Genetic Markers Associated with Ferroptosis in Cardiovascular Diseases
Abstract
1. Introduction
2. Literature Screening Methods
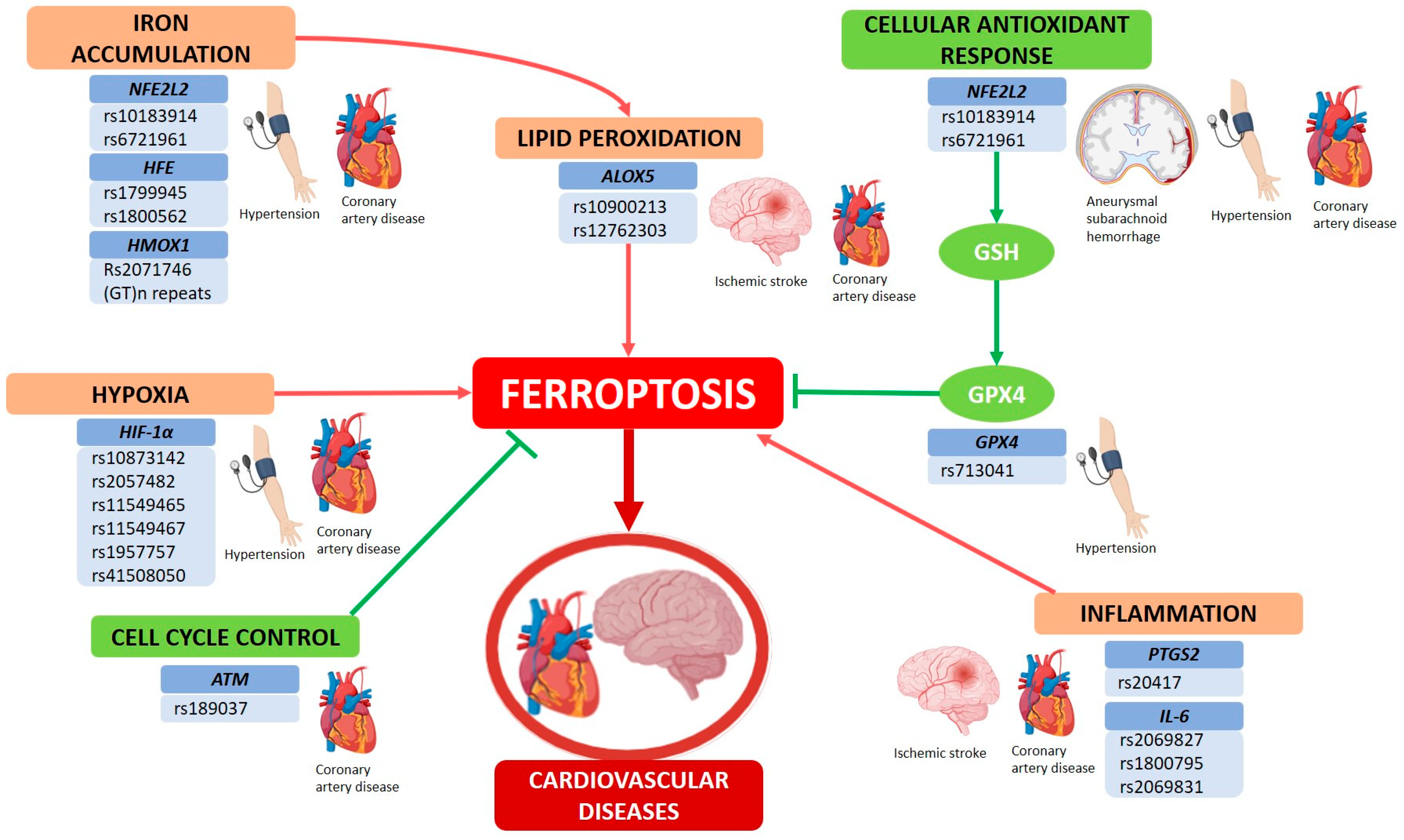
3. Molecular Mechanisms of Ferroptosis and Cardiovascular Diseases
4. Genetic Variants and Ferroptosis Pathways in CVDs
4.1. NFE2L2
4.2. HFE (Homeostatic Iron Regulator)
4.3. HMOX1 (Heme Oxygenase 1)
4.4. HIF-1α (Hypoxia Inducible Factor 1 Subunit Alpha)
4.5. ALOX5 (Arachidonate 5-Lipoxygenase)
4.6. GPX4 (Glutathione Peroxidase 4)
4.7. PTGS2 (Prostaglandin Endoperoxide Synthase 2)
4.8. IL-6 (Interleukin-6)
4.9. Ataxia-Telangiectasia Mutated (ATM)
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cole, L.; Kramer, P.R. Cardiovascular Disease. In Human Physiology, Biochemistry and Basic Medicine; Elsevier: Amsterdam, The Netherlands, 2016; pp. 201–204. Available online: https://linkinghub.elsevier.com/retrieve/pii/B9780128036990000426 (accessed on 15 November 2024).
