Understanding the Impact of Oxidative Stress on Ovarian Cancer: Advances in Diagnosis and Treatment
Abstract
1. Introduction
2. OC: Etiology, Epidemiology, Risk Factors, Characteristics, and Prognosis
3. ROS: General Definition, Balance and Levels of ROS, and Positive Role of ROS
3.1. Introduction to ROS
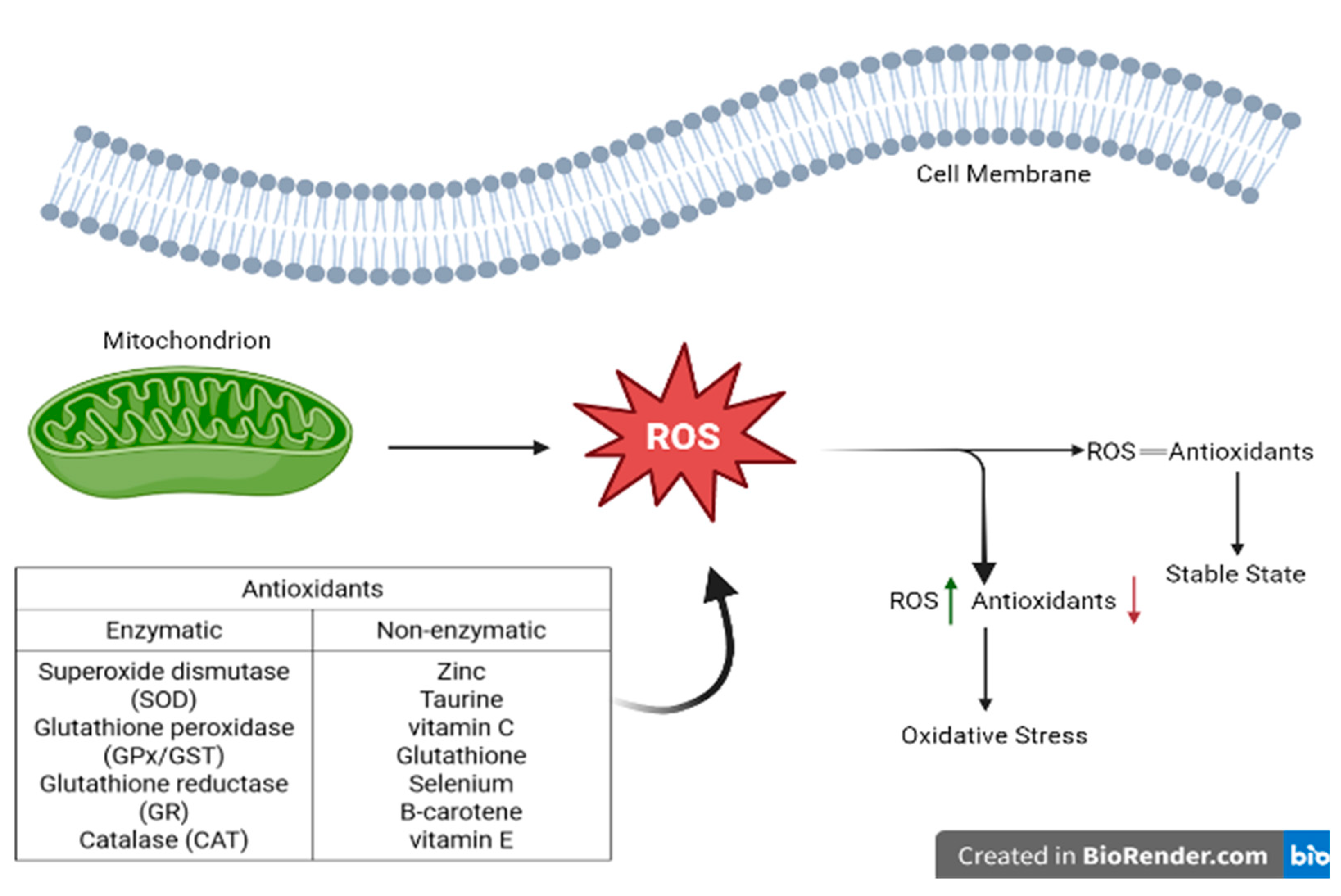
3.2. The Balance between ROS and Antioxidants
3.3. Representation of ROS and Enzymatic and Non-Enzymatic Antioxidants Balance in Healthy Cells
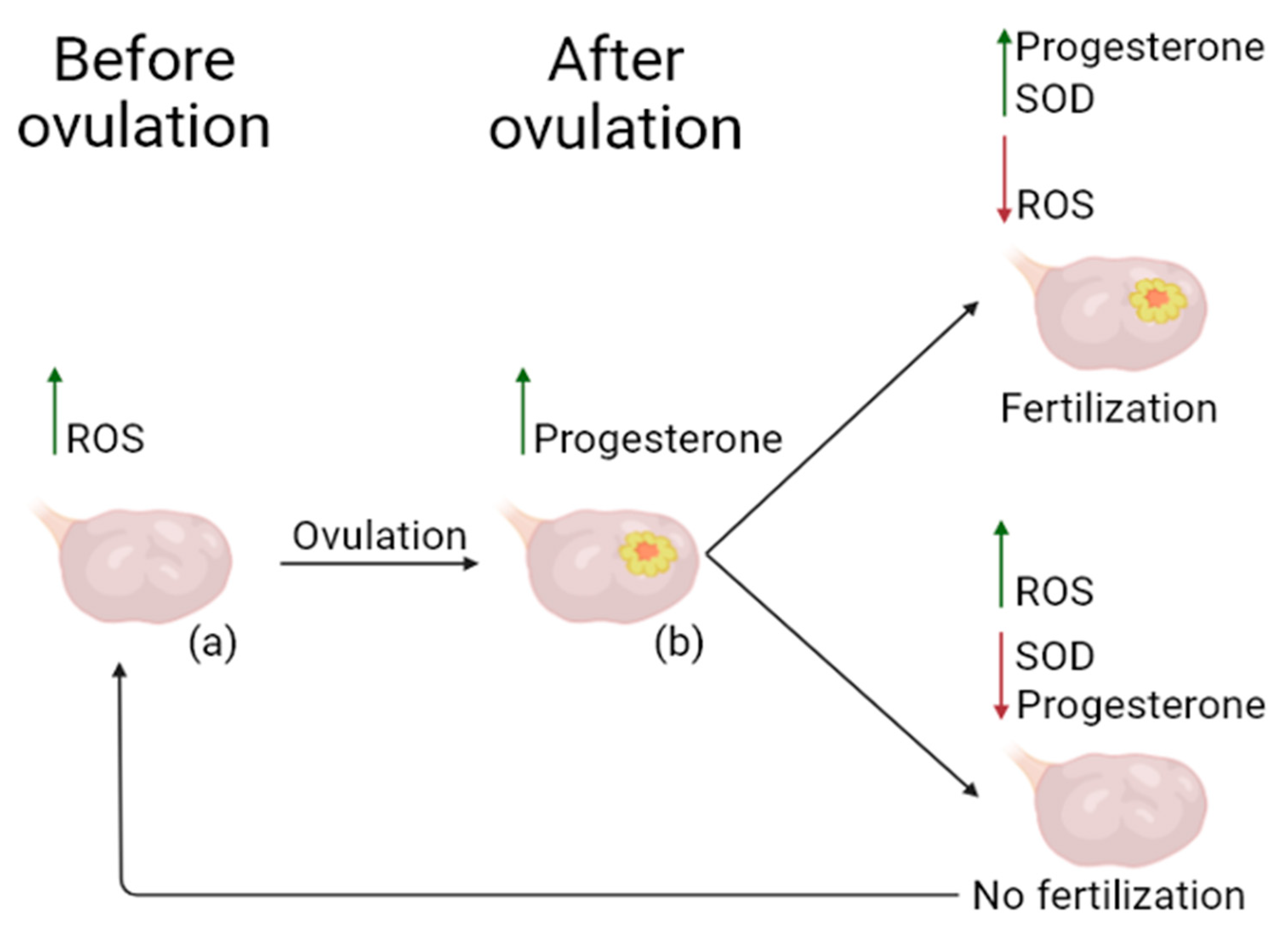
3.4. Beneficial Role of ROS in Physiological Processes of the Healthy Ovary, and Use of Potential Antioxidants in OS Reduction
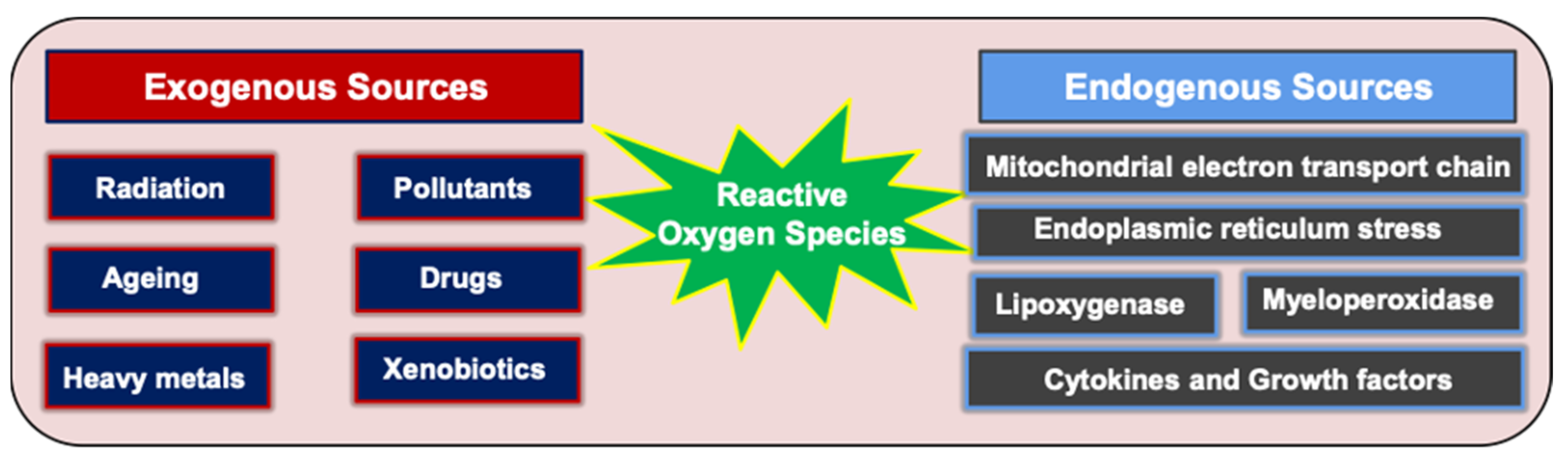
4. Generation of ROS, the Role of OS in OC Pathogenesis, and the Clinical Impact of ROS
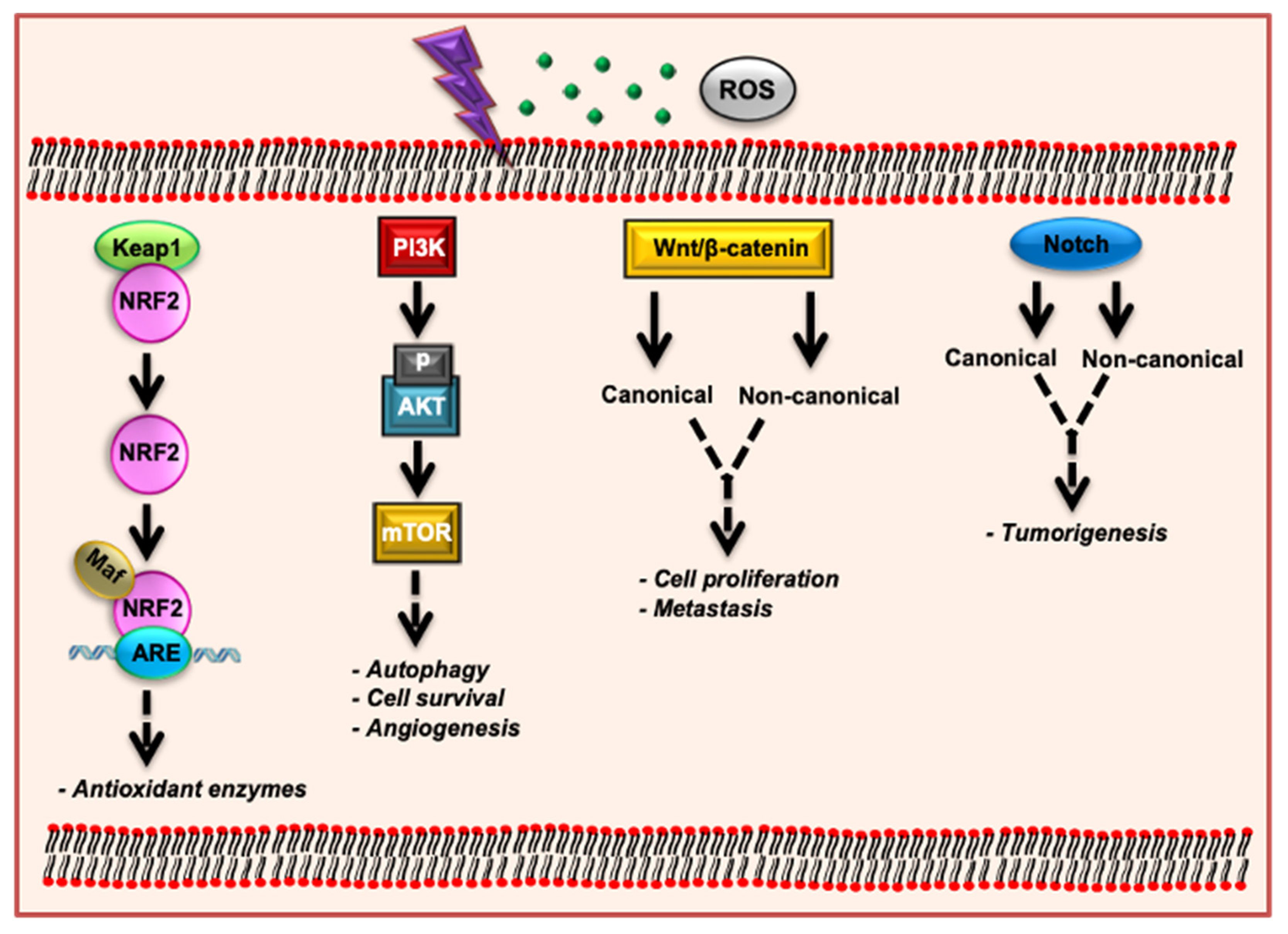
5. OS and Signaling Pathways
5.1. Keap1-Nrf2-ARE Signaling Pathway
5.2. PI3K/AKT/mTOR Signaling Pathway
5.3. Wnt/β-Catenin Signaling Pathway
5.4. Notch Signaling Pathway
6. Diagnostic Methods
7. Role of OS Inducers as Therapeutic Agents in OC Therapy, Treatment Options for OC, and the Role of Oxidative Stress in Chemotherapy Resistance
8. Future Perspectives for OC Diagnosis and Treatment
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Arora, T.; Mullangi, S.; Lekkala, M.R. Ovarian Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Morand, S.; Devanaboyina, M.; Staats, H.; Stanbery, L.; Nemunaitis, J. Ovarian Cancer Immunotherapy and Personalized Medicine. Int. J. Mol. Sci. 2021, 22, 6532. [Google Scholar] [CrossRef]
- Stewart, C.; Ralyea, C.; Lockwood, S. Ovarian cancer: An integrated review. In Seminars in Oncology Nursing; WB Saunders: Philadelphia, PA, USA, 2019; pp. 151–156. [Google Scholar]
- Lheureux, S.; Gourley, C.; Vergote, I.; Oza, A.M. Epithelial ovarian cancer. Lancet 2019, 393, 1240–1253. [Google Scholar] [CrossRef]
- Akter, S.; Rahman, M.A.; Hasan, M.N.; Akhter, H.; Noor, P.; Islam, R.; Shin, Y.; Rahman, M.H.; Gazi, M.S.; Huda, M.N.; et al. Recent Advances in Ovarian Cancer: Therapeutic Strategies, Potential Biomarkers, and Technological Improvements. Cells 2022, 11, 650. [Google Scholar] [CrossRef] [PubMed]
- Garg, V.; Oza, A.M. Treatment of Ovarian Cancer Beyond PARP Inhibition: Current and Future Options. Drugs 2023, 83, 1365–1385. [Google Scholar] [CrossRef]
- Zapardiel, I.; Diestro, M.; Aletti, G. Conservative treatment of early stage ovarian cancer: Oncological and fertility outcomes. Eur. J. Surg. Oncol. 2014, 40, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Roett, M.A.; Evans, P. Ovarian cancer: An overview. Am. Fam. Phys. 2009, 80, 609–616. [Google Scholar]
- Cirillo, P.D.R.; Margiotti, K.; Fabiani, M.; Barros-Filho, M.C.; Sparacino, D.; Cima, A.; Longo, S.A.; Cupellaro, M.; Mesoraca, A.; Giorlandino, C. Multi-analytical test based on serum miRNAs and proteins quantification for ovarian cancer early detection. PLoS ONE 2021, 16, e0255804. [Google Scholar] [CrossRef]
- Zhang, R.; Siu, M.K.Y.; Ngan, H.Y.S.; Chan, K.K.L. Molecular Biomarkers for the Early Detection of Ovarian Cancer. Int. J. Mol. Sci. 2022, 23, 12041. [Google Scholar] [CrossRef]
- Clarke, A.; Chang, Y.M.; McPherson, K. Removing organs “just in case”—Is prophylactic removal of the ovaries a good thing? J. Epidemiol. Community Health 2006, 60, 186–187. [Google Scholar] [CrossRef]
- Garzon, S.; Laganà, A.S.; Casarin, J.; Raffaelli, R.; Cromi, A.; Franchi, M.; Barra, F.; Alkatout, I.; Ferrero, S.; Ghezzi, F. Secondary and tertiary ovarian cancer recurrence: What is the best management? Gland Surg. 2020, 9, 1118–1129. [Google Scholar] [CrossRef] [PubMed]
- Oranratanaphan, S.; Wanishpongpan, S.; Termrungruanglert, W.; Triratanachat, S. Assessment of Diagnostic Values among CA-125, RMI, HE4, and ROMA for Cancer Prediction in Women with Nonfunctional Ovarian Cysts. Obstet. Gynecol. Int. 2018, 2018, 7821574. [Google Scholar] [CrossRef]
- Renjen, P.N.; Chaudhari, D.M.; Shilpi, U.S.; Zutshi, D.; Ahmad, K. Paraneoplastic Cerebellar Degeneration Associated With Ovarian Adenocarcinoma: A Case Report and Review of Literature. Ann. Indian Acad. Neurol. 2018, 21, 311–314. [Google Scholar] [CrossRef] [PubMed]
- National Academies of Sciences and Committee on the State of the Science in Ovarian Cancer Research. Ovarian Cancers: Evolving Paradigms in Research and Care; National Academies Press: Washington, DC, USA, 2016. [Google Scholar]
- Zheng, M.; Hu, Y.; Liu, O.; Li, S.; Wang, Y.; Li, X.; Liu, J.; Yang, Q.; Li, X.; Lin, B. Oxidative Stress Response Biomarkers of Ovarian Cancer Based on Single-Cell and Bulk RNA Sequencing. Oxidative Med. Cell. Longev. 2023, 2023, 1261039. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Silva, E. Cancer statistics, 2024: Mixed results in gynecologic oncology. Int. J. Gynecol. Cancer 2024, 34, 964. [Google Scholar] [CrossRef]
- Jayson, G.C.; Kohn, E.C.; Kitchener, H.C.; Ledermann, J.A. Ovarian cancer. Lancet 2014, 384, 1376–1388. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Rooth, C. Ovarian cancer: Risk factors, treatment and management. Br. J. Nurs. 2013, 22, S23–S30. [Google Scholar] [CrossRef]
- Zamwar, U.M.; Anjankar, A.P. Aetiology, Epidemiology, Histopathology, Classification, Detailed Evaluation, and Treatment of Ovarian Cancer. Cureus 2022, 14, e30561. [Google Scholar] [CrossRef]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2017, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Murali, R.; Balasubramaniam, V.; Srinivas, S.; Sundaram, S.; Venkatraman, G.; Warrier, S.; Dharmarajan, A.; Gandhirajan, R.K. Deregulated Metabolic Pathways in Ovarian Cancer: Cause and Consequence. Metabolites 2023, 13, 560. [Google Scholar] [CrossRef] [PubMed]
