolizumab and Durvalumab have been proposed as first-line therapies in combination with etoposide and a platinum-based chemotherapy regimen. The improvement in median OS was 12–13 months [62,63]. In a very recent study, adjuvant therapy with Durvalumab led to significantly longer overall survival and PFS than placebo among patients with limited-stage small-cell lung cancer [64].
4.3. Gastrointestinal Malignancies
Malignancies of the gastrointestinal tract are among the most common cancer types. Finally, when we speak of stomach or gastric cancer, it is notoriously a disease with a survival ceiling, hence, in its advanced stage, it almost sits squarely on conventional chemotherapy. The addition of ICIS may be promising. Nivolumab and Pembrolizumab have been administered in combination with classical chemotherapy or Trastuzumab for HER2+ tumors and have been approved as second-line treatments for advanced or metastatic gastric and esophageal tumors [65,66,67,68,69]. In March 2025, the FDA granted traditional approval to Pembrolizumab in combination with Trastuzumab and classical chemotherapy for HER2+ tumors of gastric or gastroesophageal junction adenocarcinoma expressing PD-L1 (CPS ≥ 1) [70]. A new PD-1 inhibitor named Tislelizumab has been promising in combination with chemotherapy in advanced HER-2 negative gastric cancer [71]. Durvalumab plus chemotherapy is also a promising treatment for patients with resectable gastric cancer [72].
For metastatic colorectal cancer patients, the median 5-year OS is approximately 14.7%. Currently, all ICIs approved for the treatment of colorectal cancers have been limited to the subset of patients with microsatellite instability-high/mismatch repair deficient (MSI-H/dMMR) metastatic colorectal cancer (mCRC) [73]. Ipilimumab, in combination with Nivolumab, as well as Pembrolizumab, was subsequently approved for unresectable/metastatic MSI-H/dMMR metastatic colorectal cancers [73,74]. Recently, in patients with locally advanced dMMR colon cancer, neoadjuvant Nivolumab plus Ipilimumab showed an acceptable safety profile and led to a significant response in a high proportion of these patients [75].
Regarding patients with advanced hepatocellular carcinoma, both Nivolumab and Pembrolizumab have been approved for patients with advanced hepatocellular carcinoma (HCC); Pembrolizumab has been administered in patients previously treated with Sorafenib [76]. Other drugs that have been implicated in disease treatment include Nivolumab plus Ipilimumab as second-line treatment [77], and more recently Atezolizumab in combination with Bevacizumab (approved as first-line therapy in 2020), as well as Tremelimumab in combination with Durvalumab; these combinations have been approved due to the significant improvement in OS compared to previous treatments which included Sorafenib [78,79]. In 2023, Camrelizumab in combination with Rivoceranib showed a significant and clinically meaningful benefit in PFS and OS compared with Sorafenib for patients with unresectable hepatocellular carcinoma, presenting a new and effective first-line treatment option [80].
4.4. Gynecological Malignancies
Although the incidence of cervical cancer has reduced in recent years due to cancer screening programs and vaccination against the human papillomavirus (HPV), cervical cancer remains a significant cause of mortality particularly in developing nations. Several studies have demonstrated relatively high PD-1/PD-L1 expression in cervical tumors, suggesting their potential as targets for immune checkpoint blockade. Since 2018, Pembrolizumab has been approved for the treatment of recurrent/metastatic cervical cancer expressing PD-L1 (combined positive score, CPS ≥ 1), either as monotherapy or in combination with chemotherapy. Its combination with platinum-based chemotherapy ± Bevacizumab demonstrated a significant survival benefit [81].
Regarding advanced endometrial cancer in 2021, the FDA granted accelerated approval for Dostarlimab (anti-PD-1) in the second-line setting, following standard platinum-based chemotherapy, for patients with dMMR/MSI-H endometrial cancers due to a significant overall response rate in these cases [82]. Indeed, improvements in survival and the manageable safety profile support the favorable benefit–risk profile for Dostarlimab plus Carboplatin–Paclitaxel in patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer [83]. Pembrolizumab has also been approved as monotherapy or in combination with the oral TKI Lenvatinib, as this agent has also improved the objective response rate (ORR) [84]. Carboplatin–Paclitaxel plus Durvalumab followed by maintenance Durvalumab with or without Olaparib demonstrated a statistically significant and clinically meaningful PFS benefit in patients with advanced or recurrent endometrial cancer [85].
4.5. Genitourinary Malignancies
Renal cell carcinoma (RCC) comprises 90% of all kidney cancers, with clear cell being the most common subtype. Approximately 33% of patients with RCC present with advanced or metastatic disease at the time of diagnosis. Although RCC demonstrates resistance to chemotherapy, it is comparatively more sensitive to immunotherapy and antiangiogenics than other cancer types. Nivolumab was the first FDA-approved agent, and three ICIs have also been approved as first-line therapies for advanced clear-cell RCC, in combination with either another ICI or with antiangiogenic inhibitors. These combinations comprise Nivolumab + Ipilimumab, Pembrolizumab + Axitinib, Avelumab + Axitinib, Nivolumab + Cabozantinib, and Pembrolizumab + Lenvatinib [86,87,88,89,90]. As no direct head-to-head trial has compared dual immune checkpoint inhibition with checkpoint blockade anti-angiogenic TKI combinations, first-line therapy selection remains an area of ongoing debate.
Furthermore, for advanced urothelial carcinoma, the current standard, first-line therapy is platinum-based chemotherapy, while Pembrolizumab has also been approved as first-line therapy for platinum-ineligible advanced/metastatic cases [91]. Along with Pembrolizumab, Avelumab and Nivolumab have also been implicated as possible first- or second-line therapy with/or without chemotherapy as treatment modalities for advanced disease cases [92,93].
4.6. Head and Neck Squamous Cell Carcinomas (HNSCC)
HNSCC includes malignancies of the oral cavity, oropharynx, hypopharynx, and larynx, for which initial treatment involves local therapeutic intervention. However, in cases of recurrent/metastatic HNSCC, the outcomes remain poor following classical chemotherapy. ICIs demonstrate both manageable safety profiles and improved overall survival compared to previous standard treatments. Pembrolizumab is currently approved as first-line therapy for unresectable recurrent/metastatic HNSCC in combination with chemotherapy, showing improved survival in patients with PD-L1 positive HNSCC (CPS ≥ 1) [94]. A randomized, open-label, multi-centred, phase III clinical trial showed that adjuvant PD-1 blockade with Camrelizumab significantly improved event-free survival with manageable toxicities, highlighting its potential role in the management of locoregionally advanced NPC [95]. A novel PD-1 inhibitor, Tislelizumab, demonstrated promising efficacy and tolerability in patients with head and neck cancers in both clinical trials and real-world studies [96]. Another PD-1 inhibitor, Toripalimab, may be a highly promising therapy for the treatment of locoregionally advanced nasopharyngeal carcinoma and was recently approved by FDA as first-line therapy [97].
4.7. Other Cancers
Regarding breast cancers, triple-negative breast cancer (TNBC) is an aggressive tumor that is difficult to treat due to a lack of targeted agents. Chemotherapy is the standard-of-care for systemic treatment and includes taxanes or platinum-based agents. However, these tumors can become rapidly resistant to chemotherapy. In 2019, Atezolizumab and more recently Pembrolizumab have been approved for the treatment of these tumors in combination with chemotherapy, showing improvement in PFS [98,99].
For lymphomas, Nivolumab and Pembrolizumab have been approved as second-line therapies for relapsed/refractory classical Hodgkin lymphoma after autologous HSCT, with Pembrolizumab as a third-line therapy for relapsed/refractory primary mediastinal B-cell lymphoma [100,101].
5. ICIs Pharmacokinetics—Pharmacodynamics
Intravenous ICIs are distributed and metabolized by various routes. Their extensive binding to target antigens in the plasma or tissues reduces the circulation of free drug and increases the volume of distribution. Transvascular transport of free ICIs is principally driven by convection, which is modulated by factors such as organ perfusion and endothelial permeability. Within tissues, ICIs become distributed by means of diffusion and convection. The neonatal Fc receptor (FcRn) plays a crucial role in preventing the lysosomal degradation of these drugs, facilitating their recycling into circulation, and hence prolonging their half-life (t½ 6–27 days). This prolonged tissue exposure may therefore increase treatment effects without the necessity for frequent drug administration [102]. Due to their high polarity, mAbs exhibit limited distribution into peripheral tissues. An important mechanism influencing distribution is the high binding affinity to target antigens, since mAbs localize and stay in microenvironments where their antigens are expressed. Concurrently, tumor size decreases in response to therapy, with altered tumor vascularization and blood flow, which may further alter mAb distribution patterns. Therefore, the steady-state volume distribution (Vss) for mAbs remains low and approximately equal to plasma volume [103].
Furthermore, it has been shown that the FcRn is subject to genetic influence based on a variable number of tandem repeats in the promoter region of the FcRn gene (FCGRT). An increased number of tandem repeats has been shown to increase its expression. As FcRn is responsible for salvaging IgG, reduced expression is thought to result in lower serum concentration and increased clearance via alternative mechanisms [104]. Fc isoforms are important determinants of Fcγ receptor (FcγR)-mediated interactions, wherein the IgG1 subtype displays a higher affinity for FcγR than the IgG4 subtype. Although the IgG1 Fc component of the PD-L1 inhibitor has been tailored to be less susceptible to a specific interaction, the absence of this modification may also explain the relatively short half-life of Avelumab compared with other ICIs [103].
Conversely, the generation of antibodies against ICIs can enhance their clearance from the body. The primary mechanism of ICI clearance remains proteolytic catabolism, which takes place in both plasma and peripheral tissues. ICI clearance may follow linear or nonlinear kinetics, depending on various physiological factors. Nonetheless, the dominant mechanism is nonspecific degradation within plasma and tissues, which is not actually impaired by conditions such as age, hepatic impairment and renal failure [105].
Another important route of elimination is receptor-mediated endocytosis, whereby the high-affinity interaction between ICIs and their cell surface targets facilitates internalization and degradation. This pathway contributes to nonlinear clearance, which is dose-dependent and influenced by target receptor saturation [105,106]. For example, Durvalumab and Pembrolizumab at doses of 3 and 0.3 mg/kg, respectively, present nonlinear clearance due to the saturation of receptors, while an absence of nonlinear clearance among other ICIs may indicate another route of clearance or saturation kinetics. Furthermore, clearance can also occur through humoral and cell-mediated degradation pathways of the immune system. The formation of anti-drug antibodies (ADAs) promotes the endocytic uptake and degradation of ICIs, thereby increasing their clearance. Under combinational therapies, ADAs might exert a greater impact on ICI pharmacokinetics compared to monotherapies. For example, in patients receiving concomitant Ipilimumab, the formation of ADAs against Nivolumab increased significantly (from 10 to 21.9%), resulting in a 24% increase in Nivolumab clearance [107]. An additional route of endocytotic degradation might be facilitated by direct interaction between the Fc component of ICIs and FcγRs on phagocytic cells of the immune system [105].
With the exception of Ipilimumab, ICIs exhibit time-varying clearance, which is often influenced by disease burden—clearance rates tend to decline as tumor load decreases [108,109]. Cachexia contributes to accelerated protein degradation, potentially including ICIs. However, this effect may be reversed as patients respond to treatment, as observed with Durvalumab and Pembrolizumab [109,110,111].
While ICIs typically demonstrate favorable tolerability, dose-limiting toxicities—including severe immune-related adverse events—have occasionally been observed [112]. Antibody-dependent cellular cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) are immune mechanisms triggered by the interaction between the Fc region of ICIs and the immune system components. These mechanisms may lead to depletion of target cells [113]. Their activation is largely influenced by the antibody isotype. In particular, IgG1 antibodies are capable of inducing both ADCC and CDC, functioning as “classical deleters” of intratumoral regulatory T cells (Tregs) due to their ability to induce cytotoxic immune responses. In contrast, IgG4 antibodies act as receptor blockers, antagonizing inhibitory signals on T cells [112]. In clinical practice, unmodified IgG1 ICIs, such as Avelumab, are associated with increased incidence of infusion-related reactions. However, Treg depletion did not occur in the tumor microenvironments of patients treated with Ipilimumab or Tremelimumab [114], indicating that ADCC observed in preclinical models may have limited relevance in clinical practice [102].
Optimizing the pharmacokinetic evaluation of ICIs may improve dosing strategies, leading to better management of drug toxicities and cost-effectiveness. ICIs are generally well-tolerated, with minimal dose-limiting toxicities reported. The establishment of a therapeutic window within the efficacy range can avert dispensable expenses. For example, in the case of Nivolumab, the exposure–response curve reaches a plateau below the marketed doses, indicating the potential for dose minimization or extension of the dose interval [115]. This supports the role of therapeutic drug monitoring (TDM) in reducing treatment costs [116]. The ICI (PD-1, PD-L1) pharmacokinetics [117,118,119,120,121,122,123,124] are shown in Table 2 and can be found in the FDA database (www.fda.gov).

Table 2.
The ICI (PD-1, PD-L1) pharmacokinetics. t½: elimination half-life (days); CL: clearance (L/day); Vc: volume of central compartment (L); Vp: volume of peripheral compartment (L); Q: inter-compartmental clearance (L/day); IIV (CV%): inter-individual variability expressed as coefficient of variation (%); CLlinear: clearance of linear elimination (in drugs with linear kinetics).
6. Pharmacogenomics—Pharmacogenetics
Although ICIs have changed the treatment strategy for various types of cancer, there is significant interpatient variability in the response to treatment and the overall survival, as well as in drug toxicities and the appearance of immune-related adverse events (irAEs) [125]. It is well known that germline genetic variations may be implicated. These genetic variations can either be single-nucleotide polymorphisms (SNPs) or structural variants, such as gene rearrangements, deletions, and amplifications. Thus, predicting which patients will respond to ICI treatment and which are at risk of severe adverse effects remains a significant challenge [126,127,128]. Many studies have focused on identifying predictive biomarkers for ICI treatment [128]. Pharmacogenetics (or pharmacogenomic) are genetic markers which could potently be used in the evaluation of interpatient variability [129,130].
SNPs are common germline DNA sequence variations that occur at a single nucleotide position and are present in at least 1% of the population. Several SNPs associated with ICI therapy are shown in Table 3. Several genome-wide association studies (GWASs) have been performed in order to generate clusters of SNPs, which could be relevant for ICI outcome [131,132,133]. While GWASs serve as a powerful approach for generating biomarker-based hypotheses, they often require large patient populations, which complicates the validation of results in other cohorts. Therefore, prospective validation is often missing. For example, in a case–control cohort of 89 melanoma patients treated with ICIs, a GWAS identified 30 SNPs which were significantly correlated with an increased risk of immune-related adverse events (irAEs) [132]. Several of these SNPs were located in genes associated with auto-immune disorders, including SEMA5A which has been linked to rheumatoid arthritis [132]. Several GWASs and follow-up validation studies have identified an SNP located on an IL7 intron (rs16906115) [131], which was significantly associated with the occurrence of all-grade irAEs [134].

Table 3.
SNPs associated with ICI therapy.
For ICI treatment specifically, genetic variations are studied in three domains: (i) genes encoding receptors targeted by ICIs, (ii) genes implicated in autoimmune pathways, and (iii) polymorphisms within the human leukocyte antigen (HLA) system.
6.1. SNPs Within the PD-1 Pathway
Three SNPs for PDCD1, which encode PD-1, (SNPs 804C > T (rs2227981), 889G > A (rs10204525) and 7146A > G (rs11568821)), are the most investigated, although none are deemed suitable for application in routine clinical care. The first one, 804C > T (rs2227981), is considered to affect PD-1 gene transcription and receptor expression on T cells. In a cohort of 119 melanoma patients treated with anti-PD-1 drugs, carriers of the T allele demonstrated a shorter OS compared to wild-type individuals; however, this finding was not confirmed in other cancer cohorts [138,140,142,144]. A non-significant trend was seen towards reduced progression-free survival (PFS) in homozygous variant carriers (TT genotype) [135]. Moreover, T allele carriers may have lower PD-1 expression in CD4 + T cells [137].
Considering that 804C > T polymorphism is located within the promoter region of PDCD1, it is plausible that this SNP alters PDCD1 transcription, leading to decreased PD-1 expression [142,149].
For the SNP 889G > A (rs10204525), wild-type patients were found to have higher-frequency and greater-severity (≥3) irAEs compared to homozygous variant genotypes in a retrospective study from Japan in RCC patients [138]; this finding was not confirmed in other Caucasian populations [130,138,149].
Finally, the third SNP, 7146A > G (rs11568821), located in an enhancer region of intron 4 of PDCD1 regulating gene transcription, was associated with an improved PFS, making it an interesting target for validation in upcoming prospective studies [135].
6.2. SNPs Within the PD-L1 Receptor Gene
Regarding the PD-L1 receptor, the two most studied SNPs (rs2282055 and rs2890658) have not been associated with toxicity or survival outcomes [140,141,142,144]. However, other SNPs might predict response to anti-PD-L1 therapies. In a cohort of 108 patients with NSCLC treated with Nivolumab, the SNPs rs1411262 and especially rs822339 were associated with longer PFS and OS [143], as well as higher frequency incidence of immune-related hypothyroidism [143,144]. These findings align with previous observations that patients who develop irAEs might have better clinical outcomes compared to those without irAEs [150]. Moreover, the predictive value of these SNPs was further confirmed in a Japanese study involving 222 patients with advanced RCC [142]. Both variants were significantly associated with improved PFS. While the G/G genotype of CD274 rs4143815 has been associated with elevated PD-L1 expression and favorable ICI responses in some studies, other cohorts have reported inconsistent or null associations, indicating the need for further validation in diverse populations [140]. It has also been suggested in several studies that PD-L1 rs4143815, located in the 3′ untranslated region (UTR), can influence the expression of PD-L1, thus driving tumor cell immune escape [151]. The C allele has been shown to increase production of PD-L1 by attenuating miR-570 [152]. Consequently, the C/C genotype has an inferior clinical response to paclitaxel—cisplatin chemotherapy but contradicting results and a lack of research specificity require further insight into the effects in anti-PD-1 therapy [153]. In addition, rs822336 has been significantly associated with treatment response and survival outcomes. Patients with the C/C genotype exhibit better ORR, PFS and OS compared to those with G/C and G/G genotypes. In the presence of allele C, PD-L1 transcription is regulated by the binding of both C/EBPβ and NFIC, whereas the G allele allows regulation by C/EBPβ alone. Moreover, a significant correlation between the presence of allele C in rs822336 and that of allele T in rs2282055 was observed. rs822336 and rs2282055 are located within PD-L1 promoter/enhancer and intron region, respectively. Presence of allele G in rs2282055 was associated with better ORR and PFS as compared to allele T [145]. However, another study has shown better PFS outcome for T/T rs2282055 in the TPS negative population [139]. Thus, these SNPs may serve as promising predictive biomarkers for ICI therapy; however, further prospective validation is necessary to confirm their clinical effect.