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.H.; Fefelova, N.; Pamarthi, S.H.; Gwathmey, J.K. Molecular Mechanisms of Ferroptosis and Relevance to Cardiovascular Disease. Cells 2022, 11, 2726. Available online: https://www.mdpi.com/2073-4409/11/17/2726 (accessed on 15 November 2024). [CrossRef] [PubMed]
- Castro-Juárez, C.; Cabrera-Pivaral, C.; Ramírez-García, S.; García-Sierra, L.; Morales-Pérez, L.; Ramírez-Concepción, H.R. Risk factors for cardiovascular disease in Mexican adults. Rev. Médica MD 2018, 9, 153–162. [Google Scholar]
- Rajagopalan, S.; Landrigan, P.J. Pollution and the Heart. N. Engl. J. Med. 2021, 385, 1881–1892. Available online: http://www.nejm.org/doi/10.1056/NEJMra2030281 (accessed on 1 October 2024). [CrossRef]
- Safdar, M.; Ullah, M.; Wahab, A.; Hamayun, S.; Ur Rehman, M.M.; Khan, M.A.; Khan, S.U.; Ullah, A.; Din, F.U.; Awan, U.A.; et al. Genomic insights into heart health: Exploring the genetic basis of cardiovascular disease. Curr. Probl. Cardiol. 2024, 49, 102182. [Google Scholar] [CrossRef]
- McPherson, R.; Tybjaerg-Hansen, A. Genetics of Coronary Artery Disease. Circ. Res. 2016, 118, 564–578. Available online: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.115.306566 (accessed on 18 November 2024). [CrossRef]
- Vrablik, M.; Dlouha, D.; Todorovova, V.; Stefler, D.; Hubacek, J.A. Genetics of Cardiovascular Disease: How Far Are We from Personalized CVD Risk Prediction and Management? Int. J. Mol. Sci. 2021, 22, 4182. Available online: https://www.mdpi.com/1422-0067/22/8/4182 (accessed on 18 November 2024). [CrossRef]
- Roberts, R.; Marian, A.J.; Dandona, S.; Stewart, A.F.R. Genomics in Cardiovascular Disease. J. Am. Coll. Cardiol. 2013, 61, 2029–2037. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0735109713011054 (accessed on 18 November 2024). [CrossRef]
- Malik, R.; Chauhan, G.; Traylor, M.; Sargurupremraj, M.; Okada, Y.; Mishra, A.; Rutten-Jacobs, L.; Giese, A.K.; van der Laan, S.W.; Gretarsdottir, S.; et al. Multiancestry genome-wide association study of 520,000 subjects identifies 32 loci associated with stroke and stroke subtypes. Nat. Genet. 2018, 50, 524–537. Available online: https://www.nature.com/articles/s41588-018-0058-3 (accessed on 18 November 2024). [CrossRef]
- Manuscript, A. A comprehensive 1000 Genomes–based genome-wide association meta-analysis of coronary artery disease. Nat. Genet. 2015, 47, 1121–1130. Available online: https://www.nature.com/articles/ng.3396 (accessed on 18 November 2024).
- Nelson, C.P.; Goel, A.; Butterworth, A.S.; Kanoni, S.; Webb, T.R.; Marouli, E.; Zeng, L.; Ntalla, I.; Lai, F.Y.; Hopewell, J.C.; et al. Association analyses based on false discovery rate implicate new loci for coronary artery disease. Nat. Genet. 2017, 49, 1385–1391. Available online: https://www.nature.com/articles/ng.3913 (accessed on 18 November 2024). [CrossRef] [PubMed]
- Van der Harst, P.; Verweij, N. Identification of 64 Novel Genetic Loci Provides an Expanded View on the Genetic Architecture of Coronary Artery Disease. Circ. Res. 2018, 122, 433–443. Available online: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.117.312086 (accessed on 18 November 2024). [CrossRef] [PubMed]
- Bakker, M.K.; van der Spek, R.A.A.; van Rheenen, W.; Morel, S.; Bourcier, R.; Hostettler, I.C.; Alg, V.S.; van Eijk, K.R.; Koido, M.; Akiyama, M.; et al. Genome-wide association study of intracranial aneurysms identifies 17 risk loci and genetic overlap with clinical risk factors. Nat. Genet. 2020, 52, 1303–1313. Available online: https://www.nature.com/articles/s41588-020-00725-7 (accessed on 24 November 2024). [CrossRef]
- Hoffmann, T.J.; Ehret, G.B.; Nandakumar, P.; Ranatunga, D.; Schaefer, C.; Kwok, P.Y.; Iribarren, C.; Chakravarti, A.; Risch, N. Genome-wide association analyses using electronic health records identify new loci influencing blood pressure variation. Nat. Genet. 2017, 49, 54–64. Available online: https://www.nature.com/articles/ng.3715 (accessed on 24 November 2024). [CrossRef] [PubMed]