- Meinhold-Heerlein, I.; Bauerschlag, D.; Hilpert, F.; Dimitrov, P.; Sapinoso, L.M.; Orlowska-Volk, M.; Bauknecht, T.; Park, T.-W.; Jonat, W.; Jacobsen, A.; et al. Molecular and prognostic distinction between serous ovarian carcinomas of varying grade and malignant potential. Oncogene 2005, 24, 1053–1065. [Google Scholar] [CrossRef]
- Singer, G.; Oldt, R., III.; Cohen, Y.; Wang, B.G.; Sidransky, D.; Kurman, R.J.; Shih, I.-M. Mutations in BRAF and KRAS Characterize the Development of Low-Grade Ovarian Serous Carcinoma. J. Natl. Cancer Inst. 2003, 95, 484–486. [Google Scholar] [CrossRef] [PubMed]
- Suszynska, M.; Ratajska, M.; Kozlowski, P. BRIP1, RAD51C, and RAD51D mutations are associated with high susceptibility to ovarian cancer: Mutation prevalence and precise risk estimates based on a pooled analysis of ~30,000 cases. J. Ovarian Res. 2020, 13, 50. [Google Scholar] [CrossRef] [PubMed]
- Reid, B.M.; Permuth, J.B.; Sellers, T.A. Epidemiology of ovarian cancer: A review. Cancer Biol. Med. 2017, 14, 9–32. [Google Scholar] [CrossRef]
- Matulonis, U.A.; Sood, A.K.; Fallowfield, L.; Howitt, B.E.; Sehouli, J.; Karlan, B.Y. Ovarian cancer. Nat. Rev. Dis. Primers 2016, 2, 16061. [Google Scholar] [CrossRef]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Huber, D.; Seitz, S.; Kast, K.; Emons, G.; Ortmann, O. Use of oral contraceptives in BRCA mutation carriers and risk for ovarian and breast cancer: A systematic review. Arch. Gynecol. Obstet. 2020, 301, 875–884. [Google Scholar] [CrossRef]
- Goldzieher, J.W.; Stanczyk, F.Z. Oral contraceptives and individual variability of circulating levels of ethinyl estradiol and progestins. Contraception 2008, 78, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Lacey, J.; James, V.; Mink, P.J.; Lubin, J.H.; Sherman, M.E.; Troisi, R.; Hartge, P.; Schatzkin, A.; Schairer, C. Menopausal Hormone Replacement Therapy and Risk of Ovarian Cancer. JAMA 2002, 288, 334–341. [Google Scholar] [CrossRef]
- Orchard, S.G.; Lockery, J.E.; Broder, J.C.; Ernst, M.E.; Espinoza, S.; Gibbs, P.; Wolfe, R.; Polekhina, G.; Zoungas, S.; Loomans-Kropp, H.A.; et al. Association of metformin, aspirin, and cancer incidence with mortality risk in adults with diabetes. JNCI Cancer Spectr. 2023, 7, pkad017. [Google Scholar] [CrossRef]
- Hensley, K.; Floyd, R.A. Reactive oxygen species and protein oxidation in aging: A look back, a look ahead. Arch. Biochem. Biophys. 2002, 397, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Fruehauf, J.P.; Meyskens, F.L., Jr. Reactive Oxygen Species: A Breath of Life or Death? Clin. Cancer Res. 2007, 13, 789–794. [Google Scholar] [CrossRef]
- Ciani, F.; Cocchia, N.; d’Angelo, D.; Tafuri, S. Influence of ROS on ovarian functions. In New Discoveries in Embryology; BoD—Books on Demand: Norderstedt, Germany, 2015; pp. 41–73. [Google Scholar]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [PubMed]
- Aldred, E.M. Pharmacology: A Handbook for Complementary Healthcare Professionals; Elsevier Health Sciences: York, UK, 2008. [Google Scholar]
- Fujii, J.; Iuchi, Y.; Okada, F. Fundamental roles of reactive oxygen species and protective mechanisms in the female reproductive system. Reprod. Biol. Endocrinol. 2005, 3, 43. [Google Scholar] [CrossRef]
- Singh, R.; Manna, P.P. Reactive oxygen species in cancer progression and its role in therapeutics. Explor. Med. 2022, 3, 43–57. [Google Scholar] [CrossRef]
- Lushchak, V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem.-Biol. Interact. 2014, 224, 164–175. [Google Scholar] [CrossRef]
- Al-Dalaen, S.M.; Al-Qtaitat, A.I. Oxidative stress versus antioxidants. Am. J. Biosci. Bioeng. 2014, 7, 60–71. [Google Scholar]
- Patekar, D.; Kheur, S.; Bagul, N.; Kulkarni, M.; Mahalle, A.; Ingle, Y.; Dhas, V. Antioxidant defence system. Oral Maxillofac. Pathol. J. 2013, 4, 309. [Google Scholar]
- Moussa, Z.; Judeh, Z.; Ahmed, S.A. Nonenzymatic exogenous and endogenous antioxidants. Free. Radic. Med. Biol. 2019, 1, 11–22. [Google Scholar]
- Owoade, A.O.; Olorunnisola, A. The supportive role of dietary antioxidants in antioxidant defence system. Adv. Life Sci. Technol. 2019, 73, 53–59. [Google Scholar]
- Irato, P.; Santovito, G. Enzymatic and Non-Enzymatic Molecules with Antioxidant Function. Antioxidants 2021, 10, 579. [Google Scholar] [CrossRef] [PubMed]
- Jeeva, J.S.; Sunitha, J.; Ananthalakshmi, R.; Rajkumari, S.; Ramesh, M.; Krishnan, R. Enzymatic antioxidants and its role in oral diseases. J. Pharm. Bioallied Sci. 2015, 7, S331–S333. [Google Scholar] [CrossRef]
- Asakura, H.; Kitahora, T. Antioxidants in Inflammatory Bowel Disease, Ulcerative Colitis, and Crohn. Bioactive Food as Dietary Interventions for Liver and Gastrointestinal Disease: Bioactive Foods in Chronic Disease States; Academic Press: Cambridge, MA, USA, 2013; p. 37. [Google Scholar]
- Katiyar, S.K.; Afaq, F.; Mukhtar, H. Effects of solar radiation on detoxification mechanisms in the skin. In Comprehensive Series in Photosciences; Elsevier: Amsterdam, The Netherlands, 2001; Volume 3, pp. 419–436. [Google Scholar]
- Mir, R.A.; Khah, M.A. Recent progress in enzymatic antioxidant defense system in plants against different environmental stresses. In Improving Stress Resilience in Plants; Elsevier: Amsterdam, The Netherlands, 2024; pp. 203–224. [Google Scholar]
- Janků, M.; Luhová, L.; Petřivalský, M. On the Origin and Fate of Reactive Oxygen Species in Plant Cell Compartments. Antioxidants 2019, 8, 105. [Google Scholar] [CrossRef]
- Yang, Y.; Bazhin, A.V.; Werner, J.; Karakhanova, S. Reactive oxygen species in the immune system. Int. Rev. Immunol. 2013, 32, 249–270. [Google Scholar] [CrossRef]
