Water-Soluble Cu(II) Complexes with Polypyridyl Ligands: Anticancer Activity and DNA Interaction
Abstract
1. Introduction
2. Materials and Methods
2.1. General Methods
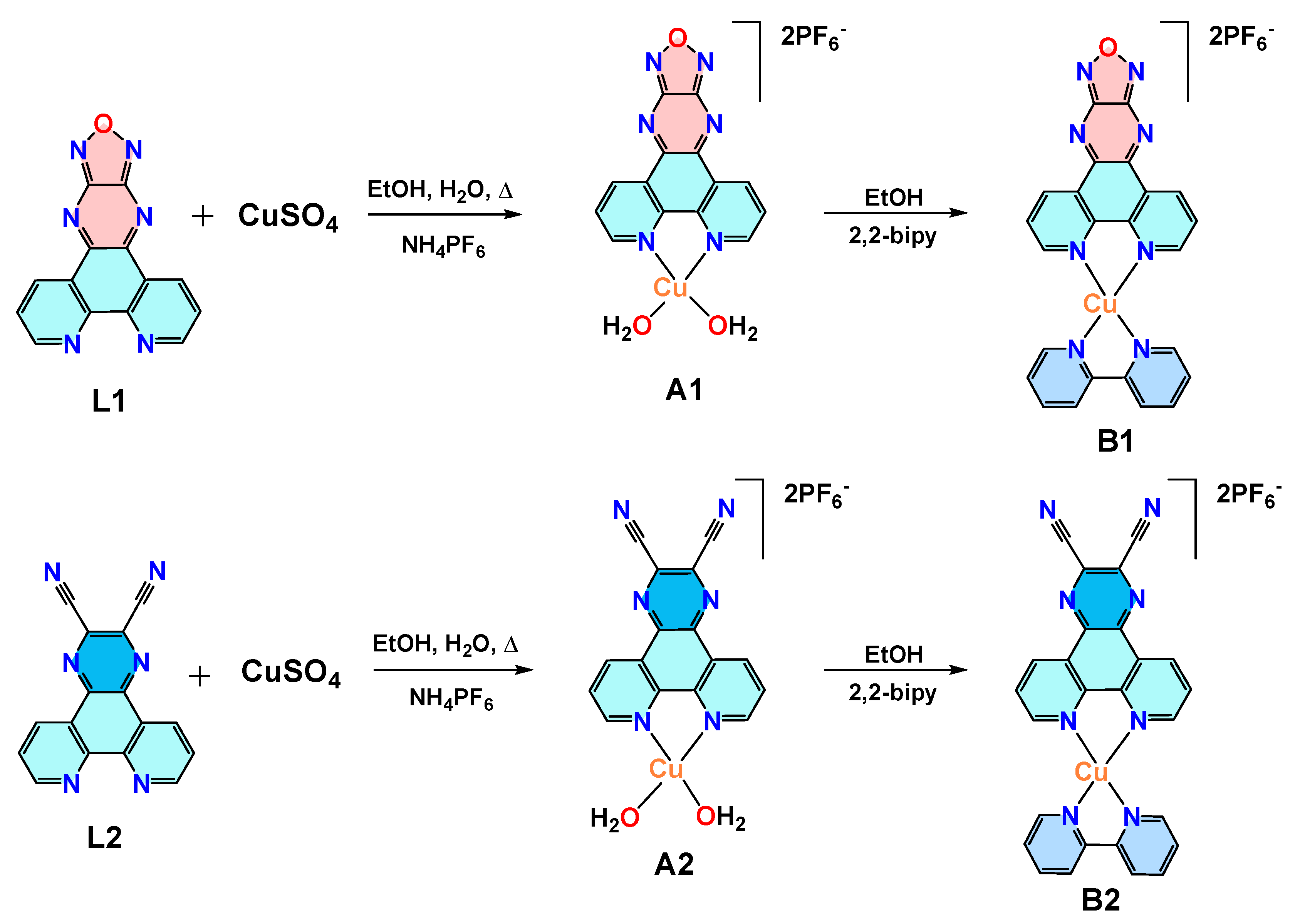
2.2. Synthesis of Ligands L1 and L2
2.3. Synthesis of Complex [Cu(L1)(H2O)2](PF6)2 (A1) and [Cu(L2)(H2O)2](PF6)2 (A2)
2.4. Synthesis of Complex [Cu(L1)(bipy)](PF6)2 (B1) and [Cu(L2)(bipy)](PF6)2 (B2)
2.5. Molecular Docking
2.6. Cell Viability Assay
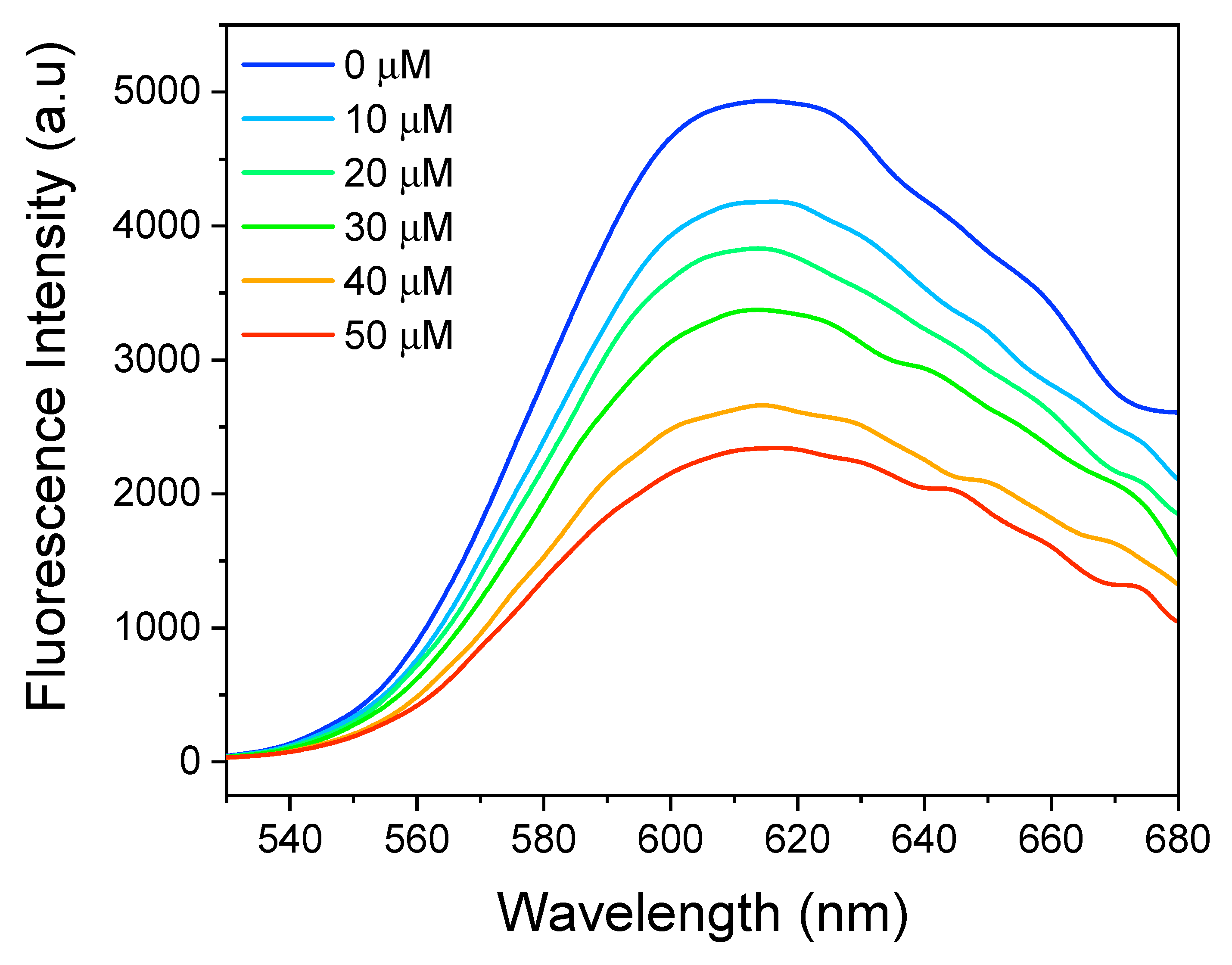
2.7. DNA Interaction Study Using Ethidium Bromide
3. Results
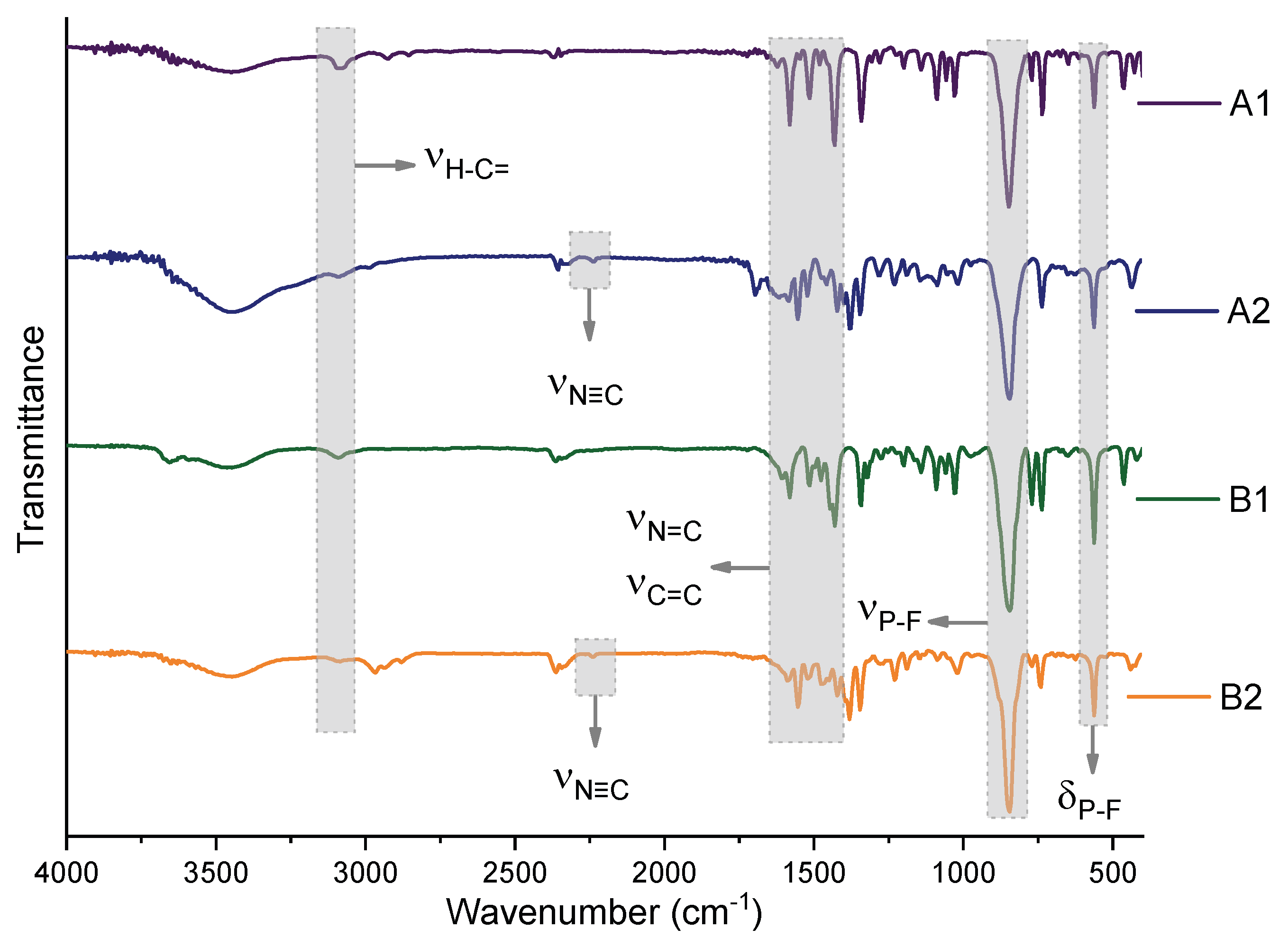
3.1. Synthesis and Characterization
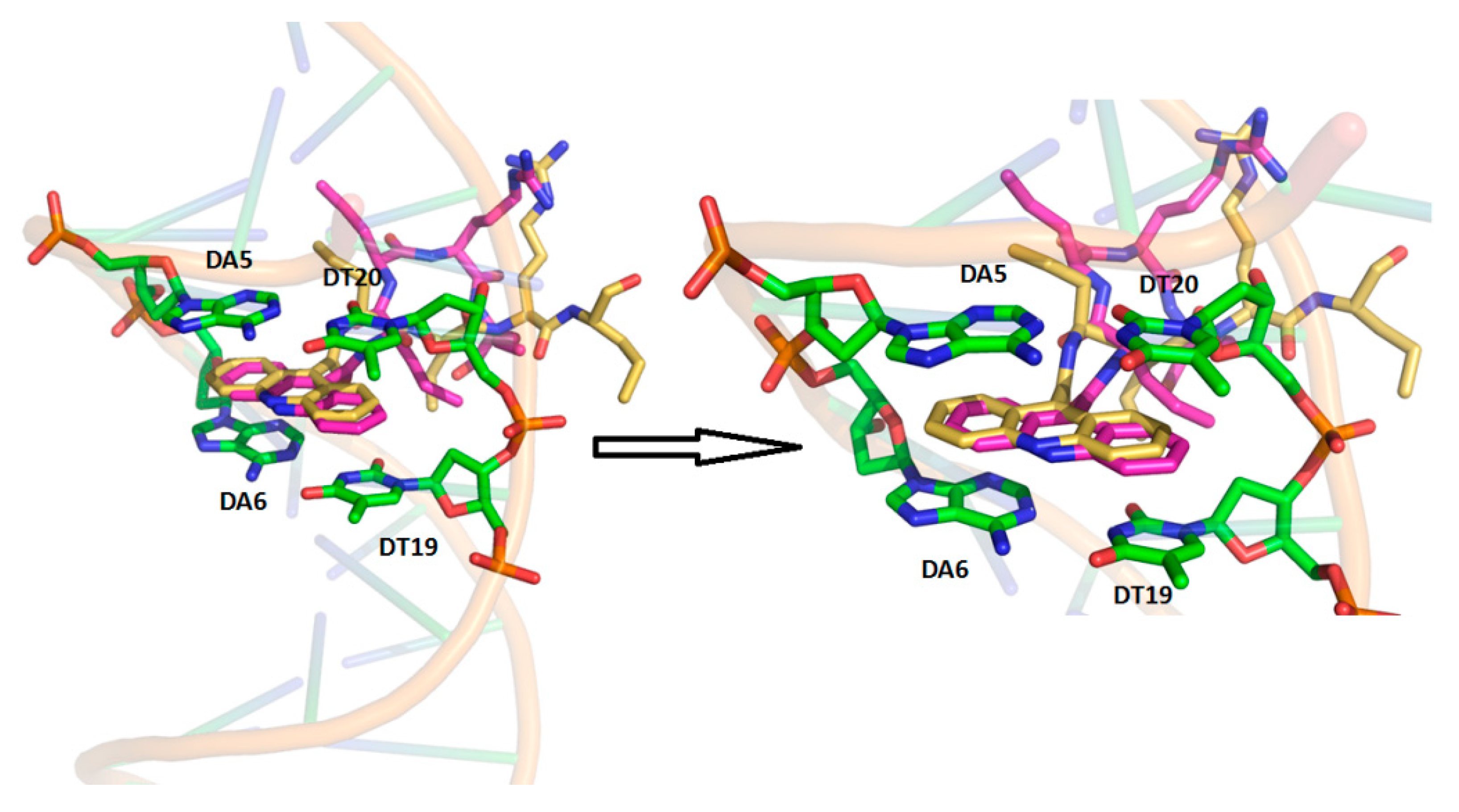
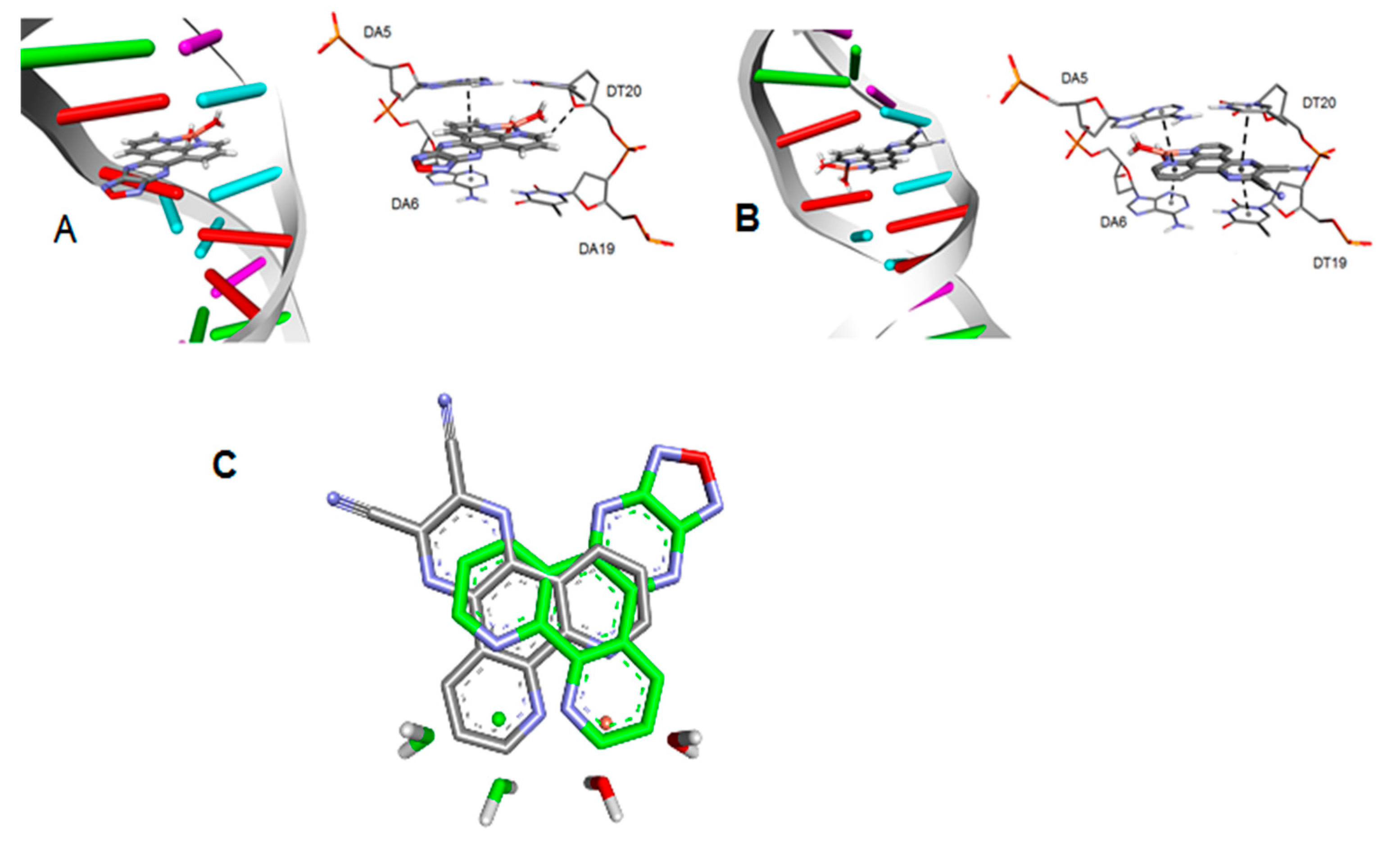
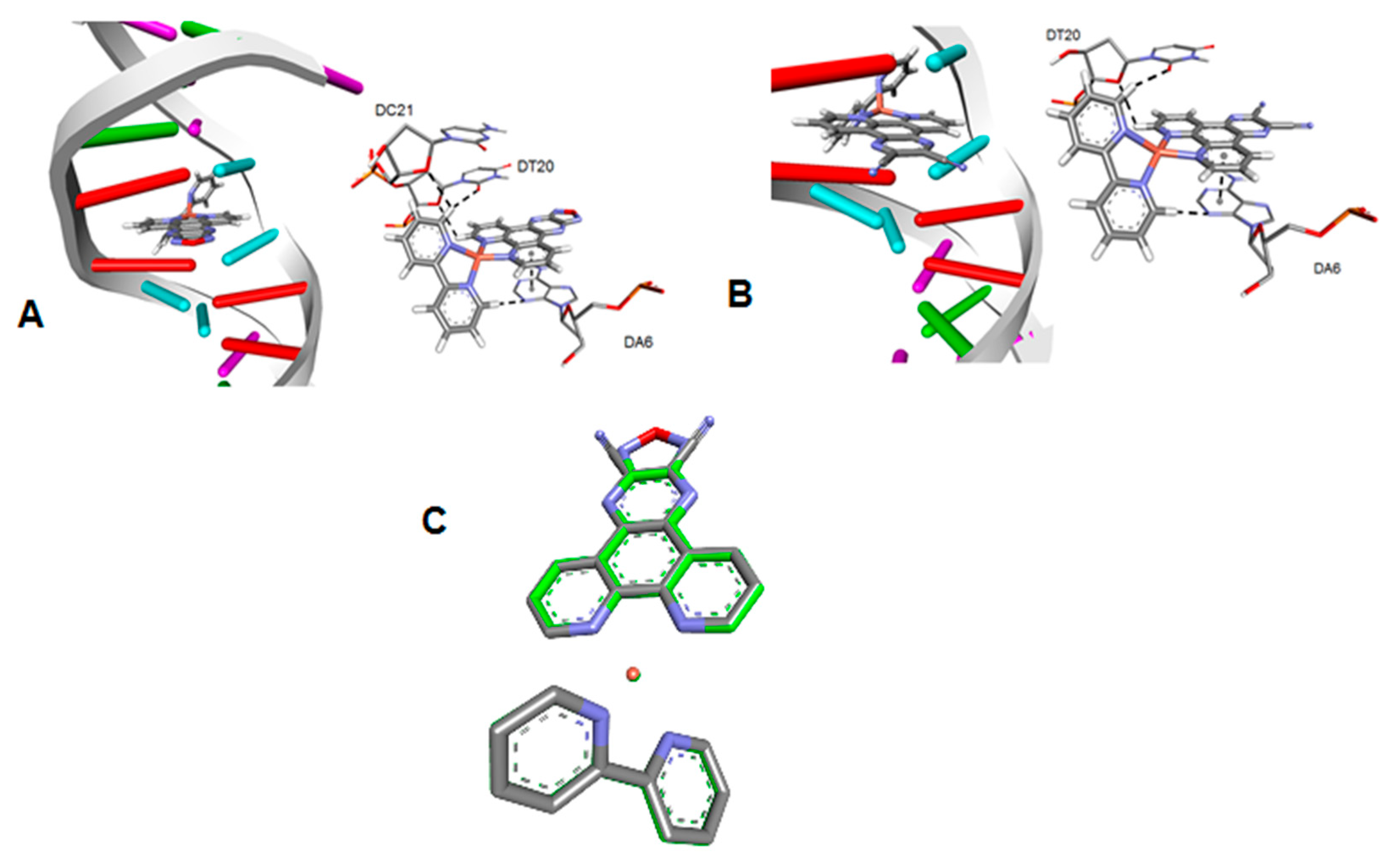
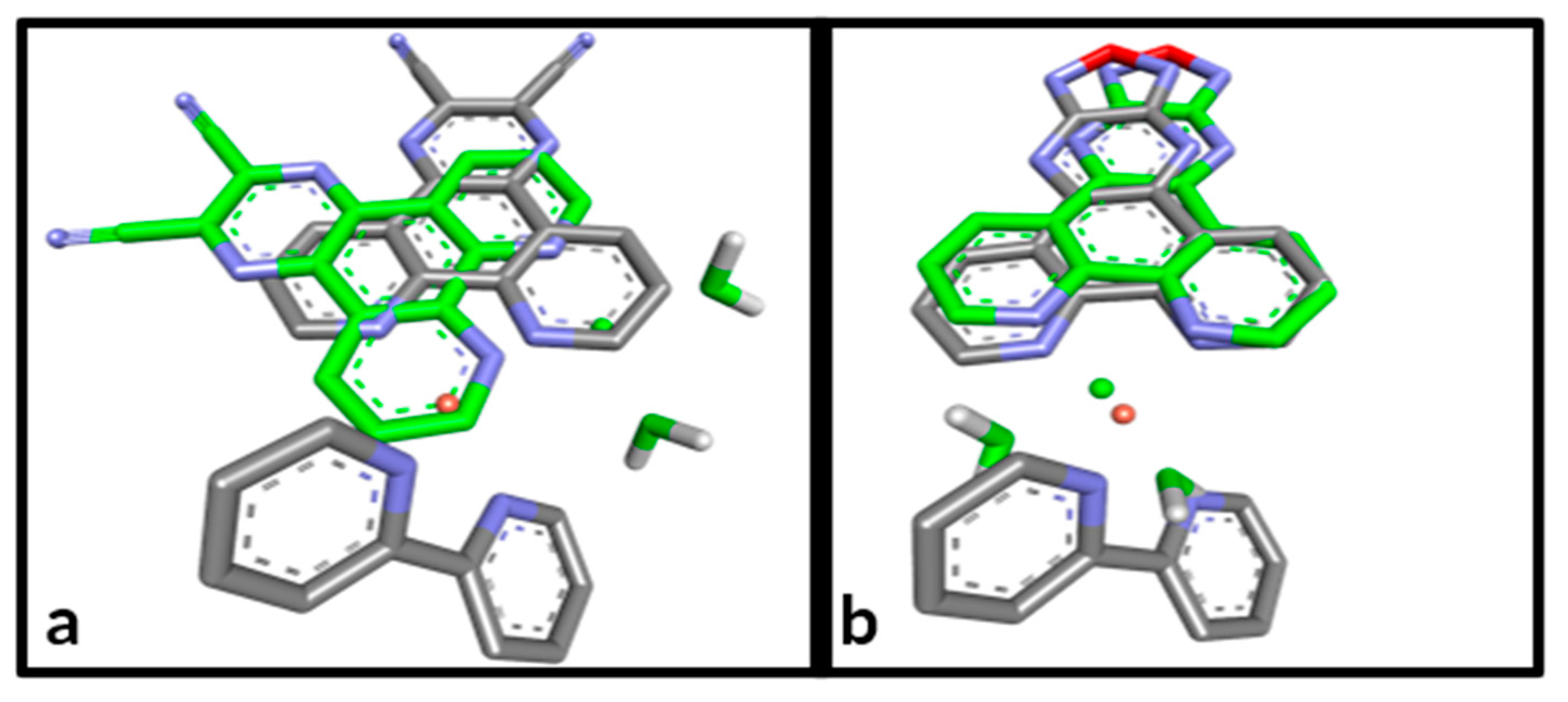
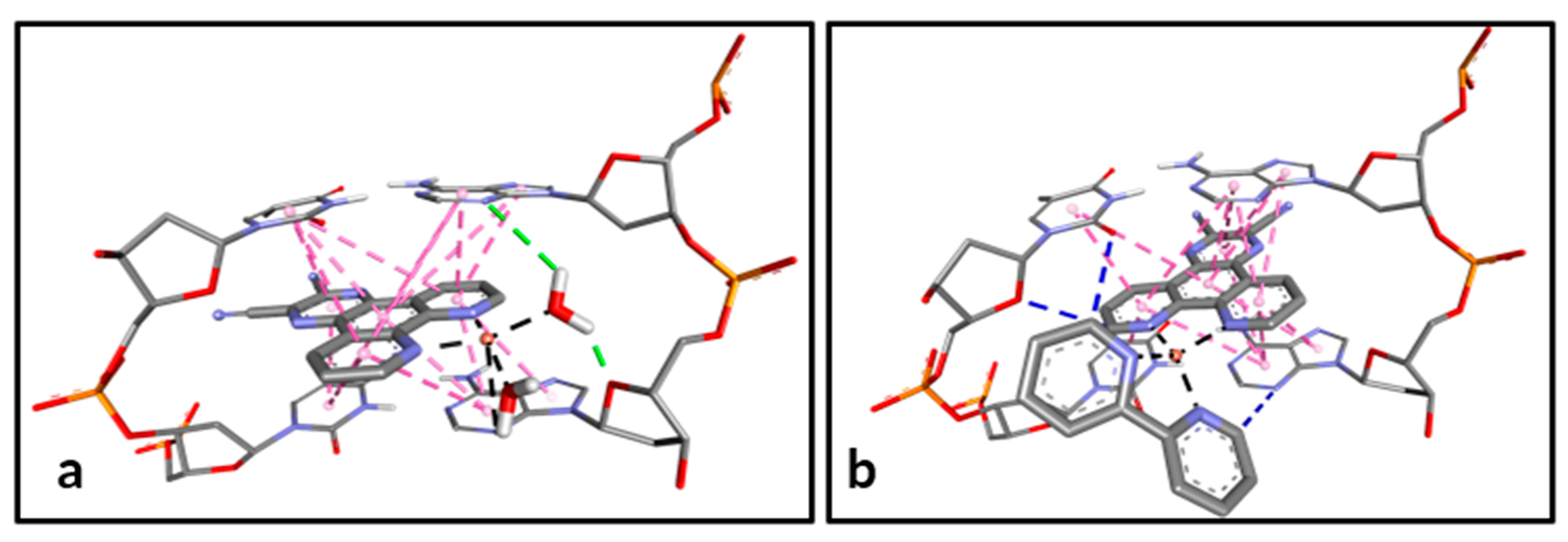
3.2. Molecular Docking
3.3. Cytotoxicity Assay
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Silva, D.A.; De Luca, A.; Squitti, R.; Rongioletti, M.; Rossi, L.; Machado, C.M.; Cerchiaro, G. Copper in tumors and the use of copper-based compounds in cancer treatment. J. Inorg. Biochem. 2022, 226, 111634. [Google Scholar] [CrossRef]
- Guan, D.; Zhao, L.; Shi, X.; Ma, X.; Chen, Z. Copper in cancer: From pathogenesis to therapy. Biomed. Pharmacother. 2023, 163, 114791. [Google Scholar] [CrossRef] [PubMed]
