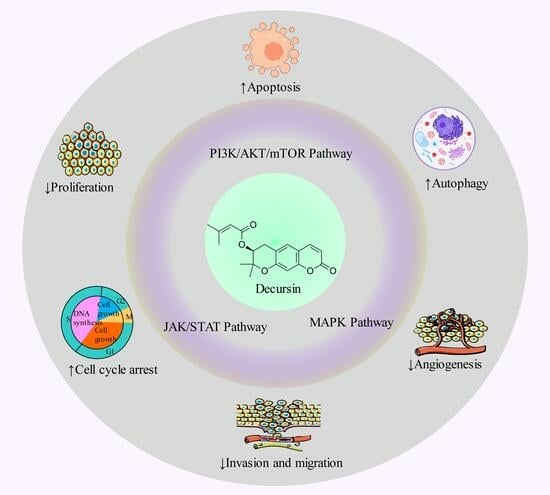
Anticancer Efficacy of Decursin: A Comprehensive Review with Mechanistic Insights
Abstract
1. Introduction
2. Materials and Methods
2.1. Literature Search Stratagem
2.2. Inclusion and Exclusion Criteria
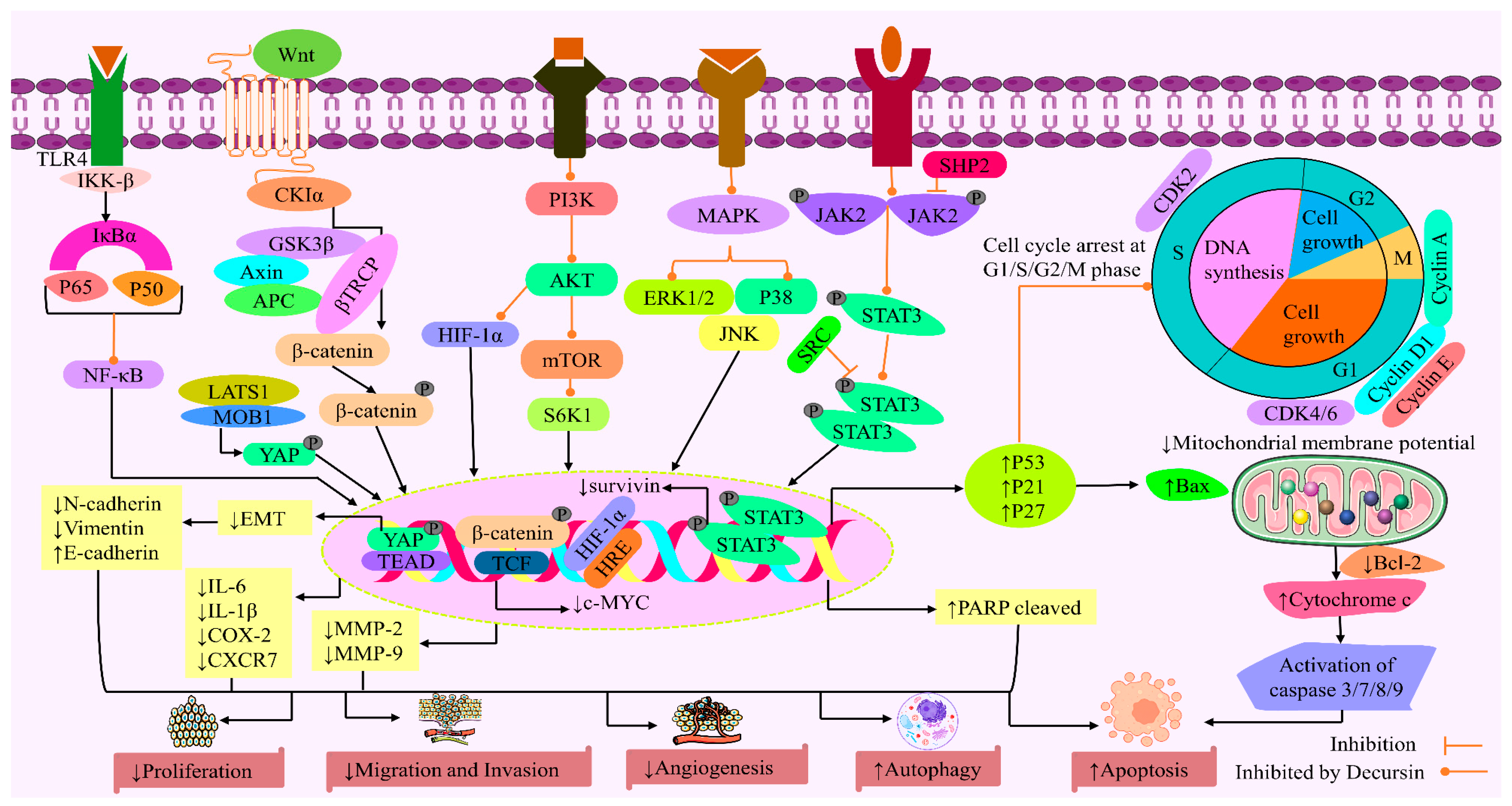
3. Anticancer Activity of Decursin: Mechanistic Analysis
3.1. Cytotoxicity
3.2. Cell Cycle Arrest
3.3. Apoptotic Cell Death
3.4. Inhibition of Cancer Cell Proliferation
3.5. Inhibition of Invasion and Migration
3.6. Inhibition of Angiogenesis
3.7. Autophagy
4. Pharmacokinetics
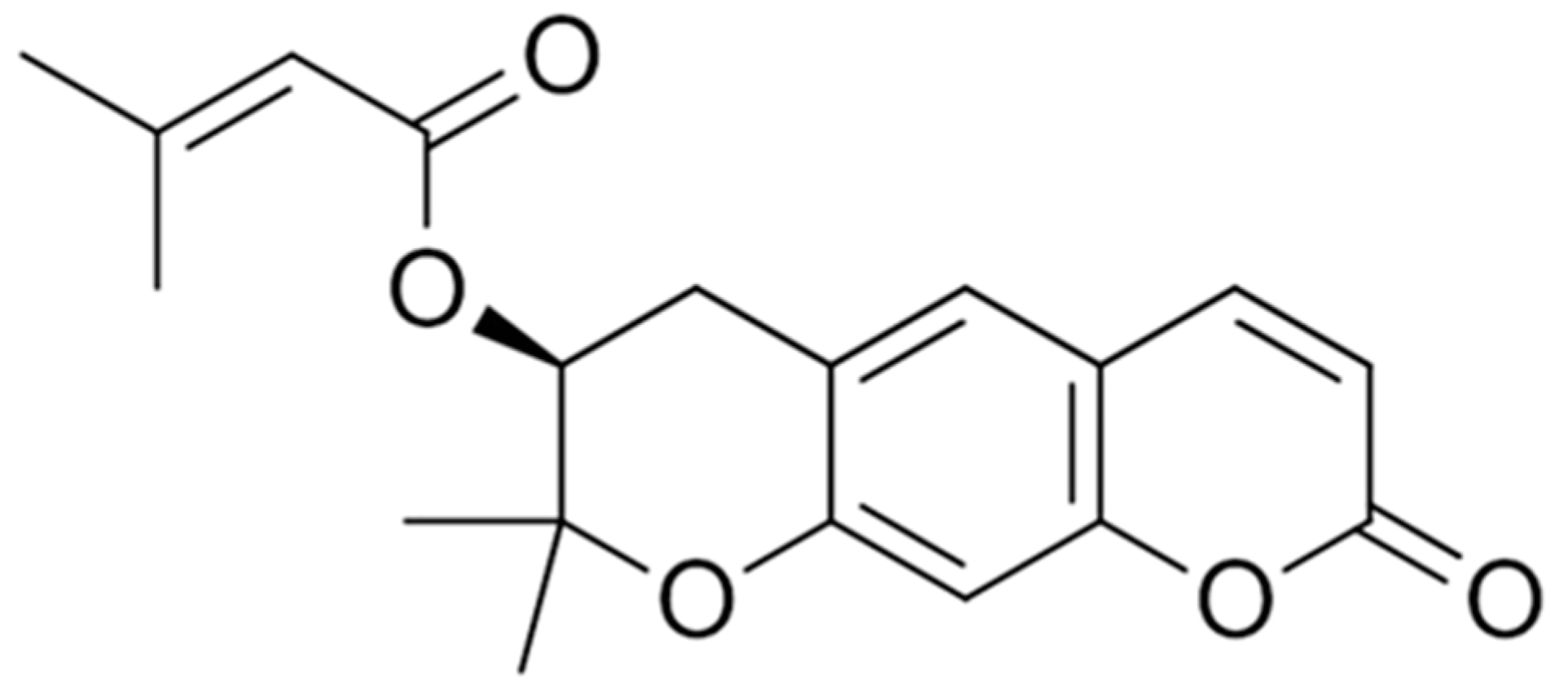
Extraction and Isolation of Decursin
5. Conclusions and Future Direction
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACC | Acetyl-CoA carboxylase |
| APC | Adenomatous polyposis coli |
| Bax | BCL2-associated X protein |
| CDK | Cyclin-dependent kinase |
| CDK2 | Cyclin-dependent kinase 2 |
| c-MYC | Cellular Myc |
| COX | Cyclooxygenase |
| CTR | Catenin response transcription |
| EGFR | Epidermal growth factor receptor |
| EKR | Extracellular signal-regulated kinases |
| EMT | Epithelial–mesenchymal transition |
| ERK1/2 | Extracellular signal-regulated kinase 1/2 |
| FAS | Fatty acid synthase |
| HIF-1α | Hypoxia-inducible factor 1-alpha |
| IKK-β | Inhibitor of nuclear factor kappa-B kinase subunit beta |
| IL | Interleukin |
| JAK | Janus-activated kinase |
| JNK | c-Jun N-terminal kinase |
| LATS1 | Large tumor suppressor kinase 1 |
| LLC | Lewis lung cancer |
| LPS | Lipopolysaccharide |
| MAP | Mitogen-activated protein |
| MAPK | Mitogen-activated protein kinase |
| MMP | Matrix metalloproteinase |
| mTOR | Mammalian target of rapamycin |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B-cells |
| PI3K | Phosphoinositide 3-kinase |
| PPAR | Peroxisome proliferator-activated receptor |
| SHP2 | Src homology 2 domain-containing phosphatase 2 |
| STAT | Signal transduction and transcription activation |
| STAT3 | Signal transducer and activator of transcription 3 |
| TCF | T-cell factor |
| YAP | Yes-associated protein |
| TEAD | Transcriptional enhancer factor domain |
References
- Bhuia, M.S.; Chowdhury, R.; Sonia, F.A.; Biswas, S.; Ferdous, J.; El-Nashar, H.A.S.; El-Shazly, M.; Islam, M.T. Efficacy of Rotundic Acid and Its Derivatives as Promising Natural Anticancer Triterpenoids: A Literature-based Study. Chem. Biodivers. 2024, 21, e202301492. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Amend, S.R.; Austin, R.H.; Gatenby, R.A.; Hammarlund, E.U.; Pienta, K.J. Updating the Definition of Cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef]
- Ma, D.; Zou, B.; Cai, G.; Hu, X.; Liu, J.O. Total Synthesis of the Cyclodepsipeptide Apratoxin A and Its Analogues and Assessment of Their Biological Activities. Chem. Eur. J. 2006, 12, 7615–7626. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Katzke, V.A.; Kaaks, R.; Kühn, T.J. Lifestyle and cancer risk. Can. J. 2015, 21, 104–110. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Wasim Bin, H.; Mohammad Al-Masum, M. State of Cancer Care in Bangladesh. The Daily Star. Available online: https://www.thedailystar.net/supplements/world-cancer-day-2022/news/state-cancer-care-bangladesh-2953926 (accessed on 20 January 2024).
- Danaei, G.; Vander Hoorn, S.; Lopez, A.D.; Murray, C.J.L.; Ezzati, M. Causes of Cancer in the World: Comparative Risk Assessment of Nine Behavioural and Environmental Risk Factors. Lancet 2005, 366, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Gherghi, I.C.; Girousi, S.T.; Voulgaropoulos, A.; Tzimou-Tsitouridou, R. Interaction of the Mutagen Ethidium Bromide with DNA, Using a Carbon Paste Electrode and a Hanging Mercury Drop Electrode. Anal. Chim. Acta 2004, 505, 135–144. [Google Scholar] [CrossRef]
- Trichopoulos, D.; Li, F.P.; Hunter, D.J. What Causes Cancer? Sci. Am. 1996, 275, 80–87. [Google Scholar] [CrossRef]
- Isikhan, V.; Güner, P.; Kömürcü, S.; Özet, A.; Arpaci, F.; Öztürk, B. The Relationship between Disease Features and Quality of Life in Patients with Cancer—I. Cancer Nurs. 2001, 24, 490–495. [Google Scholar] [CrossRef]
- Islam, M.T.; Sarkar, C.; Hossain, R.; Bhuia, M.S.; Mardare, I.; Kulbayeva, M.; Ydyrys, A.; Calina, D.; Habtemariam, S.; Kieliszek, M. Therapeutic Strategies for Rheumatic Diseases and Disorders: Targeting Redox Imbalance and Oxidative Stress. Biomed. Pharmacother. 2023, 164, 114900. [Google Scholar] [CrossRef]