- Liang, J.; Gao, Y.; Feng, Z.; Zhang, B.; Na, Z.; Li, D. Reactive oxygen species and ovarian diseases: Antioxidant strategies. Redox Biol. 2023, 62, 102659. [Google Scholar] [CrossRef]
- Shkolnik, K.; Tadmor, A.; Ben-Dor, S.; Nevo, N.; Galiani, D.; Dekel, N. Reactive oxygen species are indispensable in ovulation. Proc. Natl. Acad. Sci. USA 2011, 108, 1462–1467. [Google Scholar] [CrossRef]
- Sugino, N. Roles of reactive oxygen species in the corpus luteum. Anim. Sci. J. 2006, 77, 556–565. [Google Scholar] [CrossRef]
- Sugino, N.; Takiguchi, S.; Kashida, S.; Karube, A.; Nakamura, Y.; Kato, H. Superoxide dismutase expression in the human corpus luteum during the menstrual cycle and in early pregnancy. Mol. Hum. Reprod. 2000, 6, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Stocco, C.; Telleria, C.; Gibori, G. The Molecular Control of Corpus Luteum Formation, Function, and Regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef]
- Millea, P.J. N-acetylcysteine: Multiple clinical applications. Am. Fam. Phys. 2009, 80, 265–269. [Google Scholar]
- Anastasi, E.; Scaramuzzino, S.; Viscardi, M.F.; Viggiani, V.; Piccioni, M.G.; Cacciamani, L.; Merlino, L.; Angeloni, A.; Muzii, L.; Porpora, M.G. Efficacy of N-Acetylcysteine on Endometriosis-Related Pain, Size Reduction of Ovarian Endometriomas, and Fertility Outcomes. Int. J. Environ. Res. Public Health 2023, 20, 4686. [Google Scholar] [CrossRef]
- Delneste, Y.; Jeannin, P.; Potier, L.; Romero, P.; Bonnefoy, J.Y. N-acetyl-L-cysteine exhibits antitumoral activity by increasing tumor necrosis factor alpha-dependent T-cell cytotoxicity. Blood 1997, 90, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Brum, G.; Carbone, T.; Still, E.; Correia, V.; Szulak, K.; Calianese, D.; Best, C.; Cammarata, G.; Higgins, K.; Ji, F.; et al. N-acetylcysteine potentiates doxorubicin-induced ATM and p53 activation in ovarian cancer cells. Int. J. Oncol. 2013, 42, 211–218. [Google Scholar] [CrossRef]
- Raeeszadeh, M.; Saleh Hosseini, S.M.; Amiri, A.A. Impact of Co-Administration of N-Acetylcysteine and Vitamin E on Cyclophosphamide-Induced Ovarian Toxicity in Female Rats. J. Toxicol. 2022, 2022, 9073405. [Google Scholar] [CrossRef]
- Kiremitli, T.; Kiremitli, S.; Akselim, B.; Yilmaz, B.; Mammadov, R.; Tor, I.H.; Yazici, G.N.; Gulaboglu, M. Protective effect of Coenzyme Q10 on oxidative ovarian and uterine damage induced by methotrexate in rats. Hum. Exp. Toxicol. 2021, 40, 1537–1544. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Wang, Q.; Guo, W.; Hao, B.; Shi, X.; Lu, Y.; Wong, C.W.; Ma, V.W.; Yip, T.T.; Au, J.S.; Hao, Q. Mechanistic study of TRPM2-Ca2+-CAMK2-BECN1 signaling in oxidative stress-induced autophagy inhibition. Autophagy 2016, 12, 1340–1354. [Google Scholar] [CrossRef]
- Berlett, B.S.; Stadtman, E.R. Protein oxidation in aging, disease, and oxidative stress. J. Biol. Chem. 1997, 272, 20313–20316. [Google Scholar] [CrossRef] [PubMed]
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive oxygen species: From health to disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, L.B.; Chandel, N.S. Mitochondrial reactive oxygen species and cancer. Cancer Metab. 2014, 2, 17. [Google Scholar] [CrossRef] [PubMed]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef]
- Jelic, M.D.; Mandic, A.D.; Maricic, S.M.; Srdjenovic, B.U. Oxidative stress and its role in cancer. J. Cancer Res. Ther. 2021, 17, 22–28. [Google Scholar] [CrossRef]
- Marí-Alexandre, J.; Carcelén, A.P.; Agababyan, C.; Moreno-Manuel, A.; García-Oms, J.; Calabuig-Fariñas, S.; Gilabert-Estellés, J. Interplay Between MicroRNAs and Oxidative Stress in Ovarian Conditions with a Focus on Ovarian Cancer and Endometriosis. Int. J. Mol. Sci. 2019, 20, 5322. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Hart, P.C.; Mao, M.; De Abreu, A.L.P.; Ansenberger-Fricano, K.; Ekoue, D.N.; Ganini, D.; Kajdacsy-Balla, A.; Diamond, A.M.; Minshall, R.D.; Consolaro, M.E. MnSOD upregulation sustains the Warburg effect via mitochondrial ROS and AMPK-dependent signalling in cancer. Nat. Commun. 2015, 6, 6053. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.; Bae, J.-S. ROS homeostasis and metabolism: A critical liaison for cancer therapy. Exp. Mol. Med. 2016, 48, e269. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-l.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839. [Google Scholar] [CrossRef] [PubMed]
- Chiarugi, P.; Pani, G.; Giannoni, E.; Taddei, L.; Colavitti, R.; Raugei, G.; Symons, M.; Borrello, S.; Galeotti, T.; Ramponi, G. Reactive oxygen species as essential mediators of cell adhesion: The oxidative inhibition of a FAK tyrosine phosphatase is required for cell adhesion. J. Cell Biol. 2003, 161, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Nakajima, M.; Morimoto, K. Relationship between the intracellular reactive oxygen species and the induction of oxidative DNA damage in human neutrophil-like cells (vol 17, pg 1543, 1996). Carcinogenesis 1997, 18, 1683. [Google Scholar]
- Douma, S.; Van Laar, T.; Zevenhoven, J.; Meuwissen, R.; Van Garderen, E.; Peeper, D.S. Suppression of anoikis and induction of metastasis by the neurotrophic receptor TrkB. Nature 2004, 430, 1034–1039. [Google Scholar] [CrossRef] [PubMed]
- Sunkari, S.; Thatikonda, S.; Pooladanda, V.; Challa, V.S.; Godugu, C. Protective effects of ambroxol in psoriasis like skin inflammation: Exploration of possible mechanisms. Int. Immunopharmacol. 2019, 71, 301–312. [Google Scholar] [CrossRef]