- Palmeira-Mello, M.V.; Caballero, A.B.; Lopez-Espinar, A.; Guedes, G.P.; Caubet, A.; de Souza, A.M.T.; Lanznaster, M.; Gamez, P. DNA-interacting properties of two analogous square-planar cis-chlorido complexes: Copper versus palladium. JBIC J. Biol. Inorg. Chem. 2021, 26, 727–740. [Google Scholar] [CrossRef] [PubMed]
- Erxleben, A. Interactions of copper complexes with nucleic acids. Coord. Chem. Rev. 2018, 360, 92–121. [Google Scholar] [CrossRef]
- Oliveira, C.; Oliveira, A.; Pelinski, L.; Cailliau, K. Complexos de cobre como agentes anticancerígenos direcionados às topoisomerases I e II. Cânceres 2020, 12, 2863. [Google Scholar]
- Ghorbanpour, M.; Shayanfar, A.; Soltani, B. Copper pyrazole complexes as potential anticancer agents: Evaluation of cytotoxic response against cancer cells and their mechanistic action at the molecular level. Coord. Chem. Rev. 2024, 498, 215459. [Google Scholar] [CrossRef]
- Nurmamat, M.; Yan, H.; Wang, R.; Zhao, H.; Li, Y.; Wang, X.; Nurmaimaiti, K.; Kurmanjiang, T.; Luo, D.; Baodi, J.; et al. Novel copper (II) complex with a 4-acylpyrazolone derivative and coligand induce apoptosis in liver cancer cells. ACS Med. Chem. Lett. 2021, 12, 467–476. