An Overview of Sargassum Seaweed as Natural Anticancer Therapy
Abstract
1. Introduction
- Induction or initiation: DNA mutations appear that turn healthy cells into cancer cells: uncontrolled division, capacity for local invasion, and distant spread.
- Cancer ‘in situ’: increase in the number of cancer cells in the organ or tissue in which it originates, producing the primary tumor.
- Local invasion: extension of the primary tumor to neighboring structures, invading them and giving rise to the first symptoms.
- Distant invasion or metastasis: the cancerous cells enter the bloodstream and spread to other organs, giving rise to secondary tumors.
- Carcinoma: this is the most common cancer of epithelial origin and is capable of affecting organs or secretory glands. There are two subtypes of carcinoma, adenocarcinoma and squamous cell carcinoma.
- Sarcoma: cancer that forms in connective and supportive tissues such as bone tissue and soft tissues, including muscle, adipose tissue, lymphatic vessels, blood vessels, tendons, and fibrous tissue.
- Myeloma: cancer that starts in plasma cells (a type of white blood cell) in the bone marrow, forming tumors in many bones and preventing the production of healthy blood cells.
- Leukemia: cancer derived from blood-forming bone marrow tissue or its precursors. This type of cancer does not form solid tumors; instead, abnormal white blood cells (leukemic cells) accumulate in the blood, displacing normal blood cells in the blood.
- Lymphoma: cancer of the lymphatic system that specifically affects B-cells, which unlike leukemia produces solid tumors that involve lymph nodes or other organs in the body.
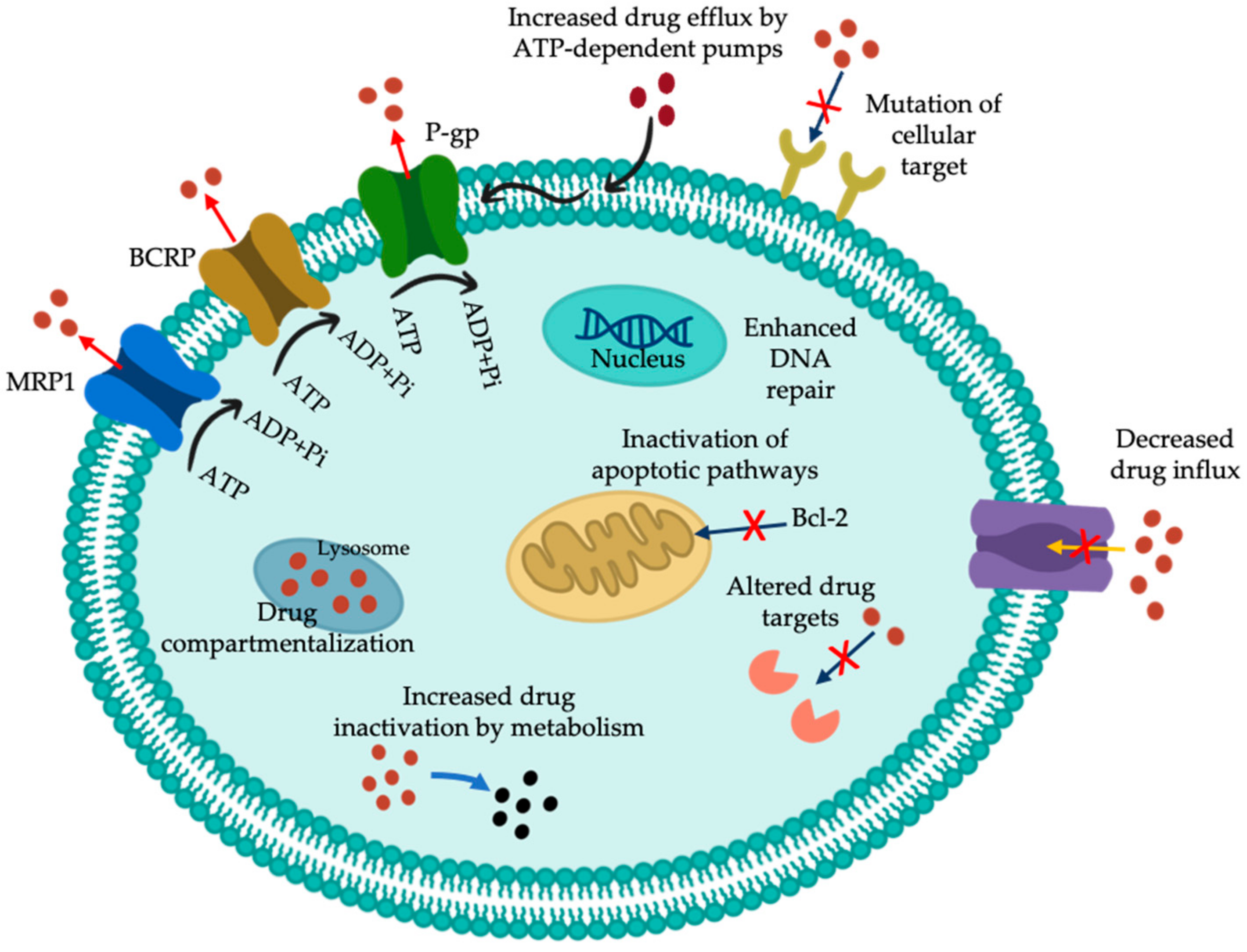
2. Multidrug-Resistance (MDR) Activity
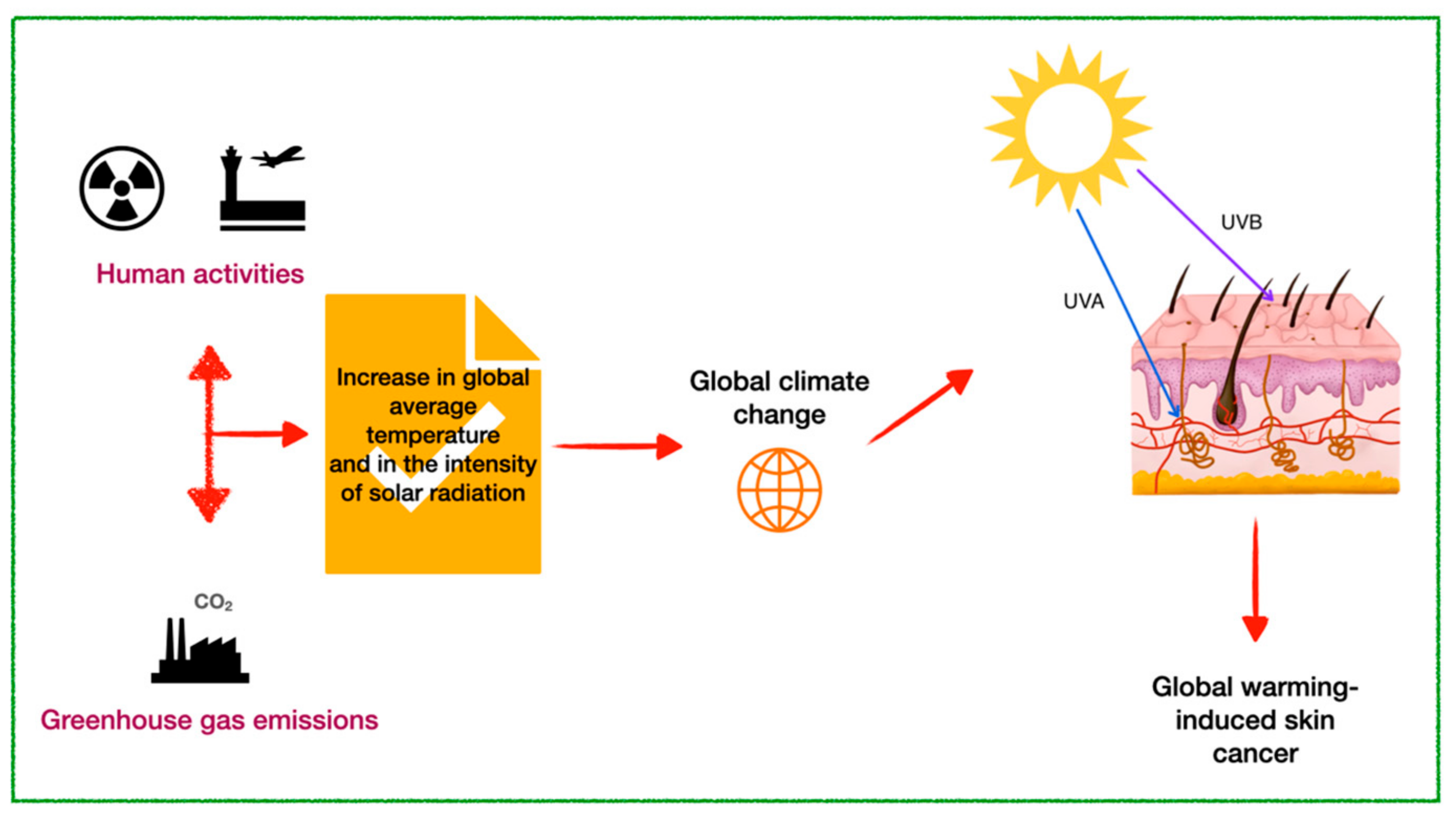
3. Skin Cancer Linked to Global Warming
4. Glycolipids Involved in MDR
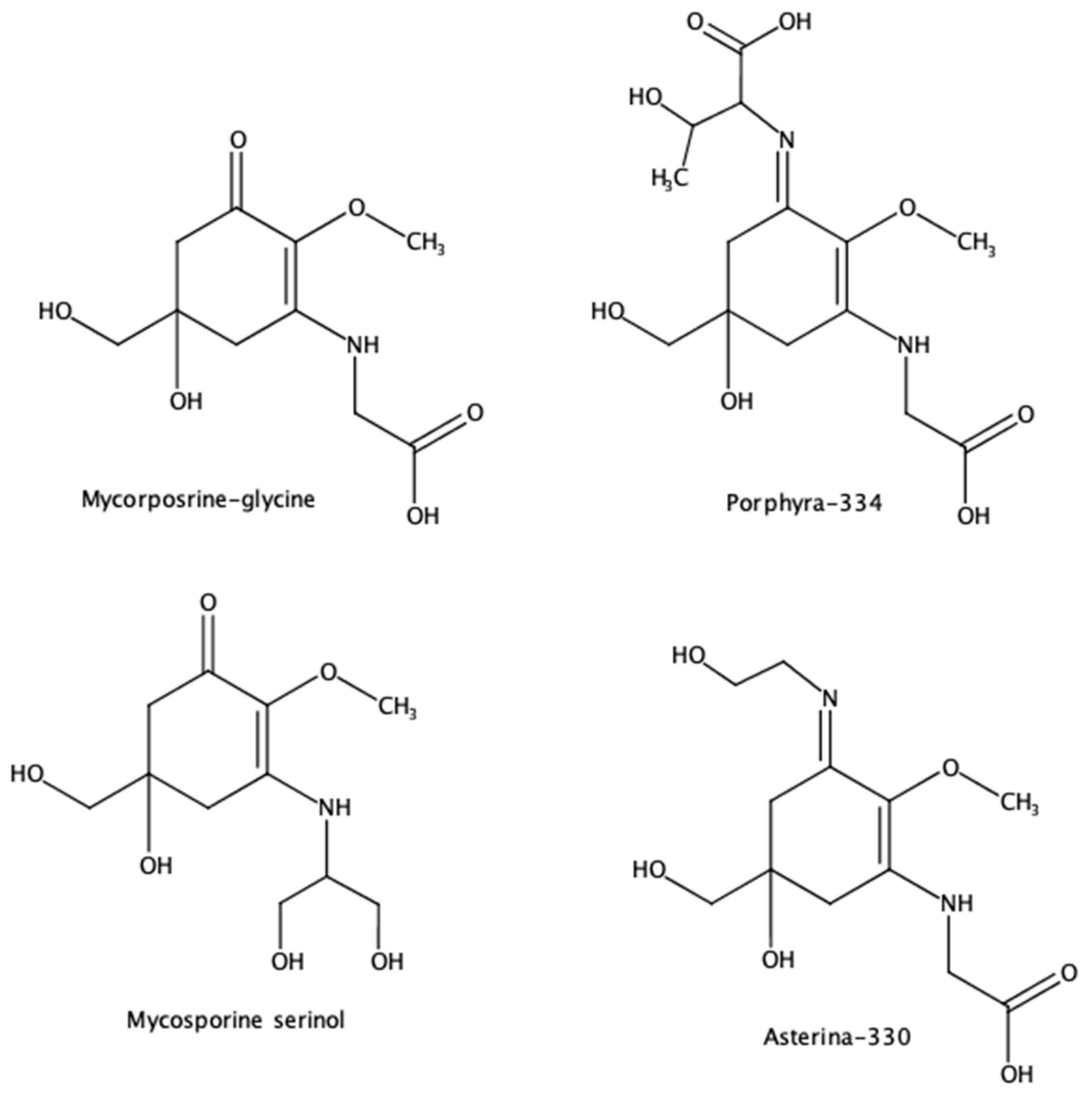
5. Mycosporin-like Amino Acids (MAAs) Against Global Warming-Induced Skin Cancer
| Main MAAs Evaluated | Application | References |
|---|---|---|
| Mycosporin-serinol and porphyra-334, shinorine | Topical sunscreens | de la Coba et al. [97] |
| Mycosporin-glutamicol, mycosporin-glutaminol-glucoside, mycosporin-serinol, mycosporin-taurine, palythine, palythine-threonine-sulphate, porphyra-334, and usujirene | Antioxidant potential | Browne et al. [98] |
| Shinorine | Commercial sunscreen formulation | Candelo and Llewellyn [99] |
| Porphyra-334, shinorine, asterina-330, palythine, and mycosporine-glycine | Natural antioxidant | de la Coba et al. [87] |
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 8 August 2024).
- Wild, C.; Weiderpass, E.; Stewart, B. World Cancer Report: Cancer Research for Cancer Prevention; International Agency for Research on Cancer: Lyon, France, 2020. [Google Scholar]
- National Cancer Institute. Understanding Cancer. Available online: https://www.cancer.gov/about-cancer/understanding (accessed on 25 November 2024).
- National Cancer Institute. SEER Training Modules: Cancer As a Disease. Available online: https://training.seer.cancer.gov/disease/ (accessed on 28 November 2024).
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef]
- Thirumaran, R.; Prendergast, G.C.; Gilman, P.B. Chapter 7—Cytotoxic Chemotherapy in Clinical Treatment of Cancer. In Cancer Immunotherapy; Prendergast, G.C., Jaffee, E.M., Eds.; Academic Press: Burlington, VT, USA, 2007; pp. 101–116. [Google Scholar]