- Thatikonda, S.; Pooladanda, V.; Tokala, R.; Nagula, S.; Godugu, C. Niclosamide inhibits epithelial-mesenchymal transition with apoptosis induction in BRAF/ NRAS mutated metastatic melanoma cells. Toxicol. In Vitro 2023, 89, 105579. [Google Scholar] [CrossRef]
- Caneba, C.A.; Yang, L.; Baddour, J.; Curtis, R.; Win, J.; Hartig, S.; Marini, J.; Nagrath, D. Nitric oxide is a positive regulator of the Warburg effect in ovarian cancer cells. Cell Death Dis. 2014, 5, e1302. [Google Scholar] [CrossRef]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Dodson, M.; Castro-Portuguez, R.; Zhang, D.D. NRF2 plays a critical role in mitigating lipid peroxidation and ferroptosis. Redox Biol. 2019, 23, 101107. [Google Scholar] [CrossRef]
- Redza-Dutordoir, M.; Averill-Bates, D.A. Activation of apoptosis signalling pathways by reactive oxygen species. Biochim. Biophys. Acta 2016, 1863, 2977–2992. [Google Scholar] [CrossRef]
- Ding, D.-N.; Xie, L.-Z.; Shen, Y.; Li, J.; Guo, Y.; Fu, Y.; Liu, F.-Y.; Han, F.-J. Insights into the Role of Oxidative Stress in Ovarian Cancer. Oxidative Med. Cell. Longev. 2021, 2021, 8388258. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, M.; Anil Kumar, N.V.; Zucca, P.; Varoni, E.M.; Dini, L.; Panzarini, E.; Rajkovic, J.; Tsouh Fokou, P.V.; Azzini, E.; Peluso, I.; et al. Lifestyle, Oxidative Stress, and Antioxidants: Back and Forth in the Pathophysiology of Chronic Diseases. Front. Physiol. 2020, 11, 694. [Google Scholar] [CrossRef]
- Franco, R.; Schoneveld, O.; Georgakilas, A.G.; Panayiotidis, M.I. Oxidative stress, DNA methylation and carcinogenesis. Cancer Lett. 2008, 266, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Che, L.; Gao, K.; Wang, L.; Yang, X.; Wen, X.; Li, M.; Jiang, Z. Taurine alleviates oxidative stress in porcine mammary epithelial cells by stimulating the Nrf2-MAPK signaling pathway. Food Sci. Nutr. 2023, 11, 1736–1746. [Google Scholar] [CrossRef] [PubMed]
- Rojo de la Vega, M.; Chapman, E.; Zhang, D.D. NRF2 and the Hallmarks of Cancer. Cancer Cell 2018, 34, 21–43. [Google Scholar] [CrossRef]
- Kerins, M.J.; Ooi, A. A catalogue of somatic NRF2 gain-of-function mutations in cancer. Sci. Rep. 2018, 8, 12846. [Google Scholar] [CrossRef]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Holtzclaw, W.D.; Wakabayashi, N. Keap1, the sensor for electrophiles and oxidants that regulates the phase 2 response, is a zinc metalloprotein. Biochemistry 2005, 44, 6889–6899. [Google Scholar] [CrossRef]
- Sun, Z.; Wu, T.; Zhao, F.; Lau, A.; Birch, C.M.; Zhang, D.D. KPNA6 (Importin α7)-mediated nuclear import of Keap1 represses the Nrf2-dependent antioxidant response. Mol. Cell. Biol. 2011, 31, 1800–1811. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef]
- Klaunig, J.E.; Kamendulis, L.M.; Hocevar, B.A. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 2010, 38, 96–109. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Koul, S.; Khandrika, L.; Meacham, R.B.; Koul, H.K. Oxidative stress is inherent in prostate cancer cells and is required for aggressive phenotype. Cancer Res. 2008, 68, 1777–1785. [Google Scholar] [CrossRef]
- Mateescu, B.; Batista, L.; Cardon, M.; Gruosso, T.; de Feraudy, Y.; Mariani, O.; Nicolas, A.; Meyniel, J.P.; Cottu, P.; Sastre-Garau, X.; et al. miR-141 and miR-200a act on ovarian tumorigenesis by controlling oxidative stress response. Nat. Med. 2011, 17, 1627–1635. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Gibson, T.B.; Robinson, F.; Silvestro, L.; Pearson, G.; Xu, B.; Wright, A.; Vanderbilt, C.; Cobb, M.H. MAP kinases. Chem. Rev. 2001, 101, 2449–2476. [Google Scholar] [CrossRef]
- Innocenti, M.; Frittoli, E.; Ponzanelli, I.; Falck, J.R.; Brachmann, S.M.; Di Fiore, P.P.; Scita, G. Phosphoinositide 3-kinase activates Rac by entering in a complex with Eps8, Abi1, and Sos-1. J. Cell Biol. 2003, 160, 17–23. [Google Scholar] [CrossRef]
- Pimienta, G.; Pascual, J. Canonical and alternative MAPK signaling. Cell Cycle 2007, 6, 2628–2632. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Cheng, J.C.; Chang, H.M.; Leung, P.C. COX2 and PGE2 mediate EGF-induced E-cadherin-independent human ovarian cancer cell invasion. Endocr. Relat. Cancer 2014, 21, 533–543. [Google Scholar] [CrossRef][Green Version]
- Fruman, D.A.; Rommel, C. PI3K and cancer: Lessons, challenges and opportunities. Nat. Rev. Drug Discov. 2014, 13, 140–156. [Google Scholar] [CrossRef]
- Levine, D.A.; Bogomolniy, F.; Yee, C.J.; Lash, A.; Barakat, R.R.; Borgen, P.I.; Boyd, J. Frequent mutation of the PIK3CA gene in ovarian and breast cancers. Clin. Cancer Res. 2005, 11, 2875–2878. [Google Scholar] [CrossRef]
- Philp, A.J.; Campbell, I.G.; Leet, C.; Vincan, E.; Rockman, S.P.; Whitehead, R.H.; Thomas, R.J.; Phillips, W.A. The phosphatidylinositol 3′-kinase p85alpha gene is an oncogene in human ovarian and colon tumors. Cancer Res. 2001, 61, 7426–7429. [Google Scholar]
- Sakamoto, K.; Iwasaki, K.; Sugiyama, H.; Tsuji, Y. Role of the tumor suppressor PTEN in antioxidant responsive element-mediated transcription and associated histone modifications. Mol. Biol. Cell 2009, 20, 1606–1617. [Google Scholar] [CrossRef] [PubMed]
- van der Reest, J.; Lilla, S.; Zheng, L.; Zanivan, S.; Gottlieb, E. Proteome-wide analysis of cysteine oxidation reveals metabolic sensitivity to redox stress. Nat. Commun. 2018, 9, 1581. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef]
- Kinross, K.M.; Montgomery, K.G.; Kleinschmidt, M.; Waring, P.; Ivetac, I.; Tikoo, A.; Saad, M.; Hare, L.; Roh, V.; Mantamadiotis, T.; et al. An activating Pik3ca mutation coupled with Pten loss is sufficient to initiate ovarian tumorigenesis in mice. J. Clin. Investig. 2012, 122, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Arend, R.C.; Londoño-Joshi, A.I.; Straughn, J.M., Jr.; Buchsbaum, D.J. The Wnt/β-catenin pathway in ovarian cancer: A review. Gynecol. Oncol. 2013, 131, 772–779. [Google Scholar] [CrossRef]