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.S.; Reena, V.N. Design, synthesis, crystal structure, antitumour and antimicrobial evaluation of a novel substituted pyrazole and its some metal complexes. Mater. Today Proc. 2022, 62, 5427–5433. [Google Scholar] [CrossRef]
- Teles, R.H.G.; Graminha, A.E.; Rivera-Cruz, C.M.; Nakahata, D.H.; Formiga, A.L.B.; Corbi, P.P.; Figueiredo, M.L.; Cominetti, M.R. Copper transporter 1 affinity as a delivery strategy to improve the cytotoxic profile of rationally designed copper (II) complexes for cancer treatment. Toxicol. Vitr. 2020, 67, 104922. [Google Scholar] [CrossRef]
- Bollu, V.S.; Bathini, T.; Barui, A.K.; Roy, A.; Ragi, N.C.; MalOth, S.; Sripadi, P.; Sreedhar, B.; Nagababu, P.; Patra, C.R. Design of DNA-intercalators based copper (II) complexes, investigation of their potential anti-cancer activity and sub-chronic toxicity. Mater. Sci. Eng. C 2019, 105, 110079. [Google Scholar] [CrossRef]
- Masuri, S.; Vaňhara, P.; Cabiddu, M.G.; Moráň, L.; Havel, J.; Cadoni, E.; Pivetta, T. Copper (II) phenanthroline-based complexes as potential anticancer drugs: A walkthrough on the mechanisms of action. Molecules 2021, 27, 49. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Oliveira, R.L.; Oliveira, M.; Soerjomataram, I.; Oliveira, A.; Bray, F. Estatísticas Globais do Câncer 2020: Estimativas GLOBOCAN de incidência e