- Tabarés-Seisdedos, R.; Dumont, N.; Baudot, A.; Valderas, J.M.; Climent, J.; Valencia, A.; Crespo-Facorro, B.; Vieta, E.; Gómez-Beneyto, M.; Martínez, S. No Paradox, No Progress: Inverse Cancer Comorbidity in People with Other Complex Diseases. Lancet Oncol. 2011, 12, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Sutherland, R.M. Cell and Environment Interactions in Tumor Microregions: The Multicell Spheroid Model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P. Physiological Mechanisms of Treatment Resistance. In The Impact of Tumor Biology on Cancer Treatment and Multidisciplinary Strategies; Springer: Berlin/Heidelberg, Germany, 2009; pp. 273–290. [Google Scholar]
- Vaupel, P.; Kallinowski, F.; Okunieff, P. Blood Flow, Oxygen and Nutrient Supply, and Metabolic Microenvironment of Human Tumors: A Review. Cancer Res. 1989, 49, 6449–6465. [Google Scholar] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian Cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Lee, J.H.; Machtay, M.; Unger, L.D.; Weinstein, G.S.; Weber, R.S.; Chalian, A.A.; Rosenthal, D.I. Prophylactic Gastrostomy Tubes in Patients Undergoing Intensive Irradiation for Cancer of the Head and Neck. Arch. Otolaryngol. Neck Surg. 1998, 124, 871–875. [Google Scholar] [CrossRef]
- Sourati, A.; Ameri, A.; Malekzadeh, M. Acute Side Effects of Radiation Therapy: A Guide to Management, 1st ed.; Springer Cham: Berlin/Heidelberg, Germany, 2017; p. 227. [Google Scholar]
- Hanna, E.; Alexiou, M.; Morgan, J.; Badley, J.; Maddox, A.M.; Penagaricano, J.; Fan, C.-Y.; Breau, R.; Suen, J. Intensive Chemoradiotherapy as a Primary Treatment for Organ Preservation in Patients with Advanced Cancer of the Head and Neck: Efficacy, Toxic Effects, and Limitations. Arch. Otolaryngol. Neck Surg. 2004, 130, 861–867. [Google Scholar] [CrossRef]
- Beg, S.; Rahman, M.; Jain, A.; Saini, S.; Midoux, P.; Pichon, C.; Ahmad, F.J.; Akhter, S. Nanoporous Metal Organic Frameworks as Hybrid Polymer–Metal Composites for Drug Delivery and Biomedical Applications. Drug Discov. Today 2017, 22, 625–637. [Google Scholar] [CrossRef]
- Hale, K.J. Monosaccharides: Use in the Asymmetric Synthesis of Natural Products. In Second Supplements to the 2nd Edition of Rodd’s Chemistry of Carbon Compounds; Elsevier: Amsterdam, The Netherlands, 1991; pp. 315–435. [Google Scholar]
- Agarwal, A.; DeNunzio, N.J.; Ahuja, D.; Hirsch, A.E. Beyond the Standard Curriculum: A Review of Available Opportunities for Medical Students to Prepare for a Career in Radiation Oncology. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 39–44. [Google Scholar] [CrossRef]
- Ali Khan, M.; El-Kersh, D.M.; Islam, M.S.; Ara Khan, S.; Kamli, H.; Sarkar, C.; Bhuia, M.S.; Islam, T.; Chandra Shill, M.; Gobe, G.C. Mikania Micrantha Kunth: An Ethnopharmacological Treasure Trove of Therapeutic Potential. Chem. Biodivers. 2023, 20, e202300392. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.P.; Pandotra, P.; Kushwaha, M.; Khan, S.; Sharma, R.; Gupta, S. Alkaloids: A Source of Anticancer Agents from Nature. Stud. Nat. Prod. Chem. 2015, 46, 341–445. [Google Scholar]
- Kashyap, D.; Garg, V.K.; Goel, N. Intrinsic and Extrinsic Pathways of Apoptosis: Role in Cancer Development and Prognosis. Adv. Protein Chem. Struct. Biol. 2021, 125, 73–120. [Google Scholar]
- Chandra Shill, M.; El-Nashar, H.A.S.; Prova Mollick, P.; Nath Acharyya, R.; Afrin, S.; Hossain, H.; Halder, S.; Torequl Islam, M.; Bhuia, M.S.; Reza, H.M. Longevity Spinach (Gynura Procumbens) Ameliorated Oxidative Stress and Inflammatory Mediators in Cisplatin-induced Organ Dysfunction in Rats: Comprehensive in Vivo and in Silico Studies. Chem. Biodivers. 2024, 21, e202301719. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Lee, J.H.; Kim, E.; Kim, W.; Suk, K.; Kim, J.; Song, G.Y.; Lee, W. A Novel Derivative of Decursin, CSL-32, Blocks Migration and Production of Inflammatory Mediators and Modulates PI3K and NF-κB Activities in HT1080 Cells. Cell Biol. Int. 2012, 36, 683–688. [Google Scholar] [CrossRef]
- Scholten, D.J.; Canals, M.; Maussang, D.; Roumen, L.; Smit, M.J.; Wijtmans, M.; De Graaf, C.; Vischer, H.F.; Leurs, R. Pharmacological Modulation of Chemokine Receptor Function. Br. J. Pharmacol. 2012, 165, 1617–1643. [Google Scholar] [CrossRef]
- Shehzad, A.; Parveen, S.; Qureshi, M.; Subhan, F.; Lee, Y.S. Decursin and Decursinol Angelate: Molecular Mechanism and Therapeutic Potential in Inflammatory Diseases. Inflamm. Res. 2018, 67, 209–218. [Google Scholar] [CrossRef]
- Graña, X.; Reddy, E.P. Cell Cycle Control in Mammalian Cells: Role of Cyclins, Cyclin Dependent Kinases (CDKs), Growth Suppressor Genes and Cyclin-Dependent Kinase Inhibitors (CKIs). Oncogene 1995, 11, 211–220. [Google Scholar]
- Yim, D.; Singh, R.P.; Agarwal, C.; Lee, S.; Chi, H.; Agarwal, R. A Novel Anticancer Agent, Decursin, Induces G1 Arrest and Apoptosis in Human Prostate Carcinoma Cells. Cancer Res. 2005, 65, 1035–1044. [Google Scholar] [CrossRef]