- Warren, H.R.; Evangelou, E.; Cabrera, C.P.; Gao, H.; Ren, M.; Mifsud, B.; Ntalla, I.; Surendran, P.; Liu, C.; Cook, J.P.; et al. Genome-wide association analysis identifies novel blood pressure loci and offers biological insights into cardiovascular risk. Nat. Genet. 2017, 49, 403–415. Available online: https://www.nature.com/articles/ng.3768 (accessed on 24 November 2024). [CrossRef]
- Evangelou, E.; Warren, H.R.; Mosen-Ansorena, D.; Mifsud, B.; Pazoki, R.; Gao, H.; Ntritsos, G.; Dimou, N.; Cabrera, C.P.; Karaman, I.; et al. Genetic analysis of over 1 million people identifies 535 new loci associated with blood pressure traits. Nat. Genet. 2018, 50, 1412–1425. Available online: https://www.nature.com/articles/s41588-018-0205-x (accessed on 5 December 2024). [CrossRef]
- Giri, A.; Hellwege, J.N.; Keaton, J.M.; Park, J.; Qiu, C.; Warren, H.R.; Torstenson, E.S.; Kovesdy, C.P.; Sun, Y.V.; Wilson, O.D.; et al. Trans-ethnic association study of blood pressure determinants in over 750,000 individuals. Nat. Genet. 2019, 51, 51–62. Available online: https://www.nature.com/articles/s41588-018-0303-9 (accessed on 5 December 2024). [CrossRef]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. Available online: https://www.physiology.org/doi/10.1152/physrev.00022.2018 (accessed on 12 November 2024). [CrossRef]
- Qin, S.; Zhu, C.; Chen, C.; Sheng, Z.; Cao, Y. An emerging double-edged sword role of ferroptosis in cardiovascular disease (Review). Int. J. Mol. Med. 2024, 55, 16. Available online: http://www.spandidos-publications.com/10.3892/ijmm.2024.5457 (accessed on 12 November 2024). [CrossRef]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Jiang, X.; Stockwell, B.R.; Conrad, M. Ferroptosis: Mechanisms, biology and role in disease. Nat. Rev. Mol. Cell Biol. 2021, 22, 266–282. Available online: https://www.nature.com/articles/s41580-020-00324-8 (accessed on 8 November 2024). [CrossRef] [PubMed]
- Deng, X.; Chu, W.; Zhang, H.; Peng, Y. Nrf2 and Ferroptosis: A New Research Direction for Ischemic Stroke. Cell. Mol. Neurobiol. 2023, 43, 3885–3896. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Wu, M.; Wang, Z.; Wang, J. Ferroptosis: From regulation of lipid peroxidation to the treatment of diseases. Cell Biol. Toxicol. 2023, 39, 827–851. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kang, R.; Tang, D. Signaling pathways and defense mechanisms of ferroptosis. FEBS J. 2022, 289, 7038–7050. Available online: https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.16059 (accessed on 10 November 2024). [CrossRef]
- Cruz-Gregorio, A.; Amezcua-Guerra, L.M.; Fisher-Bautista, B.; Romero-Beltrán, A.; Fonseca-Camarillo, G. The Protective Role of Interleukin-37 in Cardiovascular Diseases through Ferroptosis Modulation. Int. J. Mol. Sci. 2024, 25, 9758. Available online: https://www.mdpi.com/1422-0067/25/18/9758 (accessed on 16 September 2024). [CrossRef]
- Kolwicz, S.C.; Purohit, S.; Tian, R. Cardiac Metabolism and its Interactions With Contraction, Growth, and Survival of Cardiomyocytes. Circ. Res. 2013, 113, 603–616. Available online: https://www.ahajournals.org/doi/10.1161/CIRCRESAHA.113.302095 (accessed on 5 November 2024). [CrossRef]
- Wang, Y.; Mohsen, A.W.; Mihalik, S.J.; Goetzman, E.S.; Vockley, J. Evidence for Physical Association of Mitochondrial Fatty Acid Oxidation and Oxidative Phosphorylation Complexes. J. Biol. Chem. 2010, 285, 29834–29841. Available online: https://linkinghub.elsevier.com/retrieve/pii/S002192581989018X (accessed on 5 November 2024). [CrossRef]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. Available online: https://www.nature.com/articles/s41556-018-0124-1 (accessed on 5 November 2024). [CrossRef]
- Vargas-Mendoza, N.; Angeles-Valencia, M.; Morales-González, Á.; Madrigal-Santillán, E.O.; Morales-Martínez, M.; Madrigal-Bujaidar, E.; Álvarez-González, I.; Gutiérrez-Salinas, J.; Esquivel-Chirino, C.; Chamorro-Cevallos, G.; et al. Oxidative Stress, Mitochondrial Function and Adaptation to Exercise: New Perspectives in Nutrition. Life 2021, 11, 1269. Available online: https://www.mdpi.com/2075-1729/11/11/1269 (accessed on 14 November 2024). [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Arancibia-Hernández, Y.L.; Hernández-Cruz, E.Y.; Pedraza-Chaverri, J. RONS and Oxidative Stress: An Overview of Basic Concepts. Oxygen 2022, 2, 437–478. Available online: https://www.mdpi.com/2673-9801/2/4/30 (accessed on 6 November 2024). [CrossRef]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. Available online: https://www.nature.com/articles/nrm3801 (accessed on 5 November 2024). [CrossRef] [PubMed]