mortalidade em todo o mundo para 36 cânceres em 185 países. CA Câncer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.S.; Pereira, G.B.S.; Oliveira, G.P.; Lima, M.A.; Araujo-neto, J.H.; Pinto, L.S.; Forim, M.R.; Zanetti, R.D.; Netto, A.V.G.; Castellano, E.E.; et al. Synthesis and characterization of silver(I) complexes bearing phenanthroline derivatives as ligands: Cytotoxicity and DNA interaction evaluation. Inorg. Chem. Commun. 2021, 131, 108757. [Google Scholar] [CrossRef]
- Malinina, L.; Soler-López, M.; Aymamí, J.; Subirana, J.A. Intercalation of an Acridine—Peptide Drug in an AA/TT Base Step in the Crystal Structure of [d (CGCGAATTCGCG)] 2 with Six Duplexes and Seven Mg2+ Ions in the Asymmetric Unit. Biochemistry 2002, 41, 9341–9348. [Google Scholar] [CrossRef] [PubMed]
- Waszkowycz, B. Towards improving compound selection in structure-based virtual screening. Drug Discov. Today 2008, 13, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Pagadala, N.S.; Syed, K.; Tuszynski, J. Software for molecular docking: A review. Biophys. Rev. 2017, 9, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Delano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, Venezuela, 2002. [Google Scholar]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular nonbonded contact distances in organic