- Jung, Y.S.; Park, J.I. Wnt signaling in cancer: Therapeutic targeting of Wnt signaling beyond β-catenin and the destruction complex. Exp. Mol. Med. 2020, 52, 183–191. [Google Scholar] [CrossRef]
- Wiese, K.E.; Nusse, R.; van Amerongen, R. Wnt signalling: Conquering complexity. Development 2018, 145, dev165902. [Google Scholar] [CrossRef] [PubMed]
- Groden, J.; Thliveris, A.; Samowitz, W.; Carlson, M.; Gelbert, L.; Albertsen, H.; Joslyn, G.; Stevens, J.; Spirio, L.; Robertson, M.; et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell 1991, 66, 589–600. [Google Scholar] [CrossRef]
- van Schie, E.H.; van Amerongen, R. Aberrant WNT/CTNNB1 Signaling as a Therapeutic Target in Human Breast Cancer: Weighing the Evidence. Front. Cell Dev. Biol. 2020, 8, 25. [Google Scholar] [CrossRef]
- Gatcliffe, T.A.; Monk, B.J.; Planutis, K.; Holcombe, R.F. Wnt signaling in ovarian tumorigenesis. Int. J. Gynecol. Cancer 2008, 18, 954–962. [Google Scholar] [CrossRef]
- Wu, R.; Zhai, Y.; Fearon, E.R.; Cho, K.R. Diverse mechanisms of beta-catenin deregulation in ovarian endometrioid adenocarcinomas. Cancer Res. 2001, 61, 8247–8255. [Google Scholar] [PubMed]
- Nusse, R.; Clevers, H. Wnt/β-Catenin Signaling, Disease, and Emerging Therapeutic Modalities. Cell 2017, 169, 985–999. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.L.; Hough, R.; Bernaudo, S.; Peng, C. Wnt/β-catenin signalling in ovarian cancer: Insights into its hyperactivation and function in tumorigenesis. J. Ovarian Res. 2019, 12, 122. [Google Scholar] [CrossRef]
- Wu, Y.; Antony, S.; Meitzler, J.L.; Doroshow, J.H. Molecular mechanisms underlying chronic inflammation-associated cancers. Cancer Lett. 2014, 345, 164–173. [Google Scholar] [CrossRef]
- Yang, H.Y.; Shen, J.X.; Wang, Y.; Liu, Y.; Shen, D.Y.; Quan, S. Tankyrase Promotes Aerobic Glycolysis and Proliferation of Ovarian Cancer through Activation of Wnt/β-Catenin Signaling. Biomed. Res. Int. 2019, 2019, 2686340. [Google Scholar] [CrossRef]
- Radtke, F.; Raj, K. The role of Notch in tumorigenesis: Oncogene or tumour suppressor? Nat. Rev. Cancer 2003, 3, 756–767. [Google Scholar] [CrossRef]
- El-Sehemy, A.; Chang, A.C.; Azad, A.K.; Gupta, N.; Xu, Z.; Steed, H.; Karsan, A.; Fu, Y. Notch activation augments nitric oxide/soluble guanylyl cyclase signaling in immortalized ovarian surface epithelial cells and ovarian cancer cells. Cell Signal 2013, 25, 2780–2787. [Google Scholar] [CrossRef] [PubMed]
- Rose, S.L.; Kunnimalaiyaan, M.; Drenzek, J.; Seiler, N. Notch 1 signaling is active in ovarian cancer. Gynecol. Oncol. 2010, 117, 130–133. [Google Scholar] [CrossRef]
- Park, J.T.; Chen, X.; Tropè, C.G.; Davidson, B.; Shih, I.M.; Wang, T.L. Notch3 overexpression is related to the recurrence of ovarian cancer and confers resistance to carboplatin. Am. J. Pathol. 2010, 177, 1087–1094. [Google Scholar] [CrossRef]
- Gupta, N.; Xu, Z.; El-Sehemy, A.; Steed, H.; Fu, Y. Notch3 induces epithelial-mesenchymal transition and attenuates carboplatin-induced apoptosis in ovarian cancer cells. Gynecol. Oncol. 2013, 130, 200–206. [Google Scholar] [CrossRef]
- Lu, C.; Bonome, T.; Li, Y.; Kamat, A.A.; Han, L.Y.; Schmandt, R.; Coleman, R.L.; Gershenson, D.M.; Jaffe, R.B.; Birrer, M.J.; et al. Gene alterations identified by expression profiling in tumor-associated endothelial cells from invasive ovarian carcinoma. Cancer Res. 2007, 67, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Elias, K.M.; Guo, J.; Bast, R.C., Jr. Early Detection of Ovarian Cancer. Hematol. Oncol. Clin. N. Am. 2018, 32, 903–914. [Google Scholar] [CrossRef] [PubMed]
- Sundar, S.; Neal, R.D.; Kehoe, S. Diagnosis of ovarian cancer. BMJ 2015, 351, h4443. [Google Scholar] [CrossRef] [PubMed]
- Charkhchi, P.; Cybulski, C.; Gronwald, J.; Wong, F.O.; Narod, S.A.; Akbari, M.R. CA125 and Ovarian Cancer: A Comprehensive Review. Cancers 2020, 12, 3730. [Google Scholar] [CrossRef]
- Matte, I.; Garde-Granger, P.; Bessette, P.; Piché, A. Ascites from ovarian cancer patients stimulates MUC16 mucin expression and secretion in human peritoneal mesothelial cells through an Akt-dependent pathway. BMC Cancer 2019, 19, 406. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, S.; Jin, Y.; Zhao, Y.; Wang, Y. Roles of CA125 in diagnosis, prediction, and oncogenesis of ovarian cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188503. [Google Scholar] [CrossRef]
- Timmerman, D.; Ameye, L.; Fischerova, D.; Epstein, E.; Melis, G.B.; Guerriero, S.; Van Holsbeke, C.; Savelli, L.; Fruscio, R.; Lissoni, A.A.; et al. Simple ultrasound rules to distinguish between benign and malignant adnexal masses before surgery: Prospective validation by IOTA group. BMJ 2010, 341, c6839. [Google Scholar] [CrossRef]
- Skates, S.J. Ovarian cancer screening: Development of the risk of ovarian cancer algorithm (ROCA) and ROCA screening trials. Int. J. Gynecol. Cancer 2012, 22 (Suppl. S1), S24–S26. [Google Scholar] [CrossRef]
- Folkins, A.K.; Jarboe, E.A.; Saleemuddin, A.; Lee, Y.; Callahan, M.J.; Drapkin, R.; Garber, J.E.; Muto, M.G.; Tworoger, S.; Crum, C.P. A candidate precursor to pelvic serous cancer (p53 signature) and its prevalence in ovaries and fallopian tubes from women with BRCA mutations. Gynecol. Oncol. 2008, 109, 168–173. [Google Scholar] [CrossRef]
- Nelson, S.J.; Kurhanewicz, J.; Vigneron, D.B.; Larson, P.E.; Harzstark, A.L.; Ferrone, M.; Van Criekinge, M.; Chang, J.W.; Bok, R.; Park, I. Metabolic imaging of patients with prostate cancer using hyperpolarized [1-13C] pyruvate. Sci. Transl. Med. 2013, 5, ra108–ra198. [Google Scholar] [CrossRef]