- Choi, S.-R.; Lee, J.-H.; Kim, J.-Y.; Park, K.-W.; Jeong, I.-Y.; Shim, K.-H.; Lee, M.-K.; Seo, K.-I. Decursin from Angelica Gigas Nakai Induces Apoptosis in RC-58T/h/SA# 4 Primary Human Prostate Cancer Cells via a Mitochondria-Related Caspase Pathway. Food Chem. Toxicol. 2011, 49, 2517–2523. [Google Scholar] [PubMed]
- Choi, Y.J.; Kim, D.H.; Kim, S.J.; Kim, J.; Jeong, S.-I.; Chung, C.H.; Yu, K.-Y.; Kim, S.-Y. Decursin Attenuates Hepatic Fibrogenesis through Interrupting TGF-Beta-Mediated NAD (P) H Oxidase Activation and Smad Signaling in Vivo and in Vitro. Life Sci. 2014, 108, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Jin, W.; Ho, N.A.; Hong, J.; Kim, Y.J.; Shin, Y.; Lee, H.; Suh, J.-W. Decursin and Decursinol Angelate Improve Wound Healing by Upregulating Transcription of Genes Encoding Extracellular Matrix Remodeling Proteins, Inflammatory Cytokines, and Growth Factors in Human Keratinocytes. Biochem. Biophys. Res. Commun. 2018, 499, 979–984. [Google Scholar] [CrossRef]
- Kim, J.H.; Jeong, S.-J.; Kwon, H.-Y.; Park, S.Y.; Lee, H.-J.; Lee, H.-J.; Lieske, J.C.; Kim, S.-H. Decursin Prevents Cisplatin-Induced Apoptosis via the Enhancement of Antioxidant Enzymes in Human Renal Epithelial Cells. Biol. Pharm. Bull. 2010, 33, 1279–1284. [Google Scholar] [CrossRef]
- Kim, W.-J.; Lee, S.-J.; Choi, Y.D.; Moon, S.-K. Decursin Inhibits Growth of Human Bladder and Colon Cancer Cells via Apoptosis, G1-Phase Cell Cycle Arrest and Extracellular Signal-Regulated Kinase Activation. Int. J. Mol. Med. 2010, 25, 635–641. [Google Scholar]
- Taechowisan, T.; Chanaphat, S.; Ruensamran, W.; Phutdhawong, W.S. Antibacterial Activity of Decursin from Streptomyces Sp. GMT-8; an Endophyte in Zingiber Officinale Rosc. J. Appl. Pharm. Sci. 2013, 3, 74–78. [Google Scholar]
- Sharifi-Rad, M.; Lankatillake, C.; Dias, D.A.; Docea, A.O.; Mahomoodally, M.F.; Lobine, D.; Chazot, P.L.; Kurt, B.; Boyunegmez Tumer, T.; Catarina Moreira, A. Impact of Natural Compounds on Neurodegenerative Disorders: From Preclinical to Pharmacotherapeutics. J. Clin. Med. 2020, 9, 1061. [Google Scholar] [CrossRef]
- Chari, R.V.J. Targeted Cancer Therapy: Conferring Specificity to Cytotoxic Drugs. Acc. Chem. Res. 2008, 41, 98–107. [Google Scholar] [CrossRef]
- Setiawati, A.; Candrasari, D.S.; Setyajati, F.D.E.; Prasetyo, V.K.; Setyaningsih, D.; Hartini, Y.S. Anticancer Drug Screening of Natural Products: In Vitro: Cytotoxicity Assays, Techniques, and Challenges. Asian Pac. J. Trop. Biomed. 2022, 12, 279–289. [Google Scholar] [CrossRef]
- Ahmed, M.B.; Islam, S.U.; Lee, Y.S. Decursin Negatively Regulates LPS-Induced Upregulation of the TLR4 and JNK Signaling Stimulated by the Expression of PRP4 in Vitro. Animal Cells Syst. 2020, 24, 44–52. [Google Scholar] [CrossRef]
- Kim, B.S.; Seo, H.; Kim, H.-J.; Bae, S.M.; Son, H.-N.; Lee, Y.J.; Ryu, S.; Park, R.-W.; Nam, J.-O. Decursin from Angelica Gigas Nakai Inhibits B16F10 Melanoma Growth through Induction of Apoptosis. J. Med. Food 2015, 18, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Ki, D.; Go, S.H.; Song, Y.; Lee, D.K.; Park, J.R. Decursin Induces G1 Cell Cycle Arrest and Apoptosis through Reactive Oxygen Species-Mediated Endoplasmic Reticulum Stress in Human Colorectal Cancer Cells in In Vitro and Xenograft Models. Int. J. Mol. Sci. 2024, 25, 9939. [Google Scholar]
- Kweon, B.; Han, Y.-H.; Kee, J.-Y.; Mun, J.-G.; Jeon, H.D.; Yoon, D.H.; Choi, B.-M.; Hong, S.-H. Effect of Angelica Gigas Nakai Ethanol Extract and Decursin on Human Pancreatic Cancer Cells. Molecules 2020, 25, 2028. [Google Scholar] [CrossRef]
- Choi, H.S.; Cho, S.; Kim, M.K.; Kim, M.S.; Moon, S.H.; Kim, I.H.; Ko, S. Decursin in Angelica Gigas Nakai (AGN) Enhances Doxorubicin Chemosensitivity in NCI/ADR-RES Ovarian Cancer Cells via Inhibition of P-glycoprotein Expression. Phyther. Res. 2016, 30, 2020–2026. [Google Scholar] [CrossRef]
- Kim, J.-M.; Noh, E.-M.; Kim, M.-S.; Hwang, J.-K.; Hwang, H.-Y.; Ryu, D.-G.; Kim, H.-J.; Yu, H.-N.; You, Y.-O.; Kim, J.-S. Decursin Prevents TPA-Induced Invasion through Suppression of PKCα/P38/NF-ΚB-Dependent MMP-9 Expression in MCF-7 Human Breast Carcinoma Cells. Int. J. Oncol. 2014, 44, 1607–1613. [Google Scholar] [CrossRef]