- Jastroch, M.; Divakaruni, A.S.; Mookerjee, S.; Treberg, J.R.; Brand, M.D. Mitochondrial proton and electron leaks. Brown GC, Murphy MP, editors. Essays Biochem. 2010, 47, 53–67. Available online: https://portlandpress.com/essaysbiochem/article/doi/10.1042/bse0470053/78194/Mitochondrial-proton-and-electron-leaks (accessed on 5 November 2024). [PubMed]
- Cruz-Gregorio, A.; Aranda-Rivera, A.K. Quercetin and Ferroptosis. Life 2023, 13, 1730. Available online: https://www.mdpi.com/2075-1729/13/8/1730 (accessed on 9 November 2024). [CrossRef] [PubMed]
- Jena, A.B.; Samal, R.R.; Bhol, N.K.; Duttaroy, A.K. Cellular Red-Ox system in health and disease: The latest update. Biomed. Pharmacother. 2023, 162, 114606. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Juan, C.A.; Plou, F.J.; Pérez-Lebeña, E. Chemistry of Hydrogen Peroxide Formation and Elimination in Mammalian Cells, and Its Role in Various Pathologies. Stresses 2022, 2, 256–274. Available online: https://www.mdpi.com/2673-7140/2/3/19 (accessed on 12 November 2024). [CrossRef]
- Priya Dharshini, L.C.; Vishnupriya, S.; Sakthivel, K.M.; Rasmi, R.R. Oxidative stress responsive transcription factors in cellular signalling transduction mechanisms. Cell. Signal. 2020, 72, 109670. [Google Scholar] [CrossRef]
- Fang, X.; Wang, H.; Han, D.; Xie, E.; Yang, X.; Wei, J.; Gu, S.; Gao, F.; Zhu, N.; Yin, X.; et al. Ferroptosis as a target for protection against cardiomyopathy. Proc. Natl. Acad. Sci. USA 2019, 116, 2672–2680. Available online: https://pnas.org/doi/full/10.1073/pnas.1821022116 (accessed on 3 October 2024). [CrossRef]
- Gao, M.; Monian, P.; Quadri, N.; Ramasamy, R.; Jiang, X. Glutaminolysis and Transferrin Regulate Ferroptosis. Mol. Cell 2015, 59, 298–308. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1097276515004505 (accessed on 12 November 2024). [CrossRef]
- Tang, L.J.; Luo, X.J.; Tu, H.; Chen, H.; Xiong, X.M.; Li, N.S.; Peng, J. Ferroptosis occurs in phase of reperfusion but not ischemia in rat heart following ischemia or ischemia/reperfusion. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2021, 394, 401–410. Available online: https://link.springer.com/10.1007/s00210-020-01932-z (accessed on 12 November 2024). [CrossRef]
- Tang, L.J.; Zhou, Y.J.; Xiong, X.M.; Li, N.S.; Zhang, J.J.; Luo, X.J.; Peng, J. Ubiquitin-specific protease 7 promotes ferroptosis via activation of the p53/TfR1 pathway in the rat hearts after ischemia/reperfusion. Free Radic. Biol. Med. 2021, 162, 339–352. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xie, X.; Liao, W.; Chen, S.; Zhong, R.; Qin, J.; He, P.; Xie, J. Ferroptosis in cardiovascular disease. Biomed. Pharmacother. 2024, 170, 116057. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wang, X.; Hu, B.; Rong, S. Expression levels and clinical significance of ferroptosis-related genes in patients with myocardial infarction. Sci. Rep. 2024, 14, 1870. [Google Scholar] [CrossRef] [PubMed]
- Gaastra, B.; Duncan, P.; Bakker, M.K.; Hostettler, I.C.; Alg, V.S.; Houlden, H.; Ruigrok, Y.M.; Galea, I.; Tapper, W.; Werring, D.; et al. Genetic variation in NFE2L2 is associated with outcome following aneurysmal subarachnoid haemorrhage. Eur. J. Neurol. 2023, 30, 116–124. Available online: https://onlinelibrary.wiley.com/doi/10.1111/ene.15571 (accessed on 5 February 2025). [CrossRef]
- Shimoyama, Y.; Mitsuda, Y.; Hamajima, N.; Niwa, T. Polymorphisms of Nrf2, an antioxidative gene, are associated with blood pressure in Japanese. Nagoya J. Med. Sci. 2014, 76, 113–120. [Google Scholar]
- Sarutipaiboon, I.; Settasatian, N.; Komanasin, N.; Kukongwiriyapan, U.; Sawanyawisuth, K.; Intharaphet, P.; Senthong, V.; Settasatian, C. Association of Genetic Variations in NRF2, NQO1, HMOX1, and MT with Severity of Coronary Artery Disease and Related Risk Factors. Cardiovasc. Toxicol. 2020, 20, 176–189. [Google Scholar] [CrossRef]