- Awosika, A.O.; Below, J.; Das, J.M. Vincristine. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2025. [Google Scholar]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Abdullah, L.N.; Chow, E.K.-H. Mechanisms of chemoresistance in cancer stem cells. Clin. Transl. Med. 2013, 2, 3. [Google Scholar] [CrossRef]
- Yip, Z.T.; Quek, R.Z.B.; Huang, D. Historical biogeography of the widespread macroalga Sargassum (Fucales, phaeophyceae). J. Phycol. 2020, 56, 300–309. [Google Scholar] [CrossRef]
- Fidai, Y.A.; Dash, J.; Tompkins, E.L.; Tonon, T. A systematic review of floating and beach landing records of Sargassum beyond the Sargasso Sea. Environ. Res. Commun. 2020, 2, 122001. [Google Scholar] [CrossRef]
- Mohan, P.; Strobl, E. Tourism and marine crises: The impact of Sargassum invasion on Caribbean small island developing sates. Ocean. Coast. Manag. 2024, 251, 107091. [Google Scholar] [CrossRef]
- Farobie, O.; Amrullah, A.; Anis, L.A.; Hartulistiyoso, E.; Syaftika, N.; Saefurahman, G.; Bayu, A. Valorization of brown macroalgae Sargassum plagiophyllum for biogas production under different salinity conditions. Bioresour. Technol. Rep. 2023, 22, 101403. [Google Scholar] [CrossRef]
- Rivera-Hernández, Y.; Hernández-Eugenio, G.; Balagurusamy, N.; Espinosa-Solares, T. Sargassum-pig manure co-digestion: An alternative for bioenergy production and treating a polluting coastal waste. Renew. Energy 2022, 199, 1336–1344. [Google Scholar] [CrossRef]
- Abdool-Ghany, A.A.; Pollier, C.G.L.; Oehlert, A.M.; Swart, P.K.; Blare, T.; Moore, K.; Solo-Gabriele, H.M. Assessing quality and beneficial uses of Sargassum compost. Waste Manag. 2023, 171, 545–556. [Google Scholar] [CrossRef]
- Rossignolo, J.A.; Felicio Peres Duran, A.J.; Bueno, C.; Martinelli Filho, J.E.; Savastano Junior, H.; Tonin, F.G. Algae application in civil construction: A review with focus on the potential uses of the pelagic Sargassum spp. biomass. J. Environ. Manag. 2022, 303, 114258. [Google Scholar] [CrossRef]
- Digala, P.; Saravanan, M.; Dhanraj, M.; Pamarthi, J.; Muralidharan, S.; Narikimelli, A.; Dinakaran, K.P.; Arokiyaraj, S.; Vincent, S. Optimized extraction of sulfated polysaccharide from brown seaweed Sargassum polycystum and its evaluation for anti-cancer and wound healing potential. S. Afr. J. Botany. 2022, 151, 345–359. [Google Scholar] [CrossRef]
- Sun, Q.L.; Li, Y.; Ni, L.Q.; Li, Y.X.; Cui, Y.S.; Jiang, S.L.; Xie, E.Y.; Du, J.; Deng, F.; Dong, C.X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2020, 229, 115487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Zhang, X.; Tang, Y.; Mao, J. Composition, isolation, purification and biological activities of Sargassum fusiforme polysaccharides: A review. Carbohydr. Polym. 2020, 228, 115381. [Google Scholar] [CrossRef] [PubMed]
- Alzarea, S.I.; Elmaidomy, A.H.; Saber, H.; Musa, A.; Al-Sanea, M.M.; Mostafa, E.M.; Hendawy, O.M.; Youssif, K.A.; Alanazi, A.S.; Alharbi, M.; et al. Potential Anticancer Lipoxygenase Inhibitors from the Red Sea-Derived Brown Algae Sargassum cinereum: An In-Silico-Supported In-Vitro Study. Antibiotics 2021, 10, 416. [Google Scholar] [CrossRef]
- Jiang, H.; Kong, L.; Tang, H.; Wang, Z.; Liu, C.; Zhang, J.; Chen, Y.; Shen, J.; Zhou, Y. Study on the preparation and enzyme inhibitory activity of polyphenols from Sargassum pallidum. PLoS ONE 2024, 19, e0297434. [Google Scholar] [CrossRef] [PubMed]
