Abstract
Esophageal cancer (EC) is a very common form of cancer in developing countries, and its exponential progression is a cause for concern. Available treatments face the phenomenon of multi-drug resistance, as well as multiple disabling side effects. The number of deaths is expected to double by 2030 if nothing is done. Due to their high representativeness in plants, phenolic compounds are a potential alternative for halting the spread of this disease, which bereaves many thousands of families every year. This study aims to identify phenolic compounds with activity against esophageal cancer, assess their toxicological profiles, and explore future perspectives. To achieve this, the literature search was meticulously carried out in the Google Scholar, Scopus, Web of Sciences, and Pub-Med/Medline databases, in accordance with the PRISMA 2020 guidelines. The results show that proanthocyanidin and curcumin represent promising therapeutic options, given their significant in vitro and in vivo activity, and their safety in human subjects in clinical trials. Moscatilin, Genistein, and pristimerin have anticancer activities (≤10 µM) very close to those of doxorubicin and 5-FU, although their safety has not yet been fully established. The compounds identified in vivo exhibit highly significant activities compared with the results obtained in vitro, and are sometimes more effective than the molecules conventionally used to treat EC. Generally, with the exceptions of plumbagin, lapachol, and β-lapachone, all other molecules are relatively non-toxic to normal human cells and represent a therapeutic avenue to be explored by pharmaceutical companies in the fight against esophageal cancer. However, more detailed toxicological studies of certain molecules remain a priority.
1. Introduction
People have always used medicinal plants to prevent and treat diseases. These plants possess a multitude of secondary metabolites that, alone or in combination, provide an undeniable therapeutic alternative to current health problems [1]. This is justified by the fact that over 25% of marketed medicines are derived from plants [2]. Among the active constituents of plants, the group of phenolic compounds occupies a prominent place [3]. They are produced in plants by metabolizing phenylpropanoids (shikimic acid and pentose phosphate) [4]. Their spectrum encompasses basic phenolic molecules to extensively polymerized compounds, all having benzene rings with one or more hydroxyl substituents [5]. This group contains numerous families of compounds: flavonoids, phenolic acids, tannins, stilbenes, coumarins, anthocyanins, and lignans [6,7]. Phenolic compounds play a crucial role in plant protection by defending against pathogens and environmental stressors, such as UV radiation and drought [8]. They also contribute to plant growth regulation and defense against herbivores through their repellent and toxic properties [9]. Additionally, certain phenolic compounds attract pollinators, thus facilitating plant reproduction [10]. They are also responsible for a wide range of biological activities in human health, including anti-inflammatory, anticancer, anti-aging, antioxidant, antibacterial, antiviral activities, cardiovascular protection, and cognitive health enhancement [11,12,13].
Regarding anti-cancer activity, numerous studies have reported notable biological effects of various phenolic compounds against specific cancer types, including esophageal cancer. EC is a significant health issue due to its rising incidence and high mortality rate, with approximately 604,100 new cases and 544,076 deaths globally each year [14,15]. Its exponential progression raises concern, as the number of deaths is expected to double by 2030 according to GLOBOCAM forecasts if no action is taken [14]. Two main types of esophageal cancer have been identified: esophageal adenocarcinoma (EAC), which is prevalent in high-income countries, and esophageal squamous cell carcinoma (ESCC), a highly aggressive form that is more commonly found in developing countries. The disease is particularly troubling because of its various risk factors, including excessive alcohol consumption, smoking, and gastroesophageal reflux disease [14]. The challenge of treating esophageal cancer is heightened by the fact that many patients are diagnosed at advanced stages, which severely limits treatment options and impacts survival rates [16]. The situation is further complicated by the development of drug resistance and severe side effects associated with current treatments, leading to poor patient adherence [1]. Despite some studies showing promising in vitro and in vivo activities of certain phenolic compounds against esophageal cancer, no effective therapeutic products have yet been developed. This review aims to compile a comprehensive summary of natural phenolic compounds with activity against esophageal cancer, assess their toxicological profiles, and provide insights into future research directions. By exploring these compounds, we seek to identify potential avenues for enhancing the treatment of this challenging disease and guiding pharmaceutical development.
2. Methodology
2.1. Data Sources, Search Strategy, and Eligibility Criteria
Original works published before July 2024 were collected in Google Scholar, Scopus, Web of Science, and Medline/PubMed databases and methodically appraised according to the PRISMA 2020 guidelines (see Supplementary File S1) [17]. The search terms included “anti-esophageal cancer” OR “anti-esophageal squamous-cell carcinoma”; OR “anti-esophageal adenocarcinoma” AND “phenolic compounds” AND “biological activity” OR “pharmacological activity”. Studies were included if they evaluated the anti-esophageal cancer activity of compounds belonging to the phenolic compounds group as a primary or secondary objective. Only original published studies were included. Studies focusing on other metabolite groups were excluded from this work. Review articles, conference abstracts, and editorials were also excluded. No restrictions regarding language or date were applied to this study.
2.2. Selection Process and Data Collection
To improve the organization of the selection and review process, the search results were first exported to Endnote for duplicate removal and then transferred to the Rayyan program [18]. The authors (EJN, GTK) independently screened the titles and abstracts. Subsequently, a second independent selection was conducted by reviewing the full texts of the articles retained after the first review. Any disagreements were resolved through discussion. Data such as phenolic compounds, structure, extracted plants, and biological properties were extracted from the various studies. For biological properties, study results (IC50, therapeutic doses, etc.) were independently extracted from the studies by the authors.
2.3. Synthesis Methods
The process of synthesizing and analyzing data was methodical. It started with a general summary of the studies and then classified them to achieve a more in-depth understanding. A detailed summary table was created to provide an effective visual representation of the key findings from the included studies. Then, a narrative synthesis will be conducted, given that this is a systematic review [1].
3. Results
3.1. Search Outcomes and Studies Characteristics
The research process (see Figure 1) yielded 30 studies and 25 compounds belonging to the following groups: chalcones (Moscatilin, Isoliquiritigenin, and 3-deoxysappanchalcone); Coumarins (Osthole); polyphenolic-flavonoids (Quercetin, Icariin, Purpurogallin, 6,7,4′-Trihydroxyisoflavone, Genistein, Hesperetin, Baohuoside-I, Curcumin, 2,6-Bis Benzylidine cyclohexanone, Proanthocyanidin, Gallic acid, theaflavin-3-3′-digallate, (-)-epigallocatechin-3-gallate, Theaflavate A, and Sesamin); quinones (Lapachol, β-lapachone, Pristimerin, Plumbagin); tannin (Corilagin); xanthone (Griffipavixanthone). The studies originated from three (3) continents: Africa, Asia, and America. Asia accounts for the largest number of studies, with 19 from China, 2 each from Taiwan, the Republic of Korea, and Japan, and 1 from Iran (See Table 1). On the American continent, only the USA is represented with 3 studies. In Africa, South Africa is the only country represented with 1 study. Although the majority of studies reported the activities of compounds against the ESCC variant, two studies investigated the activities of Moscatilin and Proanthocyanidins against the EAC cancer line [19,20].