- Ivanova, T.; Churnosova, M.; Abramova, M.; Ponomarenko, I.; Reshetnikov, E.; Aristova, I.; Sorokina, I.; Churnosov, M. Risk Effects of rs1799945 Polymorphism of the HFE Gene and Intergenic Interactions of GWAS-Significant Loci for Arterial Hypertension in the Caucasian Population of Central Russia. Int. J. Mol. Sci. 2023, 24, 8309. [Google Scholar] [CrossRef]
- Lian, J.; Xu, L.; Huang, Y.; Le, Y.; Jiang, D.; Yang, X.; Xu, W.; Huang, X.; Dong, C.; Ye, M.; et al. Meta-analyses of HFE variants in coronary heart disease. Gene 2013, 527, 167–173. [Google Scholar] [CrossRef]
- Gill, D.; Del Greco, M.F.; Walker, A.P.; Srai, S.K.S.; Laffan, M.A.; Minelli, C. The Effect of Iron Status on Risk of Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 1788–1792. Available online: https://www.ahajournals.org/doi/10.1161/ATVBAHA.117.309757 (accessed on 8 February 2025). [CrossRef]
- Guo, N.; Zhang, N.; Yan, L.; Cao, X.; Wang, J.; Wang, Y. Correlation between genetic polymorphisms within the MAPK1/HIF-1/HO-1 signaling pathway and risk or prognosis of perimenopausal coronary artery disease. Clin. Cardiol. 2017, 40, 597–604. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Zhang, D.; Xu, X.; Yu, B.; Zhang, Y. The association of functional polymorphisms in genes expressed in endothelial cells and smooth muscle cells with the myocardial infarction. Hum. Genom. 2019, 13, 5. [Google Scholar] [CrossRef] [PubMed]
- Hlatky, M.A.; Quertermous, T.; Boothroyd, D.B.; Priest, J.R.; Glassford, A.J.; Myers, R.M.; Fortmann, S.P.; Iribarren, C.; Tabor, H.K.; Assimes, T.L.; et al. Polymorphisms in hypoxia inducible factor 1 and the initial clinical presentation of coronary disease. Am. Heart J. 2007, 154, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- López-Reyes, A.; Rodríguez-Pérez, J.M.; Fernández-Torres, J.; Martínez-Rodríguez, N.; Pérez-Hernández, N.; Fuentes-Gómez, A.J.; Aguilar-González, C.A.; Alvarez-León, E.; Posadas-Romero, C.; Villarreal-Molina, T.; et al. The HIF1A rs2057482 polymorphism is associated with risk of developing premature coronary artery disease and with some metabolic and cardiovascular risk factors. The Genetics of Atherosclerotic Disease (GEA) Mexican Study. Exp. Mol. Pathol. 2014, 96, 405–410. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Z.L.; Ju, C.W.; Yan, G.L.; Chen, Z.P.; Pan, X.D.; Lu, W.B.; Yao, Y.Y.; Ma, G.S. The relevance of HIF1A gene polymorphisms and primary hypertensive left ventricular hypertrophy in Chinese Han population. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8095–8100. [Google Scholar]
- Liu, D.; Liu, L.; Song, Z.; Hu, Z.; Liu, J.; Hou, D. Genetic Variations of Oxidative Stress Related Genes ALOX5, ALOX5AP and MPO Modulate Ischemic Stroke Susceptibility Through Main Effects and Epistatic Interactions in a Chinese Population. Cell Physiol. Biochem. 2017, 43, 1588–1602. [Google Scholar] [CrossRef]
- Heidari, L.; Ghaderian, S.M.H.; Vakili, H.; Salmani, T.A. Promoter methylation and functional variants in arachidonate 5-lipoxygenase and forkhead box protein O1 genes associated with coronary artery disease. J. Cell. Biochem. 2019, 120, 12360–12368. [Google Scholar] [CrossRef]
- Barbosa, P.; Abo El-Magd, N.F.; Hesketh, J.; Bermano, G. The Role of rs713041 Glutathione Peroxidase 4 (GPX4) Single Nucleotide Polymorphism on Disease Susceptibility in Humans: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2022, 23, 15762. [Google Scholar] [CrossRef]
- Yi, X.Y.; Zhou, Q.; Lin, J.; Chi, L.F.; Chi, W.Z. Interaction between alox5ap-sg13s114a/t and cox-2-765g/c increases susceptibility to cerebral infarction in a chinese population. Genet. Mol. Res. 2013, 12, 1660–1669. [Google Scholar] [CrossRef]
- Yi, X.; Lin, J.; Luo, H.; Wang, C.; Liu, Y. Genetic variants of PTGS2, TXA2R and TXAS1 are associated with carotid plaque vulnerability, platelet activation and TXA2 levels in ischemic stroke patients. PLoS ONE 2017, 12, e0180704. [Google Scholar] [CrossRef]
- Li, W.; Xu, J.; Wang, X.; Chen, J.; Zhang, C.; Sun, K.; Hui, R. Cyclooxygenase-2 (COX-2) G-765C is a protective factor for coronary artery disease but not for ischemic stroke: A meta-analysis. Atherosclerosis 2009, 207, 492–495. [Google Scholar] [CrossRef]