- Shobharani, P.; Nanishankar, V.H.; Halami, P.M.; Sachindra, N.M. Antioxidant and anticoagulant activity of polyphenol and polysaccharides from fermented Sargassum sp. Int. J. Biol. Macromol. 2014, 65, 542–548. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, S.; Yang, M.; Ding, J.; Huang, Y.; Zhu, Y.; Zhou, M.; Yan, B. Antiviral activity of a polysaccharide from Sargassum fusiforme against respiratory syncytial virus. Int. J. Biol. Macromol. 2024, 279, 135267. [Google Scholar] [CrossRef] [PubMed]
- Uma, V.S.; Usmani, Z.; Sharma, M.; Diwan, D.; Sharma, M.; Guo, M.; Tuohy, M.G.; Makatsoris, C.; Zhao, X.; Thakur, V.K.; et al. Valorisation of algal biomass to value-added metabolites: Emerging trends and opportunities. Phytochem. Rev. 2022, 22, 1015–1040. [Google Scholar] [CrossRef] [PubMed]
- Rebucci, M.; Michiels, C. Molecular aspects of cancer cell resistance to chemotherapy. Biochem. Pharmacol. 2013, 85, 1219–1226. [Google Scholar] [CrossRef]
- Raguz, S.; Yagüe, E. Resistance to chemotherapy: New treatments and novel insights into an old problem. Br. J. Cancer 2008, 99, 387–391. [Google Scholar] [CrossRef]
- Bar-Zeev, M.; Livney, Y.D.; Assaraf, Y.G. Targeted nanomedicine for cancer therapeutics: Towards precision medicine overcoming drug resistance. Drug Resist. Updates 2017, 31, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, A.C.; Saig, F.A.; Cloos, J.; Jansen, G.; Peters, G.J. How to overcome ATP-binding cassette drug efflux transporter-mediated drug resistance? Cancer Drug Resist. 2018, 1, 6–29. [Google Scholar] [CrossRef]
- Ween, M.P.; Armstrong, M.A.; Oehler, M.K.; Ricciardelli, C. The role of ABC transporters in ovarian cancer progression and chemoresistance. Crit. Rev. Oncol. Hematol. 2015, 96, 220–256. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.-T.; Li, Z.-L.; He, Z.-X.; Qiu, J.-X.; Zhou, S.-F. Molecular mechanisms for tumour resistance to chemotherapy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.L. P-glycoprotein Inhibition for Optimal Drug Delivery. Drug Target Insights 2013, 7, 27–34. [Google Scholar] [CrossRef]
- Zingales, B.; Araujo, R.G.; Moreno, M.; Franco, J.; Aguiar, P.H.; Nunes, S.L.; Silva, M.N.; Ienne, S.; Machado, C.R.; Brandao, A. A novel ABCG-like transporter of Trypanosoma cruzi is involved in natural resistance to benznidazole. Mem. Inst. Oswaldo Cruz 2015, 110, 433–444. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, A.; Sousa, E.; Vasconcelos, M.H.; Pinto, M.M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef] [PubMed]
- Nobili, S.; Landini, I.; Giglioni, B.; Mini, E. Pharmacological strategies for overcoming multidrug resistance. Curr. Drug Targets 2006, 7, 861–879. [Google Scholar] [CrossRef] [PubMed]
- Ambudkar, S.V.; Kimchi-Sarfaty, C.; Sauna, Z.E.; Gottesman, M.M. P-glycoprotein: From genomics to mechanism. Oncogene 2003, 22, 7468–7485. [Google Scholar] [CrossRef] [PubMed]
- Di Filippo, R.; Bursi, O.S.; di Maggio, R. Global warming and ozone depletion potentials caused by emissions from HFC and CFC banks due structural damage. Energy Build. 2022, 273, 112385. [Google Scholar] [CrossRef]
- Zhumadilova, A.; Zhigitova, S.; Turalina, M. The impact of greenhouse gases on climate change. Sci. Horiz. 2023, 26, 97–109. [Google Scholar] [CrossRef]
- Grigorieva, E.; Livenets, A.; Stelmakh, E. Adaptation of Agriculture to Climate Change: A Scoping Review. Climate 2023, 11, 202. [Google Scholar] [CrossRef]
- Hiatt, R.A.; Beyeler, N. Cancer and climate change. Lancet Oncol. 2020, 21, e519–e527. [Google Scholar] [CrossRef]