- Adolphi, N.L.; Butler, K.S.; Lovato, D.M.; Tessier, T.E.; Trujillo, J.E.; Hathaway, H.J.; Fegan, D.L.; Monson, T.C.; Stevens, T.E.; Huber, D.L.; et al. Imaging of Her2-targeted magnetic nanoparticles for breast cancer detection: Comparison of SQUID-detected magnetic relaxometry and MRI. Contrast Media Mol. Imaging 2012, 7, 308–319. [Google Scholar] [CrossRef] [PubMed]
- James, N.E.; Chichester, C.; Ribeiro, J.R. Beyond the Biomarker: Understanding the Diverse Roles of Human Epididymis Protein 4 in the Pathogenesis of Epithelial Ovarian Cancer. Front. Oncol. 2018, 8, 124. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.L.; Lu, Z.; Bast, R.C., Jr. The role of biomarkers in the management of epithelial ovarian cancer. Expert. Rev. Mol. Diagn. 2017, 17, 577–591. [Google Scholar] [CrossRef] [PubMed]
- Chandra, A.; Pius, C.; Nabeel, M.; Nair, M.; Vishwanatha, J.K.; Ahmad, S.; Basha, R. Ovarian cancer: Current status and strategies for improving therapeutic outcomes. Cancer Med. 2019, 8, 7018–7031. [Google Scholar] [CrossRef]
- Vang, R.; Levine, D.A.; Soslow, R.A.; Zaloudek, C.; Shih, I.M.; Kurman, R.J. Molecular Alterations of TP53 are a Defining Feature of Ovarian High-Grade Serous Carcinoma: A Rereview of Cases Lacking TP53 Mutations in The Cancer Genome Atlas Ovarian Study. Int. J. Gynecol. Pathol. 2016, 35, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.D.; Li, L.; Wang, Y.; Thoburn, C.; Afsari, B.; Danilova, L.; Douville, C.; Javed, A.A.; Wong, F.; Mattox, A.; et al. Detection and localization of surgically resectable cancers with a multi-analyte blood test. Science 2018, 359, 926–930. [Google Scholar] [CrossRef]
- Xu, S.; Fu, G.B.; Tao, Z.; OuYang, J.; Kong, F.; Jiang, B.H.; Wan, X.; Chen, K. MiR-497 decreases cisplatin resistance in ovarian cancer cells by targeting mTOR/P70S6K1. Oncotarget 2015, 6, 26457–26471. [Google Scholar] [CrossRef]
- Kan, C.W.; Hahn, M.A.; Gard, G.B.; Maidens, J.; Huh, J.Y.; Marsh, D.J.; Howell, V.M. Elevated levels of circulating microRNA-200 family members correlate with serous epithelial ovarian cancer. BMC Cancer 2012, 12, 627. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.G.; Alder, H.; et al. MicroRNA signatures in human ovarian cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef]
- Jiajie, T.; Yanzhou, Y.; Hoi-Hung, A.C.; Zi-Jiang, C.; Wai-Yee, C. Conserved miR-10 family represses proliferation and induces apoptosis in ovarian granulosa cells. Sci. Rep. 2017, 7, 41304. [Google Scholar] [CrossRef]
- Vescarelli, E.; Gerini, G.; Megiorni, F.; Anastasiadou, E.; Pontecorvi, P.; Solito, L.; De Vitis, C.; Camero, S.; Marchetti, C.; Mancini, R.; et al. MiR-200c sensitizes Olaparib-resistant ovarian cancer cells by targeting Neuropilin 1. J. Exp. Clin. Cancer Res. 2020, 39, 3. [Google Scholar] [CrossRef] [PubMed]
- Choi, P.W.; Ng, S.W. The Functions of MicroRNA-200 Family in Ovarian Cancer: Beyond Epithelial-Mesenchymal Transition. Int. J. Mol. Sci. 2017, 18, 1207. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Zhang, C.; Zhou, B.; Jiang, D. miR-183 modulated cell proliferation and apoptosis in ovarian cancer through the TGF-β/Smad4 signaling pathway. Int. J. Mol. Med. 2019, 43, 1734–1746. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, F.-L.; Liu, P.-S. MiR-151 promotes ovarian cancer through activation of akt/mTOR signaling pathway by decreasing RhoGDIA. Int. J. Clin. Exp. Pathol. 2016, 9, 11222–11229. [Google Scholar]
- Nakayama, I.; Shibazaki, M.; Yashima-Abo, A.; Miura, F.; Sugiyama, T.; Masuda, T.; Maesawa, C. Loss of HOXD10 expression induced by upregulation of miR-10b accelerates the migration and invasion activities of ovarian cancer cells. Int. J. Oncol. 2013, 43, 63–71. [Google Scholar] [CrossRef]
- Staicu, C.E.; Predescu, D.V.; Rusu, C.M.; Radu, B.M.; Cretoiu, D.; Suciu, N.; Crețoiu, S.M.; Voinea, S.C. Role of microRNAs as Clinical Cancer Biomarkers for Ovarian Cancer: A Short Overview. Cells 2020, 9, 169. [Google Scholar] [CrossRef]
- Yang, H.; Villani, R.M.; Wang, H.; Simpson, M.J.; Roberts, M.S.; Tang, M.; Liang, X. The role of cellular reactive oxygen species in cancer chemotherapy. J. Exp. Clin. Cancer Res. 2018, 37, 266. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-fluorouracil: Mechanisms of action and clinical strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Zhang, C.; Xu, C.; Gao, X.; Yao, Q. Platinum-based drugs for cancer therapy and anti-tumor strategies. Theranostics 2022, 12, 2115–2132. [Google Scholar] [CrossRef]
- Yu, Z.; Guo, J.; Hu, M.; Gao, Y.; Huang, L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano 2020, 14, 4816–4828. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.-N.; Wang, K.; Zhang, L.; Huang, Z.; Liu, J.; Zhang, Z.; Luo, M.; Lei, Y.; Peng, Y. Ketoconazole exacerbates mitophagy to induce apoptosis by downregulating cyclooxygenase-2 in hepatocellular carcinoma. J. Hepatol. 2019, 70, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Qin, S.; Chen, Y.; Zhou, L.; Yang, M.; Tang, Y.; Zuo, J.; Zhang, J.; Mizokami, A.; Nice, E.C. Inhibition of NPC1L1 disrupts adaptive responses of drug-tolerant persister cells to chemotherapy. EMBO Mol. Med. 2022, 14, e14903. [Google Scholar] [CrossRef] [PubMed]
- Shim, H.S.; Bae, C.; Wang, J.; Lee, K.-H.; Hankerd, K.M.; Kim, H.K.; Chung, J.M.; La, J.-H. Peripheral and central oxidative stress in chemotherapy-induced neuropathic pain. Mol. Pain 2019, 15, 1744806919840098. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Singh, A. Ocular adverse effects of anti-cancer chemotherapy. J. Cancer Ther. Res. 2012, 1, 5. [Google Scholar] [CrossRef]