Germline variants known to predispose individuals towards autoimmune diseases may also influence the development of irAEs [154,155]. Although numerous SNPs have been identified in GWAS and whole-exome sequencing (WES), only few demonstrated a significant clinical impact. Specifically, rs16906115 SNP in the IL7 gene, two highly linked FARP1 SNPs, rs685736 and rs643869, and finally rs4988956 within the IL1RL1 gene have shown replicable association with irAE risk [131,142]. Some studies focus on genes related to autoimmunity. For instance, in a cohort of 436 patients with metastatic melanoma tested for 25 different autoimmunity-related SNPs, the SNP rs17388568, located in a locus containing both IL2 and IL21, was significantly associated with improved response to anti-PD-1 treatment [146].
Another example of how autoimmunity is often linked to ICI outcome is found in the GZMB gene encoding the granzyme B (a serine protease), an apoptotic effector of T cells. A linkage between the levels of granzyme B and cutaneous autoimmune activity has been shown [156]; the SNP rs8192917, (128T > C) was associated with development of vitiligo in Nivolumab-treated patients with NSCLC with shorter PFS [147]. One could speculate that these variants may lead to the production of less effective granzyme B, thereby impairing the cytotoxic capabilities of T cells [147]. Additional SNPs may modulate the response to ICIs by inhibiting immune signaling pathways. For CD47, which may alter the macrophage response in ICI treatment, the SNP rs3804639 was associated with longer PFS and OS in patients with NSCLC treated with Nivolumab [148].
Finally, polygenic multivariate modeling has been attempted to estimate the large interpatient variability for a specific germline biomarker expression. By analyzing 166 SNPs across 86 genes associated with autoimmunity, multivariable models for tumor response and irAEs were able to reasonably predict both outcomes [136]. Another study tested 16.751 SNPs to calculate a polygenic risk score, originally designed to predict hypothyroidism in non-oncology patients. The polygenic score successfully predicted thyroid-related irAEs in patients treated with ICIs [157]. These findings highlight the importance of assessing multiple SNPs in polygenic models and underline the potential of pharmacogenetic models as predictive tools for ICI treatment.
6.3. HLA and Response to ICI Treatment
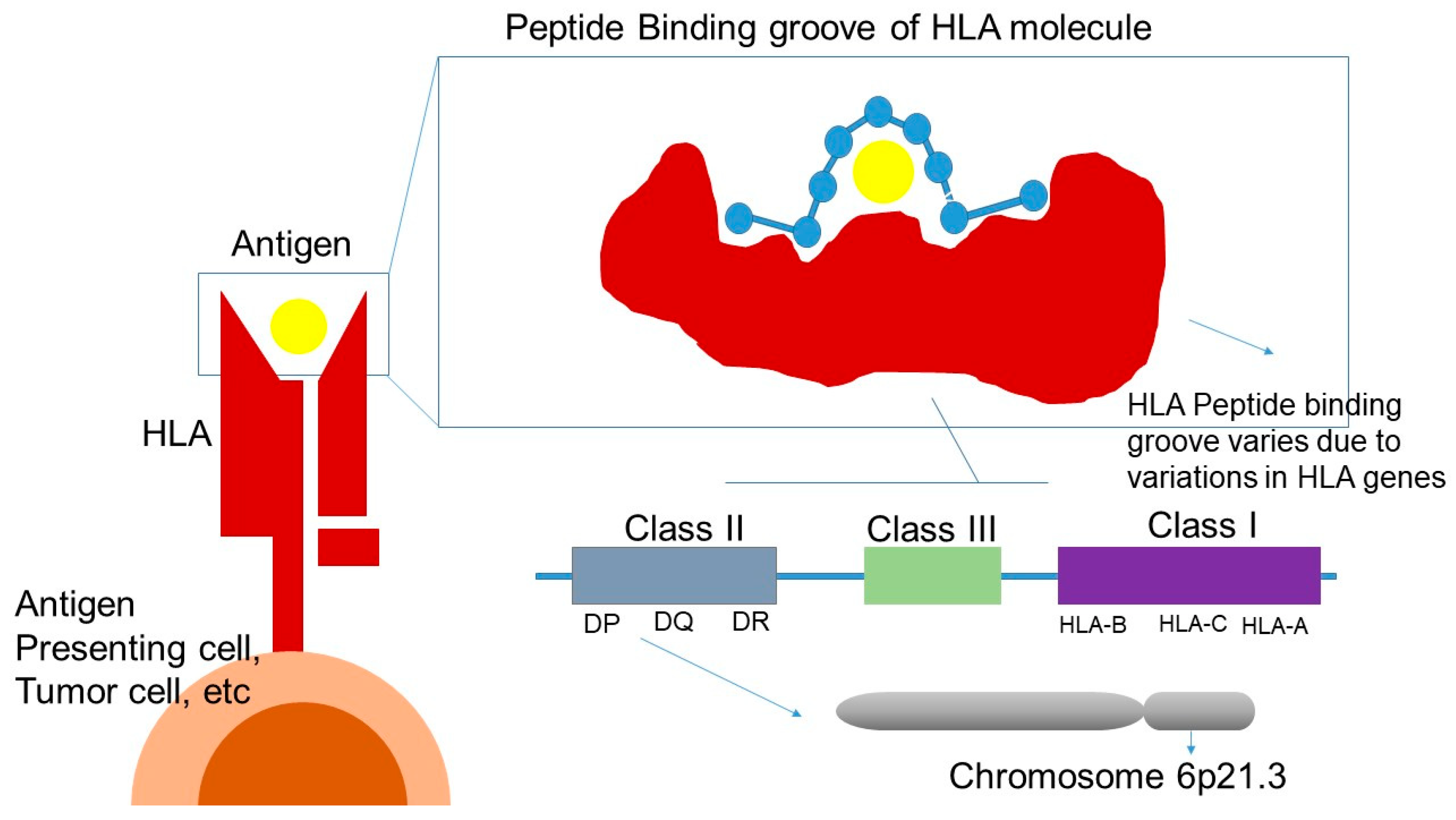
HLA consists of a highly polymorphic gene cluster located on the short arm of chromosome 6 (6p21.3) and is categorized into three classes: class I, II and III—all of which play an important role in immune system. Genetic polymorphisms of HLA molecules affect the regions encoding for the peptide-binding groove and the interaction with the TCR. As a result, these variations influence peptide-binding specificity, leading to a highly diverse repertoire of peptides presented to CD4 + and CD8 + T cells (Figure 3) [158].
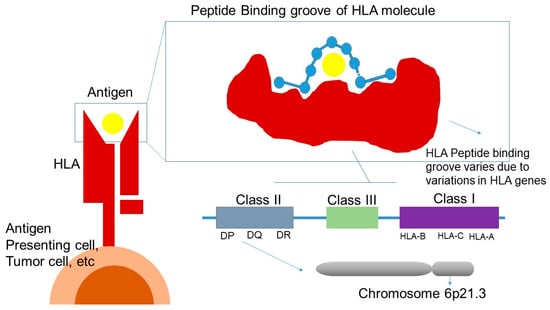
Figure 3.
HLA and response to ICI treatment. HLA: human leukocyte antigen; DP, DQ, DR: class II HLA molecules; HLA-A, HLA-B, HLA-C: class I HLA molecules; Class III: region encoding complement and other immune-related proteins.
Variations within the different HLA class I molecules determine the repertoire of peptides that can be presented to CD8+ T cells, directly affecting the diversity of cytotoxic T lymphocytes for an individual patient (i.e., the immunopeptidomes) [159]. The diversity of the HLA classes has been suggested as a predictive biomarker for response to ICI treatment, while an expression of a broader neo-antigen selection could provide a better tumor response to ICIs [160]. Individuals who are homozygous for at least one of the HLA class I alleles are hypothesized to have poorer survival compared to individuals who are heterozygous for (one of) the HLA class I alleles, due to a reduced diversity in cytotoxic T lymphocyte responses.
Two studies have demonstrated that homozygosity for one of loci A, B or C could negatively affect OS following ICI treatment [161,162]. Interestingly, in the largest study involving 1535 patients, even homozygosity at a single HLA-I locus was associated with reduced survival [161], suggesting that partial homozygosity might be clinically significant. This genetic pattern is observed in approximately 18% of all patients [161]. However, these findings have not been confirmed across all studies [163,164].
HLA-I evolutionary divergence (HED) may also influence treatment response, as it reflects the degree of sequence dissimilarity between the alleles and the resulting diversity of peptide-binding properties [165]. High HED has been associated with improved OS in patients with melanoma and NSCLC, despite the fact that all patients were heterozygous for the HLA class I alleles [165]. Similarly, high HED was correlated with more favorable PFS in RCC, as well as improved PFS and OS in gastrointestinal cancer patients [166,167]. These observations suggest that the diversity of the HLA class I molecules may influence ICI efficacy and may be used as a predictive marker across multiple tumor types [168].
The effect of HLA class II heterozygosity on survival outcomes after ICI treatment has also been investigated. However, although some trends towards associations between heterozygosity of the HLA-DRB1 locus on OS have been reported, no clinical impact has been proved to date [169].
HLA supertypes group HLA class I alleles according to their similar binding affinities. They are combinations of HLA molecules that have binding preferences for certain amino acids, resulting in an overlapping peptide binding specificity [170]. HLA-A*01 supertype was associated with prolonged PFS in metastatic NSCLC [169], which was not confirmed in other studies [161,162,171,172]. A combined HLA-A*01-HLA-A*2 haplotype was also associated with prolonged PFS [169]. On the other hand, the HLA-A*03 allele has been significantly correlated with shorter survival across multiple tumor types in patients treated with ICIs. Interestingly, this effect was not observed in patients receiving alternative therapies [170]. However, other studies have failed to replicate this finding [161,162,171,172].
Importantly, the allele frequency of HLA-A*03 varies significantly among different populations, which may explain the inconsistent findings across studies [161,162,163,164,172]. At present, only HLA-A*26 and HLA-B*27 have shown promising results as ICI biomarkers in small-scale studies and therefore merit further validation in future research [172,173]. In contrast, there is no association between HLA-C supertypes and clinical outcomes following ICI treatment [169]. Overall, the absence or presence of specific HLA alleles, including zygosity of HLA class I and II, cannot yet be used as predictive biomarkers in patients treated with ICIs.
Regarding the impact of HLA alleles on the occurrence of immune-related toxicity, a possible association between the occurrence of irAEs and HLA molecules was studied in general cohorts of patients treated with ICIs. Most of the results should be treated with caution due to marginal significance without adjusting for multiple testing. In addition, these findings may not be directly related to non-Asian populations because most studies were conducted in Japan, which has a specific incidence of HLA alleles [131].
The role of HLA class II alleles has been studied more thoroughly as this class is associated with autoimmune disease susceptibility. Different HLA-DRB1 molecules were found more frequently in patients with ICI-induced inflammatory rheumatoid arthritis [174]. This could indicate a causative mechanism of ICI-induced inflammatory arthritis and should be investigated further in a larger cohort of patients [174]. A significant association has been reported concerning the risk of developing specific irAEs with various DR-specific HLA class II alleles, such as between HLA-DR4 and the appearance of diabetes mellitus type I, HLA-DR15 with hypophysitis, and HLA-DR8 with hypothyroidism [175]. However conflicting results have been reported [176]. Interpreting studies that explore the association between HLA alleles and specific irAEs requires careful consideration of the different methods applied. In particular, HLA class II, specifically the HLA-DRB1 alleles, could play a key role in the onset of irAEs [130].
7. Drug Resistance
A considerable number of patients develop resistance to anti-PD-1/PD-L1 immunotherapy, rendering it ineffective during follow-up. Immunotherapy resistance can be categorized into primary resistance and acquired resistance, depending on the molecular processes behind them [177].
Primary resistance may be caused by impaired tumor-associated antigen presentation as well as alterations in intracellular molecular pathways in tumors affecting immune cell infiltration into tumor microenvironment (TME). The TME is a complex component, consisting of cancer cells, cancer-associated fibroblasts, immunosuppressive cells, cells that activate the immune system, and a variety of signaling molecules. TME dynamically influences the progression of tumors and impacts the treatment outcomes. An immunosuppressive TME obstructs T cell growth and activation and ultimately leads to tumor evasion. Interestingly, an irregular activation of the HGF/c-MET signaling pathway may facilitate the communication between cancer-associated fibroblasts and various immune elements, controlling PD-L1 protein expression through chemiotaxis with immunosuppressive cells. Abnormal MET activation reduces the effectiveness of anti-PD-1/PD-L1 immunotherapy [178].
Epigenetic modifications that affect antigen processing and presentation, persistent activation of the WNT/β-catenin signaling pathway, reduced T cell infiltration, and cells promoting immunosuppression are examples of primary resistance [177,179]. Immunosuppression includes deficiencies in interferon signaling and presentation of antigens, T cell immunological dysfunction, and upregulated expression of immunosuppressive molecules, as well as modifications in the level of PD-L1.
Acquired resistance occurs when a tumor is initially treated with some inhibitory effect, but the tumor later progresses or reappears. A possible explanation for this is new tumor-derived resistant mutant strains. Immune checkpoints including T cell immunoglobulin mucin 3 (TIM-3) and lymphocyte activation gene protein 3 (LAG-3) show a compensatory effect after treatment, leading to an increase in the expression of other immune surveillance pathways and consequent drug resistance [177,180]. The main resistance mechanisms in ICI treatment are immunosuppression, epigenetic alterations, microbiota alterations and metabolic abnormalities.
7.1. Tumor Antigen Deletion
HLA-I is indispensable for the recognition of tumor cells by CD8+ T cells [181]. Tumor cells can cause reduced or even complete downregulation of HLA-I expression levels through beta-2-microglobulin (B2M) deficiency or mutation, leading to failure in presenting neoantigens to tumor-infiltrating T cells (TILs), failure to activate CD8+ T cells, promotion of immune escape from the tumor, and resistance to blockade of immune checkpoint therapy [161,182]. Limited clinical data describe a small group of PD-L1 inhibitor-resistant patients exhibited B2M deficiency in contrast to those improving with no B2M changes detected [183,184]. Additionally, in multifocal HCC with intrahepatic metastases, HLA allele heterozygous deletions are associated with a higher recurrence rate in advanced cancers [185].
Immunohistology and RNA sequencing of MHC-II in tumors showed that its expression promotes increased infiltration of CD4 T cells. Tumors adapt to this change either after PD-1 immunotherapy or after tumor progression by expressing the MHC-II inhibitory receptor LAG3 (which competes with CD4 T cells for antigen presentation) or the Fc receptor-like 6 (which binds to MHC-II, directly inhibiting NK and T effector function), leading to anti-PD-1 therapy resistance [186]. It is possible for LAG-3/FCRL6 to be a target for immunotherapy.
7.2. T Cell Dysfunction
The PD-1/PD-L1 blockade may leave tumor-specific T cells activated but also causes the overexpression of other immune checkpoints such as TIM-3, which leads to inhibition of cytotoxic T-lymphocyte and Th1 cell function, reducing immunotherapeutic response [187]. In addition, PTEN, which is negatively regulated by the pathway mediated by PI3K/AKT and upregulates PD-L1 expression, is absent in a number of malignancies and may result in the development of primary immunotherapy resistance [188]. It also initiates Signal Transducer and Activator of Transcription 3 (STAT3)-mediated immunosuppression, increasing cytokines like IL-10, IL-12, IL-16, and VEGF, which suppress T cell activation and self-phagocytosis in preclinical models [189]. Finally, PD-1/PD-L1 inhibition influences the TME, where overexpression of CD38 on T cell surfaces may lead tumor cells to produce adenosine, which inhibits proliferation and function of CD8 + T cells (ineffective T cell penetration to TME) [177,190]. PTEN mRNA injection treatment in mouse tumor models lacking PTEN or PTEN mutations revealed that a substantial rise in CD8+ T cells could reverse the immunosuppressive TME [191].
Cucchiara et al., in an in silico analysis concerning genetic profiles of 644 advanced NSCLCs according to the immunotherapy response, found at least two mutations in the coding sequence of genes belonging to the chromatin remodeling pathway, and/or at least two mutations of genes involved in cell-to-cell signaling pathways. The study suggested a dependency between mutated genes and a peculiar profile of mutations in late-stage NSCLCs with immune sensitivity. The hypothesis is that somatic loss-of-function mutations in SWI/SNF-related genes and impaired cell-to-cell crosstalk may result in dysfunctional immune evasion and persistent buffering of pro-inflammatory cytokines across the TME. This inflamed state could affect TIL activity once immune checkpoint blockers are inhibited [192].
The activation of the WNT-β-collagen pathway is also linked to reduced infiltration of tumor-specific T lymphocytes, possibly due to upregulated TANK-binding kinase 1 (TBK1) and thus drug resistance [193].
7.3. Increase in Immunosuppressive Cells
An increase in immunosuppressive cells like Tregs, tumor-associated macrophages (TAMs), and other subtypes may also be implicated in drug resistance. PD-1/PD-L1 inhibitors can have an impact on Treg proliferation and function. They enhance TCR and CD28 signaling in Tregs, promoting the development of Treg-rich TMEs enhancing their inhibitory function [194]. Tregs potentially contribute to resistance against PD-L1 immunotherapy, as indicated by PD-L1 inhibitors’ capacity to restore immunity against tumors following Treg depletion.
Subtypes of Myeloid-derived suppressor cells (MDSCs) can boost Arginase-1 (Arg-1) activity, which renders immune cells insensitive or tolerant and impairs their capacity to quickly eliminate tumor cells, leading to tumor immune escape [195]. MDSCs create an immunosuppressive microenvironment by promoting tumor-derived exosomes (Exo) and Hypoxia-inducible factor-1 (HIF-1) and thereby inhibit T cell activity [196], which induces apoptosis in CD8+ T cells and enhances the inhibitory activity of T-regs [197]. Multiple preclinical studies have provided evidence that targeting MDSCs can enhance immunotherapy, and current clinical trials look into how they can be paired with PD-1/PD-L1 blockers for overcoming anti-PD-1/PD-L1 resistance [198].