- Kim, J.; Jung, J.H.; Kim, S.; Jeong, S. Decursin Exerts Anti-cancer Activity in MDA-MB-231 Breast Cancer Cells via Inhibition of the Pin1 Activity and Enhancement of the Pin1/P53 Association. Phyther. Res. 2014, 28, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Son, S.H.; Park, K.; Park, S.K.; Kim, Y.C.; Kim, Y.S.; Lee, S.; Chung, W. Decursin and Decursinol from Angelica Gigas Inhibit the Lung Metastasis of Murine Colon Carcinoma. Phyther. Res. 2011, 25, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Hu, Y.-E.; Zhao, M.-Y.; Jiang, Y.-F.; Fu, X.; You, F.-M. Decursin Affects Proliferation, Apoptosis, and Migration of Colorectal Cancer Cells through PI3K/Akt Signaling Pathway. Zhongguo Zhong yao za zhi= Zhongguo Zhongyao Zazhi = China J. Chinese Mater. Medica 2023, 48, 2334–2342. [Google Scholar]
- Kim, H.J.; Kim, S.M.; Park, K.R.; Jang, H.J.; Na, Y.S.; Ahn, K.S.; Kim, S.H.; Ahn, K.S. Decursin chemosensitizes human multiple myeloma cells through inhibition of STAT3 signaling pathway. Cancer Lett. 2011, 301, 29–37. [Google Scholar] [CrossRef]
- Ge, Y.; Yoon, S.-H.; Jang, H.; Jeong, J.-H.; Lee, Y.-M. Decursin promotes HIF-1α proteasomal degradation and immune responses in hypoxic tumour microenvironment. Phytomedicine 2020, 78, 153318. [Google Scholar] [CrossRef]
- Oh, S.T.; Lee, S.; Hua, C.; Koo, B.-S.; Pak, S.C.; Kim, D.-I.; Jeon, S.; Shin, B.A. Decursin Induces Apoptosis in Glioblastoma Cells, but Not in Glial Cells via a Mitochondria-Related Caspase Pathway. Korean J. Physiol. Pharmacol. 2019, 23, 29–35. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, H.J.; Lee, E.O.; Lee, J.H.; Lee, K.S.; Kim, K.H.; Kim, S.-H.; Lü, J. In Vivo Anti-Cancer Activity of Korean Angelica Gigas and Its Major Pyranocoumarin Decursin. Am. J. Chin. Med. 2009, 37, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Bhat, T.A.; Dheeraj, A.; Nambiar, D.K.; Singh, S.P.; Yim, D.S.; Singh, R.P. Decursin Inhibits EGFR-ERK1/2 Signaling Axis in Advanced Human Prostate Carcinoma Cells. Prostate 2023, 83, 534–546. [Google Scholar] [CrossRef]
- Song, G.-Y.; Lee, J.-H.; Cho, M.; Park, B.-S.; Kim, D.-E.; Oh, S.J. Decursin suppresses human androgen-independent PC3 prostate cancer cell proliferation by promoting the degradation of β-catenin. Mol. Pharmacol. 2007, 72, 1599–1606. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.-E.; Kim, N.; Joo, M.; Lee, M.-W.; Jeon, H.J.; Ryu, H.; Song, I.-C.; Song, G.-Y.; Lee, H.J.J. Decursin inhibits tumor growth, migration, and invasion in gastric cancer by down-regulating CXCR7 expression. Am. J. Cancer Res. 2019, 9, 2007. [Google Scholar]
- Joo, M.; Heo, J.B.; Kim, S.; Kim, N.; Jeon, H.J.; An, Y.; Song, G.-Y.; Kim, J.-M.; Lee, H.J. Decursin Inhibits Tumor Progression in Head and Neck Squamous Cell Carcinoma by Downregulating CXCR7 Expression in Vitro. Oncol. Rep. 2022, 47, 1–11. [Google Scholar]
- Kim, H.H.; Ahn, K.S.; Han, H.; Choung, S.Y.; Choi, S.-Y.; Kim, I.-H.J. Decursin and PDBu: Two PKC activators distinctively acting in the megakaryocytic differentiation of K562 human erythroleukemia cells. Leuk. Res. 2005, 29, 1407–1413. [Google Scholar] [CrossRef]
- Son, S.H.; Kim, M.-J.; Chung, W.-Y.; Son, J.-A.; Kim, Y.S.; Kim, Y.-C.; Kang, S.S.; Lee, S.-K.; Park, K.-K. Decursin and Decursinol Inhibit VEGF-Induced Angiogenesis by Blocking the Activation of Extracellular Signal-Regulated Kinase and c-Jun N-Terminal Kinase. Cancer Lett. 2009, 280, 86–92. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Wang, L.; Tan, R.; Zhu, M.; Zhong, X.; Zhang, Y.; Chen, B.; Wang, L. Decursin Inhibits the Growth of HepG2 Hepatocellular Carcinoma Cells via Hippo/YAP Signaling Pathway. Phyther. Res. 2018, 32, 2456–2465. [Google Scholar] [CrossRef]
- Ahn, Q.; Jeong, S.-J.; Lee, H.-J.; Kwon, H.-Y.; Han, I.; Kim, H.S.; Lee, H.-J.; Lee, E.-O.; Ahn, K.S.; Jung, M.-H.J.C.l. Inhibition of cyclooxygenase-2-dependent survivin mediates decursin-induced apoptosis in human KBM-5 myeloid leukemia cells. Cancer Lett. 2010, 298, 212–221. [Google Scholar] [CrossRef]
- Yam, C.H.; Fung, T.K.; Poon, R.Y.C. Cyclin A in Cell Cycle Control and Cancer. Cell. Mol. Life Sci. CMLS 2002, 59, 1317–1326. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2022, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.H.; Stoeber, K. The Cell Cycle and Cancer. J. Pathol. 2012, 226, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Bhuia, M.S.; Chowdhury, R.; Sonia, F.A.; Kamli, H.; Shaikh, A.; El-Nashar, H.A.S.; El-Shazly, M.; Islam, M.T. Anticancer Potential of the Plant-Derived Saponin Gracillin: A Comprehensive Review of Mechanistic Approaches. Chem. Biodivers. 2023, 20, e202300847. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; Harper, J.W. Anticancer Drug Targets: Cell Cycle and Checkpoint Control. J. Clin. Investig. 1999, 104, 1645–1653. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Wilairatana, P.; Ferdous, J.; Chowdhury, R.; Bappi, M.H.; Rahman, M.A.; Mubarak, M.S.; Islam, M.T. Hirsutine, an Emerging Natural Product with Promising Therapeutic Benefits: A Systematic Review. Molecules 2023, 28, 6141. [Google Scholar] [CrossRef]