- Bernhard, G.H.; Neale, R.E.; Barnes, P.W.; Neale, P.J.; Zepp, R.G.; Wilson, S.R.; Andrady, A.L.; Bais, A.F.; McKenzie, R.L.; Aucamp, P.J.; et al. Environmental effects of stratospheric ozone depletion, UV radiation and interactions with climate change: UNEP Environmental Effects Assessment Panel, update 2019. Photochem. Photobiol. Sci. 2020, 19, 542–584. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Sharma, A.; Liu, H. The Impact of Climate Change on Environmental Sustainability and Human Mortality. Environments 2023, 10, 165. [Google Scholar] [CrossRef]
- Kimlin, M.G.; Youlden, D.R.; Brodie, A.M.; DiSipio, T.; Youl, P.; Nair-Shalliker, V.; Baade, P.D. Risk of Second Primary Cancer in Survivors of In Situ Melanoma. J. Investig. Dermatol. 2019, 139, 842–847. [Google Scholar] [CrossRef]
- Yu, P.; Xu, R.; Yang, Z.; Ye, T.; Liu, Y.; Li, S.; Abramson, M.J.; Kimlin, M.; Guo, Y. Cancer and Ongoing Climate Change: Who Are the Most Affected? ACS Environ. Au 2023, 3, 5–11. [Google Scholar] [CrossRef]
- WHO. Climate and Health Country Profile—Italy; United Nations Framework Convention on Climate Change: Rio de Janeiro, Brazil, 2018. [Google Scholar]
- D’Orazio, J.; Jarrett, S.; Amaro-Ortiz, A.; Scott, T. UV radiation and the skin. Int. J. Mol. Sci. 2013, 14, 12222–12248. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Yang, T.; Yu, D.; Xiong, H.; Zhang, S. Current insights and future perspectives of ultraviolet radiation (UV) exposure: Friends and foes to the skin and beyond the skin. Environ. Int. 2024, 185, 108535. [Google Scholar] [CrossRef]
- Arnold, M.; de Vries, E.; Whiteman, D.C.; Jemal, A.; Bray, F.; Parkin, D.M.; Soerjomataram, I. Global burden of cutaneous melanoma attributable to ultraviolet radiation in 2012. Int. J. Cancer 2018, 143, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Tse, B.C.Y.; Ferguson, A.L.; Koay, Y.C.; Grau, G.E.; Don, A.S.; Byrne, S.N. Exposure to solar ultraviolet radiation establishes a novel immune suppressive lipidome in skin-draining lymph nodes. Front. Immunol. 2023, 13, 1045731. [Google Scholar]
- Jansen, R.; Wang, S.Q.; Burnett, M.; Osterwalder, U.; Lim, H.W. Photoprotection: Part I. Photoprotection by naturally occurring, physical, and systemic agents. J. Am. Acad. Dermatol. 2013, 69, 853.e1–853.e12. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.P. Ensuring the Safety of Sunscreens, and Their Efficacy in Preventing Skin Cancers: Challenges and Controversies for Clinicians, Formulators, and Regulators. Front. Med. 2019, 6, 195. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Passeron, T.; Gilaberte, Y.; Granger, C.; Leone, G.; Narda, M.; Schalka, S.; Trullas, C.; Masson, P.; Lim, H.W. Photoprotection of the future: Challenges and opportunities. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 447–454. [Google Scholar] [CrossRef]
- Baertschi, S.W.; Alsante, K.M.; Tønnesen, H.H. A Critical Assessment of the ICH Guideline on Photostability Testing of New Drug Substances and Products (Q1B): Recommendation for Revision. J. Pharm. Sci. 2010, 99, 2934–2940. [Google Scholar] [CrossRef] [PubMed]
- Deitrick, R.; Goldblatt, C. Effects of ozone levels on climate through Earth history. Clim. Past 2023, 19, 1201–1218. [Google Scholar] [CrossRef]
- Fleming, E.L.; Newman, P.A.; Liang, Q.; Daniel, J.S. The Impact of Continuing CFC-11 Emissions on Stratospheric Ozone. J. Geophys. Res. Atmos. 2020, 125, e2019JD031849. [Google Scholar] [CrossRef]
