Prospect of (Nd3+) Complexes and Its Nanoparticles as Promising Novel Anticancer Agents in Particular Targeting Breast Cancer Cell Lines
Abstract
1. Introduction
2. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marques, C. Cancer: Lessons to learn from the past. In Bone Sarcomas and Bone Metastases-From Bench to Bedside; Elsevier: Amsterdam, The Netherlands, 2022; pp. 5–15. [Google Scholar]
- Tarin, D. Causes of cancer and mechanisms of carcinogenesis. In Understanding Cancer: The Molecular Mechanisms, Biology, Pathology and Clinical Implications of Malignant Neoplasia; Springer: Berlin/Heidelberg, Germany, 2023; pp. 229–279. [Google Scholar]
- Justiz-Vaillant, A.; Gardiner, L.; Mohammed, M.; Surajbally, M.; Maharaj, L.; Ramsingh, L.; Simon, M.; Seegobin, M.; Niles, M.; Vuma, S. Narrative Literature Review on Risk Factors Involved In Breast Cancer, Brain Cancer, Colon Rectal Cancer, Gynecological Malignancy, Lung Cancer, and Prostate Cancer. Preprints 2021. [Google Scholar] [CrossRef]
- Houghton, S.C.; Hankinson, S.E. Cancer progress and priorities: Breast cancer. Cancer Epidemiol. Biomark. Prev. 2021, 30, 822–844. [Google Scholar] [CrossRef] [PubMed]
- Swallah, M.S.; Bondzie-Quaye, P.; Yu, X.; Fetisoa, M.R.; Shao, C.S.; Huang, Q. Elucidating the protective mechanism of ganoderic acid DM on breast cancer based on network pharmacology and in vitro experimental validation. Biotechnol. Appl. Biochem. 2024, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Dong, X.; Li, H.; Cao, M.; Sun, D.; He, S.; Yang, F.; Yan, X.; Zhang, S.; Li, N. Cancer statistics in China and United States, 2022: Profiles, trends, and determinants. Chin. Med. J. 2022, 135, 584–590. [Google Scholar] [CrossRef]
- Pedersen, R.N.; Esen, B.Ö.; Mellemkjær, L.; Christiansen, P.; Ejlertsen, B.; Lash, T.L.; Nørgaard, M.; Cronin-Fenton, D. The incidence of breast cancer recurrence 10-32 years after primary diagnosis. JNCI J. Natl. Cancer Inst. 2022, 114, 391–399. [Google Scholar] [CrossRef]
- Meena, K.; Kumari, S.; Mishra, S.; Saini, M.; Chauhan, J.S. The Role of Genetics and Hormones in Women’s Health. In Women’s Health: A Comprehensive Guide to Common Health Issues in Women; Bentham Science Publishers: Sharjah, United Arab Emirates, 2024; pp. 74–100. [Google Scholar]
- Mahdavi, M.; Nassiri, M.; Kooshyar, M.M.; Vakili-Azghandi, M.; Avan, A.; Sandry, R.; Pillai, S.; Lam, A.K.y.; Gopalan, V. Hereditary breast cancer; Genetic penetrance and current status with BRCA. J. Cell. Physiol. 2019, 234, 5741–5750. [Google Scholar] [CrossRef]
- Makovec, T. Cisplatin and beyond: Molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol. Oncol. 2019, 53, 148–158. [Google Scholar] [CrossRef]
- Schmidbaur, H. Metallo-Drugs: Development and Action of Anticancer Agents; De Gruyter: Berlin, Germany, 2018. [Google Scholar]
- Garreffa, E.; Arora, D. Breast cancer in the elderly, in men and during pregnancy. Surgery 2024, 42, 918–925. [Google Scholar]
- Doostmohammadi, A.; Jooya, H.; Ghorbanian, K.; Gohari, S.; Dadashpour, M. Potentials and future perspectives of multi-target drugs in cancer treatment: The next generation anti-cancer agents. Cell Commun. Signal. 2024, 22, 228. [Google Scholar] [CrossRef]
- de Siqueira, L.R.P.; de Moraes Gomes, P.A.T.; de Lima Ferreira, L.P.; de Melo Rêgo, M.J.B.; Leite, A.C.L. Multi-target compounds acting in cancer progression: Focus on thiosemicarbazone, thiazole and thiazolidinone analogues. Eur. J. Med. Chem. 2019, 170, 237–260. [Google Scholar] [CrossRef]