- Bhuia, M.S.; Chowdhury, R.; Ara, I.; Mamun, M.; Rouf, R.; Khan, M.A.; Uddin, S.J.; Shakil, M.A.K.; Habtemariam, S.; Ferdous, J. Bioactivities of Morroniside: A Comprehensive Review of Pharmacological Properties and Molecular Mechanisms. Fitoterapia 2024, 175, 105896. [Google Scholar] [CrossRef]
- Fischer, U.; Schulze-Osthoff, K. Apoptosis-Based Therapies and Drug Targets. Cell Death Differ. 2005, 12, 942–961. [Google Scholar] [CrossRef]
- Los, M.; Burek, C.J.; Stroh, C.; Benedyk, K.; Hug, H.; Mackiewicz, A. Anticancer Drugs of Tomorrow: Apoptotic Pathways as Targets for Drug Design. Drug Discov. Today 2003, 8, 67–77. [Google Scholar] [CrossRef]
- Kvansakul, M.; Hinds, M.G.J.A. The Bcl-2 family: Structures, interactions and targets for drug discovery. Apoptosis 2015, 20, 136–150. [Google Scholar] [CrossRef]
- Shi, Y. Mechanical Aspects of Apoptosome Assembly. Curr. Opin. Cell Biol. 2006, 18, 677–684. [Google Scholar] [CrossRef]
- Thushara, R.; Hemshekhar, M.; Santhosh, M.; Devaraja, S.; Kemparaju, K.; Girish, K.J. Differential action of phytochemicals on platelet apoptosis: A biological overview. Curr. Med. Chem. 2013, 20, 1018–1027. [Google Scholar] [PubMed]
- Moyer, A.; Tanaka, K.; Cheng, E.H. Apoptosis in Cancer Biology and Therapy. Annu. Rev. Pathol. 2025, 20, 303–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.-I.; Kim, N.; Joo, M.; Lee, K.-H.; Lee, M.-W.; Jeon, H.J.; Ryu, H.; Kim, J.-M.; Sul, J.-Y. Decursin Inhibits Cell Growth and Autophagic Flux in Gastric Cancer via Suppression of Cathepsin C. Am. J. Cancer Res. 2021, 11, 1304. [Google Scholar] [PubMed]
- Bhuia, M.S.; Wilairatana, P.; Chowdhury, R.; Rakib, A.I.; Kamli, H.; Shaikh, A.; Coutinho, H.D.M.; Islam, M.T. Anticancer Potentials of the Lignan Magnolin: A Systematic Review. Molecules 2023, 28, 3671. [Google Scholar] [CrossRef]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Duronio, R.J.; Xiong, Y. Signaling Pathways That Control Cell Proliferation. Cold Spring Harb. Perspect. Biol. 2013, 5, a008904. [Google Scholar] [CrossRef]
- Gu, Y.; Mohammad, I.S.; Liu, Z. Overview of the STAT-3 Signaling Pathway in Cancer and the Development of Specific Inhibitors. Oncol. Lett. 2020, 19. [Google Scholar] [CrossRef]
- Bhat, P.; Kriel, J.; Shubha Priya, B.; Basappa; Shivananju, N.S.; Loos, B. Modulating autophagy in cancer therapy: Advancements and challenges for cancer cell death sensitization. Biochem. Pharmacol. 2018, 147, 170–182. [Google Scholar] [CrossRef]
- Page, C.; Huang, M.; Jin, X.; Cho, K.; Lilja, J.; Reynolds, R.K.; Lin, J. Elevated Phosphorylation of AKT and Stat3 in Prostate, Breast, and Cervical Cancer Cells. Int. J. Oncol. 2000, 17, 23–31. [Google Scholar] [CrossRef]
- Bendris, N.; Lemmers, B.; Blanchard, J.M. Cell cycle, cytoskeleton dynamics and beyond: The many functions of cyclins and CDK inhibitors. Cell Cycle 2015, 14, 1786–1798. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, S.; DuPree, E.L.; Almasan, A. A Dual Role of Cyclin E in Cell Proliferation and Apotosis May Provide a Target for Cancer Therapy. Curr. Cancer Drug Targets 2004, 4, 65–75. [Google Scholar] [CrossRef][Green Version]
- Krakhmal, N.V.; Zavyalova, M.V.; Denisov, E.V.; Vtorushin, S.V.; Perelmuter, V.M. Cancer Invasion: Patterns and Mechanisms. Acta Naturae (aнглoязычнaя версия) 2015, 7, 17–28. [Google Scholar] [CrossRef]
- Apoptosis Lintz, M.; Muñoz, A.; Reinhart-King, C.A. The Mechanics of Single Cell and Collective Migration of Tumor Cells. J. Biomech. Eng. 2017, 139, 21005. [Google Scholar]
- Qi, X.; Sun, L.; Wan, J.; Xu, R.; He, S.; Zhu, X. Tensin4 Promotes Invasion and Migration of Gastric Cancer Cells via Regulating AKT/GSK-3β/Snail Signaling Pathway. Pathol. Pract. 2020, 216, 153001. [Google Scholar] [CrossRef]
- Zheng, H.; Takahashi, H.; Murai, Y.; Cui, Z.; Nomoto, K.; Niwa, H.; Tsuneyama, K.; Takano, Y. Expressions of MMP-2, MMP-9 and VEGF Are Closely Linked to Growth, Invasion, Metastasis and Angiogenesis of Gastric Carcinoma. Anticancer. Res. 2006, 26, 3579–3583. [Google Scholar] [PubMed]
- Cao, Z.-Q.; Wang, Z.; Leng, P. Aberrant N-Cadherin Expression in Cancer. Biomed. Pharmacother. 2019, 118, 109320. [Google Scholar] [CrossRef]
- Suciu, C.; Cîmpean, A.M.; Muresan, A.M.; Izvernariu, D.; Raica, M. E-Cadherin Expression in Invasive Breast Cancer. Rom. J. Morphol. Embryol. 2008, 49, 517–523. [Google Scholar]
- Shukla, S.; MacLennan, G.T.; Hartman, D.J.; Fu, P.; Resnick, M.I.; Gupta, S. Activation of PI3K-Akt Signaling Pathway Promotes Prostate Cancer Cell Invasion. Int. J. Cancer 2007, 121, 1424–1432. [Google Scholar] [CrossRef]