- Posadas-Sánchez, R.; López-Uribe, Á.R.; Fragoso, J.M.; Vargas-Alarcón, G. Interleukin 6 polymorphisms are associated with cardiovascular risk factors in premature coronary artery disease patients and healthy controls of the GEA Mexican study. Exp. Mol. Pathol. 2024, 136, 104886. [Google Scholar] [CrossRef] [PubMed]
- Tabrez, S.; Jabir, N.R.; Zughaibi, T.A.; Suhail, M. Association of IL-6 promoter polymorphism hotspots (− 174G/C and − 572G/C) with cardiovascular disease risk factors. Mol. Biol. Rep. 2022, 49, 2265–2272. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Liu, C.; Wu, Y.; Li, H. Correlations of IL-1 and IL-6 Gene polymorphisms with hypertrophic cardiomyopathy. Cell. Mol. Biol. 2024, 70, 61–67. Available online: https://cellmolbiol.org/index.php/CMB/article/view/5440 (accessed on 10 February 2025). [PubMed]
- Ding, X.; Yue, J.; Hao, Q.; Chen, S.; Yang, M.; Dong, B. Research on association between ataxia telangiectasia mutated (ATM) gene single nucleotide polymorphism Rs189037 C>T and essential hypertension. Shengwu Yixue Gongchengxue Zazhi/J. Biomed. Eng. 2016, 33, 741–746. [Google Scholar]
- Yamamoto-Furusho, J.K.; Fonseca-Camarillo, G. Genetic Markers Associated with Clinical Outcomes in Patients with Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 2683–2695. Available online: https://academic.oup.com/ibdjournal/article/21/11/2683-2695/4579353 (accessed on 14 February 2025). [CrossRef]
- Xiang, Y.; Song, X.; Long, D. Ferroptosis regulation through Nrf2 and implications for neurodegenerative diseases. Arch. Toxicol. 2024, 98, 579–615. [Google Scholar] [CrossRef]
- Zhang, Y.; Xin, L.; Xiang, M.; Shang, C.; Wang, Y.; Wang, Y.; Cui, X.; Lu, Y. The molecular mechanisms of ferroptosis and its role in cardiovascular disease. Biomed. Pharmacother. 2022, 145, 112423. [Google Scholar] [CrossRef]
- Peng, X.; Sun, B.; Tang, C.; Shi, C.; Xie, X.; Wang, X.; Jiang, D.; Li, S.; Jia, Y.; Wang, Y.; et al. HMOX1-LDHB interaction promotes ferroptosis by inducing mitochondrial dysfunction in foamy macrophages during advanced atherosclerosis. Dev. Cell 2024, 60, 1070–1086.e8. Available online: https://linkinghub.elsevier.com/retrieve/pii/S1534580724007330 (accessed on 10 March 2025). [CrossRef]
- Ma, C.; Wu, X.; Zhang, X.; Liu, X.; Deng, G. Heme oxygenase-1 modulates ferroptosis by fine-tuning levels of intracellular iron and reactive oxygen species of macrophages in response to Bacillus Calmette-Guerin infection. Front. Cell. Infect. Microbiol. 2022, 12, 1004148. [Google Scholar]
- Ma, L.L.; Sun, L.; Wang, Y.X.; Sun, B.H.; Li, Y.F.; Jin, Y.L. Association between HO-1 gene promoter polymorphisms and diseases (Review). Mol. Med. Rep. 2021, 25, 29. Available online: http://www.spandidos-publications.com/10.3892/mmr.2021.12545 (accessed on 15 March 2025). [CrossRef]
- Chen, Y.H.; Lin, S.J.; Lin, M.W.; Tsai, H.L.; Kuo, S.S.; Chen, J.W.; Charng, M.J.; Wu, T.C.; Chen, L.C.; Ding, Y.A.; et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum. Genet. 2002, 111, 1–8. Available online: http://link.springer.com/10.1007/s00439-002-0769-4 (accessed on 6 May 2025). [CrossRef] [PubMed]
- Li, W.; Xiang, Z.; Xing, Y.; Li, S.; Shi, S. Mitochondria bridge HIF signaling and ferroptosis blockage in acute kidney injury. Cell Death Dis. 2022, 13, 308. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Su, W.; Wei, X.; Qu, S.; Zhao, D.; Zhou, J.; Wang, Y.; Guan, Q.; Qin, C.; Xiang, J.; et al. HIF-1α drives resistance to ferroptosis in solid tumors by promoting lactate production and activating SLC1A1. Cell Rep. 2023, 42, 112945. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, Y.; Chen, Y. HIF1A gene rs10873142 polymorphism is associated with risk of chronic obstructive pulmonary disease in a Chinese Han population: A case–control study. Biosci. Rep. 2018, 38, BSR20171309. Available online: https://portlandpress.com/bioscirep/article/38/2/BSR20171309/57451/HIF1A-gene-rs10873142-polymorphism-is-associated (accessed on 17 March 2025). [CrossRef]
