An In Vitro Diacetylcurcumin Study for Periodontitis: A New Approach to Controlling Subgingival Biofilms
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of DAC

2.2. Multispecies Subgingival Biofilm Model
2.3. Metabolic Activity of Biofilm
2.4. DNA-DNA Hybridization (Checkerboard Technique)
2.5. Determination of Acute Toxic Potential in an Alternative In Vivo Model
2.6. Statistical Analysis
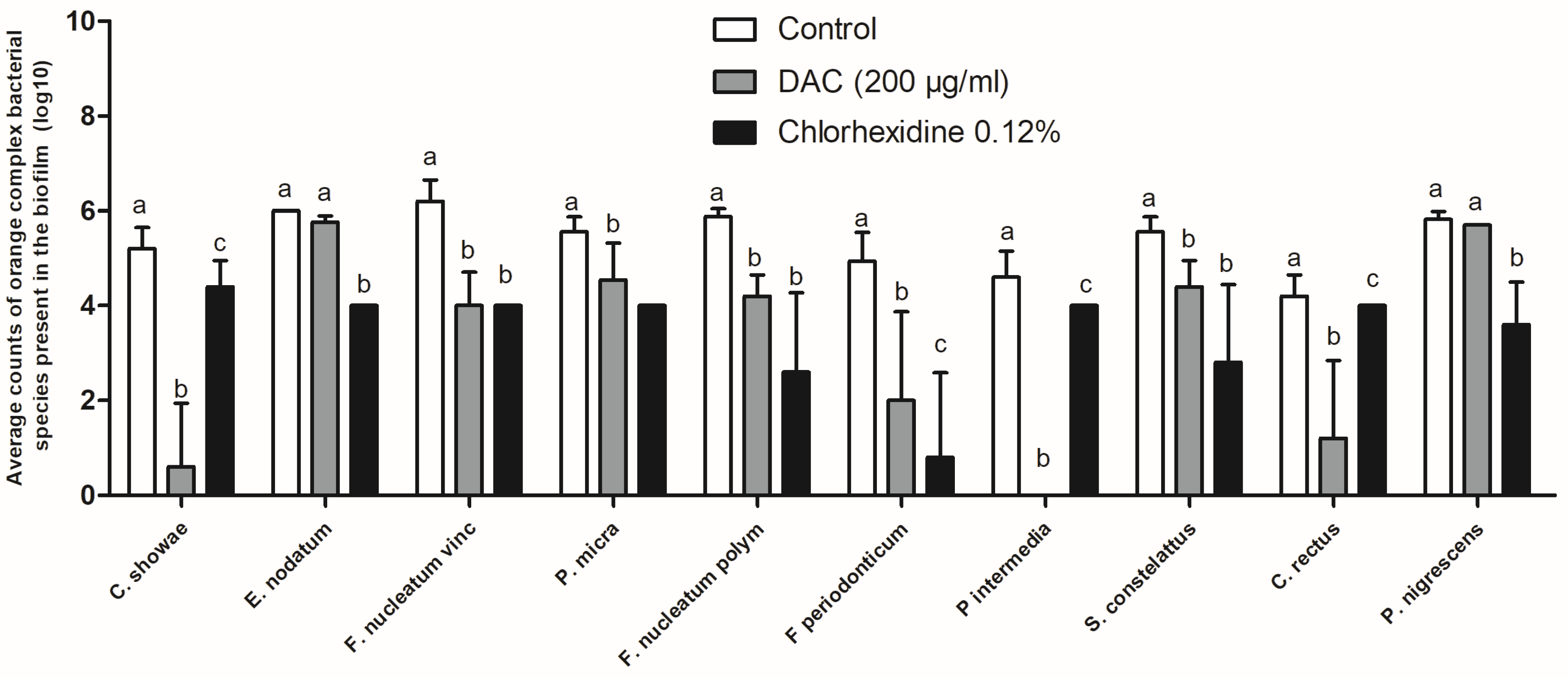
3. Results
Antibiofilm Activity of DAC
4. Discussion
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Raman, A.; Pradeep, A.R. Role of 1% alendronate gel as adjunct to mechanical therapy in the treatment of chronic periodontitis among smokers. J. Appl. Oral Sci. 2017, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89, 1475. [Google Scholar] [CrossRef] [PubMed]
- Janakiram, C.; Dye, B.A. A public health approach for prevention of periodontal disease. Periodontol 2000 2020, 84, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D.; Dzink, J.L.; Hillman, J.D. Associations between microbial species in subgingival plaque samples. Oral Microbiol. Immunol. 1988, 3, 1–7. [Google Scholar] [CrossRef]
- Aja, E.; Mangar, M.; Fletcher, H.M.; Mishra, A. Filifactor alocis: Recent Insights and Advances. J. Dent. Res. 2021, 100, 790–797. [Google Scholar] [CrossRef]
- Mishra, A.; Dou, Y.; Wang, C.; Fletcher, H.M. Filifactor alocis enhances survival of Porphyromonas gingivalis W83 in response to H2O2-induced stress. Mol. Oral Microbiol. 2024, 39, 12–26. [Google Scholar] [CrossRef]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Kumbar, V.M.; Peram, M.R.; Kugaji, M.S.; Shah, T.; Patil, S.P.; Muddapur, U.M.; Bhat, K.G. Effect of curcumin on growth, biofilm formation and virulence factor gene expression of Porphyromonas gingivalis. Odontology 2021, 109, 18–28. [Google Scholar] [CrossRef]
- Anuradha, B.R.; Bai, Y.D.; Sailaja, S.; Sudhakar, J.; Priyanka, M.; Deepika, V. Evaluation of Anti-Inflammatory Effects of Curcumin Gel as an Adjunct to Scaling and Root Planing: A Clinical Study. J. Int. Oral Health 2015, 7, 90–93. [Google Scholar]
- Peng, Y.; Ao, M.; Dong, B.; Jiang, Y.; Yu, L.; Chen, Z.; Hu, C.; Xu, R. Anti-Inflammatory Effects of Curcumin in the Inflammatory Diseases: Status, Limitations and Countermeasures. Drug Des. Dev. Ther. 2021, 15, 4503–4525. [Google Scholar] [CrossRef]
- Polaquini, C.R.; Marques, B.C.; Ayusso, G.M.; Morão, L.G.; Sardi, J.C.O.; Campos, D.L.; Silva, I.C.; Cavalca, L.B.; Scheffers, D.J.; Rosalen, P.L.; et al. Antibacterial activity of a new monocarbonyl analog of curcumin MAC 4 is associated with divisome disruption. Bioorg Chem. 2021, 109, 104668. [Google Scholar] [CrossRef] [PubMed]