- Bai, Y.; Aodeng, G.; Ga, L.; Hai, W.; Ai, J. Research Progress of Metal Anticancer Drugs. Pharmaceutics 2023, 15, 2750. [Google Scholar] [CrossRef] [PubMed]
- Leitão, M.I.P.; Morais, T.S. Tailored Metal-Based Catalysts: A New Platform for Targeted Anticancer Therapies. J. Med. Chem. 2024, 67, 16967–16990. [Google Scholar] [CrossRef] [PubMed]
- Dabrowiak, J.C. Metals in Medicine; John Wiley & Sons: Hoboken, NJ, USA, 2017. [Google Scholar]
- Abdolmaleki, S.; Aliabadi, A.; Khaksar, S. Riding the metal wave: A review of the latest developments in metal-based anticancer agents. Coord. Chem. Rev. 2024, 501, 215579. [Google Scholar] [CrossRef]
- Qader, S.M.; Muhammed, A.M.; Omer, R.A.; Abdulkareem, E.I.; Rashid, R.F. Potential of organometallic complexes in medicinal chemistry. Rev. Inorg. Chem. 2024. [Google Scholar] [CrossRef]
- Kostova, I. Lanthanides as anticancer agents. Curr. Med. Chem.-Anti-Cancer Agents 2005, 5, 591–602. [Google Scholar] [CrossRef]
- Presenjit; Chaturvedi, S.; Singh, A.; Gautam, D.; Singh, K.; Mishra, A.K. An insight into the Effect of Schiff Base and their d and f Block Metal complexes on various Cancer cell lines as Anticancer agents: A review. Anti-Cancer Agents Med. Chem.-Anti-Cancer Agents 2024, 24, 488–503. [Google Scholar] [CrossRef]
- Ngoepe, M.P.; Clayton, H.S. Metal complexes as DNA synthesis and/or repair inhibitors: Anticancer and antimicrobial agents. Pharm. Front. 2021, 3, e164–e182. [Google Scholar] [CrossRef]
- Terán, A.; Ferraro, G.; Imbimbo, P.; Sánchez-Peláez, A.E.; Monti, D.M.; Herrero, S.; Merlino, A. Steric hindrance and charge influence on the cytotoxic activity and protein binding properties of diruthenium complexes. Int. J. Biol. Macromol. 2023, 253, 126666. [Google Scholar] [CrossRef]
- Li, S.; Wang, X.X.; Li, M.; Wang, C.; Wang, F.; Zong, H.; Wang, B.; Lv, Z.; Song, N.; Liu, J. Extension of a biotic ligand model for predicting the toxicity of neodymium to wheat: The effects of pH, Ca2+ and Mg2+. Ecotoxicol. Environ. Saf. 2024, 271, 116013. [Google Scholar] [CrossRef]
- Abánades Lázaro, I.; Chen, X.; Ding, M.; Eskandari, A.; Fairen-Jimenez, D.; Giménez-Marqués, M.; Gref, R.; Lin, W.; Luo, T.; Forgan, R.S. Metal–organic frameworks for biological applications. Nat. Rev. Methods Primers 2024, 4, 42. [Google Scholar] [CrossRef]
- Elattar, R.H.; El-Malla, S.F.; Kamal, A.H.; Mansour, F.R. Applications of metal complexes in analytical chemistry: A review article. Coord. Chem. Rev. 2024, 501, 215568. [Google Scholar] [CrossRef]
- Xu, Y.; Luo, W.; Deng, H.; Hu, X.; Zhang, J.; Wang, Y. Robust antibacterial activity of rare-earth ions on planktonic and biofilm bacteria. Biomed. Mater. 2024, 19, 045014. [Google Scholar] [CrossRef]
- Ma, J.; Sun, R.; Xia, K.; Xia, Q.; Liu, Y.; Zhang, X. Design and application of fluorescent probes to detect cellular physical microenvironments. Chem. Rev. 2024, 124, 1738–1861. [Google Scholar] [CrossRef]
- Ahmad, J.; Wahab, R.; Siddiqui, M.A.; Farshori, N.N.; Saquib, Q.; Ahmad, N.; Al-Khedhairy, A.A. Neodymium oxide nanostructures and their cytotoxic evaluation in human cancer cells. J. Trace Elem. Med. Biol. 2022, 73, 127029. [Google Scholar] [CrossRef]
- Kaczmarek, M.T.; Zabiszak, M.; Nowak, M.; Jastrzab, R. Lanthanides: Schiff base complexes, applications in cancer diagnosis, therapy, and antibacterial activity. Coord. Chem. Rev. 2018, 370, 42–54. [Google Scholar] [CrossRef]