M2-type TAMs are prompted to enhance anti-inflammatory molecules like TGF-β and PGE-2, which inhibit normal antigen-presentation-induced T cell activation [199]. Vascular growth-associated factors (VEGF, IGF) and matrix metalloproteinases (MMPs) are produced by M2-type macrophages during the inflammatory response to help promote angiogenesis and tumor growth [200]. Reprogramming myeloid cells in the TME has been shown in preclinical models to help overcome resistance [201].
7.4. Drug Resistance Due to Changes in PD-L1 Expression
The oncogenic signaling pathway KRAS-ERK induces PD-L1 expression. KRAS mutations can induce the release of PD-L1 and promote apoptosis of CD3+ T cells in lung adenocarcinoma through p-ERK signaling, significantly contributing to the initial resistance to PD-1 blocking [202]. Another pathway, the JAK/STAT, may be a key pathway for the synthesis of PD-L1 and the production of tumor antigens [203]. Mutations in these genes may result in an absence of PD-L1 production and drug resistance [204].
Another oncogenic factor which alters the expression of PD-L1 on tumor cells is the oncogenic transcription factor Yin Yang 1 (YY1), a known factor overexpressed in many cancers [205]. Emerging data suggest that the transcription factor YY1 may modulate PD-L1 expression through promoter binding and chromatin remodeling, although additional mechanistic studies are warranted to establish its role across different tumor types. Multiple studies have implicated the YY1/PD-L1 axis in immune escape in melanoma, NSCLC, liver cancer, and lymphoma [206]. It has been determined that YY1 is an important regulator of PD-L1 during T cell exhaustion and promotes immune escape in prostate cancer. Additionally, YY1 is linked to tumor immune evasion through the upregulation of PD-L1 involving the p53/miR-34/PD-L1 pathway [205]. Targeting YY1 may result in a significant inhibition of cancer oncogenic activities. Various strategies are proposed to selectively target YY1 in human cancers and present a promising novel therapeutic approach for treating unresponsive cancer phenotypes. Finally, interferon gamma (IFNγ) produced by activated T cells and NK cells may strongly trigger PD-L1 expression in the tumor microenvironment. It has been shown that mutations in IFNGR1/2 or JAK1/2 components of the IFNγ signaling cascade are a common cause of acquired and primary resistance to ICI blockade therapies [207].
7.5. Epigenetic Mechanisms of Drug Resistance
Epigenetic marks, such as DNA methylation and histone post-translational modifications (histone PTMs), participate in the regulation of gene expression and chromatin structures allowing or not allowing transcriptional machinery to access DNA. Several epigenetic mechanisms are involved in resistance to the immune checkpoint inhibitors: the main ones are the modifications of histone marks and chromatin structures, alteration of DNA methylation, and changes in miRNA expression levels [208].
7.5.1. Histone Deacetylases (HDACs)
HDACs are important epigenetic regulators. Because of epigenetic silencing, they may reduce the expression of cell surface molecules required for immune system tumor identification, such as major histocompatibility complex class I (MHC-I) molecules, and co-stimulatory molecules (e.g., CD80 and CD86). HDAC inhibitors may boost the response to immunotherapy by raising levels of tumor antigens and reactivating proapoptotic genes and they are under evaluation in clinical trials with ICIs [209].
7.5.2. Histone Methyltransferases (HMT/EZH2)
EZH2 (enhancer of zeste homolog 2) may be crucial in the differentiation of Treg cells that inhibit immunological responses. EZH2 expression is associated with tumor immunogenicity and might be targeted to regulate the response to ICIs [210].
7.5.3. miRNAs in Cancers and in Resistance to ICIs
Single-stranded, noncoding short RNAs known as microRNAs (miRNAs) have the ability to adversely control gene expression, post transcription. When binding on an mRNA target, they can either degrade the target or block its translation. Numerous studies have connected different miRNAs with PD-L1 or PD-1 expression and immunotherapy resistance via altering T cell activities [211,212]. Through the induction of the epithelial-al–mesenchymal transition (EMT), altered miR expressions also influence the tumor immune response. Moreover, EMT is induced by embryonic transcription factors (including the ZEB family’s SNAIL, SLUG1, and TWIST1), which may also be reactivated in cancer cells. MiRs, like miR-200, have been shown to suppress EMT by upregulating transcription factors. Dysregulation of the miR-200/ZEB1 axis, a key regulator of the EMT, connects the EMT and PD-L1. These results imply that PD-L1 inhibitor therapy may be effective for a subset of patients whose malignant development is fueled by EMT activators [212].
7.5.4. Alteration of Tumor Immunogenicity
Epigenetic changes contribute to the TME’s remodeling, which promotes its expansion and immune system evasion and are linked to CD8+ T cell activation and differentiation. Through mutations in the chromatin remodeling complex, the SWI/SNF (SWItch/Sucrose Non-Fermentable) complexes, it has been shown that chromatin remodeling contributes to resistance to ICIs. In tumor cells, PBAF, a chromatin regulatory complex consisting of PBRM1, ARID2, and BRD7, controls chromatin accessibility for the IFN γ pathway, enhancing resistance to T cell-mediated cytotoxicity. By boosting tumor immunogenicity, PBRM1 and perhaps ARID1A inactivation restores the response to immunotherapy [213,214].
7.5.5. DNA Methylation and Anti-PD-1/PD-L1 Treatment Resistance
DNA methylation plays an important role in the development of cancer. This DNA alteration, which is linked to gene silencing, is accomplished by DNA methyltransferases (DNMTs). The control of DNMT1 by PD-L1 across the STAT3 signaling pathway causes DNA hypomethylation, which in turn leads to the in vivo development of novel drug-resistant mutant strains [215]. Overall DNA hypomethylation may potentially be a factor in the constitutive activation of cytokines, like VEGF and IL-6, which may increase immunotherapy resistance [216]. Conversely, low levels of PD-L1 are linked to general DNA hypermethylation, which is also associated with a bad prognosis for melanoma patients [217].
7.6. Further Difficulties of ICIs Treatments
Clinical trials have indicated that not all patients respond to monoclonal antibody treatment. PD-1 antibodies have a 50% response rate in melanoma therapy, while the overall response rate for other solid tumors is poor, ranging between 15% and 20% [218]. One reason for this limited efficacy is resistance, either primary or acquired, which may have an influence on the tumor-intrinsic or tumor microenvironmental factors. For example, the mutational evolution of the tumor can lead to immune evasion; thus, the timing of treatment initiation may impact the outcomes.
Resistance is not alone in limiting the clinical effectiveness of ICIs. Other factors such as poor penetration into tissues may be limiting; antibodies might not actually reach or accumulate in some tumor regions [219] and immunogenicity, where anti-drug antibodies neutralize therapeutic activity, can occur [220]. Other toxic events related to the immune system may cause autoimmune toxicities affecting multiple organs, such as the liver, lungs, gastrointestinal tract, skin, and endocrine system [221]. For example, among melanoma patients, skin toxicity was found in 34% of patients treated with Nivolumab and 39% of patients treated with Pembrolizumab [218].
8. Biomarkers in ICI Treatments in Clinical Practice
FDA-approved biomarkers used to predict ICI effectiveness include PD-L1 expression, TMB, and DNA repair deficiencies, including inadequate MMR and high microsatellite instability (MSI-H), as well as TILs [222,223].
8.1. PD-L1 Expression
PD-L1, as previously stated, is an important biomarker for anti-PD-1/PD-L1 treatment in a variety of malignancies. Immunohistochemical staining (IHC) is the most often used approach for detecting PD-L1. The US FDA and European Medicines Agency (EMA) have authorized four PD-L1 IHC antibodies (SP142, 22C3, 28-8, and SP263), as well as their staining procedures. Each antibody is authorized to target certain PD-1/PD-L1 inhibitors and cancer types. PD-L1 detection is scored using algorithms based on cell type, location, frequency, and intensity of PD-L1 expression; however, in clinical practice, three scoring algorithms are used: TPS (Tumor Proportion Score, also known as TC for tumor cells), CPS (combined positive score), and IC, for immune cells. TPS is the percentage of viable tumor cells with partial or complete membrane staining compared to all viable tumor cells (determined by positive or negative PD-L1 staining). TC represents the proportion of tumor cells stained with PD-L1. CPS is defined as the proportion of PD L1-positive cells (tumor cells, lymphocytes, and macrophages) compared to the total number of viable tumor cells. The IC indicates the proportion of the tumor occupied by PD-L1-positive immune cells of any intensity [128].
Despite diversity in detection among cancer types, PD-L1 expression influences the effectiveness of PD-1/PD-L1 inhibitors, impacting response rates (RRs), PFS, and OS. Retrospective studies and meta-analyses show that PD-L1 expression should guide the first decision between PD-1 blockade and chemotherapy in treating naïve individuals [224,225]. In all PD-L1 categories, PD-1 inhibitors often yield better results than chemotherapy for patients who have already received treatment [224], with higher PD-L1 expression being linked to improved overall survival. Additionally, PD-L1 expression and survival outcomes in advanced/metastatic non-small cell lung cancer are correlated. According to Topalian et al., there was no response from any of the 17 PD-L1 negative tumors; however, there was a strong correlation between patients with PD-L1 positive tumors and objective response to Nivolumab (9 of 25 patients, p = 0.006) [202]. Nivolumab has demonstrated a noteworthy objective response in cancers that are PD-L1 positive (≥5% on tumor cells), according to another study that examined a variety of malignancies, including melanoma, NSCLC, and renal cell carcinoma [226]. It should be underlined that while the aforementioned studies show that PD-L1 expression detection is necessary, half of the PD-L1-positive patients did not react to PD-1/PD-L1 blocking, while some PD-L1-negative patients also showed clinical benefit and therapeutic response [226].
PD-L1 staining’s significance varies depending on the type of cancer [227]. In order to administer Pembrolizumab in NSCLC (either at stage III NSCLC localized to the chest, unsuitable for local therapy, or metastatic NSCLC), the tumor must be PD-L1 positive and lack EGFR or ALK mutations, or it can be prescribed if other treatment have failed. Interestingly, in metastatic NSCLC, a beneficial effect has been recorded in combination with chemotherapy even in PD-L1 scores < 1%, although the greatest improvement is observed in patients with a PD-L1 score > 50% [228]. Furthermore, PD-L1 staining had no discernible impact on the effectiveness of Pembrolizumab in the treatment of melanoma, renal cell carcinoma, Hodgkin lymphoma, hepatocellular carcinoma, and other cancer types.
Considering Atezolizumab, PD-L1 staining is obligatory for first-line NSCLC treatment qualification. However, the drug can be administered in combination with Bevacizumab, paclitaxel, and carboplatin, when no PD-L1 has been found, for first-line treatment of metastatic non-squamous NSCLC without EGFR or ALK mutations or for metastatic NSCLC post-platinum chemotherapy. Regardless of PD-L1 expression, Atezolizumab is recommended as part of combination treatment for BRAF V600-positive small-cell lung cancer, hepatocarcinoma, and melanoma. An issue that has to be considered is the possibility of PD-L1 glycosylation and other confounding factors leading to false-negative detection of PD-L1. Glycosylation is a post-translational modification in the extracellular domain of PD-L1 that significantly modulates its stability and interaction with the immune system [229]. This alteration affects how PD-L1 functions biologically and how it may be found in tumor tissues, particularly when using conventional IHC staining, which makes diagnostic and therapy choices even more challenging. PD-L1 expression, which varies depending on treatment antibodies, staining antibodies, and scoring methods, is regrettably believed to be underreported in up to 40% of patient tissues. Therefore, the consequences of false-negative, glycosylation-induced findings are a growing issue in terms of denying patients access to appropriate ICI medication. An inclusive protocol incorporating pre-treatment of glycosylation removal before IHC staining has been developed [229]. Intriguingly, a study that included patients with advanced TNBC who had previously been diagnosed as PD-L1 negative revealed that individuals who tested positive for PD-L1 after de-glycosylation had unexpectedly favorable reactions to Atezolizumab. This implies that de-glycosylated PD-L1 may be a more reliable biomarker for gauging the effectiveness of therapy [230].
Researchers are looking at a number of confounding variables that are connected to PD-L1 expression as a biomarker. The variation in PD-L1 expression within and between tumors is a significant problem. If the biopsy does not adequately reflect the overall PD-L1 status within tumors, this heterogeneity may result in sampling mistakes. In addition to intra-tumoral heterogeneity, which occurs when PD-L1 expression differs across various parts of the same tumor, intertumoral heterogeneity occurs when PD-L1 expression differs between distinct cancers, such as the main tumor and metastatic lesions in the same patient. Furthermore, PD-L1 expression may fluctuate over time as a result of further mutations, tumor growth, and the effects of different therapeutic interventions. To conclude, it is of utmost importance to gain a better understanding of the biology of PD-L1 regulation at the transcriptional and post-translational levels, applying more comprehensive approaches to selecting cancer immunotherapies. Personalized ICI therapy will be made possible by expanding the scope of PD-L1 staining to incorporate a larger panel of biomarker, genetic, and molecular profiling, as well as taking clinical considerations into account [168].
8.2. Tumor Mutational Burden (TMB)
TMB, which serves as a metric of genetic mutations, is present in the genome of cancer cells, reflecting the mutagenic processes induced by environmental and intracellular factors. It is hypothesized that a greater TMB increases the number of neoantigens, which raises the possibility that T cells will recognize the tumor cell and eliminate it. Although this idea centers on the overall quantity of mutations present in a tumor sample, the precise standards for genetic changes in TMB vary between various approaches.
Initial TMB measurements, conducted through whole-exome sequencing (WES), included non-synonymous mutations in coding regions and omitted germline alterations by subtracting matched normal samples [231]. Next-generation sequencing (NGS) is used in clinical practice for extensive cancer gene-targeted sequencing panels [231]. FoundationOne CDx (F1CDx), Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT), Guardant360 CDx, and FoundationOne Liquid CDx (F1 Liquid CDx) are the four TMB measurement tests that the FDA has authorized. The ability to detect gene mutations in circulating cell-free DNA (cfDNA) from blood samples is another benefit of the NGS liquid biopsy test. The evaluation of TMB is crucial in practice. It has been reported that high TBM patients receiving Pembrolizumab have more favorable ORR compared to those with low TMB (29% vs. 6%, respectively) [232].
The combination of the two biomarkers, PD-L1 expression and the TMB, may identify patients most likely to respond to anti-PD-1/PD-L1 therapy, with those having high TMB and PD-L1 levels above 50% experiencing a 57% ORR and the longest PFS [233]. The application of the TMB biomarker may be improved by combining it with additional factors, according to recent research. The landscape of frameshift mutations in conjunction with TMB, particularly in cancers with low TMB, is one example. They have been found in a high proportion of patients with low TMB who might benefit from ICIs when T cell immunity is already present, without the need to distinguish between high- and low-TMB tumors [234,235].
8.3. MSI-H/dMMR
Microsatellites are repetitive DNA sequences containing 1–6 nucleotides, spread in tandem across the genome. Under normal conditions, DNA integrity and the length of microsatellites are maintained by the DNA MMR system, which rectifies base mismatches generated by DNA polymerases during replication or due to DNA damage. The presence of dMMR results in variations in microsatellite length, causing microsatellite instability (MSI) compared to matched normal DNA.
The phenotypic evidence for dMMR is the presence of MSI [236]. The most well-known hereditary disorder that causes MSI-H or dMMR tumors is Lynch syndrome, a familial trait that increases the chance of developing numerous cancers, even though high-MSI (MSI-H) can appear in a variety of cancer types. It is believed that defective DNA repair processes cause a substantial buildup of mutations in cancers classified as MSI-H and/or high-dMMR, making the tumor cells easier for the immune system to identify [236]. Consequently, dMMR and MSI-H have been emphasized in a number of publications as prognostic biomarkers for ICIs.
The FDA has authorized MSI-H as a biomarker for the use of ICIs in several cancer types [237]. Pembrolizumab was first authorized in 2017 for the treatment of pre-treated MSI-H colorectal cancer and in 2020 for the treatment of MSI-H colorectal cancer as a first-line therapy [232]. In 2023, the FDA fully approved Pembrolizumab for use in patients with MSI-H solid tumors that have previously received treatment. Pembrolizumab is also approved by the EMA for patients with PD-L1-positive metastatic cervical cancer and MSI-H or dMMR advanced colorectal, endometrial, gastric, small intestine, or biliary malignancies. An ORR of 33.3% was demonstrated by Pembrolizumab in a variety of advanced MSI-H/dMMR solid tumors [237]. These findings highlight the difficulties of depending only on TMB-H and MSI-H, even though they imply that these markers are predictive of Pembrolizumab outcomes across a range of tumor types.
The FDA granted Dostarlimab expedited approval in 2021 for solid tumors and pre-treated MSI-H endometrial cancer and full approval for first-line therapy of MSI-H endometrial cancer in 2023. Furthermore, the FDA authorized Nivolumab monotherapy in 2017 and Ipilimumab with Nivolumab in 2018 for MSI-H colorectal cancer that has previously been treated [238].
A composite predictor that includes important factors including MHC, T cell receptor repertoire, and TIL status is recommended in order to improve predictive precision [239].
8.4. Cost Effectiveness of Biomarkers
First off, the clinical use of PD-1/PD-L1 antibody medications may be restricted due to the challenges of producing monoclonal antibodies, their high cost, and their cumbersome storage and transportation. The health economic assessments of predictive biomarker testing in solid tumors treated with ICIs were examined in a recent systematic review and meta-analysis [240]. Out of the 730 screened publications, 58 were examined for this meta-analysis. NSCLC (60%) and other solid tumors (40%) were the main emphasis. Out of 35 NSCLC trials, 21 showed cost-effectiveness. Notably, when PD-L1 testing is performed before beginning Pembrolizumab as a first-line therapy, it is cost-effective at a threshold of USD 100,000/QALY in comparison to the standard of care. The affordability requirement of USD 100,000/QALY was not satisfied by any economic studies for bladder, cervical, or triple-negative breast cancers (TNBCs). Overall, the analysis points out that there is some ambiguity about ICI’s cost-effectiveness.
According to this study, ICI therapy linked to PD-L1 expression is an economical strategy, especially for renal cell carcinoma, urothelial, and non-small cell lung cancer. While the results point to difficulties in attaining cost-effectiveness for some other solid tumors, they also point to the potential benefits of predictive biomarker testing, particularly with Pembrolizumab in NSCLC. As a result, the economic impact of biomarkers prior to the selection of a particular therapy is crucial for confirming its efficacy, potential correlation with quality of life, and long-term viability. Therefore, investing in a biomarker testing approach is a wise move because it increases the patient’s chances of receiving increasingly effective therapy and reduces adverse events brought on by the administration of untargeted therapies, improving quality of life without a doubt and optimizing the use of health care resources [240].