- Sardi, J.C.O.; Polaquini, C.R.; Freires, I.A.; Galvão, L.C.C.; Lazarini, J.G.; Torrezan, G.S.; Regasini, L.O.; Rosalen, P.L. Antibacterial activity of diacetylcurcumin against Staphylococcus aureus results in decreased biofilm and cellular adhesion. J. Med. Microbiol. 2017, 66, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Sanches, C.V.G.; Sardi, J.C.O.; Terada, R.S.S.; Lazarini, J.G.; Freires, I.A.; Polaquini, C.R.; Torrezan, G.S.; Regasini, L.O.; Fujimaki, M.; Rosalen, P.L. Diacetylcurcumin: A new photosensitizer for antimicrobial photodynamic therapy in Streptococcus mutans biofilms. Biofouling 2019, 35, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Polaquini, C.R.; Torrezan, G.S.; Santos, V.R.; Nazaré, A.C.; Campos, D.L.; Almeida, L.A.; Silva, I.C.; Ferreira, H.; Pavan, F.R.; Duque, C.; et al. Antibacterial and Antitubercular Activities of Cinnamylideneacetophenones. Molecules 2017, 22, 1685. [Google Scholar] [CrossRef]
- Soares, G.M.; Teles, F.; Starr, J.R.; Feres, M.; Patel, M.; Martin, L.; Teles, R. Effects of azithromycin, metronidazole, amoxicillin, and metronidazole plus amoxicillin on an in vitro polymicrobial subgingival biofilm model. Antimicrob. Agents Chemother. 2015, 59, 2791–2798. [Google Scholar] [CrossRef]
- Megaw, J.; Thompson, T.P.; Lafferty, R.A.; Gilmore, B.F. Galleria mellonella as a novel in vivo model for assessment of the toxicity of 1-alkyl-3-methylimidazolium chloride ionic liquids. Chemosphere 2015, 139, 197–201. [Google Scholar] [CrossRef]
- Miranda, S.L.F.; Damaceno, J.T.; Faveri, M.; Figueiredo, L.C.; Soares, G.M.S.; Feres, M.; Bueno-Silva, B. In Vitro Antimicrobial Effect of Cetylpyridinium Chloride on Complex Multispecies Subgingival Biofilm. Braz. Dent. J. 2020, 31, 103–108. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Li, B.; Li, X.; Lin, H.; Zhou, Y. Curcumin as a Promising Antibacterial Agent: Effects on Metabolism and Biofilm Formation in S. mutans. Biomed. Res. Int. 2018, 2018, 4508709. [Google Scholar]
- Izui, S.; Sekine, S.; Maeda, K.; Kuboniwa, M.; Takada, A.; Amano, A.; Nagata, H. Antibacterial Activity of Curcumin Against Periodontopathic Bacteria. J. Periodontol. 2016, 87, 83–90. [Google Scholar] [CrossRef]
- Wang, Y.J.; Pan, M.H.; Cheng, A.L.; Lin, L.I.; Ho, Y.S.; Hsieh, C.Y.; Lin, J.K. Stability of curcumin in buffer solutions and characterization of its degradation products. J. Pharm. Biomed. Anal. 1997, 15, 1867–1876. [Google Scholar] [CrossRef] [PubMed]
- Kharat, M.; Du, Z.; Zhang, G.; McClements, D.J. Physical and Chemical Stability of Curcumin in Aqueous Solutions and Emulsions: Impact of pH, Temperature, and Molecular Environment. J. Agric. Food Chem. 2017, 65, 1525–1532. [Google Scholar] [CrossRef]