- Trošt Sedej, T.; Erznožnik, T.; Rovtar, J. Effect of UV radiation and altitude characteristics on the functional traits and leaf optical properties in Saxifraga hostii at the alpine and montane sites in the Slovenian Alps. Photochem. Photobiol. Sci. 2020, 19, 180–192. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, V.L.; Lim, H.W. Sunscreens in the management of photodermatoses. Ski. Ther. Lett. 2010, 15, 1–3. [Google Scholar]
- Kaddurah, H.; Braunberger, T.L.; Vellaichamy, G.; Nahhas, A.F.; Lim, H.W.; Hamzavi, I.H. The Impact of Sunlight on Skin Aging. Curr. Geriatr. Rep. 2018, 7, 228–237. [Google Scholar] [CrossRef]
- Subhadarshani, S.; Athar, M.; Elmets, C.A. Photocarcinogenesis. Curr. Dermatol. Rep. 2020, 9, 189–199. [Google Scholar] [CrossRef]
- Madan, K.; Nanda, S. In-vitro evaluation of antioxidant, anti-elastase, anti-collagenase, anti-hyaluronidase activities of safranal and determination of its sun protection factor in skin photoaging. Bioorgan. Chem. 2018, 77, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sherje, A.P. Development of resveratrol and green tea sunscreen formulation for combined photoprotective and antioxidant properties. J. Drug Deliv. Sci. Technol. 2020, 60, 102000. [Google Scholar] [CrossRef]
- Rastogi, R.P.; Sonani, R.R.; Madamwar, D. UV Photoprotectants From Algae-Synthesis and Bio-Functionalities. In Algal Green Chemistry: Recent Progress in Biotechnology; Elsevier: Amsterdam, The Netherlands, 2017; pp. 17–38. [Google Scholar]
- Torres, A.E.; Luk, K.M.; Lim, H.W. Botanicals for photoprotection. Plast. Aesthetic Res. 2020, 7, 57. [Google Scholar] [CrossRef]
- Mejía-Giraldo, J.C.; Scaiano, J.C.; Gallardo-cabrera, C.; Puertas-Mejía, M.A. Photoprotection and Photostability of a New Hybrid Biomaterial. Antioxidants 2021, 10, 1904. [Google Scholar] [CrossRef]
- Dewanjee, S.; Dua, T.K.; Bhattacharjee, N.; Das, A.; Gangopadhyay, M.; Khanra, R.; Joardar, S.; Riaz, M.; Feo, V.D.; Zia-Ul-Haq, M. Natural Products as Alternative Choices for P-Glycoprotein (P-gp) Inhibition. Molecules 2017, 22, 871. [Google Scholar] [CrossRef]
- Castañeda-Gómez, J.; Lavias-Hernández, P.; Fragoso-Serrano, M.; Lorence, A.; Pereda-Miranda, R. Acylsugar diversity in the resin glycosides from Ipomoea tricolor seeds as chemosensitizers in breast cancer cells. Phytochem. Lett. 2019, 32, 77–82. [Google Scholar] [CrossRef]
- Pham, T.H.; Thomas, R.; Schwab, C.; Martinez, M.M.; Vidal, N.P. Unraveling the neutral and polar lipidome of Nordic brown macroalgae: A sustainable source of functional lipids. Food Chem. 2024, 459, 140415. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.-s.; Wang, Z.-g. Glyceroglycolipids in marine algae: A review of their pharmacological activity. Front. Pharmacol. 2022, 13, 1008797. [Google Scholar] [CrossRef] [PubMed]
- Abdelrheem, D.A.; Rahman, A.A.; Elsayed, K.N.M.; Abd El-Mageed, H.R.; Mohamed, H.S.; Ahmed, S.A. Isolation, characterization, in vitro anticancer activity, dft calculations, molecular docking, bioactivity score, drug-likeness and admet studies of eight phytoconstituents from brown alga Sargassum platycarpum. J. Mol. Struct. 2021, 1225, 129245. [Google Scholar] [CrossRef]
- Plouguerné, E.; de Souza, L.M.; Sassaki, G.L.; Hellio, C.; Trepos, R.; da Gama, B.A.P.; Pereira, R.C.; Barreto-Bergter, E. Glycoglycerolipids From Sargassum vulgare as Potential Antifouling Agents. Front. Mar. Sci. 2020, 7, 116. [Google Scholar] [CrossRef]