- Badria, F.A.; Soliman, S.M.; Atef, S.; Islam, M.S.; Al-Majid, A.M.; Dege, N.; Ghabbour, H.A.; Ali, M.; El-Senduny, F.F.; Barakat, A. Anticancer indole-based chalcones: A structural and theoretical analysis. Molecules 2019, 24, 3728. [Google Scholar] [CrossRef]
- Yan, S.; Na, J.; Liu, X.; Wu, P. Different Targeting Ligands-Mediated Drug Delivery Systems for Tumor Therapy. Pharmaceutics 2024, 16, 248. [Google Scholar] [CrossRef]
- Swamy, P.C.A.; Sivaraman, G.; Priyanka, R.N.; Raja, S.O.; Ponnuvel, K.; Shanmugpriya, J.; Gulyani, A. Near Infrared (NIR) absorbing dyes as promising photosensitizer for photo dynamic therapy. Coord. Chem. Rev. 2020, 411, 213233. [Google Scholar] [CrossRef]
- Parker, D.; Williams, J.G. Responsive luminescent lanthanide complexes. In Metal Ions in Biological Systems; Marcel Decker: New York, NY, USA, 2003; Volume 40, pp. 233–280. [Google Scholar]
- Somerville, R.J.; Odena, C.; Obst, M.F.; Hazari, N.; Hopmann, K.H.; Martin, R. Ni (I)–alkyl complexes bearing phenanthroline ligands: Experimental evidence for CO2 insertion at Ni (I) centers. J. Am. Chem. Soc. 2020, 142, 10936–10941. [Google Scholar] [CrossRef]
- Xie, J.; Lee, S.; Chen, X. Nanoparticle-based theranostic agents. Adv. Drug Deliv. Rev. 2010, 62, 1064–1079. [Google Scholar] [CrossRef]
- Mu, Q.; Wang, H.; Zhang, M. Nanoparticles for imaging and treatment of metastatic breast cancer. Expert Opin. Drug Deliv. 2017, 14, 123–136. [Google Scholar] [CrossRef]
- Akhter, S.; Ahmad, I.; Ahmad, M.Z.; Ramazani, F.; Singh, A.; Rahman, Z.; Ahmad, F.J.; Storm, G.; Kok, R.J. Nanomedicines as cancer therapeutics: Current status. Curr. Cancer Drug Targets 2013, 13, 362–378. [Google Scholar] [CrossRef]
- Maksimović, M.; Omanović-Mikličanin, E. Towards green nanotechnology: Maximizing benefits and minimizing harm. In Proceedings of the CMBEBIH 2017: Proceedings of the International Conference on Medical and Biological Engineering 2017, Sarajevo, Bosnia and Herzegovina, 16–18 March 2017; Springer: Singapore, 2017. [Google Scholar]
- Kahlon, S.K.; Sharma, G.; Julka, J.; Kumar, A.; Sharma, S.; Stadler, F.J. Impact of heavy metals and nanoparticles on aquatic biota. Environ. Chem. Lett. 2018, 16, 919–946. [Google Scholar] [CrossRef]
- Bour, A.; Mouchet, F.; Silvestre, J.; Gauthier, L.; Pinelli, E. Environmentally relevant approaches to assess nanoparticles ecotoxicity: A review. J. Hazard. Mater. 2015, 283, 764–777. [Google Scholar] [CrossRef]
- Cârâc, A.; Boscencu, R.; Dinică, R.M.; Guerreiro, J.F.; Silva, F.; Marques, F.; Campello, M.P.C.; Moise, C.; Brîncoveanu, O.; Enăchescu, M. Synthesis, characterization and antitumor activity of two new dipyridinium ylide based lanthanide (III) complexes. Inorg. Chim. Acta 2018, 480, 83–90. [Google Scholar] [CrossRef]
- Heffeter, P.; Jakupec, M.A.; Körner, W.; Wild, S.; von Keyserlingk, N.G.; Elbling, L.; Zorbas, H.; Korynevska, A.; Knasmüller, S.; Sutterlüty, H. Anticancer activity of the lanthanum compound [tris (1, 10-phenanthroline) lanthanum (III)] trithiocyanate (KP772; FFC24). Biochem. Pharmacol. 2006, 71, 426–440. [Google Scholar] [CrossRef]
- Palizban, A.; Sadeghi-Aliabadi, H.; Abdollahpour, F. Effect of cerium lanthanide on Hela and MCF-7 cancer cell growth in the presence of transferring. Res. Pharm. Sci. 2010, 5, 119. [Google Scholar]
- Bortner, C.D.; Oldenburg, N.B.; Cidlowski, J.A. The role of DNA fragmentation in apoptosis. Trends Cell Biol. 1995, 5, 21–26. [Google Scholar] [CrossRef]