9. Discussion
Cancer immunotherapy has revolutionized the clinical landscape of oncology and brought renewed hope to the treatment of previously difficult-to-treat malignancies. Yet, more questions and challenges continue to lie ahead. The study of immune-oncologic drugs earlier during the stages of cancer disease progression, such as in the context of neoadjuvant treatment across solid tumors, should be one of the future areas of cancer immunotherapy. It should be acknowledged that there are challenges in implementing these treatments, mostly associated with drug resistance [241]. This resistance results from the intricate interaction of several dynamic processes within the TME, such as immunosuppressive networks, T cell exhaustion, and limited T cell invasion [242].
As a result, combination immunotherapies—that is, immunotherapies combined with radiation therapy, chemotherapy, and targeted therapies—are potential treatment approaches that need further investigation. Combination immunotherapy is an interesting strategy that counteracts several immuno-suppressive factors in the TME and activates several stages of the cancer-immunity cycle, but it may also enhance toxicity [243,244]. As previously mentioned, MET inhibitors may alter the TME by neutralizing the irregular expression of PD-L1 caused by MET irregularities, thereby stimulating the cancer-fighting abilities of T and NK cells, reducing the gathering and penetration of immunosuppressive cells. Ultimately, MET inhibitors may be used therapeutically in ICI combination [178].
The combination of ICI and tyrosine kinase inhibitors (TKIs) have gained therapeutic significance in cancer therapy recently due to the high efficacy of each substance group, individually and combined. The majority of available data are from clinical phase I and II trials. As of right now, ICI-TKI combos that include Nivolumab + Cabozantinib, Pembrolizumab + Axitinib or Lenvatinib, and Avelumab + Axitinib, have been authorized for advanced renal cell cancer (RCC), endometrial carcinoma (Pembrolizumab + Lenvatinib), and hepatocellular carcinoma (HCC) (Camrelizumab + Rivoceranib). The optimal ICI-TKI sequencing, cross-resistances, and increased toxicities are additional concerns that need to be addressed [245].
Many recent studies on the mechanisms of resistance to ICI therapy and its associated biomarkers show promising prospects. More clinical research is necessary since there are still difficulties in putting these into clinical practice.
Recently, new compounds have being investigated as potential substitutes for existing treatments. These substances are inhibitory small molecules. They intervene in the PD-1/PD-L1 signaling pathway, presenting different mechanisms of action. They have significant advantages such as controllable pharmacokinetic characteristics, suitability for oral administration, avoidance of immune-related SAEs, and favorable biosafety, thus presenting them as a novel monotherapy or complementary therapy [246,247,248]. Novel small-molecule inhibitors of the PD-1/PDL-1 axis include a variety of targets. Some block the binding of PD-1 and PD-L1, utilizing the physicochemical properties of amino acid residues such as AUNP-12, cyclic peptide compounds DPPA-1, TPP-1, nonpeptide-based small-molecule CA-170, BMS-202 and its derivatives, and INCB086550, INCB099280, and INCB099318 in combination with Axitinib. Other targets may include suppressing the expression level of PD-L1, like JQ1 inhibitor, eFT508 (tomivosertib), Osimertinib (AZD9291), and Platycodin D, and promoting the degradation of PD-L1, like PD-LYSO, Curcumin, and Metformin. All of these are currently in clinical or preclinical stages of research [246,247,249,250,251,252,253,254,255,256,257]. Furthermore, the development of small-molecule inhibitors of YY1 presents a promising method of YY1 inhibition. It should be noted that novel YY1 inhibition therapies include a variety of other possible treatments [205]. These molecules are shown in Table 4. There is a lot of research underway to identify effective small-molecule inhibitors, with hundreds of candidates including the BMS and INCB families.

Table 4.
Small-molecule inhibitors of PD-1, PD-L1 as possible cancer treatment.
Another factor is long noncoding RNAs. Studies have shown that lncRNAs are abnormally expressed in tumors and play an important role in tumor proliferation, angiogenesis, apoptosis, and metastasis. Lots of lncRNAs have been demonstrated to regulate the expression of key immune checkpoints, PD-1/PD-L1, CTLA-4, TIGIT, and TIM-3. One possible post-transcriptional regulatory mechanism is the lncRNA-miRNA network in the PD-1/PD-L1 pathway. LncRNAs can sponge miRNAs as competing endogenous RNA to upregulate the downstream target gene PD-L1, thus contributing to immune evasion. Several lncRNAs exert their roles in immunotherapy through modulating T cells. It was found that lncRNAs inhibit antitumor activity, decreased the proportion of and induced exhaustion of CD8+ T cells, and they play an important role in modulating oncogenic signaling pathways involved in tumor proliferation and invasion, such as the NF-κB, PI3K/AKT, and Wnt/β-catenin pathways. The preclinical data and clinical data evidence is lacking. One of the greatest challenges is developing a delivery system to deliver lncRNAs to specific targets efficiently and with lasting effects [260]. Several lncRNAs are thought to be of prognostic value by some clinical studies in advanced tumors; recently, the association of the lncRNA NEAT with a favorable response to PD1/PD-L1 therapy in melanoma and glioblastoma patients was uncovered [261,262].
It is important to take into account how age, gender, and race affect the way immune-oncologic treatments function. For instance, estrogen can influence the immune response by controlling the distribution and activity of immune cells, which is why women often show higher innate and adaptive immunological responses [263]. Furthermore, older individuals frequently respond less well to immunotherapy, which might be connected to immune system deterioration [264].
The heterogeneity in patient responses to immunotherapy is a substantial hurdle, necessitating the identification of useful biomarkers capable of predicting immunotherapeutic success. Currently, the primary biomarkers employed in clinical applications are limited to PD-L1 expression, TMB, and MSI status [265]. Although various ICI biomarkers have been employed in clinical trials, their efficacy as biomarkers in predicting ICI responders is insufficient, as indicated by the low ICI response rate. Various tumor types can differ, and each patient’s health and immunological condition may also influence ICI sensitivity or resistance, making it difficult to identify a single universal biomarker for ICIs. As a result, integrating various biomarkers may be required to better predict the success of ICI treatment. This necessitates the development of machine learning on larger datasets and more efficient methods.
In particular types of malignancies, PD-L1 can be employed to guide patient selection for immune–oncologic treatment. However, the temporal and geographical dynamics of PD-L1 have complicated its use as a biomarker in clinical settings [266]. Nonetheless, TME components are emerging as novel biomarkers, but they are frequently prognostic rather than predictive of immunotherapy response [267]. In particular types of malignancies, PD-L1 can be employed to guide patient selection for immune-oncologic treatment. However, the temporal and geographical dynamics of PD-L1 have complicated its use as a biomarker in clinical settings [266]. Nonetheless, TME components are emerging as novel biomarkers, but they are frequently prognostic rather than predictive of immunotherapy response [267].
As a result, further efforts to identify and verify novel biomarkers are required. Furthermore, non-invasive biomarkers must be created, and detection costs must be reduced in order to be cost-effective and widely used in clinical applications.
Promising Ongoing Clinical Trials
The field surrounding the inhibition of the PD-1/PD-L1 axis is rapidly evolving. New antibodies, innovative combination therapies, biomarkers, and methods of administration are currently under investigation. In this dynamic research environment, it is essential to showcase clinical trials that could advance and transform this area. The latest ongoing phase III clinical trials (referred in www.clinicaltrials.gov, accessed on 5 May 2025) are summarized in Table 5.

Table 5.
Recent ongoing clinical trials phase III (2024–2025) for ICI cancer therapy (www.clinicaltrials.gov, accessed on 1 January 2020).
10. Conclusions
Future research that focuses on the characterization of immune checkpoint mechanisms will transform the field and lead to the development of potential immunotherapies for so far untreatable malignancies.
Polygenic models may more clearly highlight variations in interpatient variability and better forecast those individuals who may see an improved potential therapeutic benefit from ICI treatment while also limiting those patients at risk of developing (severe) irAEs. The identification and confirmation of viable biomarkers for ICI therapy are crucial for moving forward with customized cancer treatment. While substantial advances have been made, research into precision immunotherapy is ongoing. Future research should focus on resolving present constraints, standardizing biomarker evaluation, and exploring novel biomarker discovery methodologies in order to maximize treatment outcomes and improve patient quality of life. In the new era of immunotherapy, researchers are looking at novel medicines that are more effective and safer.
Author Contributions
Conceptualization, I.S.V., D.-I.Z., E.G. and A.N.M.; methodology, D.-I.Z. and A.N.M.; formal analysis, D.-I.Z., A.N.M. and E.G.; investigation, A.N.M. and N.F.T.; resources, I.S.V., D.-I.Z. and A.N.M.; data curation, A.N.M. and D.-I.Z.; writing—original draft preparation, D.-I.Z., E.G., A.N.M. and N.F.T.; writing—review and editing, I.S.V., D.-I.Z., E.G., A.N.M. and N.G.; visualization, D.-I.Z.; supervision, I.S.V.; project administration, I.S.V., D.-I.Z. and A.N.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wang, J.; Chen, Q.; Shan, Q.; Liang, T.; Forde, P.; Zheng, L. Clinical development of immuno-oncology therapeutics. Cancer Lett. 2025, 617, 217616. [Google Scholar] [CrossRef]
- Sharma, P.; Allison, J.P. The future of immune checkpoint therapy. Science 2015, 348, 56–61. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Ishida, Y.; Agata, Y.; Shibahara, K.; Honjo, T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992, 11, 3887–3895. [Google Scholar] [CrossRef]
- Freeman, G.J.; Long, A.J.; Iwai, Y.; Bourque, K.; Chernova, T.; Nishimura, H.; Fitz, L.J.; Malenkovich, N.; Okazaki, T.; Byrne, M.C.; et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J. Exp. Med. 2000, 192, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Latchman, Y.; Wood, C.R.; Chernova, T.; Chaudhary, D.; Borde, M.; Chernova, I.; Iwai, Y.; Long, A.J.; Brown, J.A.; Nunes, R.; et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat. Immunol. 2001, 2, 261–268. [Google Scholar] [CrossRef] [PubMed]
- Hirano, F.; Kaneko, K.; Tamura, H.; Dong, H.; Wang, S.; Ichikawa, M.; Rietz, C.; Flies, D.B.; Lau, J.S.; Zhu, G.; et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res. 2005, 65, 1089–1096. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.R.; Drake, C.G.; Wollner, I.; Powderly, J.D.; Picus, J.; Sharfman, W.H.; Stankevich, E.; Pons, A.; Salay, T.M.; McMiller, T.L.; et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: Safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 2010, 28, 3167–3175. [Google Scholar] [CrossRef]
- Smyth, M.J.; Teng, M.W. 2018 Nobel Prize in physiology or medicine. Clin. Transl. Immunol. 2018, 7, e1041. [Google Scholar] [CrossRef]
- Schwartz, R.H. Costimulation of T lymphocytes: The role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 1992, 71, 1065–1068. [Google Scholar] [CrossRef]
- Rosenberg, J.E.; Hoffman-Censits, J.; Powles, T.; van der Heijden, M.S.; Balar, A.V.; Necchi, A.; Dawson, N.; O’Donnell, P.H.; Balmanoukian, A.; Loriot, Y.; et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016, 387, 1909–1920. [Google Scholar] [CrossRef]
- Massard, C.; Gordon, M.S.; Sharma, S.; Rafii, S.; Wainberg, Z.A.; Luke, J.; Curiel, T.J.; Colon-Otero, G.; Hamid, O.; Sanborn, R.E.; et al. Safety and Efficacy of Durvalumab (MEDI4736), an Anti-Programmed Cell Death Ligand-1 Immune Checkpoint Inhibitor, in Patients With Advanced Urothelial Bladder Cancer. J. Clin. Oncol. 2016, 34, 3119–3125. [Google Scholar] [CrossRef]
- Kaufman, H.L.; Russell, J.; Hamid, O.; Bhatia, S.; Terheyden, P.; D’Angelo, S.P.; Shih, K.C.; Lebbé, C.; Linette, G.P.; Milella, M.; et al. Avelumab in patients with chemotherapy-refractory metastatic Merkel cell carcinoma: A multicentre, single-group, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 1374–1385. [Google Scholar] [CrossRef]
- Patel, T.H.; Brewer, J.R.; Fan, J.; Cheng, J.; Shen, Y.L.; Xiang, Y.; Zhao, H.; Lemery, S.J.; Pazdur, R.; Kluetz, P.G.; et al. FDA Approval Summary: Tremelimumab in Combination with Durvalumab for the Treatment of Patients with Unresectable Hepatocellular Carcinoma. Clin. Cancer Res. 2024, 30, 269–273. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Dunmall, L.C.; Wang, Y.Y.; Fan, Z.; Cheng, Z.; Wang, Y. The dilemmas and possible solutions for CAR-T cell therapy application in solid tumors. Cancer Lett. 2024, 591, 216871. [Google Scholar] [CrossRef]
- Al Hadidi, S.; Heslop, H.E.; Brenner, M.K.; Suzuki, M. Bispecific antibodies and autologous chimeric antigen receptor T cell therapies for treatment of hematological malignancies. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 2444–2460. [Google Scholar] [CrossRef]
- Lin, P.; Lin, Y.; Mai, Z.; Zheng, Y.; Zheng, J.; Zhou, Z.; Zhao, X.; Cui, L. Targeting cancer with precision: Strategical insights into TCR-engineered T cell therapies. Theranostics 2025, 15, 300–323. [Google Scholar] [CrossRef] [PubMed]
- Lopez de Rodas, M.; Villalba-Esparza, M.; Sanmamed, M.F.; Chen, L.; Rimm, D.L.; Schalper, K.A. Biological and clinical significance of tumour-infiltrating lymphocytes in the era of immunotherapy: A multidimensional approach. Nat. Rev. Clin. Oncol. 2025, 22, 163–181. [Google Scholar] [CrossRef]
- Zhou, S.; Liu, M.; Ren, F.; Meng, X.; Yu, J. The landscape of bispecific T cell engager in cancer treatment. Biomark. Res. 2021, 9, 38. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T cell engagers: An emerging therapy for management of hematologic malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef] [PubMed]
- Fan, T.; Zhang, M.; Yang, J.; Zhu, Z.; Cao, W.; Dong, C. Therapeutic cancer vaccines: Advancements, challenges, and prospects. Signal Transduct. Target. Ther. 2023, 8, 450. [Google Scholar] [CrossRef]
- Bommareddy, P.K.; Shettigar, M.; Kaufman, H.L. Integrating oncolytic viruses in combination cancer immunotherapy. Nat. Rev. Immunol. 2018, 18, 498–513. [Google Scholar] [CrossRef]
- Chen, L. Co-inhibitory molecules of the B7-CD28 family in the control of T-cell immunity. Nat. Rev. Immunol. 2004, 4, 336–347. [Google Scholar] [CrossRef]