crystal structures: Comparison with distances expected from van der Waals radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Pinho, J.O.; da Silva, I.V.; Amaral, J.D.; Rodrigues, C.M.; Casini, A.; Soveral, G.; Gaspar, M.M. Therapeutic potential of a copper complex loaded in pH-sensitive long circulating liposomes for colon cancer management. Int. J. Pharm. 2021, 599, 120463. [Google Scholar] [CrossRef] [PubMed]
- Nafisi, S.; Saboury, A.A.; Keramat, N.; Neault, J.; Tajmir-Riahi, H. Stability and structural features of DNA intercalation with ethidium bromide, acridine orange and methylene blue. J. Mol. Struct. 2007, 827, 35–43. [Google Scholar] [CrossRef]
- Hussain, A.; AlAjmi, M.F.; Rehman, M.T.; Amir, S.; Husain, F.M.; Alsalme, A.; Siddiqui, M.A.; AlKhedhairy, A.A.; Khan, R.A. Copper (II) complexes as potential anticancer and Nonsteroidal anti-inflammatory agents: In vitro and in vivo studies. Sci. Rep. 2019, 9, 5237. [Google Scholar] [CrossRef]
- Ballance, D.G.; Bryantsev, V.S.; Ivanov, A.S.; Dai, S.; Hancock, R.D. Complexation of Lanthanides and other metal ions by the polypyridyl ligand quaterpyridine: Relation between metal ion size, chelate ring size, and complex stability. Inorganica Chim. Acta 2019, 488, 19–27. [Google Scholar] [CrossRef]