- Shirato, A.; Kikugawa, T.; Miura, N.; Tanji, N.; Takemori, N.; Higashiyama, S.; Yokoyama, M. Cisplatin resistance by induction of aldo-keto reductase family 1 member C2 in human bladder cancer cells. Oncol. Lett. 2014, 7, 674–678. [Google Scholar] [CrossRef]
- Hu, T.; Pan, C.; Zhang, T.; Ni, M.; Wang, W.; Zhang, S.; Chen, Y.; Wang, J.; Fang, Q. Nrf2 overexpression increases the resistance of acute myeloid leukemia to cytarabine by inhibiting replication factor C4. Cancer Gene Ther. 2022, 29, 1773–1790. [Google Scholar] [CrossRef]
- Agriesti, F.; Tataranni, T.; Pacelli, C.; Scrima, R.; Laurenzana, I.; Ruggieri, V.; Cela, O.; Mazzoccoli, C.; Salerno, M.; Sessa, F. Nandrolone induces a stem cell-like phenotype in human hepatocarcinoma-derived cell line inhibiting mitochondrial respiratory activity. Sci. Rep. 2020, 10, 2287. [Google Scholar] [CrossRef]
- Orr, B.; Edwards, R.P. Diagnosis and treatment of ovarian cancer. Hematol./Oncol. Clin. 2018, 32, 943–964. [Google Scholar] [CrossRef]
- Cummings, M.; Nicolais, O.; Shahin, M. Surgery in Advanced Ovary Cancer: Primary versus Interval Cytoreduction. Diagnostics 2022, 12, 988. [Google Scholar] [CrossRef]
- Bacalbasa, N.; Balescu, I.; Vilcu, M.; Dima, S.; Diaconu, C.; Iliescu, L.; Filipescu, A.; Dimitriu, M.; Brezean, I. The Risk of Para-Aortic Lymph Node Metastases in Apparent Early Stage Ovarian Cancer. Medicina 2020, 56, 108. [Google Scholar] [CrossRef]
- Kurnit, K.C.; Fleming, G.F.; Lengyel, E. Updates and New Options in Advanced Epithelial Ovarian Cancer Treatment. Obstet. Gynecol. 2021, 137, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Herrera, F.G.; Irving, M.; Kandalaft, L.E.; Coukos, G. Rational combinations of immunotherapy with radiotherapy in ovarian cancer. Lancet Oncol. 2019, 20, e417–e433. [Google Scholar] [CrossRef] [PubMed]
- Erol, A.; Niemira, M.; Krętowski, A.J. Novel Approaches in Ovarian Cancer Research against Heterogeneity, Late Diagnosis, Drug Resistance, and Transcoelomic Metastases. Int. J. Mol. Sci. 2019, 20, 2649. [Google Scholar] [CrossRef]
- Chehelgerdi, M.; Chehelgerdi, M.; Allela, O.Q.B.; Pecho, R.D.C.; Jayasankar, N.; Rao, D.P.; Thamaraikani, T.; Vasanthan, M.; Viktor, P.; Lakshmaiya, N.; et al. Progressing nanotechnology to improve targeted cancer treatment: Overcoming hurdles in its clinical implementation. Mol. Cancer 2023, 22, 169. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, Y.; Wang, P.; Zhang, J.; Liu, H.; Li, Q.; Yin, R.; Bian, C.; Peng, H.; Peng, Z. The prognostic factor for recurrence in advanced-stage high-grade serous ovarian cancer after complete clinical remission: A nested case-control study. J. Ovarian Res. 2021, 14, 179. [Google Scholar] [CrossRef]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef] [PubMed]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 protein family: Attractive targets for cancer therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef]
- Mobahat, M.; Narendran, A.; Riabowol, K. Survivin as a preferential target for cancer therapy. Int. J. Mol. Sci. 2014, 15, 2494–2516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xia, M.; Jin, K.; Wang, S.; Wei, H.; Fan, C.; Wu, Y.; Li, X.; Li, X.; Li, G.; et al. Function of the c-Met receptor tyrosine kinase in carcinogenesis and associated therapeutic opportunities. Mol. Cancer 2018, 17, 45. [Google Scholar] [CrossRef]
- Surowiak, P.; Materna, V.; Denkert, C.; Kaplenko, I.; Spaczynski, M.; Dietel, M.; Zabel, M.; Lage, H. Significance of cyclooxygenase 2 and MDR1/P-glycoprotein coexpression in ovarian cancers. Cancer Lett. 2006, 235, 272–280. [Google Scholar] [CrossRef]
- Januchowski, R.; Sterzynska, K.; Zaorska, K.; Sosinska, P.; Klejewski, A.; Brazert, M.; Nowicki, M.; Zabel, M. Analysis of MDR genes expression and cross-resistance in eight drug resistant ovarian cancer cell lines. J. Ovarian Res. 2016, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Tian, T.; Cui, Z. Targeting ovarian cancer stem cells: A new way out. Stem Cell Res. Ther. 2023, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Vella, V.; Lappano, R.; Bonavita, E.; Maggiolini, M.; Clarke, R.B.; Belfiore, A.; De Francesco, E.M. Insulin/IGF Axis and the Receptor for Advanced Glycation End Products: Role in Meta-inflammation and Potential in Cancer Therapy. Endocr. Rev. 2023, 44, 693–723. [Google Scholar] [CrossRef] [PubMed]
- Bilyk, O.; Coatham, M.; Jewer, M.; Postovit, L.M. Epithelial-to-Mesenchymal Transition in the Female Reproductive Tract: From Normal Functioning to Disease Pathology. Front. Oncol. 2017, 7, 145. [Google Scholar] [CrossRef]
- Singh, S.; Barik, D.; Arukha, A.P.; Prasad, S.; Mohapatra, I.; Singh, A.; Singh, G. Small Molecule Targeting Immune Cells: A Novel Approach for Cancer Treatment. Biomedicines 2023, 11, 2621. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vacchelli, E.; Bravo-San Pedro, J.M.; Buqué, A.; Senovilla, L.; Baracco, E.E.; Bloy, N.; Castoldi, F.; Abastado, J.P.; Agostinis, P.; et al. Classification of current anticancer immunotherapies. Oncotarget 2014, 5, 12472–12508. [Google Scholar] [CrossRef]
- Macpherson, A.M.; Barry, S.C.; Ricciardelli, C.; Oehler, M.K. Epithelial ovarian cancer and the immune system: Biology, interactions, challenges and potential advances for immunotherapy. J. Clin. Med. 2020, 9, 2967. [Google Scholar] [CrossRef]
- Drugs Approved for Ovarian, Fallopian Tube, or Primary Peritoneal Cancer. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/ovarian (accessed on 22 December 2022).