- Fomenko, S.E.; Kushnerova, N.F.; Sprygin, V.G.; Drugova, E.S.; Lesnikova, L.N.; Merzlyakov, V.Y.; Momot, T.V. Lipid Composition, Content of Polyphenols, and Antiradical Activity in Some Representatives of Marine Algae. Russ. J. Plant Physiol. 2019, 66, 942–949. [Google Scholar] [CrossRef]
- Santos, J.; Guihéneuf, F.; Fleming, G.; Chow, F.; Stengel, D. Temporal stability in lipid classes and fatty acid profiles of three seaweed species from the north-eastern coast of Brazil. Algal Res. 2019, 41, 101572. [Google Scholar] [CrossRef]
- Logvinov, S.; Gerasimenko, N.; Esipov, A.; Denisenko, V.A. Examination of the structures of several glycerolipids from marine macroalgae by NMR and GC-MS. J. Phycol. 2015, 51, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Rahelivao, M.P.; Gruner, M.; Andriamanantoanina, H.; Bauer, I.; Knölker, H.-J. Brown Algae (Phaeophyceae) from the Coast of Madagascar: Preliminary Bioactivity Studies and Isolation of Natural Products. Nat. Prod. Bioprospecting 2015, 5, 223–235. [Google Scholar] [CrossRef]
- Ma, A.-C.; Chen, Z.; Wang, T.; Song, N.; Yan, Q.; Fang, Y.-C.; Guan, H.-S.; Liu, H.-B. Isolation of the molecular species of monogalactosyldiacylglycerols from brown edible seaweed Sargassum horneri and their inhibitory effects on triglyceride accumulation in 3T3-L1 adipocytes. J. Agric. Food Chem. 2014, 62, 11157–11162. [Google Scholar] [CrossRef]
- Tsai, C.-J.; Sun Pan, B. Identification of sulfoglycolipid bioactivities and characteristic fatty acids of marine macroalgae. J. Agric. Food Chem. 2012, 60, 8404–8410. [Google Scholar] [CrossRef]
- Sanina, N.M.; Kostetsky, E.Y.; Shnyrov, V.L.; Tsybulsky, A.V.; Novikova, O.D.; Portniagina, O.Y.; Vorobieva, N.S.; Mazeika, A.N.; Bogdanov, M.V. The influence of monogalactosyldiacylglycerols from different marine macrophytes on immunogenicity and conformation of protein antigen of tubular immunostimulating complex. Biochimie 2012, 94, 1048–1056. [Google Scholar] [CrossRef] [PubMed]
- Plouguerné, E.; Ioannou, E.; Georgantea, P.; Vagias, C.; Roussis, V.; Hellio, C.; Kraffe, E.; Stiger-Pouvreau, V. Anti-microfouling activity of lipidic metabolites from the invasive brown alga Sargassum muticum (Yendo) Fensholt. Mar. Biotechnol. 2010, 12, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Kim, E.-H.; Lee, C.; Kim, M.-H.; Rho, J.-R. Two new monogalactosyl diacylglycerols from brown alga Sargassum thunbergii. Lipids 2007, 42, 395–399. [Google Scholar] [CrossRef]
- Hossain, Z.; Kurihara, H.; Hosokawa, M.; Takahashi, K. Growth inhibition and induction of differentiation and apoptosis mediated by sodium butyrate in Caco-2 cells with algal glycolipids. In Vitro Cell. Dev. Biology Anim. 2005, 41, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-González, G.; Jacobo-Herrera, N.; Zentella-Dehesa, A.; Pereda-Miranda, R. Reversal of multidrug resistance by morning glory resin glycosides in human breast cancer cells. J. Nat. Prod. 2012, 75, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Rydz, E.; Harper, A.; Leong, B.; Arrandale, V.H.; Kalia, S.; Forsman-Phillips, L.; Holness, D.L.; Tenkate, T.; Peters, C.E. Solar ultraviolet radiation exposure among outdoor workers in Alberta, Canada. Environ. Res. 2020, 189, 109902. [Google Scholar] [CrossRef]
- World Health Organization. Radiation and health. Available online: https://www.who.int/news-room/questions-and-answers/item/radiation-and-health (accessed on 10 December 2024).