- Sergeeva, N.N.; Donnier-Marechal, M.; Vaz, G.M.; Davies, A.M.; Senge, M.O. Stability and spectral properties of europium and zinc phenanthroline complexes as luminescent probes in high content cell-imaging analysis. J. Inorg. Biochem. 2011, 105, 1589–1595. [Google Scholar] [CrossRef] [PubMed]
- Hadadzadeh, H.; Mansouri, G.; Rezvani, A.; Khavasi, H.R.; Skelton, B.W.; Makha, M.; Charati, F.R. Mononuclear nickel (II) complexes coordinated by polypyridyl ligands. Polyhedron 2011, 30, 2535–2543. [Google Scholar] [CrossRef]
- Katheria, S. Ruthenium complexes as potential cancer cell growth inhibitors for targeted chemotherapy. ChemistrySelect 2022, 7, e202201645. [Google Scholar] [CrossRef]
- Carcelli, M.; Tegoni, M.; Bartoli, J.; Marzano, C.; Pelosi, G.; Salvalaio, M.; Rogolino, D.; Gandin, V. In vitro and in vivo anticancer activity of tridentate thiosemicarbazone copper complexes: Unravelling an unexplored pharmacological target. Eur. J. Med. Chem. 2020, 194, 112266. [Google Scholar] [CrossRef] [PubMed]
- Suffness, M.; Pezzuto, J.M. Assays related to cancer drug discovery. In Methods in Plant Biochemistry; Hostettmann, K., Ed.; Academic Press: London, UK, 1991; pp. 71–133. [Google Scholar]