- Sarkar, T.; Banerjee, S.; Mukherjee, S.; Hussain, A. Mitochondrial selectivity and remarkable photocytotoxicity of a ferrocenyl neodymium (III) complex of terpyridine and curcumin in cancer cells. Dalton Trans. 2016, 45, 6424–6438. [Google Scholar] [CrossRef]
- Chen, D.; Cui, Q.C.; Yang, H.; Barrea, R.A.; Sarkar, F.H.; Sheng, S.; Yan, B.; Reddy, G.P.V.; Dou, Q.P. Clioquinol, a therapeutic agent for Alzheimer’s disease, has proteasome-inhibitory, androgen receptor–suppressing, apoptosis-inducing, and antitumor activities in human prostate cancer cells and xenografts. Cancer Res. 2007, 67, 1636–1644. [Google Scholar] [CrossRef]
- Boldyrev, I.; Gaenko, G.; Moiseeva, E.; Deligeorgiev, T.; Kaloyanova, S.; Lesev, N.; Vasilev, A.; Molotkovsky, J. Europium complexes of 1, 10-phenanthrolines: Their inclusion in liposomes and cytotoxicity. Russ. J. Bioorg. Chem. 2011, 37, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Kang, M.-K.; Jung, K.-H.; Kang, S.-H.; Kim, Y.-H.; Jung, J.-C.; Lee, G.H.; Chang, Y.; Kim, T.-J. Gadolinium complex of DO3A-benzothiazole aniline (BTA) conjugate as a theranostic agent. J. Med. Chem. 2013, 56, 8104–8111. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Wang, X.; Li, T.; Aime, S.; Sadler, P.J.; Guo, Z. Platinum (II)–Gadolinium (III) Complexes as Potential Single-Molecular Theranostic Agents for Cancer Treatment. Angew. Chem. 2014, 126, 13441–13444. [Google Scholar] [CrossRef]
- Kwong, W.-L.; Sun, R.W.-Y.; Lok, C.-N.; Siu, F.-M.; Wong, S.-Y.; Low, K.-H.; Che, C.-M. An ytterbium (III) porphyrin induces endoplasmic reticulum stress and apoptosis in cancer cells: Cytotoxicity and transcriptomics studies. Chem. Sci. 2013, 4, 747–754. [Google Scholar] [CrossRef]
- Zaki, N.G.; Mahmoud, W.H.; El Kerdawy, A.M.; Abdallah, A.M.; Mohamed, G.G. Structural characterization, thermal, DFT, cytotoxicity, and antimetastatic properties of cocaine complexes with La (III), Er (III), and Yb (III). Res. Chem. Intermed. 2020, 46, 3193–3216. [Google Scholar] [CrossRef]
- Aziz, A.A.A.; Sayed, M.A. Some novel rare earth metal ions complexes: Synthesis, characterization, luminescence and biocidal efficiency. Anal. Biochem. 2020, 598, 113645. [Google Scholar]
- Tăbăcaru, A.; Dediu, A.V.B.; Dinică, R.M.; Carac, G.; Basliu, V.; Campello, M.P.C.; Silva, F.; Pinto, C.I.; Guerreiro, J.F.; Martins, M. Biological properties of a new mixed lanthanide (III) complex incorporating a dypiridinium ylide. Inorg. Chim. Acta 2020, 506, 119517. [Google Scholar] [CrossRef]
- García-Valdivia, A.A.; Cepeda, J.; Fernández, B.; Medina-O’Donnell, M.; Oyarzabal, I.; Parra, J.; Jannus, F.; Choquesillo-Lazarte, D.; García, J.A.; Lupiáñez, J.A. 5-Aminopyridine-2-carboxylic acid as appropriate ligand for constructing coordination polymers with luminescence, slow magnetic relaxation and anti-cancer properties. J. Inorg. Biochem. 2020, 207, 111051. [Google Scholar] [CrossRef]
- Campello, M.P.C.; Palma, E.; Correia, I.; Paulo, P.M.; Matos, A.; Rino, J.; Coimbra, J.; Pessoa, J.C.; Gambino, D.; Paulo, A. Lanthanide complexes with phenanthroline-based ligands: Insights into cell death mechanisms obtained by microscopy techniques. Dalton Trans. 2019, 48, 4611–4624. [Google Scholar] [CrossRef]
- Meng, T.; Liu, T.; Qin, Q.-P.; Chen, Z.-L.; Zou, H.-H.; Wang, K.; Liang, F.-P. Mitochondria-localizing dicarbohydrazide Ln complexes and their mechanism of in vitro anticancer activity. Dalton Trans. 2020, 49, 4404–4415. [Google Scholar] [CrossRef]
- Kostova, I.; Manolov, I.; Momekov, G. Cytotoxic activity of new neodymium (III) complexes of bis-coumarins. Eur. J. Med. Chem. 2004, 39, 765–775. [Google Scholar] [CrossRef]