- Souza, V.; Polaquini, C.R.; de Moraes, G.R.; Oliveira Braga, A.R.; da Silva, P.V.; da Silva, D.R.; Ribeiro Lima, F.R.; Regasini, L.O.; Cássia Orlandi Sardi, J. Diacetylcurcumin: A novel strategy against Enterococcus faecalis biofilm in root canal disinfection. Future Microbiol. 2024, 19, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Bregaint, S.; Boyer, E.; Fong, S.B.; Meuric, V.; Bonnaure-Mallet, M.; Jolivet-Gougeon, A. Porphyromonas gingivalis outside the oral cavity. Odontology 2022, 110, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Olsen, I.; Taubman, M.A.; Singhrao, S.K. Porphyromonas gingivalis suppresses adaptive immunity in periodontitis, atherosclerosis, and Alzheimer’s disease. J. Oral Microbiol. 2016, 8, 33029. [Google Scholar] [CrossRef]
- Hr, V.; Daniel, R.A.; Prabhu, A.P.S.; Basavaraju, S. Susceptibility of periodontal pathogens to a novel target-specific drug delivery system containing self-nanoemulsifying curcumin: An in vitro study. J. Adv. Periodontol. Implant. Dent. 2023, 15, 67–73. [Google Scholar] [CrossRef]
- Guru, S.R.; Reddy, K.A.; Rao, R.J.; Padmanabhan, S.; Guru, R.; Srinivasa, T.S. Comparative evaluation of 2% turmeric extract with nanocarrier and 1% chlorhexidine gel as an adjunct to scaling and root planing in patients with chronic periodontitis: A pilot randomized controlled clinical trial. J. Indian Soc. Periodontol. 2020, 24, 244–252. [Google Scholar] [CrossRef]
- Heller, D.; Silva-Boghossian, C.M.; do Souto, R.M.; Colombo, A.P. Subgingival microbial profiles of generalized aggressive and chronic periodontal diseases. Arch. Oral Biol. 2012, 57, 973–980. [Google Scholar] [CrossRef]
- Vielkind, P.; Jentsch, H.; Eschrich, K.; Rodloff, A.C.; Stingu, C.S. Prevalence of Actinomyces spp. in patients with chronic periodontitis. Int. J. Med. Microbiol. 2015, 305, 682–688. [Google Scholar] [CrossRef]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef]
- Ménard, G.; Rouillon, A.; Cattoir, V.; Donnio, P.Y. Galleria mellonella as a Suitable Model of Bacterial Infection: Past, Present and Future. Front. Cell Infect. Microbiol. 2021, 11, 782733. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.J.; Loh, J.M.; Proft, T. Galleria mellonella infection models for the study of bacterial diseases and for antimicrobial drug testing. Virulence 2016, 7, 214–229. [Google Scholar] [CrossRef] [PubMed]
- Santos, T.A.; Scorzoni, L.; Santos, A.C.; Junqueira, J.C.; Anbinder, A.L. Galleria mellonella as an experimental model for studying periodontopathogens. J. Indian Soc. Periodontol. 2020, 24, 593–596. [Google Scholar] [PubMed]





| Bacteria | ATCC | Bacteria | ATCC |
|---|---|---|---|
| Actinomyces naeslundii | ATCC12104 | Actinomyces oris | ATCC43146 |
| Actinomyces gerencseriae | ATCC23840 | Actinomyces israelii | ATCC12102 |
| Veillonella parvula | ATCC10790 | Actinomyces odontolyticus | ATCC17929 |
| Streptococcus sanguinis | ATCC10556 | Streptococcus oralis | ATCC35037 |
| Streptococcus intermedius | ATCC27335 | Streptococcus gordonii | ATCC10558 |