- Garnacho Saucedo, G.M.; Salido Vallejo, R.; Moreno Giménez, J.C. Effects of solar radiation and an update on photoprotection. An. Pediatría 2020, 92, 377.e1–377.e9. [Google Scholar] [CrossRef]
- De la Coba, F.; Aguilera, J.; de Gálvez, M.V.; Álvarez, M.; Gallego, E.; Figueroa, F.L.; Herrera, E. Prevention of the ultraviolet effects on clinical and histopathological changes, as well as the heat shock protein-70 expression in mouse skin by topical application of algal UV-absorbing compounds. J. Dermatol. Sci. 2009, 55, 161–169. [Google Scholar] [CrossRef] [PubMed]
- De la Coba, F.; Aguilera, J.; Figueroa, F.L.; de Gálvez, M.V.; Herrera, E. Antioxidant activity of mycosporine-like amino acids isolated from three red macroalgae and one marine lichen. J. Appl. Phycol. 2009, 21, 161–169. [Google Scholar] [CrossRef]
- Torres, A.; Enk, C.D.; Hochberg, M.; Srebnik, M. Porphyra-334, a potential natural source for UVA protective sunscreens. Photochem. Photobiol. Sci. 2006, 5, 432–435. [Google Scholar] [CrossRef] [PubMed]
- Wada, N.; Sakamoto, T.; Matsugo, S. Mycosporine-like amino acids and their derivatives as natural antioxidants. Antioxidants 2015, 4, 603–646. [Google Scholar] [CrossRef]
- Nishida, Y.; Saburi, W.; Miyabe, Y.; Kishimura, H.; Kumagai, Y. Characterization of Antioxidant Activity of Heated Mycosporine-like Amino Acids from Red Alga Dulse Palmaria palmata in Japan. Mar. Drugs 2022, 20, 184. [Google Scholar] [CrossRef]
- Tarasuntisuk, S.; Palaga, T.; Kageyama, H.; Waditee-Sirisattha, R. Mycosporine-2-glycine exerts anti-inflammatory and antioxidant effects in lipopolysaccharide (LPS)-stimulated RAW 264.7 macrophages. Arch. Biochem. Biophys. 2019, 662, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.M.; Alonso-Varona, A.; Palomares, T.; Zubillaga, V.; Labidi, J.; Bulone, V. Exploiting Mycosporines as Natural Molecular Sunscreens for the Fabrication of UV-Absorbing Green Materials. ACS Appl. Mater. Interfaces 2015, 7, 16558–16564. [Google Scholar] [CrossRef] [PubMed]
- Lalegerie, F.; Lajili, S.; Bedoux, G.; Taupin, L.; Stiger-Pouvreau, V.; Connan, S. Photo-protective compounds in red macroalgae from Brittany: Considerable diversity in mycosporine-like amino acids (MAAs). Mar. Environ. Res. 2019, 147, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K.P.; Long, P.F.; Young, A.R. Mycosporine-Like Amino Acids for Skin Photoprotection. Curr. Med. Chem. 2017, 25, 5512–5527. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.-S.; Hwang, J.; Park, M.; Seo, H.H.; Kim, H.-S.; Lee, J.H.; Moh, S.H.; Lee, T.-K. Anti-Inflammation Activities of Mycosporine-Like Amino Acids (MAAs) in Response to UV Radiation Suggest Potential Anti-Skin Aging Activity. Mar. Drugs 2014, 12, 5174–5187. [Google Scholar] [CrossRef]
- Orfanoudaki, M.; Hartmann, A.; Alilou, M.; Gelbrich, T.; Planchenault, P.; Derbré, S.; Schinkovitz, A.; Richomme, P.; Hensel, A.; Ganzera, M. Absolute Configuration of Mycosporine-Like Amino Acids, Their Wound Healing Properties and In Vitro Anti-Aging Effects. Mar. Drugs 2020, 18, 35. [Google Scholar] [CrossRef] [PubMed]
- De la Coba, F.; Aguilera, J.; Korbee, N.; de Gálvez, M.V.; Herrera-Ceballos, E.; Álvarez-Gómez, F.; Figueroa, F.L. UVA and UVB Photoprotective capabilities of topical formulations containing mycosporine-like amino acids (maas) through different biological effective protection factors (BEPFs). Mar. Drugs 2019, 17, 55. [Google Scholar] [CrossRef]
- Browne, N.; Otero, P.; Murray, P.; Saha, S.K. Rapid Screening for Mycosporine-like Amino Acids (MAAs) of Irish Marine Cyanobacteria and Their Antioxidant Potential. Sustainability 2023, 15, 3792. [Google Scholar] [CrossRef]
- Candelo, V.; Llewellyn, C.A. Separating and Purifying Mycosporine-like Amino Acids from Cyanobacteria for Application in Commercial Sunscreen Formulations. BioTech 2023, 12, 16. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Pichelt, F.; Castenholz, R.W. Occurrence of UV-Absorbing, Mycosporine-Like Compounds among Cyanobacterial Isolates and an Estimate of Their Screening Capacity. Appl. Environ. Microbiol. 1993, 59, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Waditee-Sirisattha, R.; Kageyama, H. Protective effects of mycosporine-like amino acid-containing emulsions on UV-treated mouse ear tissue from the viewpoints of antioxidation and antiglycation. J. Photochem. Photobiol. B 2021, 223, 112296. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, A.; Gostner, J.; Fuchs, J.E.; Chaita, E.; Aligiannis, N.; Skaltsounis, L.; Ganzera, M. Inhibition of Collagenase by Mycosporine-like Amino Acids from Marine Sources. Planta Medica 2015, 81, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Jin, X.; Jin, S.; Zhang, D.; Chen, Q.; Jin, X.; Wang, C.; Qian, G.; Ding, H. Anti-Leukemia Activity of Polysaccharide from Sargassum fusiforme via the PI3K/AKT/BAD Pathway In Vivo and In Vitro. Mar. Drugs 2023, 21, 289. [Google Scholar] [CrossRef]
- Yadav, R.; Kumaravelu, P.; Umamaheswari, S.; Subramanian, V.; Kantipudi, S.J. Evaluation of Preclinical Toxicity of Methanolic Extract of Sargassum tenerrimum using the Zebrafish Model. J. Clin. Diagn. Res. 2024, 18, 1–6. [Google Scholar] [CrossRef]

| Species | Characterization Technique | Key Features |
|---|---|---|