- Creaven, B.S.; Egan, D.A.; Kavanagh, K.; McCann, M.; Noble, A.; Thati, B.; Walsh, M. Synthesis, characterization and antimicrobial activity of a series of substituted coumarin-3-carboxylatosilver (I) complexes. Inorg. Chim. Acta 2006, 359, 3976–3984. [Google Scholar] [CrossRef]
- Patil, S.A.; Kandathil, V.; Sobha, A.; Somappa, S.B.; Feldman, M.R.; Bugarin, A.; Patil, S.A. Comprehensive review on medicinal applications of coumarin-derived imine–metal complexes. Molecules 2022, 27, 5220. [Google Scholar] [CrossRef]
- Gopinath, K.; Gnanasekar, S.; Al-Ghanim, K.A.; Nicoletti, M.; Govindarajan, M.; Arumugam, A.; Balalakshmi, C.; Thanakkasaranee, S. Fabrication of neodymium (Nd), cadmium (Cd) and Nd: Cd doped hybrid copper oxide nanocomposites: Evaluation of their antibacterial activity and cytotoxicity against human L132 cell line. Ceram. Int. 2023, 49, 29933–29947. [Google Scholar] [CrossRef]
- Chundawat, N.S.; Jadoun, S.; Zarrintaj, P.; Chauhan, N.P.S. Lanthanide complexes as anticancer agents: A review. Polyhedron 2021, 207, 115387. [Google Scholar] [CrossRef]
- Kostova, I.; Valcheva-Traykova, M. Synthesis, characterization, and antioxidant activity of a new Gd (III) complex. J. Coord. Chem. 2015, 68, 4082–4101. [Google Scholar] [CrossRef]
- Kostova, I.; Peica, N.; Kiefer, W. Theoretical and spectroscopic studies of new lanthanum (III) complex of orotic acid. Vib. Spectrosc. 2007, 44, 209–219. [Google Scholar] [CrossRef]
- Haas, K.L.; Franz, K.J. Application of metal coordination chemistry to explore and manipulate cell biology. Chem. Rev. 2009, 109, 4921–4960. [Google Scholar] [CrossRef]
- Kostova, I.; Mojžiš, J.; Chiş, V. Theoretical and Experimental Vibrational Characterization of Biologically Active Nd(III) Complex. Molecules 2021, 26, 2726. [Google Scholar] [CrossRef]
- More, M.; Joshi, P.; Mishra, Y.; Khanna, P. Metal complexes driven from Schiff bases and semicarbazones for biomedical and allied applications: A review. Mater. Today Chem. 2019, 14, 100195. [Google Scholar] [CrossRef]
- Karati, D.; Mukherjee, S.; Roy, S. An Explicative Review on the Current Advancement in Schiff Base-Metal Complexes as Anticancer Agents Evolved in the Past Decade: Medicinal Chemistry Aspects. Med. Chem. 2023, 19, 960–985. [Google Scholar] [CrossRef]
- Abd El-Halim, H.; Mohamed, G.G.; Anwar, M.N. Antimicrobial and anticancer activities of Schiff base ligand and its transition metal mixed ligand complexes with heterocyclic base. Appl. Organomet. Chem. 2018, 32, e3899. [Google Scholar] [CrossRef]
- Filippou, C.; Themistocleous, S.C.; Marangos, G.; Panayiotou, Y.; Fyrilla, M.; Kousparou, C.A.; Pana, Z.-D.; Tsioutis, C.; Johnson, E.O.; Yiallouris, A. Microbial Therapy and Breast Cancer Management: Exploring Mechanisms, Clinical Efficacy, and Integration within the One Health Approach. Int. J. Mol. Sci. 2024, 25, 1110. [Google Scholar] [CrossRef]
- Abdalla, E.M.; Abd-Allah, M. Synthesis, Characterization, Antimicrobial/Antitumor Activity of Binary and Ternary Neodymium (III) Complex with 2, 2′-((1E, 1′E)-(ethane-1, 2-diylbis (azaneylylidene)) bis (methaneylylidene)) diphenol and Imidazole. Egypt. J. Chem. 2022, 65, 735–744. [Google Scholar] [CrossRef]
- Meenakshi, P.; Kaur, K.; Bala, N.; Gupta, N.; Malik, A.K. Innovative Lanthanide Complexes: Shaping the future of cancer/tumor Chemotherapy. J. Trace Elem. Med. Biol. 2023, 80, 127277. [Google Scholar]