- Intlekofer, A.M.; Thompson, C.B. At the bench: Preclinical rationale for CTLA-4 and PD-1 blockade as cancer immunotherapy. J. Leukoc. Biol. 2013, 94, 25–39. [Google Scholar] [CrossRef]
- Boussiotis, V.A. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N. Engl. J. Med. 2016, 375, 1767–1778. [Google Scholar] [CrossRef]
- Lázár-Molnár, E.; Yan, Q.; Cao, E.; Ramagopal, U.; Nathenson, S.G.; Almo, S.C. Crystal structure of the complex between programmed death-1 (PD-1) and its ligand PD-L2. Proc. Natl. Acad. Sci. USA 2008, 105, 10483–10488. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Pauken, K.E. The diverse functions of the PD1 inhibitory pathway. Nat. Rev. Immunol. 2018, 18, 153–167. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.; Movassagh, M.; Johnson, A.; Mittler, R.S.; Galy, A. 4-1BB co-stimulation enhances human CD8(+) T cell priming by augmenting the proliferation and survival of effector CD8(+) T cells. Int. Immunol. 2002, 14, 1155–1167. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Cui, J.J.; Zhan, Y.; Ouyang, Q.Y.; Lu, Q.S.; Yang, D.H.; Li, X.P.; Yin, J.Y. Reprogramming the tumor microenvironment by genome editing for precision cancer therapy. Mol. Cancer 2022, 21, 98. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 expression by tumour-associated macrophages inhibits phagocytosis and tumour immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hogg, G.D.; DeNardo, D.G. Rethinking immune checkpoint blockade: ‘Beyond the T cell’. J. Immunother. Cancer 2021, 9, e001460. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Francisco, L.M.; Salinas, V.H.; Brown, K.E.; Vanguri, V.K.; Freeman, G.J.; Kuchroo, V.K.; Sharpe, A.H. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J. Exp. Med. 2009, 206, 3015–3029. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Judge, S.J.; Murphy, W.J.; Canter, R.J. Characterizing the Dysfunctional NK Cell: Assessing the Clinical Relevance of Exhaustion, Anergy, and Senescence. Front. Cell. Infect. Microbiol. 2020, 10, 49. [Google Scholar] [CrossRef]
- Pauken, K.E.; Sammons, M.A.; Odorizzi, P.M.; Manne, S.; Godec, J.; Khan, O.; Drake, A.M.; Chen, Z.; Sen, D.R.; Kurachi, M.; et al. Epigenetic stability of exhausted T cells limits durability of reinvigoration by PD-1 blockade. Science 2016, 354, 1160–1165. [Google Scholar] [CrossRef]
- Rajewsky, K. George Klein: 1925-2016. Proc. Natl. Acad. Sci. USA 2017, 114, 3275–3277. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Kang, K.; Chen, P.; Zeng, Z.; Li, G.; Xiong, W.; Yi, M.; Xiang, B. Regulatory mechanisms of PD-1/PD-L1 in cancers. Mol. Cancer 2024, 23, 108. [Google Scholar] [CrossRef]
- Barrios, D.M.; Do, M.H.; Phillips, G.S.; Postow, M.A.; Akaike, T.; Nghiem, P.; Lacouture, M.E. Immune checkpoint inhibitors to treat cutaneous malignancies. J. Am. Acad. Dermatol. 2020, 83, 1239–1253. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.F.; McDermott, D.F.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Combined nivolumab and ipilimumab versus ipilimumab alone in patients with advanced melanoma: 2-year overall survival outcomes in a multicentre, randomised, controlled, phase 2 trial. Lancet Oncol. 2016, 17, 1558–1568. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; van de Wiel, B.A.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Larkin, J.; Del Vecchio, M.; Mandalá, M.; Gogas, H.; Arance Fernandez, A.M.; Dalle, S.; Cowey, C.L.; Schenker, M.; Grob, J.J.; Chiarion-Sileni, V.; et al. Adjuvant Nivolumab versus Ipilimumab in Resected Stage III/IV Melanoma: 5-Year Efficacy and Biomarker Results from CheckMate 238. Clin. Cancer Res. 2023, 29, 3352–3361. [Google Scholar] [CrossRef]
- Luke, J.J.; Ascierto, P.A.; Khattak, M.A.; Rutkowski, P.; Del Vecchio, M.; Spagnolo, F.; Mackiewicz, J.; Merino, L.C.; Chiarion-Sileni, V.; Kirkwood, J.M.; et al. Pembrolizumab versus placebo as adjuvant therapy in resected stage IIB or IIC melanoma: Long-term follow-up, crossover, and rechallenge with pembrolizumab in the phase III KEYNOTE-716 study. Eur. J. Cancer 2025, 220, 115381. [Google Scholar] [CrossRef]
- Nghiem, P.; Bhatia, S.; Lipson, E.J.; Sharfman, W.H.; Kudchadkar, R.R.; Brohl, A.S.; Friedlander, P.A.; Daud, A.; Kluger, H.M.; Reddy, S.A.; et al. Durable Tumor Regression and Overall Survival in Patients With Advanced Merkel Cell Carcinoma Receiving Pembrolizumab as First-Line Therapy. J. Clin. Oncol. 2019, 37, 693–702. [Google Scholar] [CrossRef]
- Damsin, T.; Lebas, E.; Marchal, N.; Rorive, A.; Nikkels, A.F. Cemiplimab for locally advanced and metastatic basal cell carcinoma. Expert Rev. Anticancer Ther. 2022, 22, 243–248. [Google Scholar] [CrossRef]
- Yan, F.; Schmalbach, C.E. Updates in the Management of Advanced Nonmelanoma Skin Cancer. Surg. Oncol. Clin. N. Am. 2024, 33, 723–733. [Google Scholar] [CrossRef]
- Lee, A. Cosibelimab: First Approval. Drugs 2025, 85, 695–698. [Google Scholar] [CrossRef]
- Patel, S.A.; Weiss, J. Advances in the Treatment of Non-Small Cell Lung Cancer: Immunotherapy. Clin. Chest Med. 2020, 41, 237–247. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1-Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Sezer, A.; Kilickap, S.; Gümüş, M.; Bondarenko, I.; Özgüroğlu, M.; Gogishvili, M.; Turk, H.M.; Cicin, I.; Bentsion, D.; Gladkov, O.; et al. Cemiplimab monotherapy for first-line treatment of advanced non-small-cell lung cancer with PD-L1 of at least 50%: A multicentre, open-label, global, phase 3, randomised, controlled trial. Lancet 2021, 397, 592–604. [Google Scholar] [CrossRef]
- Landre, T.; Justeau, G.; Assié, J.B.; Chouahnia, K.; Davoine, C.; Taleb, C.; Chouaïd, C.; Duchemann, B. Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: A meta-analysis of randomized-controlled trials. Cancer Immunol. Immunother. CII 2022, 71, 719–726. [Google Scholar] [CrossRef]
- Chen, W.; Li, L.; Cheng, S.; Yu, J. The Efficacy of Immune Checkpoint Inhibitors vs. Chemotherapy for KRAS-Mutant or EGFR-Mutant Non-Small-Cell Lung Cancers: A Meta-Analysis Based on Randomized Controlled Trials. Dis. Markers 2022, 2022, 2631852. [Google Scholar] [CrossRef]
- Cascone, T.; Awad, M.M.; Spicer, J.D.; He, J.; Lu, S.; Sepesi, B.; Tanaka, F.; Taube, J.M.; Cornelissen, R.; Havel, L.; et al. Perioperative Nivolumab in Resectable Lung Cancer. N. Engl. J. Med. 2024, 390, 1756–1769. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.H.; Gao, S.; Chen, K.N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Paz-Ares, L.; Bernabe Caro, R.; Zurawski, B.; Kim, S.W.; Carcereny Costa, E.; Park, K.; Alexandru, A.; Lupinacci, L.; de la Mora Jimenez, E.; et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2019, 381, 2020–2031. [Google Scholar] [CrossRef]
- West, H.; McCleod, M.; Hussein, M.; Morabito, A.; Rittmeyer, A.; Conter, H.J.; Kopp, H.G.; Daniel, D.; McCune, S.; Mekhail, T.; et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019, 20, 924–937. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Kurata, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N. Engl. J. Med. 2018, 379, 2342–2350. [Google Scholar] [CrossRef]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. Perioperative Durvalumab for Resectable Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar] [CrossRef]
- Felip, E.; Altorki, N.; Zhou, C.; Csőszi, T.; Vynnychenko, I.; Goloborodko, O.; Luft, A.; Akopov, A.; Martinez-Marti, A.; Kenmotsu, H.; et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): A randomised, multicentre, open-label, phase 3 trial. Lancet 2021, 398, 1344–1357. [Google Scholar] [CrossRef]
- Horn, L.; Mansfield, A.S.; Szczęsna, A.; Havel, L.; Krzakowski, M.; Hochmair, M.J.; Huemer, F.; Losonczy, G.; Johnson, M.L.; Nishio, M.; et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2220–2229. [Google Scholar] [CrossRef]
- Goldman, J.W.; Dvorkin, M.; Chen, Y.; Reinmuth, N.; Hotta, K.; Trukhin, D.; Statsenko, G.; Hochmair, M.J.; Özgüroğlu, M.; Ji, J.H.; et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 51–65. [Google Scholar] [CrossRef]
- Cheng, Y.; Spigel, D.R.; Cho, B.C.; Laktionov, K.K.; Fang, J.; Chen, Y.; Zenke, Y.; Lee, K.H.; Wang, Q.; Navarro, A.; et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N. Engl. J. Med. 2024, 391, 1313–1327. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Shitara, K.; Moehler, M.; Garrido, M.; Salman, P.; Shen, L.; Wyrwicz, L.; Yamaguchi, K.; Skoczylas, T.; Campos Bragagnoli, A.; et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): A randomised, open-label, phase 3 trial. Lancet 2021, 398, 27–40. [Google Scholar] [CrossRef]
- Shah, M.A.; Kojima, T.; Hochhauser, D.; Enzinger, P.; Raimbourg, J.; Hollebecque, A.; Lordick, F.; Kim, S.B.; Tajika, M.; Kim, H.T.; et al. Efficacy and Safety of Pembrolizumab for Heavily Pretreated Patients with Advanced, Metastatic Adenocarcinoma or Squamous Cell Carcinoma of the Esophagus: The Phase 2 KEYNOTE-180 Study. JAMA Oncol. 2019, 5, 546–550. [Google Scholar] [CrossRef]
- Kelly, R.J.; Ajani, J.A.; Kuzdzal, J.; Zander, T.; Van Cutsem, E.; Piessen, G.; Mendez, G.; Feliciano, J.; Motoyama, S.; Lièvre, A.; et al. Adjuvant Nivolumab in Resected Esophageal or Gastroesophageal Junction Cancer. N. Engl. J. Med. 2021, 384, 1191–1203. [Google Scholar] [CrossRef]
- Rha, S.Y.; Oh, D.Y.; Yañez, P.; Bai, Y.; Ryu, M.H.; Lee, J.; Rivera, F.; Alves, G.V.; Garrido, M.; Shiu, K.K.; et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023, 24, 1181–1195. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef]
- Grieb, B.C.; Agarwal, R. HER2-Directed Therapy in Advanced Gastric and Gastroesophageal Adenocarcinoma: Triumphs and Troubles. Curr. Treat. Options Oncol. 2021, 22, 88. [Google Scholar] [CrossRef]
- Moehler, M.; Oh, D.Y.; Kato, K.; Arkenau, T.; Tabernero, J.; Lee, K.W.; Rha, S.Y.; Hirano, H.; Spigel, D.; Yamaguchi, K.; et al. First-Line Tislelizumab Plus Chemotherapy for Advanced Gastric Cancer with Programmed Death-Ligand 1 Expression ≥ 1%: A Retrospective Analysis of RATIONALE-305. Adv. Ther. 2025, 42, 2248–2268. [Google Scholar] [CrossRef]
- Yoshinami, Y.; Shoji, H. Recent advances in immunotherapy and molecular targeted therapy for gastric cancer. Future Sci. OA 2023, 9, Fso842. [Google Scholar] [CrossRef]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Overman, M.J.; McDermott, R.; Leach, J.L.; Lonardi, S.; Lenz, H.J.; Morse, M.A.; Desai, J.; Hill, A.; Axelson, M.; Moss, R.A.; et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017, 18, 1182–1191. [Google Scholar] [CrossRef] [PubMed]
- Chalabi, M.; Verschoor, Y.L.; Tan, P.B.; Balduzzi, S.; Van Lent, A.U.; Grootscholten, C.; Dokter, S.; Büller, N.V.; Grotenhuis, B.A.; Kuhlmann, K.; et al. Neoadjuvant Immunotherapy in Locally Advanced Mismatch Repair-Deficient Colon Cancer. N. Engl. J. Med. 2024, 390, 1949–1958. [Google Scholar] [CrossRef] [PubMed]
- Zhu, A.X.; Finn, R.S.; Edeline, J.; Cattan, S.; Ogasawara, S.; Palmer, D.; Verslype, C.; Zagonel, V.; Fartoux, L.; Vogel, A.; et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): A non-randomised, open-label phase 2 trial. Lancet Oncol. 2018, 19, 940–952. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Sangro, B.; Yau, T.; Crocenzi, T.S.; Kudo, M.; Hsu, C.; Kim, T.Y.; Choo, S.P.; Trojan, J.; Welling, T.H.R.; et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): An open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017, 389, 2492–2502. [Google Scholar] [CrossRef]
- Finn, R.S.; Qin, S.; Ikeda, M.; Galle, P.R.; Ducreux, M.; Kim, T.Y.; Kudo, M.; Breder, V.; Merle, P.; Kaseb, A.O.; et al. Atezolizumab plus Bevacizumab in Unresectable Hepatocellular Carcinoma. N. Engl. J. Med. 2020, 382, 1894–1905. [Google Scholar] [CrossRef]
- Sangro, B.; Chan, S.L.; Kelley, R.K.; Lau, G.; Kudo, M.; Sukeepaisarnjaroen, W.; Yarchoan, M.; De Toni, E.N.; Furuse, J.; Kang, Y.K.; et al. Four-year overall survival update from the phase III HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Ann. Oncol. 2024, 35, 448–457. [Google Scholar] [CrossRef]
- Qin, S.; Chan, S.L.; Gu, S.; Bai, Y.; Ren, Z.; Lin, X.; Chen, Z.; Jia, W.; Jin, Y.; Guo, Y.; et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet 2023, 402, 1133–1146. [Google Scholar] [CrossRef]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Hoyos Usta, E.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef]
- Oaknin, A.; Tinker, A.V.; Gilbert, L.; Samouëlian, V.; Mathews, C.; Brown, J.; Barretina-Ginesta, M.P.; Moreno, V.; Gravina, A.; Abdeddaim, C.; et al. Clinical Activity and Safety of the Anti-Programmed Death 1 Monoclonal Antibody Dostarlimab for Patients With Recurrent or Advanced Mismatch Repair-Deficient Endometrial Cancer: A Nonrandomized Phase 1 Clinical Trial. JAMA Oncol. 2020, 6, 1766–1772. [Google Scholar] [CrossRef]
- Powell, M.A.; Cibula, D.; O’Malley, D.M.; Boere, I.; Shahin, M.S.; Savarese, A.; Chase, D.M.; Gilbert, L.; Black, D.; Herrstedt, J.; et al. Efficacy and safety of dostarlimab in combination with chemotherapy in patients with dMMR/MSI-H primary advanced or recurrent endometrial cancer in a phase 3, randomized, placebo-controlled trial (ENGOT-EN6-NSGO/GOG-3031/RUBY). Gynecol. Oncol. 2025, 192, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Di Dio, C.; Bogani, G.; Di Donato, V.; Cuccu, I.; Muzii, L.; Musacchio, L.; Scambia, G.; Lorusso, D. The role of immunotherapy in advanced and recurrent MMR deficient and proficient endometrial carcinoma. Gynecol. Oncol. 2023, 169, 27–33. [Google Scholar] [CrossRef]
- Westin, S.N.; Moore, K.; Chon, H.S.; Lee, J.Y.; Thomes Pepin, J.; Sundborg, M.; Shai, A.; de la Garza, J.; Nishio, S.; Gold, M.A.; et al. Durvalumab Plus Carboplatin/Paclitaxel Followed by Maintenance Durvalumab With or Without Olaparib as First-Line Treatment for Advanced Endometrial Cancer: The Phase III DUO-E Trial. J. Clin. Oncol. 2024, 42, 283–299. [Google Scholar] [CrossRef]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef] [PubMed]
- Rini, B.I.; Plimack, E.R.; Stus, V.; Gafanov, R.; Hawkins, R.; Nosov, D.; Pouliot, F.; Alekseev, B.; Soulières, D.; Melichar, B.; et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1116–1127. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Penkov, K.; Haanen, J.; Rini, B.; Albiges, L.; Campbell, M.T.; Venugopal, B.; Kollmannsberger, C.; Negrier, S.; Uemura, M.; et al. Avelumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2019, 380, 1103–1115. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Powles, T.; Burotto, M.; Escudier, B.; Bourlon, M.T.; Zurawski, B.; Oyervides Juárez, V.M.; Hsieh, J.J.; Basso, U.; Shah, A.Y.; et al. Nivolumab plus Cabozantinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2021, 384, 829–841. [Google Scholar] [CrossRef]
- Motzer, R.; Alekseev, B.; Rha, S.Y.; Porta, C.; Eto, M.; Powles, T.; Grünwald, V.; Hutson, T.E.; Kopyltsov, E.; Méndez-Vidal, M.J.; et al. Lenvatinib plus Pembrolizumab or Everolimus for Advanced Renal Cell Carcinoma. N. Engl. J. Med. 2021, 384, 1289–1300. [Google Scholar] [CrossRef]
- Powles, T.; Csőszi, T.; Özgüroğlu, M.; Matsubara, N.; Géczi, L.; Cheng, S.Y.; Fradet, Y.; Oudard, S.; Vulsteke, C.; Morales Barrera, R.; et al. Pembrolizumab alone or combined with chemotherapy versus chemotherapy as first-line therapy for advanced urothelial carcinoma (KEYNOTE-361): A randomised, open-label, phase 3 trial. Lancet Oncol. 2021, 22, 931–945. [Google Scholar] [CrossRef] [PubMed]
- Powles, T.; Park, S.H.; Voog, E.; Caserta, C.; Valderrama, B.P.; Gurney, H.; Kalofonos, H.; Radulović, S.; Demey, W.; Ullén, A.; et al. Avelumab Maintenance Therapy for Advanced or Metastatic Urothelial Carcinoma. N. Engl. J. Med. 2020, 383, 1218–1230. [Google Scholar] [CrossRef]