| S. cinereum [21] | UV and NMR | The researchers found a glucopyranosylglycerol type glycolipid that can inhibit the growth of HepG2, MCF-7, and Caco-2 cells. The compound selectively inhibits the enzymes 5-LOX and 15-LOX, which are involved in the development of several types of cancer. In silico tests, including docking, MDS, and free energy binding, reveal that the amphipathic character of the compound is crucial in the interaction with the active sites of both enzymes, which explains its moderate antiproliferative activity. |
| S. platycarpum [70] | NMR, EI/MS, and GC/MS | A glycolipid of the MGDG-type, which was previously reported, exhibited significant cytotoxic activity against HepG-2 cells when compared to the standard 5-fluorouracil. |
| S. vulgare [71] | NMR, ESI-MS, and CID-MS | The studies showed the chemical characterization of one MGDG (3), one DGDG (4), and six SQDGs (5–10). |
| S. pallidum [72] | GLC | The study of the lipid complex of the extract showed that glycolipids constituted 41.5% of the total lipids. The analysis of fatty acids revealed that the C16:0, C18:2, and C20:4 acids are the most prevalent. Additionally, the extract contains a significant amount of n-6 PUFA, representing 41.3%, with C18 to C20 carbon atoms. |
| S. vulgare [73] | GC/MSD | The extract showed high levels of SFA, with the C16:0 and C14:0 acids being the most prominent. Among the PUFAs, C18:2 and C18:3 acids stood out, while MUFAs mainly contained the C18:1 acid. The lipid analysis did not show any significant differences between the different collection times. |
| S. pallidum [74] | GC-MS and NMR | The chloroform/methanol extract contained different types of glycerolipids, which were identified through analysis of C-H coupling using NMR. Chemical shifts revealed the presence of galactolipids (MGMG, MGDG, and DGDG) and sulfoglycolipids (SQDG), especially the hydrophilic part. The fatty acid composition of the glycerol lipids was investigated. The main fatty acids in MGMG were the C18:2, C16:0, and C20:5 acids; in MGDG, C16:0 and C18:2; in DGDG, C20:5 and C18:3s; and in SQDG, C16:0. |
| S. incisifolium [75] | UV-VIS, ATR, NMR, GC, and ESI-MS | Extraction with ethyl acetate resulted in the isolation of two glycolipid-enriched fractions, one of which was identified as MGDG. The sugar residue was identified as β-D-galactose and the fatty acid chains were identified as the C18:3 and C16:0 acids, which were reported for the first time for this species. |
| S. horneri [76] | NMR, GC-FID, HPLC−MS/MS, and ESI−QITMS | A total of ten molecular species of MGDGs were characterized by comparing the NMR spectra with previously reported data. The MGDGs identified in the present study mainly contained the C14:0 and C16:0 SFAs in the sn-1 position and the C18 and C16 UFAs at the sn-2 position of the glycerol backbone. |
| S. crassifolium and S. cristaefolium [77] | GC | Analysis of the FA composition of the extract revealed that C16:0 acid was the predominant SFA, while the most prominent UFAs were C18:1, C18:4, C20:4, and C20:5. |
| S. pallidum [78] | GC | The polar lipids were characterized at two different collection times (summer and winter), and the main constituents identified were MGDG, DGDG, and SQDG. During the summer season, C16:0, C18:2, C20:4, and C20:5 were the most abundant FAs for MGDG and DGDG. However, for SQDG, the predominant FAs were C16:0 and C17:0. In summer, MGDG and DGDG had a higher proportion of SFA and MUFA, while SQDG showed this trend in winter. Additionally, PUFAs were more abundant in winter for all glycolipid types. |
| S. muticum [79] | GC-MS and NMR | The chloroform extract was purified, resulting in twelve fractions containing both saturated and unsaturated linear hydrocarbons. Upon analyzing a subfraction in detail, MGDG was identified. The FA analysis of this galactolipid showed that the C14:0, C16:0, and C18:1 acids were the most abundant. In addition, the study found that SFAs were more prevalent than MUFAs and 1PUFAs. |
| S. thunbergia [80] | CID-MS/MS and NMR | Four MGDGs were isolated from the polar fraction of the methanolic extract. Compounds 21 and 22 were isolated for the first time, while the others were identified by comparing their spectral and physical data with previously reported compounds. |
| S. horneri [81] | GC | Conventional chromatography techniques were used to identify DGDG and SQDG. The lipid analysis revealed that the main FAs in DGDG were C16:0, C18:4, C20:4, and C20:5, while those in SQDG were the C16:0, C18:1, C18:4, C20:1, C20:4, and C20:5 acids. Glycolipids showed a potent ability to inhibit Caco-2 growth when co-administered with NaBT (an anticancer drug). In addition, the glycolipids had no toxic effects on normal human colon cell lines. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Losada, K.J.; Gallego-Villada, M.; Puertas-Mejía, M.A. An Overview of Sargassum Seaweed as Natural Anticancer Therapy. Future Pharmacol. 2025, 5, 5. https://doi.org/10.3390/futurepharmacol5010005
Muñoz-Losada KJ, Gallego-Villada M, Puertas-Mejía MA. An Overview of Sargassum Seaweed as Natural Anticancer Therapy. Future Pharmacology. 2025; 5(1):5. https://doi.org/10.3390/futurepharmacol5010005
Chicago/Turabian StyleMuñoz-Losada, Kelly Johanna, Manuela Gallego-Villada, and Miguel Angel Puertas-Mejía. 2025. "An Overview of Sargassum Seaweed as Natural Anticancer Therapy" Future Pharmacology 5, no. 1: 5. https://doi.org/10.3390/futurepharmacol5010005
APA StyleMuñoz-Losada, K. J., Gallego-Villada, M., & Puertas-Mejía, M. A. (2025). An Overview of Sargassum Seaweed as Natural Anticancer Therapy. Future Pharmacology, 5(1), 5. https://doi.org/10.3390/futurepharmacol5010005