- de Oliveira Neto, J.G.; Viana, J.R.; Abreu, K.R.; Butarelli, A.L.A.; dos Santos, A.P.A.; Lage, M.R.; de Sousa, F.F.; Souto, E.B.; dos Santos, A.O. Antitumor neodymium(III) complex with 1,10-phenanthroline and nitrate ligands: A comprehensive experimental-theoretical study, in silico pharmacokinetic and cytotoxic properties. J. Mol. Struct. 2025, 1321, 139757. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Zhou, L.X.; Lin, J.L.; Zhang, S.W. Syntheses and Crystal Structures of Ln (phen)2(NO3)3 with Ln = Pr, Nd, Sm, Eu, Dy, and phen = 1, 10-phenanthroline. Z. Für Anorg. Allg. Chem. 2001, 627, 1643–1646. [Google Scholar] [CrossRef]
- Măciucă, A.-M.; Munteanu, A.-C.; Uivarosi, V. Quinolone complexes with lanthanide ions: An insight into their analytical applications and biological activity. Molecules 2020, 25, 1347. [Google Scholar] [CrossRef]
- Kostova, I.; Trendafilova, N.; Mihaylov, T. Theoretical and spectroscopic studies of pyridyl substituted bis-coumarins and their new neodymium (III) complexes. Chem. Phys. 2005, 314, 73–84. [Google Scholar] [CrossRef]
- Raju, L.; Rajkumar, E. Coordination compounds of iron, ruthenium and osmium. In Photochemistry and Photophysics of Coordination Compounds; Elsevier: Amsterdam, The Netherlands, 2023; pp. 135–203. [Google Scholar]
- Carbonati, T.; Cionti, C.; Cosaert, E.; Nimmegeers, B.; Meroni, D.; Poelman, D. NIR emitting GdVO4: Nd nanoparticles for bioimaging: The role of the synthetic pathway. J. Alloys Compd. 2021, 862, 158413. [Google Scholar] [CrossRef]
- Abo-Rehab, R.S.; Kasim, E.A.; Farhan, N.; Tolba, M.S.; Shehata, M.R.; Abdalla, E.M. Synthesis, characterization, anticancer, antibacterial, antioxidant, DFT, and molecular docking of novel La (III), Ce (III), Nd (III), and Dy (III) lanthanide complexes with Schiff base derived from 2-aminobenzothiazole and coumarin. Appl. Organomet. Chem. 2024, 38, e7622. [Google Scholar] [CrossRef]
- Wong, W.K.; Yang, X.; Jones, R.A.; Rivers, J.H.; Lynch, V.; Lo, W.K.; Xiao, D.; Oye, M.M.; Holmes, A.L. Multinuclear luminescent schiff-base Zn− Nd sandwich complexes. Inorg. Chem. 2006, 45, 4340–4345. [Google Scholar] [CrossRef]
- Madanhire, T.; Davids, H.; Pereira, M.C.; Hosten, E.C.; Abrahams, A.r. Lanthanide complexes with N-(2, 6-dimethylphenyl) oxamate: Synthesis, characterisation and cytotoxicity. Polyhedron 2020, 184, 114561. [Google Scholar] [CrossRef]
- Caporale, A.; Palma, G.; Mariconda, A.; Del Vecchio, V.; Iacopetta, D.; Parisi, O.I.; Sinicropi, M.S.; Puoci, F.; Arra, C.; Longo, P. Synthesis and antitumor activity of new group 3 metallocene complexes. Molecules 2017, 22, 526. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Muthulakshmi, V. Green synthesis of ionic liquid mediated neodymium oxide nanoparticles by Andrographis paniculata leaves extract for effective bio-medical applications. J. Environ. Chem. Eng. 2021, 9, 104716. [Google Scholar] [CrossRef]
- Dang, Y.; Bai, J.; Lou, K.; Yang, R.; Gao, Y.; Tian, H.; Li, J.; Lin, L.; Lv, R.; Wang, P. Intraoperative Surgical Margin Assessment by NIR-II Imaging with Urine Excretable Nd-Based Nanoprobe in Breast Cancers. Adv. Funct. Mater. 2024, 34, 2311673. [Google Scholar] [CrossRef]
- Ansari, A.A.; Khan, A.; Alam, M.; Siddiqui, M.A.; Ahmad, N.; Alkhedhairy, A.A. Optically active neodymium hydroxide surface-functionalized mesoporous silica micro-cocoons for biomedical applications. Colloids Surf. B Biointerfaces 2020, 189, 110877. [Google Scholar] [CrossRef]