| Streptococcus mitis | ATCC49456 | Aggregatibacter actinomycetemcomitans | ATCC29523 |
| Capnocytophaga ochracea | ATCC33596 | Capnocytophaga gingivalis | ATCC33624 |
| Eikenella corrodens | ATCC23834 | Capnocytophaga sputigena | ATCC33612 |
| Streptococcus constellatus | ATCC27823 | Eubacterium nodatum | ATCC33099 |
| Fusobacterium nucleatum vincentii | ATCC49256 | Parvimonas micra | ATCC33270 |
| Fusobacterium nucleatum polymorphum | ATCC10953 | Fusobacterium nucleatum nucleatum | ATCC25586 |
| Campylobacter showae | ATCC51146 | Capnocytophaga sputigena | ATCC33612 |
| Campylobacter gracilis | ATCC33236 | Leptotrichia buccalis | ATCC14201 |
| Campylobacter rectus | ATCC33238 | Fusobacterium periodonticum | ATCC33693 |
| Prevotella intermedia | ATCC25611 | Porphyromonas gingivalis | ATCC33277 |
| Tannerella forsythia | ATCC43037 | Prevotella melaninogenica | ATCC25845 |
| Prevotella nigrescens | ATCC33563 | Eubacterium saburreum | ATCC33271 |
| Streptococcus anginosus | ATCC33397 | Selenomonas noxia | ATCC43541 |
| Neisseria mucosa | ATCC19696 | Propionibacterium acnes | ATCC11827 |
| Gemella morbillorum | ATCC27824 | Streptococcus mutans | ATCC25175 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aires da Silva, V.A.; Bueno-Silva, B.; Figueiredo, L.C.; Tiemi Macedo, T.; Aguiar da Silva, L.D.; Chagas Chaves de Oliveira Junior, H.; Polaquini, C.R.; Regasini, L.O.; Sardi, J.d.C.O. An In Vitro Diacetylcurcumin Study for Periodontitis: A New Approach to Controlling Subgingival Biofilms. Future Pharmacol. 2025, 5, 19. https://doi.org/10.3390/futurepharmacol5020019
Aires da Silva VA, Bueno-Silva B, Figueiredo LC, Tiemi Macedo T, Aguiar da Silva LD, Chagas Chaves de Oliveira Junior H, Polaquini CR, Regasini LO, Sardi JdCO. An In Vitro Diacetylcurcumin Study for Periodontitis: A New Approach to Controlling Subgingival Biofilms. Future Pharmacology. 2025; 5(2):19. https://doi.org/10.3390/futurepharmacol5020019
Chicago/Turabian StyleAires da Silva, Valdo Antonio, Bruno Bueno-Silva, Luciene Cristina Figueiredo, Tatiane Tiemi Macedo, Lucas Daylor Aguiar da Silva, Helio Chagas Chaves de Oliveira Junior, Carlos Roberto Polaquini, Luís Octávio Regasini, and Janaina de Cássia Orlandi Sardi. 2025. "An In Vitro Diacetylcurcumin Study for Periodontitis: A New Approach to Controlling Subgingival Biofilms" Future Pharmacology 5, no. 2: 19. https://doi.org/10.3390/futurepharmacol5020019
APA StyleAires da Silva, V. A., Bueno-Silva, B., Figueiredo, L. C., Tiemi Macedo, T., Aguiar da Silva, L. D., Chagas Chaves de Oliveira Junior, H., Polaquini, C. R., Regasini, L. O., & Sardi, J. d. C. O. (2025). An In Vitro Diacetylcurcumin Study for Periodontitis: A New Approach to Controlling Subgingival Biofilms. Future Pharmacology, 5(2), 19. https://doi.org/10.3390/futurepharmacol5020019