- Chen, Y.-C. Beware of docking! Trends Pharmacol. Sci. 2015, 36, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tang, T.; Yuan, Y.; Li, N.; Wang, X.; Guan, J. Copper and Copper Complexes in Tumor Therapy. ChemMedChem 2024, 19, e202400060. [Google Scholar] [CrossRef] [PubMed]
- Machado, P.H.A.; Paixão, D.A.; Lino, R.C.; de Souza, T.R.; de Souza Bontempo, N.J.; Sousa, L.M.; Van Petten de Vasconcelos Azevedo, F.; Orsolin, P.C.; Lima, P.; Martins, I.C.; et al. A selective CuII complex with 4-fluorophenoxyacetic acid hydrazide and phenanthroline displays DNA-cleaving and pro-apoptotic properties in cancer cells. Sci. Rep. 2021, 11, 24450. [Google Scholar] [CrossRef]
| Complex | Score | sp2 C-H⋯O | π–π | Total Contacts |
|---|---|---|---|---|
| A1 | 70.75 | 1 | 2 | 3 |
| A2 | 77.85 | 0 | 4 | 4 |
| B1 | 75.68 | 4 | 1 | 5 |
| B2 | 77.61 | 3 | 1 | 4 |
| DU 145 | MCF-7 | PNT-2 | SI * | SI ** | |
|---|---|---|---|---|---|
| L1 | 3.12 ± 0.06 | 1.44 ± 0.15 | 1.59 ± 0.06 | 0.51 | 1.10 |
| L2 | 5.83 ± 0.26 | 23.26 ± 0.85 | 8.67 ± 0.10 | 1.48 | 0.37 |
| A1 | 0.32 ± 0.03 | 0.40 ± 0.02 | 0.49 ± 0.06 | 1.53 | 1.22 |
| A2 | 2.36 ± 0.05 | 4.52 ± 0.37 | 9.15 ± 0.54 | 3.87 | 2.02 |
| B1 | 0.37 ± 0.04 | 0.47 ± 0.01 | 2.01 ± 0.05 | 5.43 | 4.27 |
| B2 | 3.66 ± 0.91 | 4.33 ± 0.15 | 5.51 ± 0.34 | 1.50 | 1.27 |