- Yang, Z.; Ji, Y.; Jia, Q.; Feng, Y.; Ji, R.; Bai, M.; Yan, H.; Sun, F.; Zhang, R.; Wang, Z. Real-time detection and resection of sentinel lymph node metastasis in breast cancer through a rare earth nanoprobe based NIR-IIb fluorescence imaging. Mater. Today Bio 2024, 28, 101166. [Google Scholar] [CrossRef]
- Alexander, A.; Pillai, A.S.; Manikantan, V.; Varalakshmi, G.S.; Akash, B.A.; Enoch, I.V. Magnetic and luminescent neodymium-doped carbon dot-cyclodextrin polymer nanocomposite as an anticancer drug-carrier. Mater. Lett. 2022, 313, 131830. [Google Scholar] [CrossRef]
- Bheeram, V.R.; Dadhich, A.S.; Nagumantri, R.; Rentala, S.; Saha, A.; Mukkamala, S.B. Gamma ray enhanced Vis-NIR photoluminescence and cytotoxicity of biocompatible silica coated Nd3+ doped GdPO4 nanophosphors. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2019, 440, 11–18. [Google Scholar] [CrossRef]
- Jin, W.; Wang, Q.; Wu, M.; Li, Y.; Tang, G.; Ping, Y.; Chu, P.K. Lanthanide-integrated supramolecular polymeric nanoassembly with multiple regulation characteristics for multidrug-resistant cancer therapy. Biomaterials 2017, 129, 83–97. [Google Scholar] [CrossRef] [PubMed]
- Jafarirad, S.; Hammami Torghabe, E.; Rasta, S.H.; Salehi, R. A novel non-invasive strategy for low-level laser-induced cancer therapy by using new Ag/ZnO and Nd/ZnO functionalized reduced graphene oxide nanocomposites. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. S2), 800–816. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Ye, J.; Ren, S.; Wang, G.; Lv, J.; Zhang, S.; Che, Y.; Li, Y.; Chen, B.; Ning, G. Temperature Feedback-Controlled Photothermal/Photodynamic/Chemodynamic Combination Cancer Therapy Based on NaGdF4: Er, Yb@ NaGdF4: Nd@ Cu-BIF Nanoassemblies. Adv. Healthc. Mater. 2020, 9, 2001205. [Google Scholar] [CrossRef]
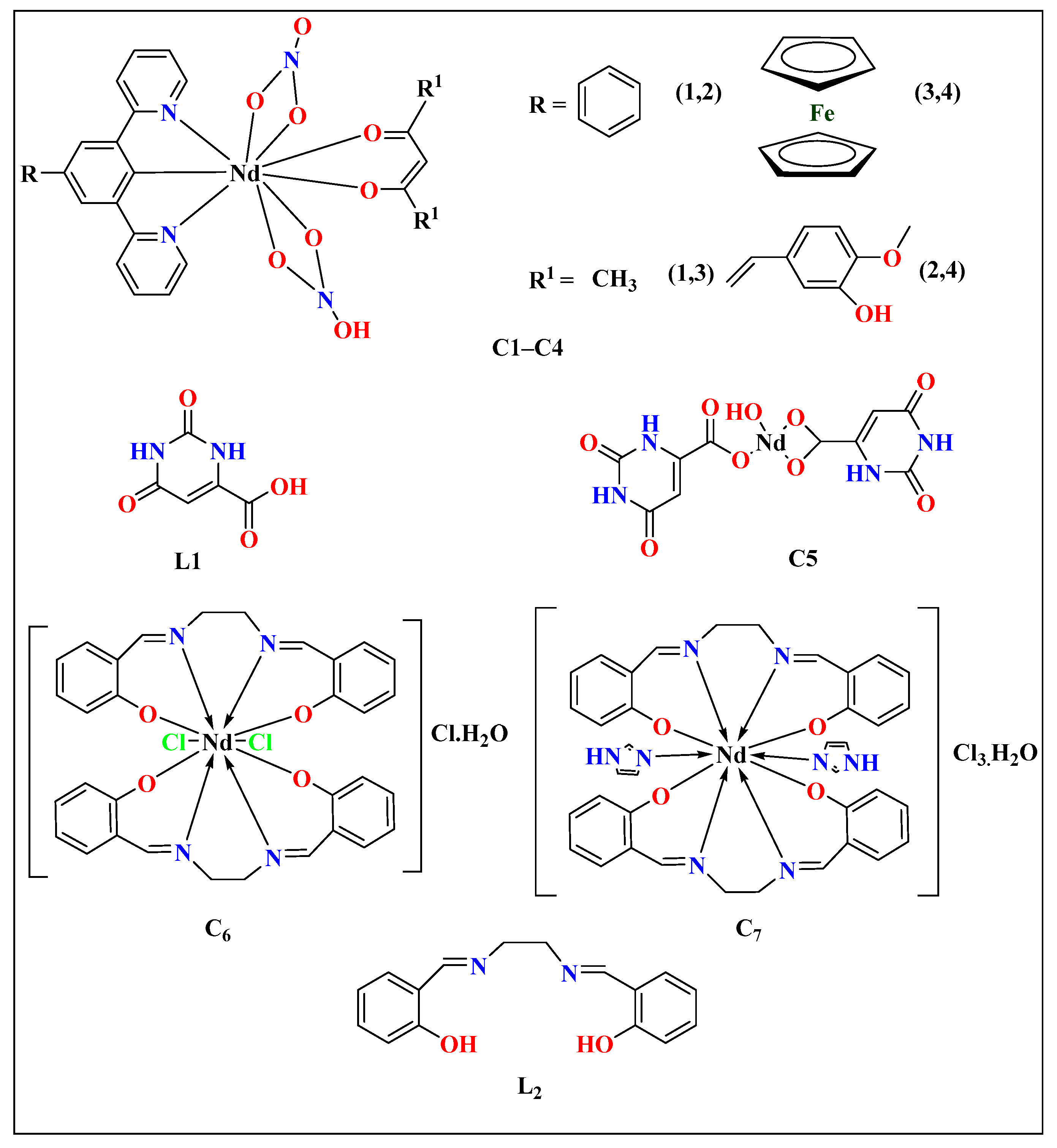
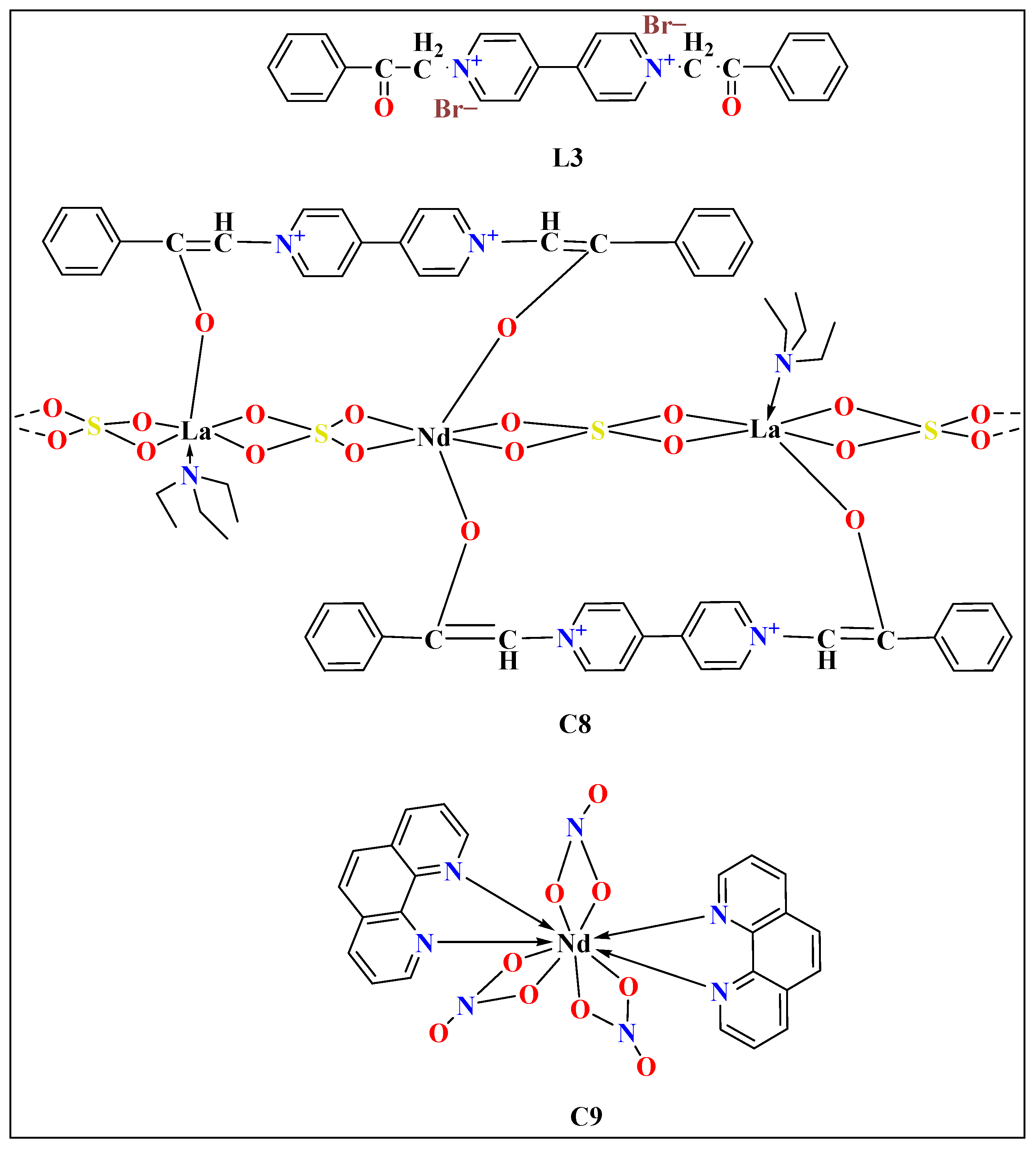
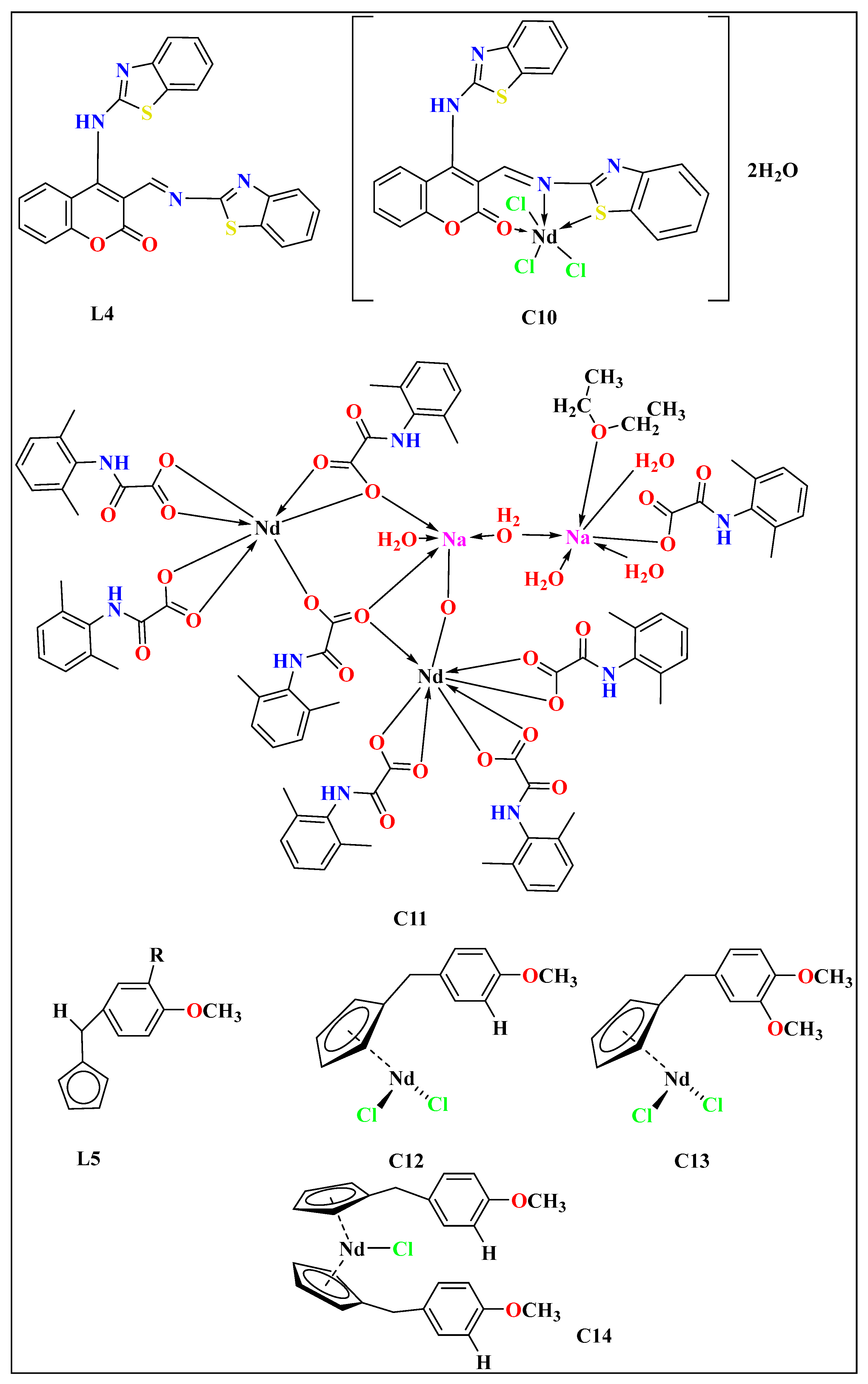
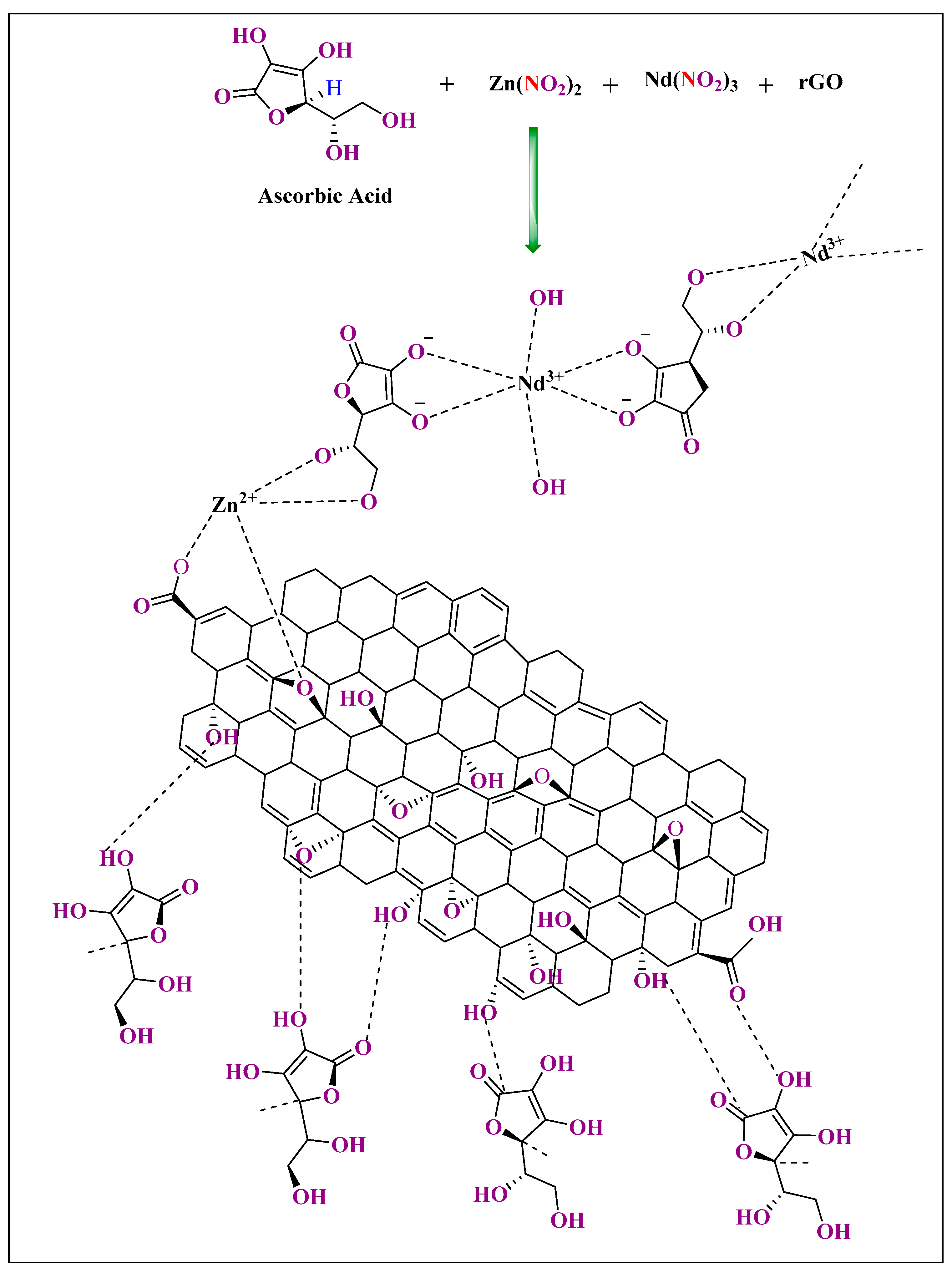
| Lanthanide Metals | Cell Line | Mode of Action | References |
|---|---|---|---|
| La3+, Nd3+ | MCF7 | Apoptotic cell death | [42] |
| La3+ | MCF7 and MDA-MB-231 | DNA-laddering phenomenon | [43] |
| La3+ | MDA-MB435 | DNA intercalation | [44] |
| Ce3+ | MDA-MB-231 breast cancer cells, MCF-7 | Mechanism of action remains unclarified DNA cleavage | [45,46] |
| Pr3+, Er3+ and Yb3+ | Human breast cancer (MCF7), and cervical (HeLa) | Programmed cell death | [47,48] |
| La3+ | HeLa and MCF-7 cells | Complex accumulates within the mitochondria of HeLa cells and induces apoptosis, cleaves plasmid DNA | [49,50] |
| Eu3+, Gd3+, Nd3+, Sm3+ and Tb3+ | HeLa and MCF-7 cells | Complex accumulates within the mitochondria of HeLa cells and induces apoptosis, cleaves plasmid DNA | [51] |
| Eu3+ and Tb3+ | MDA-MB-231 (mammary cancer) and PC-3 (prostate carcinoma) cell lines, HBL-100 human breast carcinoma cells, and MCF7 cell lines | Complex and ct-DNA binding, Liposomes, anti- | [52] |
| Gd3+ | Human breast cancer MCF-7 | angiogenic activity | [53] |
| Pr3+, Er3+ and Yb3+ | human breast cancer (MCF7) | DNA fragmentation | [54] |
| La3+, Er3+ and Yb3+ | MCF-7 | Elevated the cellular levels of caspase-3 and caspase-9 | [55] |
| La3+, Sm3+ and Yb3+ | human breast cancer (MCF-7) cell lines | Intercalate into the double-stranded DNA (or) bind to the phosphate group of the DNA backbone | [56] |
| La3+ and Nd3+ | Ovarian (A2780), breast (MCF7) | Caspase activation, DNA fragmentation, | [57] |
| Ce3+, Nd3+, Gd3+ and Er3+ | MCF-7 | [56] |