- Zhao, Y.; Adjei, A.A. Targeting Angiogenesis in Cancer Therapy: Moving beyond Vascular Endothelial Growth Factor. Oncologist 2015, 20, 660–673. [Google Scholar] [CrossRef]
- Kieran, M.W.; Kalluri, R.; Cho, Y.-J. The VEGF Pathway in Cancer and Disease: Responses, Resistance, and the Path Forward. Cold Spring Harb. Perspect. Med. 2012, 2, a006593. [Google Scholar] [CrossRef] [PubMed]
- Abhinand, C.S.; Raju, R.; Soumya, S.J.; Arya, P.S.; Sudhakaran, P.R. VEGF-A/VEGFR2 Signaling Network in Endothelial Cells Relevant to Angiogenesis. J. Cell Commun. Signal. 2016, 10, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Cheng, Y.; Liu, Q.; Bao, J.; Yang, J.-M. Autophagic Pathways as New Targets for Cancer Drug Development. Acta Pharmacol. Sin. 2010, 31, 1154–1164. [Google Scholar] [CrossRef]
- Tanida, I.; Ueno, T.; Kominami, E. LC3 and Autophagy. Autophagosome Phagosome 2008, 445, 77–88. [Google Scholar] [CrossRef]
- Carew, J.S.; Kelly, K.R.; Nawrocki, S.T. Autophagy as a Target for Cancer Therapy: New Developments. Cancer Manag. Res. 2012, 4, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; He, S.; Ma, B. Autophagy and Autophagy-Related Proteins in Cancer. Mol. Cancer 2020, 19, 12. [Google Scholar] [CrossRef]
- Sharma, P.; Patel, N.; Prasad, B.; Varma, M.V.S. Pharmacokinetics: Theory and Application in Drug Discovery and Development. In Drug Discovery and Development; Poduri, R., Ed.; Springer: Singapore, 2021; pp. 297–355. [Google Scholar] [CrossRef]
- Lucas, A.J.; Sproston, J.L.; Barton, P.; Riley, R.J. Estimating Human ADME Properties, Pharmacokinetic Parameters and Likely Clinical Dose in Drug Discovery. Expert. Opin. Drug Discov. 2019, 14, 1313–1327. [Google Scholar] [CrossRef]
- Hasan, R.; Alshammari, A.; Albekairi, N.A.; Bhuia, M.S.; Afroz, M.; Chowdhury, R.; Khan, M.A.; Ansari, S.A.; Ansari, I.A.; Mubarak, M.S. Antiemetic Activity of Abietic Acid Possibly through the 5HT3 and Muscarinic Receptors Interaction Pathways. Sci. Rep. 2024, 14, 6642. [Google Scholar] [CrossRef]
- Ferdous, J.; Bhuia, M.S.; Chowdhury, R.; Rakib, A.I.; Aktar, M.A.; Al Hasan, M.S.; Melo Coutinho, H.D.; Islam, M.T. Pharmacological Activities of Plant-derived Fraxin with Molecular Mechanisms: A Comprehensive Review. Chem. Biodivers. 2024, 21, e202301615. [Google Scholar] [CrossRef]
- Wu, F.; Zhou, Y.; Li, L.; Shen, X.; Chen, G.; Wang, X.; Liang, X.; Tan, M.; Huang, Z. Computational Approaches in Preclinical Studies on Drug Discovery and Development. Front. Chem. 2020, 8, 726. [Google Scholar] [CrossRef]
- Luo, Z.; Yu, G.; Han, X.; Yang, T.; Ji, Y.; Huang, H.; Wang, G.; Liu, Y.; Sun, W. Prediction of the Pharmacokinetics and Pharmacodynamics of Topiroxostat in Humans by Integrating the Physiologically Based Pharmacokinetic Model with the Drug-Target Residence Time Model. Biomed. Pharmacother. 2020, 121, 109660. [Google Scholar] [CrossRef]
- Chowdhury, R.; Bhuia, M.S.; Rakib, A.I.; Hasan, R.; Coutinho, H.D.M.; Araújo, I.M.; de Menezes, I.R.A.; Islam, M.T. Assessment of Quercetin Antiemetic Properties: In Vivo and in Silico Investigations on Receptor Binding Affinity and Synergistic Effects. Plants 2023, 12, 4189. [Google Scholar] [CrossRef]
- Gallo, J.M.; Vicini, P.; Orlansky, A.; Li, S.; Zhou, F.; Ma, J.; Pulfer, S.; Bookman, M.A.; Guo, P. Pharmacokinetic Model-Predicted Anticancer Drug Concentrations in Human Tumors. Clin. Cancer Res. 2004, 10, 8048–8058. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Talmadge, J.E.; Singh, R.K.; Fidler, I.J.; Raz, A. Murine models to evaluate novel and conventional therapeutic strategies for cancer. Am. J. Pathol. 2007, 170, 793–804. [Google Scholar] [CrossRef] [PubMed]
- Mahat, B.; Chae, J.; Baek, I.; Song, G.; Song, J.; Cho, S.; Kwon, K. Physicochemical Characterization and Toxicity of Decursin and Their Derivatives from Angelica Gigas. Biol. Pharm. Bull. 2012, 35, 1084–1090. [Google Scholar] [CrossRef]
- Park, Y.; Park, P.S.; Jeong, D.H.; Sim, S.; Kim, N.; Park, H.; Jeon, K.S.; Um, Y.; Kim, M.-J. The Characteristics of the Growth and the Active Compounds of Angelica Gigas Nakai in Cultivation Sites. Plants 2020, 9, 823. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhang, J.; Xing, C.; Kim, S.-H.; Lü, J. Single Oral Dose Pharmacokinetics of Decursin, Decursinol Angelate, and Decursinol in Rats. Planta Med. 2013, 79, 275–280. [Google Scholar] [CrossRef]
- Mahat, B.; Chae, J.; Baek, I.; Song, G.; Song, J.; Ma, J.; Kwon, K. Biopharmaceutical Characterization of Decursin and Their Derivatives for Drug Discovery. Drug Dev. Ind. Pharm. 2013, 39, 1523–1530. [Google Scholar] [CrossRef]