| Cisplatin | 15.0 ± 1.40 | 19.90 ± 4.20 | 11.74 ± 1.20 | 0.78 | 0.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos, H.F.; da Silva, N.N.P.; Pereira, G.B.S.; Lima, M.A.; Nascimento-Júnior, N.M.; de Farias, R.L.; Akinyemi, A.O.; Rocha, F.V. Water-Soluble Cu(II) Complexes with Polypyridyl Ligands: Anticancer Activity and DNA Interaction. Future Pharmacol. 2025, 5, 10. https://doi.org/10.3390/futurepharmacol5010010
dos Santos HF, da Silva NNP, Pereira GBS, Lima MA, Nascimento-Júnior NM, de Farias RL, Akinyemi AO, Rocha FV. Water-Soluble Cu(II) Complexes with Polypyridyl Ligands: Anticancer Activity and DNA Interaction. Future Pharmacology. 2025; 5(1):10. https://doi.org/10.3390/futurepharmacol5010010
Chicago/Turabian Styledos Santos, Herisson F., Nádija N. P. da Silva, George B. S. Pereira, Mauro A. Lima, Nailton M. Nascimento-Júnior, Renan L. de Farias, Amos O. Akinyemi, and Fillipe V. Rocha. 2025. "Water-Soluble Cu(II) Complexes with Polypyridyl Ligands: Anticancer Activity and DNA Interaction" Future Pharmacology 5, no. 1: 10. https://doi.org/10.3390/futurepharmacol5010010
APA Styledos Santos, H. F., da Silva, N. N. P., Pereira, G. B. S., Lima, M. A., Nascimento-Júnior, N. M., de Farias, R. L., Akinyemi, A. O., & Rocha, F. V. (2025). Water-Soluble Cu(II) Complexes with Polypyridyl Ligands: Anticancer Activity and DNA Interaction. Future Pharmacology, 5(1), 10. https://doi.org/10.3390/futurepharmacol5010010