| Complex | Ligand | Geometry | Pathway | Doses Assay (IC50 = μm) | Time | Cell Line | Ref. | |
|---|---|---|---|---|---|---|---|---|
| C1, C2 | - | Distorted square anti-prismatic | MTT assay | Light | Dark | - | MCF-7 | [46] |
| 53.1 ± 2.5 (62.6 ± 2.8) | 80.3 ± 2.1 (94.5 ± 3.1) | |||||||
| - | - | MTT assay | 4.2 ± 0.8 (9.6 ± 1.2) | >50 (>50) | - | MCF-7 | ||
| C3, C4 | - | Tricapped trigonal prismatic | MTT assay | 13.2 ± 1.6 (19.9 ± 1.8) | >50 (>50 | - | MCF-7 | |
| - | - | MTT assay | 0.7 ± 0.2 (2.1 ± 0.6) | >50 (>50) | - | MCF-7 | ||
| C5 | L1 | Distorted pentagonal bipyramidal | MTT assay | MCF-7 (IC50 = 25) MDA-MB-231 (IC50 = 30) | 72 h | MCF-7, MDA-MB-231 | [23] | |
| C6 | L2 | Distorted octahedral | MTT assay | - | - | MDA-MB231 | [71] | |
| C7 | Distorted octahedral | MTT assay | - | - | MDA-MB231 | |||
| C8 | L3 | Distorted dodecahedral | Hoechst nuclei staining assay | 1.6 ± 0.4 for L3 45 ± 18 for Cisplatin | 24 h | MCF-7 | [54] | |
| 0.3 ± 0.2 for L3 20 ± 6 for Cisplatin | 48 h | |||||||
| C9 | - | Distorted bicapped square antiprismatic | MTT assay | 0.3 ± 0.2 for MCF-7 | 48 h | MCF-7 cells | [80] | |
| C10 | L4 | Dodecahedral | MTT assay | 0.861 ± 0.544 | 24 h | MCF-7 | [79] | |
| C11 | - | Icosahedral | MTT assay | 46.8 ± 6.46 | 24 h | MCF-7 | [81] | |
| C12 | L5 | - | MTT assay | 6 ± 50 | 48 h | MDA. MB231 | [82] | |
| C13 | L5 | - | MTT assay | - | - | MDA. MB231 | ||
| C14 | L5 | - | MTT assay | - | - | MDA. MB231 | ||
| Nd-Nanoparticles | Synthesis Method | Dose Assay (IC50) | Cell Line | Pathway | Modal | Cell Viability | Ref. |
|---|---|---|---|---|---|---|---|
| Nd2O3-IL | Green Synthesis Method | 63 μg/mL | MCF-7 | MTT assay | - | 25.82% | [83] |
| Sio2@Nd(OH)3 | Sol–gel process | 25 μg/mL | MCF-7 A-549 | MTT assay | - | 75% | [85] |
| Renps@HA | Thermal decomposition method | 50 μg mL−1 | MCF-7 MCF-10A MDA-MB-231 | MTT assay | In vivo | 95% | [86] |
| Nd-doped C-dots | Hydrothermal method | 10 μg/mL 3.1 ± 0.4 | MCF-7 | MTT assay | In vitro | 86.3% | [87] |
| Gdpo4:Nd3+@sio2 | Solution combustion method | 25 µg/mL | PC-3 MCF-7 | MTT assay | - | - | [88] |
| PCD/siRNA/Nd-PC | - | 34.0 µg/mL | MCF-7 ADR cells | MTT assay | In vitro and in vivo | - | [89] |
| Nd-zno/rgo ncs | Hydrothermal process | 25 µg/mL | MCF-7 | MTT assay | In vitro | 80% | [90] |
| Nagdf4:Nd@Cu(II) | Thermal decomposition method | 400 µg/mL | Hela MCF-7 | MTT assay | In vitro and in vivo | 12% | [91] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abuilaiwi, F.A. Prospect of (Nd3+) Complexes and Its Nanoparticles as Promising Novel Anticancer Agents in Particular Targeting Breast Cancer Cell Lines. Future Pharmacol. 2025, 5, 4. https://doi.org/10.3390/futurepharmacol5010004
Abuilaiwi FA. Prospect of (Nd3+) Complexes and Its Nanoparticles as Promising Novel Anticancer Agents in Particular Targeting Breast Cancer Cell Lines. Future Pharmacology. 2025; 5(1):4. https://doi.org/10.3390/futurepharmacol5010004
Chicago/Turabian StyleAbuilaiwi, Faraj Ahmad. 2025. "Prospect of (Nd3+) Complexes and Its Nanoparticles as Promising Novel Anticancer Agents in Particular Targeting Breast Cancer Cell Lines" Future Pharmacology 5, no. 1: 4. https://doi.org/10.3390/futurepharmacol5010004
APA StyleAbuilaiwi, F. A. (2025). Prospect of (Nd3+) Complexes and Its Nanoparticles as Promising Novel Anticancer Agents in Particular Targeting Breast Cancer Cell Lines. Future Pharmacology, 5(1), 4. https://doi.org/10.3390/futurepharmacol5010004