- van der Heijden, M.S.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine-Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Oridate, N.; Takahashi, S.; Tanaka, K.; Shimizu, Y.; Fujimoto, Y.; Matsumoto, K.; Yokota, T.; Yamazaki, T.; Takahashi, M.; Ueda, T.; et al. First-line pembrolizumab with or without chemotherapy for recurrent or metastatic head and neck squamous cell carcinoma: 5-year follow-up of the Japanese population of KEYNOTE-048. Int. J. Clin. Oncol. 2024, 29, 1825–1839. [Google Scholar] [CrossRef]
- Liang, Y.L.; Liu, X.; Shen, L.F.; Hu, G.Y.; Zou, G.R.; Zhang, N.; Chen, C.B.; Chen, X.Z.; Zhu, X.D.; Yuan, Y.W.; et al. Adjuvant PD-1 Blockade With Camrelizumab for Nasopharyngeal Carcinoma: The DIPPER Randomized Clinical Trial. J. Am. Med. Assoc. 2025. [Google Scholar] [CrossRef]
- Zheng, B.; Huang, Z.; Liu, W.; Zhao, D.; Xu, X.; Xiao, S.; Sun, Y.; Wang, W. A real-world evaluation of tislelizumab in patients with head and neck cancer. Transl. Cancer Res. 2024, 13, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.L.; Li, X.Y.; Yang, J.H.; Wen, D.X.; Guo, S.S.; Liu, L.T.; Li, Y.F.; Luo, M.J.; Xie, S.Y.; Liang, Y.J.; et al. Neoadjuvant and adjuvant toripalimab for locoregionally advanced nasopharyngeal carcinoma: A randomised, single-centre, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2024, 25, 1563–1575. [Google Scholar] [CrossRef]
- Schmid, P.; Rugo, H.S.; Adams, S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Henschel, V.; Molinero, L.; Chui, S.Y.; et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): Updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020, 21, 44–59. [Google Scholar] [CrossRef] [PubMed]
- Cetin, B.; Gumusay, O. Pembrolizumab for Early Triple-Negative Breast Cancer. N. Engl. J. Med. 2020, 382, e108. [Google Scholar] [CrossRef]
- Younes, A.; Santoro, A.; Shipp, M.; Zinzani, P.L.; Timmerman, J.M.; Ansell, S.; Armand, P.; Fanale, M.; Ratanatharathorn, V.; Kuruvilla, J.; et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: A multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016, 17, 1283–1294. [Google Scholar] [CrossRef]
- Armand, P.; Rodig, S.; Melnichenko, V.; Thieblemont, C.; Bouabdallah, K.; Tumyan, G.; Özcan, M.; Portino, S.; Fogliatto, L.; Caballero, M.D.; et al. Pembrolizumab in Relapsed or Refractory Primary Mediastinal Large B-Cell Lymphoma. J. Clin. Oncol. 2019, 37, 3291–3299. [Google Scholar] [CrossRef]
- Centanni, M.; Moes, D.; Trocóniz, I.F.; Ciccolini, J.; van Hasselt, J.G.C. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin. Pharmacokinet. 2019, 58, 835–857. [Google Scholar] [CrossRef]
- Yan, T.; Yu, L.; Shangguan, D.; Li, W.; Liu, N.; Chen, Y.; Fu, Y.; Tang, J.; Liao, D. Advances in pharmacokinetics and pharmacodynamics of PD-1/PD-L1 inhibitors. Int. Immunopharmacol. 2023, 115, 109638. [Google Scholar] [CrossRef]
- Sachs, U.J.; Socher, I.; Braeunlich, C.G.; Kroll, H.; Bein, G.; Santoso, S. A variable number of tandem repeats polymorphism influences the transcriptional activity of the neonatal Fc receptor alpha-chain promoter. Immunology 2006, 119, 83–89. [Google Scholar] [CrossRef]
- Keizer, R.J.; Huitema, A.D.; Schellens, J.H.; Beijnen, J.H. Clinical pharmacokinetics of therapeutic monoclonal antibodies. Clin. Pharmacokinet. 2010, 49, 493–507. [Google Scholar] [CrossRef]
- Wang, W.; Wang, E.Q.; Balthasar, J.P. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558. [Google Scholar] [CrossRef]
- Zhao, X.; Suryawanshi, S.; Hruska, M.; Feng, Y.; Wang, X.; Shen, J.; Vezina, H.E.; McHenry, M.B.; Waxman, I.M.; Achanta, A.; et al. Assessment of nivolumab benefit-risk profile of a 240-mg flat dose relative to a 3-mg/kg dosing regimen in patients with advanced tumors. Ann. Oncol. 2017, 28, 2002–2008. [Google Scholar] [CrossRef]
- Liu, C.; Yu, J.; Li, H.; Liu, J.; Xu, Y.; Song, P.; Liu, Q.; Zhao, H.; Xu, J.; Maher, V.E.; et al. Association of time-varying clearance of nivolumab with disease dynamics and its implications on exposure response analysis. Clin. Pharmacol. Ther. 2017, 101, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, J.; Liu, C.; Liu, J.; Subramaniam, S.; Zhao, H.; Blumenthal, G.M.; Turner, D.C.; Li, C.; Ahamadi, M.; et al. Time dependent pharmacokinetics of pembrolizumab in patients with solid tumor and its correlation with best overall response. J. Pharmacokinet. Pharmacodyn. 2017, 44, 403–414. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E. Understanding cachexia as a cancer metabolism syndrome. Oncogenesis 2016, 5, e200. [Google Scholar] [CrossRef] [PubMed]
- Baverel, P.G.; Dubois, V.F.S.; Jin, C.Y.; Zheng, Y.; Song, X.; Jin, X.; Mukhopadhyay, P.; Gupta, A.; Dennis, P.A.; Ben, Y.; et al. Population Pharmacokinetics of Durvalumab in Cancer Patients and Association With Longitudinal Biomarkers of Disease Status. Clin. Pharmacol. Ther. 2018, 103, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Beers, S.A.; Glennie, M.J.; White, A.L. Influence of immunoglobulin isotype on therapeutic antibody function. Blood 2016, 127, 1097–1101. [Google Scholar] [CrossRef]
- Kretschmer, A.; Schwanbeck, R.; Valerius, T.; Rösner, T. Antibody Isotypes for Tumor Immunotherapy. Transfus. Med. Hemother. 2017, 44, 320–326. [Google Scholar] [CrossRef]
- Sharma, A.; Subudhi, S.K.; Blando, J.; Scutti, J.; Vence, L.; Wargo, J.; Allison, J.P.; Ribas, A.; Sharma, P. Anti-CTLA-4 Immunotherapy Does Not Deplete FOXP3(+) Regulatory T Cells (Tregs) in Human Cancers. Clin. Cancer Res. 2019, 25, 1233–1238. [Google Scholar] [CrossRef]
- Agrawal, S.; Feng, Y.; Roy, A.; Kollia, G.; Lestini, B. Nivolumab dose selection: Challenges, opportunities, and lessons learned for cancer immunotherapy. J. Immunother. Cancer 2016, 4, 72. [Google Scholar] [CrossRef]
- Oude Munnink, T.H.; Henstra, M.J.; Segerink, L.I.; Movig, K.L.; Brummelhuis-Visser, P. Therapeutic drug monitoring of monoclonal antibodies in inflammatory and malignant disease: Translating TNF-α experience to oncology. Clin. Pharmacol. Ther. 2016, 99, 419–431. [Google Scholar] [CrossRef]
- Stroh, M.; Winter, H.; Marchand, M.; Claret, L.; Eppler, S.; Ruppel, J.; Abidoye, O.; Teng, S.L.; Lin, W.T.; Dayog, S.; et al. Clinical Pharmacokinetics and Pharmacodynamics of Atezolizumab in Metastatic Urothelial Carcinoma. Clin. Pharmacol. Ther. 2017, 102, 305–312. [Google Scholar] [CrossRef]
- Bajaj, G.; Wang, X.; Agrawal, S.; Gupta, M.; Roy, A.; Feng, Y. Model-Based Population Pharmacokinetic Analysis of Nivolumab in Patients With Solid Tumors. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ahamadi, M.; Freshwater, T.; Prohn, M.; Li, C.H.; de Alwis, D.P.; de Greef, R.; Elassaiss-Schaap, J.; Kondic, A.; Stone, J.A. Model-Based Characterization of the Pharmacokinetics of Pembrolizumab: A Humanized Anti-PD-1 Monoclonal Antibody in Advanced Solid Tumors. CPT Pharmacomet. Syst. Pharmacol. 2017, 6, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Paccaly, A.J.; Rippley, R.K.; Davis, J.D.; DiCioccio, A.T. Population pharmacokinetic characteristics of cemiplimab in patients with advanced malignancies. J. Pharmacokinet. Pharmacodyn. 2021, 48, 479–494. [Google Scholar] [CrossRef]
- Melhem, M.; Hanze, E.; Lu, S.; Alskär, O.; Visser, S.; Gandhi, Y. Population pharmacokinetics and exposure-response of anti-programmed cell death protein-1 monoclonal antibody dostarlimab in advanced solid tumours. Br. J. Clin. Pharmacol. 2022, 88, 4142–4154. [Google Scholar] [CrossRef]
- Budha, N.; Wu, C.Y.; Tang, Z.; Yu, T.; Liu, L.; Xu, F.; Gao, Y.; Li, R.; Zhang, Q.; Wan, Y.; et al. Model-based population pharmacokinetic analysis of tislelizumab in patients with advanced tumors. CPT Pharmacomet. Syst. Pharmacol. 2023, 12, 95–109. [Google Scholar] [CrossRef]
- Wang, C.Y.; Sheng, C.C.; Ma, G.L.; Xu, D.; Liu, X.Q.; Wang, Y.Y.; Zhang, L.; Cui, C.L.; Xu, B.H.; Song, Y.Q.; et al. Population pharmacokinetics of the anti-PD-1 antibody camrelizumab in patients with multiple tumor types and model-informed dosing strategy. Acta Pharmacol. Sin. 2021, 42, 1368–1375. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Epling, D.; Dolphin, N.; Paccaly, A.; Conrado, D.; Davis, J.D.; Al-Huniti, N. Population pharmacokinetics modeling and exposure-response analyses of cemiplimab in patients with recurrent or metastatic cervical cancer. CPT Pharmacomet. Syst. Pharmacol. 2022, 11, 1458–1471. [Google Scholar] [CrossRef] [PubMed]
- Shek, D.; Read, S.A.; Ahlenstiel, G.; Piatkov, I. Pharmacogenetics of anticancer monoclonal antibodies. Cancer Drug Resist. 2019, 2, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Michot, J.M.; Bigenwald, C.; Champiat, S.; Collins, M.; Carbonnel, F.; Postel-Vinay, S.; Berdelou, A.; Varga, A.; Bahleda, R.; Hollebecque, A.; et al. Immune-related adverse events with immune checkpoint blockade: A comprehensive review. Eur. J. Cancer 2016, 54, 139–148. [Google Scholar] [CrossRef]
- Blank, C.U.; Haanen, J.B.; Ribas, A.; Schumacher, T.N. CANCER IMMUNOLOGY. The “cancer immunogram”. Science 2016, 352, 658–660. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Hsu, J.M.; Sun, L.; Wang, S.C.; Hung, M.C. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep. Med. 2024, 5, 101621. [Google Scholar] [CrossRef] [PubMed]
- Heersche, N.; Veerman, G.D.M.; de With, M.; Bins, S.; Assaraf, Y.G.; Dingemans, A.C.; van Schaik, R.H.N.; Mathijssen, R.H.J.; Jansman, F.G.A. Clinical implications of germline variations for treatment outcome and drug resistance for small molecule kinase inhibitors in patients with non-small cell lung cancer. Drug Resist. Updates 2022, 62, 100832. [Google Scholar] [CrossRef]
- de Joode, K.; Heersche, N.; Basak, E.A.; Bins, S.; van der Veldt, A.A.M.; van Schaik, R.H.N.; Mathijssen, R.H.J. Review—The impact of pharmacogenetics on the outcome of immune checkpoint inhibitors. Cancer Treat. Rev. 2024, 122, 102662. [Google Scholar] [CrossRef]
- Groha, S.; Alaiwi, S.A.; Xu, W.; Naranbhai, V.; Nassar, A.H.; Bakouny, Z.; El Zarif, T.; Saliby, R.M.; Wan, G.; Rajeh, A.; et al. Germline variants associated with toxicity to immune checkpoint blockade. Nat. Med. 2022, 28, 2584–2591. [Google Scholar] [CrossRef]
- Abdel-Wahab, N.; Diab, A.; Yu, R.K.; Futreal, A.; Criswell, L.A.; Tayar, J.H.; Dadu, R.; Shannon, V.; Shete, S.S.; Suarez-Almazor, M.E. Genetic determinants of immune-related adverse events in patients with melanoma receiving immune checkpoint inhibitors. Cancer Immunol. Immunother. CII 2021, 70, 1939–1949. [Google Scholar] [CrossRef]
- Montaudié, H.; Beranger, G.E.; Reinier, F.; Nottet, N.; Martin, H.; Picard-Gauci, A.; Troin, L.; Ballotti, R.; Passeron, T. Germline variants in exonic regions have limited impact on immune checkpoint blockade clinical outcomes in advanced melanoma. Pigment. Cell Melanoma Res. 2021, 34, 978–983. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.A.; Watson, R.A.; Tong, O.; Ye, W.; Nassiri, I.; Gilchrist, J.J.; de Los Aires, A.V.; Sharma, P.K.; Koturan, S.; Cooper, R.A.; et al. IL7 genetic variation and toxicity to immune checkpoint blockade in patients with melanoma. Nat. Med. 2022, 28, 2592–2600. [Google Scholar] [CrossRef]
- Parakh, S.; Musafer, A.; Paessler, S.; Witkowski, T.; Suen, C.; Tutuka, C.S.A.; Carlino, M.S.; Menzies, A.M.; Scolyer, R.A.; Cebon, J.; et al. PDCD1 Polymorphisms May Predict Response to Anti-PD-1 Blockade in Patients With Metastatic Melanoma. Front. Immunol. 2021, 12, 672521. [Google Scholar] [CrossRef]
- Refae, S.; Gal, J.; Ebran, N.; Otto, J.; Borchiellini, D.; Peyrade, F.; Chamorey, E.; Brest, P.; Milano, G.; Saada-Bouzid, E. Germinal Immunogenetics predict treatment outcome for PD-1/PD-L1 checkpoint inhibitors. Investig. N. Drugs 2020, 38, 160–171. [Google Scholar] [CrossRef]
- de With, M.; Hurkmans, D.P.; Oomen-de Hoop, E.; Lalouti, A.; Bins, S.; El Bouazzaoui, S.; van Brakel, M.; Debets, R.; Aerts, J.; van Schaik, R.H.N.; et al. Germline Variation in PDCD1 Is Associated with Overall Survival in Patients with Metastatic Melanoma Treated with Anti-PD-1 Monotherapy. Cancers 2021, 13, 1370. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Numakura, K.; Hatakeyama, S.; Muto, Y.; Sekine, Y.; Sasagawa, H.; Kashima, S.; Yamamoto, R.; Koizumi, A.; Nara, T.; et al. Severe Immune-Related Adverse Events in Patients Treated with Nivolumab for Metastatic Renal Cell Carcinoma Are Associated with PDCD1 Polymorphism. Genes 2022, 13, 1204. [Google Scholar] [CrossRef]
- Suminaga, K.; Nomizo, T.; Yoshida, H.; Ozasa, H. The impact of PD-L1 polymorphisms on the efficacy of immune checkpoint inhibitors depends on the tumor proportion score: A retrospective study. J. Cancer Res. Clin. Oncol. 2025, 151, 61. [Google Scholar] [CrossRef] [PubMed]
- Nomizo, T.; Ozasa, H.; Tsuji, T.; Funazo, T.; Yasuda, Y.; Yoshida, H.; Yagi, Y.; Sakamori, Y.; Nagai, H.; Hirai, T.; et al. Clinical Impact of Single Nucleotide Polymorphism in PD-L1 on Response to Nivolumab for Advanced Non-Small-Cell Lung Cancer Patients. Sci. Rep. 2017, 7, 45124. [Google Scholar] [CrossRef]
- Del Re, M.; Cucchiara, F.; Rofi, E.; Fontanelli, L.; Petrini, I.; Gri, N.; Pasquini, G.; Rizzo, M.; Gabelloni, M.; Belluomini, L.; et al. A multiparametric approach to improve the prediction of response to immunotherapy in patients with metastatic NSCLC. Cancer Immunol. Immunother. CII 2021, 70, 1667–1678. [Google Scholar] [CrossRef]
- Shiota, M.; Miyake, H.; Takahashi, M.; Oya, M.; Tsuchiya, N.; Masumori, N.; Matsuyama, H.; Obara, W.; Shinohara, N.; Fujimoto, K.; et al. Effect of genetic polymorphisms on outcomes following nivolumab for advanced renal cell carcinoma in the SNiP-RCC trial. Cancer Immunol. Immunother. CII 2023, 72, 1903–1915. [Google Scholar] [CrossRef]
- Funazo, T.Y.; Nomizo, T.; Ozasa, H.; Tsuji, T.; Yasuda, Y.; Yoshida, H.; Sakamori, Y.; Nagai, H.; Hirai, T.; Kim, Y.H. Clinical impact of low serum free T4 in patients with non-small cell lung cancer treated with nivolumab. Sci. Rep. 2019, 9, 17085. [Google Scholar] [CrossRef]
- Yoshida, H.; Nomizo, T.; Ozasa, H.; Tsuji, T.; Funazo, T.; Yasuda, Y.; Ajimizu, H.; Yamazoe, M.; Kuninaga, K.; Ogimoto, T.; et al. PD-L1 polymorphisms predict survival outcomes in advanced non-small-cell lung cancer patients treated with PD-1 blockade. Eur. J. Cancer 2021, 144, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Polcaro, G.; Liguori, L.; Manzo, V.; Chianese, A.; Donadio, G.; Caputo, A.; Scognamiglio, G.; Dell’Annunziata, F.; Langella, M.; Corbi, G.; et al. rs822336 binding to C/EBPβ and NFIC modulates induction of PD-L1 expression and predicts anti-PD-1/PD-L1 therapy in advanced NSCLC. Mol. Cancer 2024, 23, 63. [Google Scholar] [CrossRef] [PubMed]
- Chat, V.; Ferguson, R.; Simpson, D.; Kazlow, E.; Lax, R.; Moran, U.; Pavlick, A.; Frederick, D.; Boland, G.; Sullivan, R.; et al. Autoimmune genetic risk variants as germline biomarkers of response to melanoma immune-checkpoint inhibition. Cancer Immunol. Immunother. CII 2019, 68, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Hurkmans, D.P.; Basak, E.A.; Schepers, N.; Oomen-De Hoop, E.; Van der Leest, C.H.; El Bouazzaoui, S.; Bins, S.; Koolen, S.L.W.; Sleijfer, S.; Van der Veldt, A.A.M.; et al. Granzyme B is correlated with clinical outcome after PD-1 blockade in patients with stage IV non-small-cell lung cancer. J. Immunother. Cancer 2020, 8. [Google Scholar] [CrossRef]