- Kim, S.-J.; Ko, S.-M.; Choi, E.-J.; Ham, S.-H.; Kwon, Y.-D.; Lee, Y.-B.; Cho, H.-Y. Simultaneous Determination of Decursin, Decursinol Angelate, Nodakenin, and Decursinol of Angelica Gigas Nakai in Human Plasma by UHPLC-MS/MS: Application to Pharmacokinetic Study. Molecules 2018, 23, 1019. [Google Scholar] [CrossRef]
- Choi, H.; Yoon, J.-H.; Youn, K.; Jun, M. Decursin Prevents Melanogenesis by Suppressing MITF Expression through the Regulation of PKA/CREB, MAPKs, and PI3K/Akt/GSK-3β Cascades. Biomed. Pharmacother. 2022, 147, 112651. [Google Scholar] [CrossRef]
- Park, H.S.; Kim, B.; Oh, J.-H.; Kim, Y.C.; Lee, Y.-J. First-Pass Metabolism of Decursin, a Bioactive Compound of Angelica Gigas, in Rats. Planta Med. 2012, 78, 909–913. [Google Scholar] [CrossRef]
- Zhang, J.; Li, L.; Hale, T.W.; Chee, W.; Xing, C.; Jiang, C.; Lü, J. Single Oral Dose Pharmacokinetics of Decursin and Decursinol Angelate in Healthy Adult Men and Women. PLoS ONE 2015, 10, e0114992. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-M.; Kim, T.-H.; Park, Y.-J.; Kim, I.-H.; Kang, J.-S. Evaluation of the genotoxicity of decursin and decursinol angelate produced by Angelica gigas Nakai. J. Mol. Cell Toxicol. 2009, 5, 83–87. [Google Scholar]
- Muralikrishnan, A.; Sekar, M.; Kumarasamy, V.; Gan, S.H.; Ravi, S.; Subramaniyan, V.; Wong, L.S.; Wu, Y.S.; Khattulanuar, F.S.; Mat Rani, N.N.I. Chemistry, Pharmacology and Therapeutic Potential of Decursin: A Promising Natural Lead for New Drug Discovery and Development. Dru Des. Dev. Therap. 2024, 18, 3741–3763. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Yuan, Q.; Jiang, H.; Wu, L.; Xie, Y.; Zhang, X.; Li, L. A comprehensive review of the anticancer effects of decursin. Front. Pharmacol. 2024, 15, 1303412. [Google Scholar] [CrossRef]
- Hwang, J.T.; Kim, S.H.; Hur, H.J.; Kim, H.J.; Park, J.H.; Sung, M.J.; Yang, H.J.; Ryu, S.Y.; Kim, Y.S.; Cha, M.R.; et al. Decursin, an active compound isolated from Angelica gigas, inhibits fat accumulation, reduces adipocytokine secretion and improves glucose tolerance in mice fed a high-fat diet. Phytotherap Res. 2012, 26, 633–638. [Google Scholar] [CrossRef]
- Li, L.; Zhang, J.; Shaik, A.A.; Zhang, Y.; Wang, L.; Xing, C.; Kim, S.H.; Lü, J. Quantitative determination of decursin, decursinol angelate, and decursinol in mouse plasma and tumor tissue using liquid-liquid extraction and HPLC. Planta Med. 2012, 78, 252–259. [Google Scholar] [CrossRef]
| Cancer Type | Test System | Dose/ Concentration/(R/A) | IC50 | Results/Possible Mechanism | References |
|---|---|---|---|---|---|
| Breast cancer | MCF-7 cells, in vitro | 1–50 µM | - | ↓ TPA-induce MMP-9, cell invasion, ↓ NF-κB, PKCα, MAPK | [49] |
| MDA-MB-231, MDA-MB-453, MDAMB-157, MCF-7, and MCF-10A cells, in vitro | 80 µM | - | ↑ G1 arrest, cell cycle arrest, p53 protein, ↓ Cyclin D1 level, protein expression, Pin1 | [50] | |
| Bladder and colon cancer | Human urinary bladder cancer 235J cells and colon cancer HCT116 cells, in vitro | 50 and 100 µM | - | ↑ Cytotoxicity, apoptosis, cytochrome c, caspase -3 and Bax, protein levels of p21waf1, cytoplasmic DNA-histone complex, ERK, ↓ Bcl-2, cyclin D1, cyclin E, CDK-2,4, cell cycle, cell growth, proliferation | [38] |
| Colorectal cancer | CT-26 colon carcinoma cells, in vitro | 10–20 µM | - | ↓ Proliferation, invasion, MMP-2 and MMP-9, formation of tumor nodules, ERK, JNK, growth of cancer cells, ↑ lung weight | [51] |
| HT29 and HCT116, in vitro | 10–90 μmol/L | - | ↑ Apoptosis, Bax, expression of E-cadherin, p53, EMT ↓ Colony number, cell proliferation, Bcl-2, wound healing, expression of N-cadherin, vimentin, expression of PI3K, Akt | [52] | |
| Skin cancer | U266, MM.1S, and ARH77 cells, in vitro | 40–160µM | - | ↑ Apoptosis, caspase-3, -8, and -9, tumor growth, cytotoxicity, cleavage of procaspase-8, procaspase-9 ↓ Cyclin D1, bcl-2, Bcl-xL, survivin, VEGF, STAT3, JAK2, interleukin-6, angiogenesis | [53] |
| B16F10 and NIH-3T3 cells, in vitro | 20–100 µM | - | ↑ Apoptosis, cytotoxicity, Bax, phosphorylation of p38, caspase-3, ↓ Proliferation, ERK, Bcl-2, tumor growth, tumor weight | [45] | |
| In vivo tumor and histological assay in male C57BL/6J mice, n = 6 | 10 mg/kg (i.p.) | ||||
| (B16-F10) cells, in vitro studies | 10 µM | - | ↓ IL-6, IL-1β, TLR4, and NF-κB, Akt, JNK, ERK, and p-ERK | [44] | |
| Lung cancer | HEK-293 cells, A549 cancer cells, in vitro studies | 10–50 µM | - | ↑ Apoptosis, oxygen-dependant hydroxylation and ubiquitination, ↓ HIF-1, HIF-1α and PD-L1, mRNA expression, proliferation, invasion | [54] |
| C57BL/6 mice, in vivo studies (n = 5) | 10 mg/kg (i.p.) | ↓ Tumor growth | |||