- Davis-Perry, S.; Hernandez, E.; Houck, K.L.; Shank, R. Melphalan for the treatment of patients with recurrent epithelial ovarian cancer. Am. J. Clin. Oncol. 2003, 26, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Carboplatin. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/carboplatin (accessed on 21 March 2023).
- Collins, I.M.; Roberts-Thomson, R.; Faulkner, D.; Rischin, D.; Friedlander, M.; Mileshkin, L. Carboplatin dosing in ovarian cancer: Problems and pitfalls. Int. J. Gynecol. Cancer 2011, 21, 1213–1218. [Google Scholar] [CrossRef]
- Handolias, D.; Quinn, M.; Foo, S.; Mileshkin, L.; Grant, P.; Dutu, G.; Rischin, D. Oral cyclophosphamide in recurrent ovarian cancer. Asia Pac. J. Clin. Oncol. 2016, 12, e154–e160. [Google Scholar] [CrossRef]
- Lihua, P.; Chen, X.Y.; Wu, T.X. Topotecan for ovarian cancer. Cochrane Database Syst. Rev. 2008, 2008, Cd005589. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cui, M.; Liu, K. Therapeutic strategies to overcome cisplatin resistance in ovarian cancer. Eur. J. Med. Chem. 2022, 232, 114205. [Google Scholar] [CrossRef] [PubMed]
- Doxorubicin Hydrochloride. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/doxorubicinhydrochloride (accessed on 9 June 2023).
- Gemcitabine Hydrochloride. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/gemcitabinehydrochloride (accessed on 14 September 2023).
- Weaver, B.A. How Taxol/paclitaxel kills cancer cells. Mol. Biol. Cell 2014, 25, 2677–2681. [Google Scholar] [CrossRef] [PubMed]
- Nakai, H.; Matsumura, N. The roles and limitations of bevacizumab in the treatment of ovarian cancer. Int. J. Clin. Oncol. 2022, 27, 1120–1126. [Google Scholar] [CrossRef]
- Bevacizumab. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/bevacizumab (accessed on 8 December 2023).
- Mirvetuximab Soravtansine-gynx. Available online: https://www.cancer.gov/about-cancer/treatment/drugs/mirvetuximab-soravtansine-gynx (accessed on 24 April 2024).
- Dilawari, A.; Shah, M.; Ison, G.; Gittleman, H.; Fiero, M.H.; Shah, A.; Hamed, S.S.; Qiu, J.; Yu, J.; Manheng, W.; et al. FDA Approval Summary: Mirvetuximab Soravtansine-Gynx for FRα-Positive, Platinum-Resistant Ovarian Cancer. Clin. Cancer Res. 2023, 29, 3835–3840. [Google Scholar] [CrossRef]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef]
- Dockery, L.E.; Gunderson, C.C.; Moore, K.N. Rucaparib: The past, present, and future of a newly approved PARP inhibitor for ovarian cancer. Onco. Targets Ther. 2017, 10, 3029–3037. [Google Scholar] [CrossRef]
- Ueland, F.R. A Perspective on Ovarian Cancer Biomarkers: Past, Present and Yet-To-Come. Diagnostics 2017, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Karamahmutoglu, H.; Elitas, M.; Yuce, M.; Budak, H. THROUGH THE LOOKING GLASS: Real-Time Imaging in Brachypodium Roots and Osmotic Stress Analysis. Plants 2019, 8, 14. [Google Scholar] [CrossRef]
- Schmid, B.C.; Oehler, M.K. New perspectives in ovarian cancer treatment. Maturitas 2014, 77, 128–136. [Google Scholar] [CrossRef]
- Gogineni, V.; Morand, S.; Staats, H.; Royfman, R.; Devanaboyina, M.; Einloth, K.; Dever, D.; Stanbery, L.; Aaron, P.; Manning, L.; et al. Current Ovarian Cancer Maintenance Strategies and Promising New Developments. J. Cancer 2021, 12, 38–53. [Google Scholar] [CrossRef] [PubMed]
- Rocconi, R.P.; Ghamande, S.A.; Barve, M.A.; Stevens, E.E.; Aaron, P.; Stanbery, L.; Bognar, E.; Manning, L.; Nemunaitis, J.J.; O’Malley, D.M.; et al. Maintenance vigil immunotherapy in newly diagnosed advanced ovarian cancer: Efficacy assessment of homologous recombination proficient (HRP) patients in the phase IIb VITAL trial. J. Clin. Oncol. 2021, 39, 5502. [Google Scholar] [CrossRef]
- Wang, X.; Chang, W.C.; Wong, C.W.; Colcher, D.; Sherman, M.; Ostberg, J.R.; Forman, S.J.; Riddell, S.R.; Jensen, M.C. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 2011, 118, 1255–1263. [Google Scholar] [CrossRef]
- Rob, L.; Cibula, D.; Knapp, P.; Mallmann, P.; Klat, J.; Minar, L.; Bartos, P.; Chovanec, J.; Valha, P.; Pluta, M.; et al. Safety and efficacy of dendritic cell-based immunotherapy DCVAC/OvCa added to first-line chemotherapy (carboplatin plus paclitaxel) for epithelial ovarian cancer: A phase 2, open-label, multicenter, randomized trial. J. Immunother. Cancer 2022, 10, e003190. [Google Scholar] [CrossRef]
- Xiao, Y.F.; Jie, M.M.; Li, B.S.; Hu, C.J.; Xie, R.; Tang, B.; Yang, S.M. Peptide-Based Treatment: A Promising Cancer Therapy. J. Immunol. Res. 2015, 2015, 761820. [Google Scholar] [CrossRef]
- Hoare, J.; Campbell, N.; Carapuça, E. Oncolytic virus immunotherapies in ovarian cancer: Moving beyond adenoviruses. Porto Biomed. J. 2018, 3, e7. [Google Scholar] [CrossRef]
- Pal, S.; Sharma, A.; Mathew, S.P.; Jaganathan, B.G. Targeting cancer-specific metabolic pathways for developing novel cancer therapeutics. Front. Immunol. 2022, 13, 955476. [Google Scholar] [CrossRef] [PubMed]
- Basudan, A.M. The Role of Immune Checkpoint Inhibitors in Cancer Therapy. Clin. Pract. 2022, 13, 22–40. [Google Scholar] [CrossRef] [PubMed]
- Pirš, B.; Škof, E.; Smrkolj, V.; Smrkolj, Š. Overview of Immune Checkpoint Inhibitors in Gynecological Cancer Treatment. Cancers 2022, 14, 631. [Google Scholar] [CrossRef]
- Rinne, N.; Christie, E.L.; Ardasheva, A.; Kwok, C.H.; Demchenko, N.; Low, C.; Tralau-Stewart, C.; Fotopoulou, C.; Cunnea, P. Targeting the PI3K/AKT/mTOR pathway in epithelial ovarian cancer, therapeutic treatment options for platinum-resistant ovarian cancer. Cancer Drug Resist. 2021, 4, 573–595. [Google Scholar] [CrossRef]
- Adinolfi, S.; Patinen, T.; Jawahar Deen, A.; Pitkanen, S.; Harkonen, J.; Kansanen, E.; Kublbeck, J.; Levonen, A.L. The KEAP1-NRF2 pathway: Targets for therapy and role in cancer. Redox Biol. 2023, 63, 102726. [Google Scholar] [CrossRef] [PubMed]
- Perez-Fidalgo, J.A.; Ortega, B.; Simon, S.; Samartzis, E.P.; Boussios, S. NOTCH signalling in ovarian cancer angiogenesis. Ann. Transl. Med. 2020, 8, 1705. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meshkovska, Y.; Abramov, A.; Mahira, S.; Thatikonda, S. Understanding the Impact of Oxidative Stress on Ovarian Cancer: Advances in Diagnosis and Treatment. Future Pharmacol. 2024, 4, 651-675. https://doi.org/10.3390/futurepharmacol4030035
Meshkovska Y, Abramov A, Mahira S, Thatikonda S. Understanding the Impact of Oxidative Stress on Ovarian Cancer: Advances in Diagnosis and Treatment. Future Pharmacology. 2024; 4(3):651-675. https://doi.org/10.3390/futurepharmacol4030035
Chicago/Turabian StyleMeshkovska, Yeva, Artem Abramov, Shaheen Mahira, and Sowjanya Thatikonda. 2024. "Understanding the Impact of Oxidative Stress on Ovarian Cancer: Advances in Diagnosis and Treatment" Future Pharmacology 4, no. 3: 651-675. https://doi.org/10.3390/futurepharmacol4030035
APA StyleMeshkovska, Y., Abramov, A., Mahira, S., & Thatikonda, S. (2024). Understanding the Impact of Oxidative Stress on Ovarian Cancer: Advances in Diagnosis and Treatment. Future Pharmacology, 4(3), 651-675. https://doi.org/10.3390/futurepharmacol4030035