- Wu, L.F.; Xu, G.P.; Zhao, Q.; Zhou, L.J.; Wang, D.; Chen, W.X. The association between hypoxia inducible factor 1 subunit alpha gene rs2057482 polymorphism and cancer risk: A meta-analysis. BMC Cancer 2019, 19, 1123. Available online: https://bmccancer.biomedcentral.com/articles/10.1186/s12885-019-6329-2 (accessed on 17 March 2025). [CrossRef]
- Islam, M.S.; Jesmin. Exploring the Correlation Between Hypoxia, HIF1A Variants, and Breast Cancer in Different Ethnicities, and Bangladeshi Women: Through ELISA and Integrative Multi-Omics Analysis. Biomark Insights 2024, 19, 11772719241278176. Available online: https://journals.sagepub.com/doi/10.1177/11772719241278176 (accessed on 17 March 2025). [CrossRef]
- Wang, W.; Wu, B.Q.; Chen, G.B.; Zhou, Y.; Li, Z.H.; Zhang, J.L.; Ding, Y.L.; Zhang, P.; Wang, J.Q. Hypoxia-inducible factor-1α rs11549465 C>T and rs11549467 G>A gene polymorphisms are associated with an increased risk of digestive cancers in Asians. J. Cancer Res. Ther. 2018, 14 (Suppl. 1), S46–S53. Available online: https://journals.lww.com/01363817-201814001-00008 (accessed on 17 March 2025). [CrossRef]
- Feng, C.C.; Ye, Q.L.; Zhu, Y.; Leng, R.X.; Chen, G.M.; Yang, J.; Cen, H.; Yang, X.K.; Li, R.; Xu, W.D.; et al. Lack of association between the polymorphisms of hypoxia-inducible factor 1A (HIF1A) gene and SLE susceptibility in a Chinese population. Immunogenetics 2014, 66, 9–13. Available online: http://link.springer.com/10.1007/s00251-013-0743-4 (accessed on 20 March 2025). [CrossRef]
- Lou, Y.; Ma, M.; Jiang, Y.; Xu, H.; Gao, Z.; Gao, L.; Wang, Y. Ferroptosis: A new strategy for traditional Chinese medicine treatment of stroke. Biomed. Pharmacother. 2022, 156, 113806. [Google Scholar] [CrossRef]
- Song, S.; Su, Z.; Kon, N.; Chu, B.; Li, H.; Jiang, X.; Luo, J.; Stockwell, B.R.; Gu, W. ALOX5-mediated ferroptosis acts as a distinct cell death pathway upon oxidative stress in Huntington’s disease. Genes. Dev. 2023, 37, 204–217. [Google Scholar] [CrossRef]
- Sun, Q.Y.; Zhou, H.H.; Mao, X.Y. Emerging Roles of 5-Lipoxygenase Phosphorylation in Inflammation and Cell Death. Oxid. Med. Cell. Longev. 2019, 2019, 2749173. Available online: https://www.hindawi.com/journals/omcl/2019/2749173/ (accessed on 27 March 2025). [CrossRef] [PubMed]
- Ma, T.; Du, J.; Zhang, Y.; Wang, Y.; Wang, B.; Zhang, T. GPX4-independent ferroptosis—A new strategy in disease’s therapy. Cell Death Discov. 2022, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Chistiakov, D.A.; Melnichenko, A.A.; Grechko, A.V.; Myasoedova, V.A.; Orekhov, A.N. Potential of anti-inflammatory agents for treatment of atherosclerosis. Exp. Mol. Pathol. 2018, 104, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Fratta Pasini, A.M.; Stranieri, C.; Busti, F.; Di Leo, E.G.; Girelli, D.; Cominacini, L. New Insights into the Role of Ferroptosis in Cardiovascular Diseases. Cells 2023, 12, 867. Available online: https://www.mdpi.com/2073-4409/12/6/867 (accessed on 6 October 2024). [CrossRef]
- Chen, X.; Comish, P.B.; Tang, D.; Kang, R. Characteristics and Biomarkers of Ferroptosis. Front. Cell Dev. Biol. 2021, 9, 637162. Available online: https://www.frontiersin.org/articles/10.3389/fcell.2021.637162/full (accessed on 15 November 2024). [CrossRef]
- Grebenciucova, E.; VanHaerents, S. Interleukin 6: At the interface of human health and disease. Front. Immunol. 2023, 14, 1255533. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2023.1255533/full (accessed on 27 March 2025). [CrossRef]
- Chen, Y.; Fang, Z.M.; Yi, X.; Wei, X.; Jiang, D.S. The interaction between ferroptosis and inflammatory signaling pathways. Cell Death Dis. 2023, 14, 205. [Google Scholar] [CrossRef]
- Amezcua-Castillo, E.; González-Pacheco, H.; Sáenz-San Martín, A.; Méndez-Ocampo, P.; Gutierrez-Moctezuma, I.; Massó, F.; Sierra-Lara, D.; Springall, R.; Rodríguez, E.; Arias-Mendoza, A.; et al. C-Reactive Protein: The Quintessential Marker of Systemic Inflammation in Coronary Artery Disease—Advancing toward Precision Medicine. Biomedicines 2023, 11, 2444. [Google Scholar] [CrossRef]
- Hoffman-Andrews, L. The known unknown: The challenges of genetic variants of uncertain significance in clinical practice. J. Law. Biosci. 2017, 4, 648–657. Available online: https://academic.oup.com/jlb/article/4/3/648/4820755 (accessed on 6 May 2025). [CrossRef]