| Glioblastoma cancer | U87 cells, glioblastoma cells, in vitro | 10–200 µM | 49.01 µM | ↑ Apoptosis, apoptotic bodies, phosphorylated JNK, p38, caspase-3, -7, and -9, sub-G1 DNA population, PARP-1 ↓ Bcl-2, CDK-4 cyclin D1 | [55] |
| Ovarian cancer | Ovarian cancer cells, in vitro studies | 5–50 µg/mL | 23 μg/mL, 8 μg/mL | ↑ Caspase-3, -8 and -9, cleaved PARP level, apoptosis ↓ Proliferation of NCI/ADR-RES, P-glycoprotein expression | [48] |
| Prostate cancer | Human DU145, PC-3 prostate cancer and LLC cell lines, in vivo | 30–100 mg/kg (i.p and p.o.) | - | ↑ Apoptosis ↓ Tumor volume, cell proliferation, angiogenesis | [56] |
| Mouse LLC allograft tumor and human PC-3 and DU145 xenograft models in E right flank of C57BL/6 mice, in vivo (n = 5–16) | |||||
| Pancreatic cancer | Pancreatic ductal adenocarcinoma cells (PANC-1 and MIA PaCa-2), in vitro | 20–60 µM | - | ↑ Cytotoxicity, G0/G1 phase arrest, apoptosis, caspase-3, poly (ADP-ribose) polymerase (PARP) cleavage ↓ Colony formation, cyclin D1, CDK4, p38 phosphorylation, Proliferation, MMP-2, MMP-9 | [47] |
| Prostate and lung cancer | DU145 and 22Rv1 cells, in vitro studies | 25–100 µM | - | ↑ Cell cycle arrest, apoptosis, p107 and p130, growth inhibition, cell death, ↓ CDK-2 and CDK-4, EGFR, ERK1/2, cell proliferation, number of surviving colonies, E2F-3, E2F-4 and E2F-5, growth of androgen, EGF ligand | [57] |
| PC-3 cells, in vitro | 50–200 µM | - | ↓ Expression of cyclin D1 and c-myc, beta-catenin, growth of PC3, Wnt/β-catenin pathway | [58] | |
| DU145, PC-3, and LNCaP cells, in vitro | 25–100 µmol/L | - | ↑ Binding of CDK inhibitor (CDKI) with CDK, apoptosis, caspase-3, -9, cell death, a Strong G1 Arrest, protein levels of Cip1/p21, Kip1/p27 Levels, ↓ CDK2,4,6, cyclin D1, and cyclin E, Cell growth, | [34] | |
| Gastric cancer | In vitro and vivo studies human gastric cancer cell lines-SNU484 and SNU216 | 20–40 µM | - | ↓ Cell growth, migration, invasion, survival, cell proliferation, expression of CXCR7, Bcl-2, c-Myc expression | [59] |
| Head and neck cancer | HNSCC cell line, in vitro studies | 50–100 µM | - | ↑ Stress fiber formation, G0/G1 cell cycle arrest, ↓ CXCR7 expression, cell proliferation, migration, invasion, cyclin A, cyclin E, and CDK2, cell growth, S phase and G2/M phase, cell motility, STAT-3 phosphorylation, c-MYC expression | [60] |
| Blood and bone marrow cancer | K562 cells, in vitro | 50 µM | - | ↓ Bleb formation, megakaryocytic differentiation, translocation of PKCα and βII, binding of PDBu to PKC, PDBu, | [61] |
| - | HUVEC and mice, in vitro | 1–20 µM | - | ↓ Proliferation, migration, capillary-tube formation, microvessel formation, P-ERK, p-JNK, angiogenesis | [62] |
| HepG2 cells In vitro studies | 5–80 µM | - | ↑ LATS1 and βTRCP, apoptosis ↓ Growth of HepG2 cells, cell proliferation, cell cycle | [63] | |
| Leukemia | leukemic KBM-5 cells, in vitro studies | 10–80 µM | - | ↑ Apoptosis, ↓ COX-2, surviving. | [64] |
| Gastric cancer | SNU216, NCI-N87 cells, in vitro | - | - | ↑ Cell cycle arrest, LC3-II levels, DNA synthesis ↓ Cell growth, autophagic flux, the expression of lysosomal protein cathepsin C (CTSC), cell proliferation, E2F3, growth of spheroids, patient-derived gastric organoids, autophagy, CDK4/6, | [39] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eity, T.A.; Bhuia, M.S.; Chowdhury, R.; Ali, M.A.; Khatun, M.M.; Sheikh, S.; Al Hasan, M.S.; Hasan, R.; Neto, I.C.P.; Araújo, I.M.; et al. Anticancer Efficacy of Decursin: A Comprehensive Review with Mechanistic Insights. Future Pharmacol. 2025, 5, 17. https://doi.org/10.3390/futurepharmacol5020017
Eity TA, Bhuia MS, Chowdhury R, Ali MA, Khatun MM, Sheikh S, Al Hasan MS, Hasan R, Neto ICP, Araújo IM, et al. Anticancer Efficacy of Decursin: A Comprehensive Review with Mechanistic Insights. Future Pharmacology. 2025; 5(2):17. https://doi.org/10.3390/futurepharmacol5020017
Chicago/Turabian StyleEity, Tanzila Akter, Md. Shimul Bhuia, Raihan Chowdhury, Md. Arman Ali, Mst Muslima Khatun, Salehin Sheikh, Md. Sakib Al Hasan, Rubel Hasan, Ivo Cavalcante Pita Neto, Isaac Moura Araújo, and et al. 2025. "Anticancer Efficacy of Decursin: A Comprehensive Review with Mechanistic Insights" Future Pharmacology 5, no. 2: 17. https://doi.org/10.3390/futurepharmacol5020017
APA StyleEity, T. A., Bhuia, M. S., Chowdhury, R., Ali, M. A., Khatun, M. M., Sheikh, S., Al Hasan, M. S., Hasan, R., Neto, I. C. P., Araújo, I. M., Coutinho, H. D. M., & Islam, M. T. (2025). Anticancer Efficacy of Decursin: A Comprehensive Review with Mechanistic Insights. Future Pharmacology, 5(2), 17. https://doi.org/10.3390/futurepharmacol5020017