- Ogimoto, T.; Ozasa, H.; Yoshida, H.; Nomizo, T.; Funazo, T.; Yoshida, H.; Hashimoto, K.; Hosoya, K.; Yamazoe, M.; Ajimizu, H.; et al. CD47 polymorphism for predicting nivolumab benefit in patients with advanced non-small-cell lung cancer. Oncol. Lett. 2023, 26, 364. [Google Scholar] [CrossRef]
- Bins, S.; Basak, E.A.; El Bouazzaoui, S.; Koolen, S.L.W.; Oomen-de Hoop, E.; van der Leest, C.H.; van der Veldt, A.A.M.; Sleijfer, S.; Debets, R.; van Schaik, R.H.N.; et al. Association between single-nucleotide polymorphisms and adverse events in nivolumab-treated non-small cell lung cancer patients. Br. J. Cancer 2018, 118, 1296–1301. [Google Scholar] [CrossRef]
- Haratani, K.; Hayashi, H.; Chiba, Y.; Kudo, K.; Yonesaka, K.; Kato, R.; Kaneda, H.; Hasegawa, Y.; Tanaka, K.; Takeda, M.; et al. Association of Immune-Related Adverse Events with Nivolumab Efficacy in Non-Small-Cell Lung Cancer. JAMA Oncol. 2018, 4, 374–378. [Google Scholar] [CrossRef]
- Lee, S.Y.; Jung, D.K.; Choi, J.E.; Jin, C.C.; Hong, M.J.; Do, S.K.; Kang, H.G.; Lee, W.K.; Seok, Y.; Lee, E.B.; et al. Functional polymorphisms in PD-L1 gene are associated with the prognosis of patients with early stage non-small cell lung cancer. Gene 2017, 599, 28–35. [Google Scholar] [CrossRef]
- Wang, W.; Li, F.; Mao, Y.; Zhou, H.; Sun, J.; Li, R.; Liu, C.; Chen, W.; Hua, D.; Zhang, X. A miR-570 binding site polymorphism in the B7-H1 gene is associated with the risk of gastric adenocarcinoma. Hum. Genet. 2013, 132, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Jung, D.K.; Choi, J.E.; Jin, C.C.; Hong, M.J.; Do, S.K.; Kang, H.G.; Lee, W.K.; Seok, Y.; Lee, E.B.; et al. PD-L1 polymorphism can predict clinical outcomes of non-small cell lung cancer patients treated with first-line paclitaxel-cisplatin chemotherapy. Sci. Rep. 2016, 6, 25952. [Google Scholar] [CrossRef]
- Khan, Z.; Hammer, C.; Carroll, J.; Di Nucci, F.; Acosta, S.L.; Maiya, V.; Bhangale, T.; Hunkapiller, J.; Mellman, I.; Albert, M.L.; et al. Genetic variation associated with thyroid autoimmunity shapes the systemic immune response to PD-1 checkpoint blockade. Nat. Commun. 2021, 12, 3355. [Google Scholar] [CrossRef]
- Postow, M.A.; Sidlow, R.; Hellmann, M.D. Immune-Related Adverse Events Associated with Immune Checkpoint Blockade. N. Engl. J. Med. 2018, 378, 158–168. [Google Scholar] [CrossRef]
- Russo, V.; Klein, T.; Lim, D.J.; Solis, N.; Machado, Y.; Hiroyasu, S.; Nabai, L.; Shen, Y.; Zeglinski, M.R.; Zhao, H.; et al. Granzyme B is elevated in autoimmune blistering diseases and cleaves key anchoring proteins of the dermal-epidermal junction. Sci. Rep. 2018, 8, 9690. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Martucci, V.L.; Quandt, Z.; Groha, S.; Murray, M.H.; Lovly, C.M.; Rizvi, H.; Egger, J.V.; Plodkowski, A.J.; Abu-Akeel, M.; et al. Immunotherapy-Mediated Thyroid Dysfunction: Genetic Risk and Impact on Outcomes with PD-1 Blockade in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2021, 27, 5131–5140. [Google Scholar] [CrossRef] [PubMed]
- Pierini, F.; Lenz, T.L. Divergent Allele Advantage at Human MHC Genes: Signatures of Past and Ongoing Selection. Mol. Biol. Evol. 2018, 35, 2145–2158. [Google Scholar] [CrossRef]
- Paul, S.; Weiskopf, D.; Angelo, M.A.; Sidney, J.; Peters, B.; Sette, A. HLA class I alleles are associated with peptide-binding repertoires of different size, affinity, and immunogenicity. J. Immunol. 2013, 191, 5831–5839. [Google Scholar] [CrossRef]
- Cuppens, K.; Baas, P.; Geerdens, E.; Cruys, B.; Froyen, G.; Decoster, L.; Thomeer, M.; Maes, B. HLA-I diversity and tumor mutational burden by comprehensive next-generation sequencing as predictive biomarkers for the treatment of non-small cell lung cancer with PD-(L)1 inhibitors. Lung Cancer 2022, 170, 1–10. [Google Scholar] [CrossRef]
- Chowell, D.; Morris, L.G.T.; Grigg, C.M.; Weber, J.K.; Samstein, R.M.; Makarov, V.; Kuo, F.; Kendall, S.M.; Requena, D.; Riaz, N.; et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 2018, 359, 582–587. [Google Scholar] [CrossRef]
- Abed, A.; Calapre, L.; Lo, J.; Correia, S.; Bowyer, S.; Chopra, A.; Watson, M.; Khattak, M.A.; Millward, M.; Gray, E.S. Prognostic value of HLA-I homozygosity in patients with non-small cell lung cancer treated with single agent immunotherapy. J. Immunother. Cancer 2020, 8, e001620. [Google Scholar] [CrossRef]
- Chhibber, A.; Huang, L.; Zhang, H.; Xu, J.; Cristescu, R.; Liu, X.; Mehrotra, D.V.; Shen, J.; Shaw, P.M.; Hellmann, M.D.; et al. Germline HLA landscape does not predict efficacy of pembrolizumab monotherapy across solid tumor types. Immunity 2022, 55, 56–64.e54. [Google Scholar] [CrossRef]
- Negrao, M.V.; Lam, V.K.; Reuben, A.; Rubin, M.L.; Landry, L.L.; Roarty, E.B.; Rinsurongkawong, W.; Lewis, J.; Roth, J.A.; Swisher, S.G.; et al. PD-L1 Expression, Tumor Mutational Burden, and Cancer Gene Mutations Are Stronger Predictors of Benefit from Immune Checkpoint Blockade than HLA Class I Genotype in Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2019, 14, 1021–1031. [Google Scholar] [CrossRef]
- Chowell, D.; Krishna, C.; Pierini, F.; Makarov, V.; Rizvi, N.A.; Kuo, F.; Morris, L.G.T.; Riaz, N.; Lenz, T.L.; Chan, T.A. Evolutionary divergence of HLA class I genotype impacts efficacy of cancer immunotherapy. Nat. Med. 2019, 25, 1715–1720. [Google Scholar] [CrossRef]
- Lee, C.H.; DiNatale, R.G.; Chowell, D.; Krishna, C.; Makarov, V.; Valero, C.; Vuong, L.; Lee, M.; Weiss, K.; Hoen, D.; et al. High Response Rate and Durability Driven by HLA Genetic Diversity in Patients with Kidney Cancer Treated with Lenvatinib and Pembrolizumab. Mol. Cancer Res. MCR 2021, 19, 1510–1521. [Google Scholar] [CrossRef]
- Lu, Z.; Chen, H.; Jiao, X.; Wang, Y.; Wu, L.; Sun, H.; Li, S.; Gong, J.; Li, J.; Zou, J.; et al. Germline HLA-B evolutionary divergence influences the efficacy of immune checkpoint blockade therapy in gastrointestinal cancer. Genome Med. 2021, 13, 175. [Google Scholar] [CrossRef]
- Yin, X.; Song, Y.; Deng, W.; Blake, N.; Luo, X.; Meng, J. Potential predictive biomarkers in antitumor immunotherapy: Navigating the future of antitumor treatment and immune checkpoint inhibitor efficacy. Front. Oncol. 2024, 14, 1483454. [Google Scholar] [CrossRef]
- Correale, P.; Saladino, R.E.; Giannarelli, D.; Giannicola, R.; Agostino, R.; Staropoli, N.; Strangio, A.; Del Giudice, T.; Nardone, V.; Altomonte, M.; et al. Distinctive germline expression of class I human leukocyte antigen (HLA) alleles and DRB1 heterozygosis predict the outcome of patients with non-small cell lung cancer receiving PD-1/PD-L1 immune checkpoint blockade. J. Immunother. Cancer 2020, 8, e000733. [Google Scholar] [CrossRef]
- Naranbhai, V.; Viard, M.; Dean, M.; Groha, S.; Braun, D.A.; Labaki, C.; Shukla, S.A.; Yuki, Y.; Shah, P.; Chin, K.; et al. HLA-A*03 and response to immune checkpoint blockade in cancer: An epidemiological biomarker study. Lancet Oncol. 2022, 23, 172–184. [Google Scholar] [CrossRef]
- Takahashi, S.; Narita, S.; Fujiyama, N.; Hatakeyama, S.; Kobayashi, T.; Kato, R.; Naito, S.; Sakatani, T.; Kashima, S.; Koizumi, A.; et al. Impact of germline HLA genotypes on clinical outcomes in patients with urothelial cancer treated with pembrolizumab. Cancer Sci. 2022, 113, 4059–4069. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, Y.; Zhang, B.; Wang, X.; Mo, H.; Jiao, Y.; Xu, J.; Huang, J. Prognostic and predictive impact of neutrophil-to-lymphocyte ratio and HLA-I genotyping in advanced esophageal squamous cell carcinoma patients receiving immune checkpoint inhibitor monotherapy. Thorac. Cancer 2022, 13, 1631–1641. [Google Scholar] [CrossRef] [PubMed]
- Ishida, Y.; Otsuka, A.; Tanaka, H.; Levesque, M.P.; Dummer, R.; Kabashima, K. HLA-A*26 Is Correlated With Response to Nivolumab in Japanese Melanoma Patients. J. Investig. Dermatol. 2017, 137, 2443–2444. [Google Scholar] [CrossRef]
- Cappelli, L.C.; Dorak, M.T.; Bettinotti, M.P.; Bingham, C.O.; Shah, A.A. Association of HLA-DRB1 shared epitope alleles and immune checkpoint inhibitor-induced inflammatory arthritis. Rheumatology 2019, 58, 476–480. [Google Scholar] [CrossRef]
- Akturk, H.K.; Couts, K.L.; Baschal, E.E.; Karakus, K.E.; Van Gulick, R.J.; Turner, J.A.; Pyle, L.; Robinson, W.A.; Michels, A.W. Analysis of Human Leukocyte Antigen DR Alleles, Immune-Related Adverse Events, and Survival Associated With Immune Checkpoint Inhibitor Use Among Patients With Advanced Malignant Melanoma. JAMA Netw. Open 2022, 5, e2246400. [Google Scholar] [CrossRef]
- Hasan Ali, O.; Berner, F.; Bomze, D.; Fässler, M.; Diem, S.; Cozzio, A.; Jörger, M.; Früh, M.; Driessen, C.; Lenz, T.L.; et al. Human leukocyte antigen variation is associated with adverse events of checkpoint inhibitors. Eur. J. Cancer 2019, 107, 8–14. [Google Scholar] [CrossRef]
- Chen, L.; Diao, L.; Yang, Y.; Yi, X.; Rodriguez, B.L.; Li, Y.; Villalobos, P.A.; Cascone, T.; Liu, X.; Tan, L.; et al. CD38-Mediated Immunosuppression as a Mechanism of Tumor Cell Escape from PD-1/PD-L1 Blockade. Cancer Discov. 2018, 8, 1156–1175. [Google Scholar] [CrossRef]
- Xia, Y.; Huang, C.; Zhong, M.; Zhong, H.; Ruan, R.; Xiong, J.; Yao, Y.; Zhou, J.; Deng, J. Targeting HGF/c-MET signaling to regulate the tumor microenvironment: Implications for counteracting tumor immune evasion. Cell Commun. Signal. CCS 2025, 23, 46. [Google Scholar] [CrossRef]
- Zhang, G. Regulatory T-cells-related signature for identifying a prognostic subtype of hepatocellular carcinoma with an exhausted tumor microenvironment. Front. Immunol. 2022, 13, 975762. [Google Scholar] [CrossRef]
- Morad, G.; Helmink, B.A.; Sharma, P.; Wargo, J.A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 2021, 184, 5309–5337. [Google Scholar] [CrossRef]
- McGranahan, N.; Rosenthal, R.; Hiley, C.T.; Rowan, A.J.; Watkins, T.B.K.; Wilson, G.A.; Birkbak, N.J.; Veeriah, S.; Van Loo, P.; Herrero, J.; et al. Allele-Specific HLA Loss and Immune Escape in Lung Cancer Evolution. Cell 2017, 171, 1259–1271.e1211. [Google Scholar] [CrossRef]
- Jhunjhunwala, S.; Hammer, C.; Delamarre, L. Antigen presentation in cancer: Insights into tumour immunogenicity and immune evasion. Nat. reviews. Cancer 2021, 21, 298–312. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Jiao, Y.J.; Chen, J.H.; Rooney, M.S.; Barzily-Rokni, M.; Eliane, J.P.; Bjorgaard, S.L.; Hammond, M.R.; Vitzthum, H.; Blackmon, S.M.; et al. Resistance to checkpoint blockade therapy through inactivation of antigen presentation. Nat. Commun. 2017, 8, 1136. [Google Scholar] [CrossRef]
- Le, D.T.; Durham, J.N.; Smith, K.N.; Wang, H.; Bartlett, B.R.; Aulakh, L.K.; Lu, S.; Kemberling, H.; Wilt, C.; Luber, B.S.; et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science 2017, 357, 409–413. [Google Scholar] [CrossRef]
- Dong, L.Q.; Peng, L.H.; Ma, L.J.; Liu, D.B.; Zhang, S.; Luo, S.Z.; Rao, J.H.; Zhu, H.W.; Yang, S.X.; Xi, S.J.; et al. Heterogeneous immunogenomic features and distinct escape mechanisms in multifocal hepatocellular carcinoma. J. Hepatol. 2020, 72, 896–908. [Google Scholar] [CrossRef]
- Johnson, D.B.; Nixon, M.J.; Wang, Y.; Wang, D.Y.; Castellanos, E.; Estrada, M.V.; Ericsson-Gonzalez, P.I.; Cote, C.H.; Salgado, R.; Sanchez, V.; et al. Tumor-specific MHC-II expression drives a unique pattern of resistance to immunotherapy via LAG-3/FCRL6 engagement. JCI Insight 2018, 3, e120360. [Google Scholar] [CrossRef]
- Koyama, S.; Akbay, E.A.; Li, Y.Y.; Herter-Sprie, G.S.; Buczkowski, K.A.; Richards, W.G.; Gandhi, L.; Redig, A.J.; Rodig, S.J.; Asahina, H.; et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 2016, 7, 10501. [Google Scholar] [CrossRef] [PubMed]
- Chow, J.T.; Salmena, L. Recent advances in PTEN signalling axes in cancer. Fac. Rev. 2020, 9, 31. [Google Scholar] [CrossRef]
- Dong, Y.; Richards, J.A.; Gupta, R.; Aung, P.P.; Emley, A.; Kluger, Y.; Dogra, S.K.; Mahalingam, M.; Wajapeyee, N. PTEN functions as a melanoma tumor suppressor by promoting host immune response. Oncogene 2014, 33, 4632–4642. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Role of adenosine A2b receptor overexpression in tumor progression. Life Sci. 2016, 166, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Crunkhorn, S. Reactivating PTEN promotes antitumour immunity. Nat. Rev. Drug Discov. 2021, 20, 588. [Google Scholar] [CrossRef]
- Cucchiara, F.; Crucitta, S.; Petrini, I.; de Miguel Perez, D.; Ruglioni, M.; Pardini, E.; Rolfo, C.; Danesi, R.; Del Re, M. Gene-network analysis predicts clinical response to immunotherapy in patients affected by NSCLC. Lung Cancer 2023, 183, 107308. [Google Scholar] [CrossRef]
- Trujillo, J.A.; Luke, J.J.; Zha, Y.; Segal, J.P.; Ritterhouse, L.L.; Spranger, S.; Matijevich, K.; Gajewski, T.F. Secondary resistance to immunotherapy associated with β-catenin pathway activation or PTEN loss in metastatic melanoma. J. Immunother. Cancer 2019, 7, 295. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef]
- Veglia, F.; Sanseviero, E.; Gabrilovich, D.I. Myeloid-derived suppressor cells in the era of increasing myeloid cell diversity. Nat. reviews. Immunol. 2021, 21, 485–498. [Google Scholar] [CrossRef]
- Poggio, M.; Hu, T.; Pai, C.C.; Chu, B.; Belair, C.D.; Chang, A.; Montabana, E.; Lang, U.E.; Fu, Q.; Fong, L.; et al. Suppression of Exosomal PD-L1 Induces Systemic Anti-tumor Immunity and Memory. Cell 2019, 177, 414–427.e413. [Google Scholar] [CrossRef]
- Yang, Y.; Li, C.W.; Chan, L.C.; Wei, Y.; Hsu, J.M.; Xia, W.; Cha, J.H.; Hou, J.; Hsu, J.L.; Sun, L.; et al. Exosomal PD-L1 harbors active defense function to suppress T cell killing of breast cancer cells and promote tumor growth. Cell Res. 2018, 28, 862–864. [Google Scholar] [CrossRef] [PubMed]
- Hou, A.; Hou, K.; Huang, Q.; Lei, Y.; Chen, W. Targeting Myeloid-Derived Suppressor Cell, a Promising Strategy to Overcome Resistance to Immune Checkpoint Inhibitors. Front. Immunol. 2020, 11, 783. [Google Scholar] [CrossRef]
- Kuang, D.M.; Zhao, Q.; Peng, C.; Xu, J.; Zhang, J.P.; Wu, C.; Zheng, L. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 2009, 206, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Sprinzl, M.F.; Galle, P.R. Immune control in hepatocellular carcinoma development and progression: Role of stromal cells. Semin. Liver Dis. 2014, 34, 376–388. [Google Scholar] [CrossRef] [PubMed]
- De Henau, O.; Rausch, M.; Winkler, D.; Campesato, L.F.; Liu, C.; Cymerman, D.H.; Budhu, S.; Ghosh, A.; Pink, M.; Tchaicha, J.; et al. Overcoming resistance to checkpoint blockade therapy by targeting PI3Kγ in myeloid cells. Nature 2016, 539, 443–447. [Google Scholar] [CrossRef]
- Topalian, S.L.; Hodi, F.S.; Brahmer, J.R.; Gettinger, S.N.; Smith, D.C.; McDermott, D.F.; Powderly, J.D.; Carvajal, R.D.; Sosman, J.A.; Atkins, M.B.; et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 2012, 366, 2443–2454. [Google Scholar] [CrossRef]
- Song, T.L.; Nairismägi, M.L.; Laurensia, Y.; Lim, J.Q.; Tan, J.; Li, Z.M.; Pang, W.L.; Kizhakeyil, A.; Wijaya, G.C.; Huang, D.C.; et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood 2018, 132, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Formisano, L.; Gonzalez-Ericsson, P.I.; Sanchez, V.; Dean, P.T.; Opalenik, S.R.; Sanders, M.E.; Cook, R.S.; Arteaga, C.L.; Johnson, D.B.; et al. Melanoma response to anti-PD-L1 immunotherapy requires JAK1 signaling, but not JAK2. Oncoimmunology 2018, 7, e1438106. [Google Scholar] [CrossRef] [PubMed]
- Dillen, A.; Bui, I.; Jung, M.; Agioti, S.; Zaravinos, A.; Bonavida, B. Regulation of PD-L1 Expression by YY1 in Cancer: Therapeutic Efficacy of Targeting YY1. Cancers 2024, 16, 1237. [Google Scholar] [CrossRef]