| Gene | Official Name | Pathway Associated with Ferroptosis | SNP | Cardiovascular Disease | OR | Reference |
|---|---|---|---|---|---|---|
| NFE2L2 | NFE2 like bZIP transcription factor 2 | Cellular antioxidant response to iron accumulation and and lipid peroxidation | rs10183914 | Aneurysmal subarachnoid hemorrhage | Risk OR 1.33 | [44] |
| rs6721961 | Blood pressure | Risk *** | [45] | |||
| Coronary artery disease | Risk OR 5.07 | [46] | ||||
| HFE | Homeostatic iron regulator | Iron accumulation | rs1799945 | Arterial hypertension | Risk 2.53 | [47] |
| Coronary heart disease | Risk 1.06 | [48] | ||||
| rs1800562 | Iron status on coronary artery disease | Protective *** | [49] | |||
| HMOX1 | Heme oxygenase 1 | rs2071746 | Perimenopausal coronary artery disease | Protective OR 0.67 | [50] | |
| (GT)n repeat (S > L) | Perimenopausal coronary artery disease | Risk OR 1.34 | [50] | |||
| HIF-1α | Hypoxia inducible factor 1 subunit alpha | Hypoxia | rs10873142 | Perimenopausal coronary artery disease | Protective OR 0.56 | [50] |
| Perimenopausal coronary artery disease ** | Risk OR 1.24 ** | [50] | ||||
| Myocardial infarction | Risk OR 1.28 | [51] | ||||
| Coronary disease | Risk *** | [52] | ||||
| rs2057482 | Perimenopausal coronary artery disease | Protective OR 0.71 | [50] | |||
| Premature coronary artery disease | Protective OR 0.616 | [53] | ||||
| Myocardial infarction | Risk OR 1.27 | [51] | ||||
| rs11549465 | Myocardial infarction | Protective OR 0.84 | [51] | |||
| Hypertensive left ventricular hypertrophy | Risk OR 1.26 | [54] | ||||
| Coronary artery disease | Risk *** | [52] | ||||
| rs11549467 | Hypertensive left ventricular hypertrophy | Risk OR 1.21 | [54] | |||
| rs1957757 | Hypertensive left ventricular hypertrophy | Risk OR 1.20 | [54] | |||
| rs41508050 | Coronary disease | Risk *** | [52] | |||
| ALOX5 | Arachidonate 5-lipoxygenase | Lipid peroxidation | rs10900213 | Ischemic stroke | Risk OR 1.991 | [55] |
| rs12762303 | Coronary artery disease | Risk OR 1.36 | [56] | |||
| GPX4 | Glutathione peroxidase 4 | Cellular antioxidant response | rs713041 | Arterial hypertension | Risk OR 4.19 | [57] |
| PTGS2 | Prostaglandin-endoperoxide synthase 2 | Inflammation | rs20417 | Cerebral infarction | Risk OR 2.84 * | [58] |
| Ischemic stroke | Risk OR 1.94 | [59] | ||||
| Ischemic stroke | Risk OR 1.11 | [60] | ||||
| Coronary artery disease | Protective OR 0.80 | [60] | ||||
| IL-6 | Interleukin -6 | rs2069827 | Obesity and premature coronary artery disease | Protective OR 0.57 | [61] | |
| Hypertriglyceridemia on premature coronary artery disease | Protective OR 0.72 | [61] | ||||
| rs1800795 | Obesity and premature coronary artery disease | Protective OR 0.40 | [61] | |||
| Hypertriglyceridemia on premature coronary artery disease | Protective OR 0.57 | [61] | ||||
| Cardiovascular disease risk factor | Risk *** | [62] | ||||
| rs2069831 | Left ventricular ejection fraction | Risk *** | [63] | |||
| ATM | Ataxia-Telangiectasia Mutated | Cell cycle control | rs189037 | Coronary artery disease | Protective OR 0.47 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fisher-Bautista, B.; Fonseca-Camarillo, G.; Cruz-Gregorio, A. Genetic Markers Associated with Ferroptosis in Cardiovascular Diseases. Future Pharmacol. 2025, 5, 37. https://doi.org/10.3390/futurepharmacol5030037
Fisher-Bautista B, Fonseca-Camarillo G, Cruz-Gregorio A. Genetic Markers Associated with Ferroptosis in Cardiovascular Diseases. Future Pharmacology. 2025; 5(3):37. https://doi.org/10.3390/futurepharmacol5030037
Chicago/Turabian StyleFisher-Bautista, Brandon, Gabriela Fonseca-Camarillo, and Alfredo Cruz-Gregorio. 2025. "Genetic Markers Associated with Ferroptosis in Cardiovascular Diseases" Future Pharmacology 5, no. 3: 37. https://doi.org/10.3390/futurepharmacol5030037
APA StyleFisher-Bautista, B., Fonseca-Camarillo, G., & Cruz-Gregorio, A. (2025). Genetic Markers Associated with Ferroptosis in Cardiovascular Diseases. Future Pharmacology, 5(3), 37. https://doi.org/10.3390/futurepharmacol5030037