- Li, M.; Wei, J.; Xue, C.; Zhou, X.; Chen, S.; Zheng, L.; Duan, Y.; Deng, H.; Xiong, W.; Tang, F.; et al. Dissecting the roles and clinical potential of YY1 in the tumor microenvironment. Front. Oncol. 2023, 13, 1122110. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.S.; Zaretsky, J.M.; Escuin-Ordinas, H.; Garcia-Diaz, A.; Hu-Lieskovan, S.; Kalbasi, A.; Grasso, C.S.; Hugo, W.; Sandoval, S.; Torrejon, D.Y.; et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017, 7, 188–201. [Google Scholar] [CrossRef]
- Dawson, M.A.; Kouzarides, T. Cancer epigenetics: From mechanism to therapy. Cell 2012, 150, 12–27. [Google Scholar] [CrossRef]
- Conte, M.; De Palma, R.; Altucci, L. HDAC inhibitors as epigenetic regulators for cancer immunotherapy. Int. J. Biochem. Cell Biol. 2018, 98, 65–74. [Google Scholar] [CrossRef]
- Wang, D.; Quiros, J.; Mahuron, K.; Pai, C.C.; Ranzani, V.; Young, A.; Silveria, S.; Harwin, T.; Abnousian, A.; Pagani, M.; et al. Targeting EZH2 Reprograms Intratumoral Regulatory T Cells to Enhance Cancer Immunity. Cell Rep. 2018, 23, 3262–3274. [Google Scholar] [CrossRef]
- Keir, M.E.; Butte, M.J.; Freeman, G.J.; Sharpe, A.H. PD-1 and its ligands in tolerance and immunity. Annu. Rev. Immunol. 2008, 26, 677–704. [Google Scholar] [CrossRef]
- Perrier, A.; Didelot, A.; Laurent-Puig, P.; Blons, H.; Garinet, S. Epigenetic Mechanisms of Resistance to Immune Checkpoint Inhibitors. Biomolecules 2020, 10, 1061. [Google Scholar] [CrossRef]
- Pan, D.; Kobayashi, A.; Jiang, P.; Ferrari de Andrade, L.; Tay, R.E.; Luoma, A.M.; Tsoucas, D.; Qiu, X.; Lim, K.; Rao, P.; et al. A major chromatin regulator determines resistance of tumor cells to T cell-mediated killing. Science 2018, 359, 770–775. [Google Scholar] [CrossRef]
- Shen, J.; Ju, Z.; Zhao, W.; Wang, L.; Peng, Y.; Ge, Z.; Nagel, Z.D.; Zou, J.; Wang, C.; Kapoor, P.; et al. ARID1A deficiency promotes mutability and potentiates therapeutic antitumor immunity unleashed by immune checkpoint blockade. Nat. Med. 2018, 24, 556–562. [Google Scholar] [CrossRef]
- Yuan, Y.; Adam, A.; Zhao, C.; Chen, H. Recent Advancements in the Mechanisms Underlying Resistance to PD-1/PD-L1 Blockade Immunotherapy. Cancers 2021, 13, 663. [Google Scholar] [CrossRef] [PubMed]
- Emran, A.A.; Chatterjee, A.; Rodger, E.J.; Tiffen, J.C.; Gallagher, S.J.; Eccles, M.R.; Hersey, P. Targeting DNA Methylation and EZH2 Activity to Overcome Melanoma Resistance to Immunotherapy. Trends Immunol. 2019, 40, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Madore, J.; Strbenac, D.; Vilain, R.; Menzies, A.M.; Yang, J.Y.; Thompson, J.F.; Long, G.V.; Mann, G.J.; Scolyer, R.A.; Wilmott, J.S. PD-L1 Negative Status is Associated with Lower Mutation Burden, Differential Expression of Immune-Related Genes, and Worse Survival in Stage III Melanoma. Clin. Cancer Res. 2016, 22, 3915–3923. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef] [PubMed]
- Perez, H.L.; Cardarelli, P.M.; Deshpande, S.; Gangwar, S.; Schroeder, G.M.; Vite, G.D.; Borzilleri, R.M. Antibody-drug conjugates: Current status and future directions. Drug Discov. Today 2014, 19, 869–881. [Google Scholar] [CrossRef]
- Naidoo, J.; Page, D.B.; Li, B.T.; Connell, L.C.; Schindler, K.; Lacouture, M.E.; Postow, M.A.; Wolchok, J.D. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 2015, 26, 2375–2391. [Google Scholar] [CrossRef]
- Spain, L.; Diem, S.; Larkin, J. Management of toxicities of immune checkpoint inhibitors. Cancer Treat. Rev. 2016, 44, 51–60. [Google Scholar] [CrossRef]
- Wang, Y.; Tong, Z.; Zhang, W.; Zhang, W.; Buzdin, A.; Mu, X.; Yan, Q.; Zhao, X.; Chang, H.H.; Duhon, M.; et al. FDA-Approved and Emerging Next Generation Predictive Biomarkers for Immune Checkpoint Inhibitors in Cancer Patients. Front. Oncol. 2021, 11, 683419. [Google Scholar] [CrossRef] [PubMed]
- Havel, J.J.; Chowell, D.; Chan, T.A. The evolving landscape of biomarkers for checkpoint inhibitor immunotherapy. Nat. Rev. Cancer 2019, 19, 133–150. [Google Scholar] [CrossRef]
- Man, J.; Millican, J.; Mulvey, A.; Gebski, V.; Hui, R. Response Rate and Survival at Key Timepoints With PD-1 Blockade vs Chemotherapy in PD-L1 Subgroups: Meta-Analysis of Metastatic NSCLC Trials. JNCI Cancer Spectr. 2021, 5, pkab012. [Google Scholar] [CrossRef]
- Vallejo, J.; Singh, H.; Larkins, E.; Drezner, N.; Ricciuti, B.; Mishra-Kalyani, P.; Tang, S.; Beaver, J.A.; Awad, M.M. Impact of Increasing PD-L1 Levels on Outcomes to PD-1/PD-L1 Inhibition in Patients With NSCLC: A Pooled Analysis of 11 Prospective Clinical Trials. Oncologist 2024, 29, 422–430. [Google Scholar] [CrossRef]
- Taube, J.M.; Klein, A.; Brahmer, J.R.; Xu, H.; Pan, X.; Kim, J.H.; Chen, L.; Pardoll, D.M.; Topalian, S.L.; Anders, R.A. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin. Cancer Res. 2014, 20, 5064–5074. [Google Scholar] [CrossRef]
- Zdrenka, M.; Kowalewski, A.; Ahmadi, N.; Sadiqi, R.U.; Chmura, Ł.; Borowczak, J.; Maniewski, M.; Szylberg, Ł. Refining PD-1/PD-L1 assessment for biomarker-guided immunotherapy: A review. Biomol. Biomed. 2024, 24, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, L.; Rodríguez-Abreu, D.; Gadgeel, S.; Esteban, E.; Felip, E.; De Angelis, F.; Domine, M.; Clingan, P.; Hochmair, M.J.; Powell, S.F.; et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 378, 2078–2092. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.H.; Wang, Y.N.; Xia, W.; Chen, C.H.; Rau, K.M.; Ye, L.; Wei, Y.; Chou, C.K.; Wang, S.C.; Yan, M.; et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019, 36, 168–178.e164. [Google Scholar] [CrossRef]
- Mei, J.; Xu, J.; Yang, X.; Gu, D.; Zhou, W.; Wang, H.; Liu, C. A comparability study of natural and deglycosylated PD-L1 levels in lung cancer: Evidence from immunohistochemical analysis. Mol. Cancer 2021, 20, 11. [Google Scholar] [CrossRef]
- Meléndez, B.; Van Campenhout, C.; Rorive, S.; Remmelink, M.; Salmon, I.; D’Haene, N. Methods of measurement for tumor mutational burden in tumor tissue. Transl. Lung Cancer Res. 2018, 7, 661–667. [Google Scholar] [CrossRef]
- Marcus, L.; Fashoyin-Aje, L.A.; Donoghue, M.; Yuan, M.; Rodriguez, L.; Gallagher, P.S.; Philip, R.; Ghosh, S.; Theoret, M.R.; Beaver, J.A.; et al. FDA Approval Summary: Pembrolizumab for the Treatment of Tumor Mutational Burden-High Solid Tumors. Clin. Cancer Res. 2021, 27, 4685–4689. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of High Tumor Mutation Burden in Non-Small Cell Lung Cancers With Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef]
- Florou, V.; Floudas, C.S.; Maoz, A.; Naqash, A.R.; Norton, C.; Tan, A.C.; Sokol, E.S.; Frampton, G.; Soares, H.P.; Puri, S.; et al. Real-world pan-cancer landscape of frameshift mutations and their role in predicting responses to immune checkpoint inhibitors in cancers with low tumor mutational burden. J. Immunother. Cancer 2023, 11, e007440. [Google Scholar] [CrossRef]
- Georgoulias, G.; Zaravinos, A. Genomic landscape of the immunogenicity regulation in skin melanomas with diverse tumor mutation burden. Front. Immunol. 2022, 13, 1006665. [Google Scholar] [CrossRef] [PubMed]
- Bonneville, R.; Krook, M.A.; Kautto, E.A.; Miya, J.; Wing, M.R.; Chen, H.Z.; Reeser, J.W.; Yu, L.; Roychowdhury, S. Landscape of Microsatellite Instability Across 39 Cancer Types. JCO Precis. Oncol. 2017, 2017, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Marabelle, A.; O’Malley, D.M.; Hendifar, A.E.; Ascierto, P.A.; Motola-Kuba, D.; Penel, N.; Cassier, P.A.; Bariani, G.; De Jesus-Acosta, A.; Doi, T.; et al. Pembrolizumab in microsatellite-instability-high and mismatch-repair-deficient advanced solid tumors: Updated results of the KEYNOTE-158 trial. Nat. Cancer 2025, 6, 253–258. [Google Scholar] [CrossRef]
- Fan, A.; Wang, B.; Wang, X.; Nie, Y.; Fan, D.; Zhao, X.; Lu, Y. Immunotherapy in colorectal cancer: Current achievements and future perspective. Int. J. Biol. Sci. 2021, 17, 3837–3849. [Google Scholar] [CrossRef]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Ueno, N.T.; et al. Validation of cancer-type-dependent benefit from immune checkpoint blockade in TMB-H tumors identified by the FoundationOne CDx assay. Ann. Oncol. 2022, 33, 1204–1206. [Google Scholar] [CrossRef]
- Mucherino, S.; Lorenzoni, V.; Triulzi, I.; Del Re, M.; Orlando, V.; Capuano, A.; Danesi, R.; Turchetti, G.; Menditto, E. Cost-Effectiveness of Treatment Optimisation with Biomarkers for Immunotherapy in Solid Tumours: A Systematic Review. Cancers 2024, 16, 995. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhang, C.; Wang, H. Immune-checkpoint inhibitor resistance in cancer treatment: Current progress and future directions. Cancer Lett. 2023, 562, 216182. [Google Scholar] [CrossRef] [PubMed]
- Bell, H.N.; Zou, W. Beyond the Barrier: Unraveling the Mechanisms of Immunotherapy Resistance. Annu. Rev. Immunol. 2024, 42, 521–550. [Google Scholar] [CrossRef]
- Heinhuis, K.M.; Ros, W.; Kok, M.; Steeghs, N.; Beijnen, J.H.; Schellens, J.H.M. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann. Oncol. 2019, 30, 219–235. [Google Scholar] [CrossRef] [PubMed]
- Moya-Horno, I.; Viteri, S.; Karachaliou, N.; Rosell, R. Combination of immunotherapy with targeted therapies in advanced non-small cell lung cancer (NSCLC). Ther. Adv. Med. Oncol. 2018, 10, 1758834017745012. [Google Scholar] [CrossRef]
- Starzer, A.M.; Wolff, L.; Popov, P.; Kiesewetter, B.; Preusser, M.; Berghoff, A.S. The more the merrier? Evidence and efficacy of immune checkpoint- and tyrosine kinase inhibitor combinations in advanced solid cancers. Cancer Treat. Rev. 2024, 125, 102718. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Li, S.C.; He, Q.J.; Yang, B.; Cao, J. Small molecule inhibitors targeting the PD-1/PD-L1 signaling pathway. Acta Pharmacol. Sin. 2021, 42, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Uzar, W.; Kaminska, B.; Rybka, H.; Skalniak, L.; Magiera-Mularz, K.; Kitel, R. An updated patent review on PD-1/PD-L1 antagonists (2022-present). Expert Opin. Ther. Pat. 2024, 34, 627–650. [Google Scholar] [CrossRef]
- Hao, X.; Chen, Z.; Li, H.; Wei, M.; Zuo, Z.; Su, Q. Small-Molecule Drugs in Immunotherapy. Mini Rev. Med. Chem. 2023, 23, 1341–1359. [Google Scholar] [CrossRef]
- Li, C.; Zhang, N.; Zhou, J.; Ding, C.; Jin, Y.; Cui, X.; Pu, K.; Zhu, Y. Peptide Blocking of PD-1/PD-L1 Interaction for Cancer Immunotherapy. Cancer Immunol. Res. 2018, 6, 178–188. [Google Scholar] [CrossRef]
- Kamijo, H.; Sugaya, M.; Takahashi, N.; Oka, T.; Miyagaki, T.; Asano, Y.; Sato, S. BET bromodomain inhibitor JQ1 decreases CD30 and CCR4 expression and proliferation of cutaneous T-cell lymphoma cell lines. Arch. Dermatol. Res. 2017, 309, 491–497. [Google Scholar] [CrossRef]
- Liu, M.; Cao, Z.; Zhang, R.; Chen, Y.; Yang, X. Injectable Supramolecular Hydrogel for Locoregional Immune Checkpoint Blockade and Enhanced Cancer Chemo-Immunotherapy. ACS Appl. Mater. Interfaces 2021, 13, 33874–33884. [Google Scholar] [CrossRef]
- Shafi, H.; Lora, A.J.; Donow, H.M.; Dickinson, S.E.; Wondrak, G.T.; Chow, H.S.; Curiel-Lewandrowski, C.; Mansour, H.M. Comprehensive Advanced Physicochemical Characterization and In Vitro Human Cell Culture Assessment of BMS-202: A Novel Inhibitor of Programmed Cell Death Ligand. Pharmaceutics 2024, 16, 1409. [Google Scholar] [CrossRef]
- Sasikumar, P.G.; Sudarshan, N.S.; Adurthi, S.; Ramachandra, R.K.; Samiulla, D.S.; Lakshminarasimhan, A.; Ramanathan, A.; Chandrasekhar, T.; Dhudashiya, A.A.; Talapati, S.R.; et al. PD-1 derived CA-170 is an oral immune checkpoint inhibitor that exhibits preclinical anti-tumor efficacy. Commun. Biol. 2021, 4, 699. [Google Scholar] [CrossRef]
- Chen, S.; Wang, Q.; Ming, S.; Zheng, H.; Hua, B.; Yang, H.S. Platycodin D induces apoptosis through JNK1/AP-1/PUMA pathway in non-small cell lung cancer cells: A new mechanism for an old compound. Front. Pharmacol. 2022, 13, 1045375. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yao, H.; Li, C.; Shi, H.; Lan, J.; Li, Z.; Zhang, Y.; Liang, L.; Fang, J.Y.; Xu, J. HIP1R targets PD-L1 to lysosomal degradation to alter T cell-mediated cytotoxicity. Nat. Chem. Biol. 2019, 15, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Salih, D.J.; Barsoom, S.H.; Ahmed, G.F.; Hussien, S.Q.; Al Ismaeel, Q.; Alasady, A.A.B.; Alsalim, T.A.; Salih, A.M. Curcumin inhibits IFN-γ induced PD-L1 expression via reduction of STAT1 Phosphorylation in A549 non-small cell lung cancer cells. Saudi Pharm. J. SPJ Off. Publ. Saudi Pharm. Soc. 2025, 33, 16. [Google Scholar] [CrossRef] [PubMed]
- Amato, L.; De Rosa, C.; Di Guida, G.; Sepe, F.; Ariano, A.; Capaldo, S.; Ul Haq, F.; Di Liello, A.; Tuccillo, C.; Lucà, S.; et al. Addition of metformin to anti-PD-1/PD-L1 drugs activates anti-tumor immune response in peripheral immune cells of NSCLC patients. Cell Death Dis. 2025, 16, 286. [Google Scholar] [CrossRef]
- Ferrario, C.; Mackey, J.; Gelmon, K.A.; Levasseur, N.; Sorensen, P.H.; Oo, H.Z.; Negri, G.L.; Tse, V.W.L.; Spencer, S.E.; Cheng, G.; et al. Phase Ib Pharmacodynamic Study of the MNK Inhibitor Tomivosertib (eFT508) Combined with Paclitaxel in Patients With Refractory Metastatic Breast Cancer. Clin. Cancer Res. 2025, 31, 491–502. [Google Scholar] [CrossRef]
- Riess, J.W.; de Langen, A.J.; Ponce, S.; Goldberg, S.B.; Piotrowska, Z.; Goldman, J.W.; Le, X.; Cho, B.C.; Yoneshima, Y.; Ambrose, H.; et al. ORCHARD: Osimertinib Plus Necitumumab in Patients with Epidermal Growth Factor Receptor-Mutated Advanced Non-Small Cell Lung Cancer With a Secondary Epidermal Growth Factor Receptor Alteration Whose Disease Had Progressed on First-Line Osimertinib. JCO Precis. Oncol. 2025, 9, e2400818. [Google Scholar] [CrossRef]
- Pan, X.; Li, C.; Feng, J. The role of LncRNAs in tumor immunotherapy. Cancer Cell Int. 2023, 23, 30. [Google Scholar] [CrossRef]
- Zhou, J.G.; Liang, B.; Liu, J.G.; Jin, S.H.; He, S.S.; Frey, B.; Gu, N.; Fietkau, R.; Hecht, M.; Ma, H.; et al. Identification of 15 lncRNAs Signature for Predicting Survival Benefit of Advanced Melanoma Patients Treated with Anti-PD-1 Monotherapy. Cells 2021, 10, 977. [Google Scholar] [CrossRef]
- Toker, J.; Iorgulescu, J.B.; Ling, A.L.; Villa, G.R.; Gadet, J.; Parida, L.; Getz, G.; Wu, C.J.; Reardon, D.A.; Chiocca, E.A.; et al. Clinical Importance of the lncRNA NEAT1 in Cancer Patients Treated with Immune Checkpoint Inhibitors. Clin. Cancer Res. 2023, 29, 2226–2238. [Google Scholar] [CrossRef]
- Diao, M.; Wang, Y.; Wu, S.; He, S.; Liao, Y. Estrogen, estrogen receptor and the tumor microenvironment of NSCLC. Int. J. Cancer 2025, 156, 1501–1508. [Google Scholar] [CrossRef]
- Ontiveros, C.O.; Murray, C.E.; Crossland, G.; Curiel, T.J. Considerations and Approaches for Cancer Immunotherapy in the Aging Host. Cancer Immunol. Res. 2023, 11, 1449–1461. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J.; Crown, J. Biomarkers for Predicting Response to Immunotherapy with Immune Checkpoint Inhibitors in Cancer Patients. Clin. Chem. 2019, 65, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a biomarker of response to immune-checkpoint inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef] [PubMed]
- Chennamadhavuni, A.; Abushahin, L.; Jin, N.; Presley, C.J.; Manne, A. Risk Factors and Biomarkers for Immune-Related Adverse Events: A Practical Guide to Identifying High-Risk Patients and Rechallenging Immune Checkpoint Inhibitors. Front. Immunol. 2022, 13, 779691. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).