Figure 1.
Study selection diagram.

Table 1.
Description of included studies.
3.2. Description Phenolic Compounds with Anticancer Activities against Esophageal Cancer
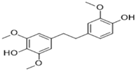
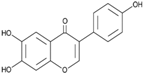
3.2.1. Moscatilin
Moscatilin or “Dendrophenol” (C17H20O5, Mol. wt. = 304.34 g mol−1) is a chalcone-bibenzyl derivative isolated from the orchid Dendrobium loddigesii. This plant is mainly found in Laos, Vietnam, and China [49]. Despite its multiple biological properties, it has activities against ESCC lines CE81T/VGH (IC50 = 7.0 µM) and EAC lines BE3 (IC50 = 6.7 µM) [19]. In vivo, moscatilin at a dose of 50 mg/kg reduces tumor mass by almost 50% in mice artificially induced with the CE81T/VGH line [21]. Chen et al. [19] revealed that the activity of this substance involves induction of apoptosis (by increasing early apoptotic cells such as PI− and annexin-V+), increased caspase activity, and cell cycle arrest in G2/M phase (through increased Plk1 and cyclin B1 expression, as well as increased phosphorylation of Cdc25c) in ESCC and EAC cells. Moscatilin is non-toxic to normal non-immortalized human oral fibroblast cells [49] and non-tumoral mammary epithelial cells [50]. Similarly, repeated use of moscatilin over 28 days does not lead to renal and hepatic complications [21]. See Table 2 for a summary of biological activities.

Table 2.
Summary of in vitro and in vivo anticancer efficacy of phenolic compounds.
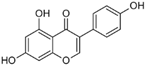
3.2.2. Isoliquiritigenin
Isoliquiritigenin is a chalcone-flavonoid isolated from Glycyrrhiza glabra, a plant native to southern Europe and Asia [51]. In vitro, this molecule inhibited cell growth and proliferation in ESCC KYSE140, KYSE520, and TE1 cancer cells [22]. Isoliquiritigenin exerts its anti-ESCC activity at several levels: induces cell cycle arrest at G0/G1 phase by reducing cyclin D1 expression; down-regulates AP-1 family proteins (Jun and Fos); and significantly reduces EGFR activation and its downstream signaling pathway Akt and ERK1/2 [22]. In vitro toxicology studies of Isoliquiritigenin on a broad range of human normal cells have demonstrated its safety on MCF-10A cells from the breast at concentration ≤ 100 µM [52,53] and H184B5F5/M10 at concentration ≤ 10 µM, HELF from lung [54], AML-12 Hepatocyte at concentration < 5 µM [55], T-HESCs from the uterus Endometrium at concentration ≤ 75 µM [56], GES-1 from the stomach at dose < 20 µM [57], HUVEC Endothelia [58], SG from the mouth (IC50 = 386.3 ± 29.7 µM) at concentration ≤ 400 µM [59], IEC-6 from the small intestine at concentration ≤ 100 µM [60], and H22 from the brain (neuroprotective) [61].
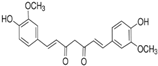
3.2.3. 3-Deoxysappanchalcone (3-DSC)
3-deoxysappanchalcone is a chalcone found in Caesalpinia sappan L. (Leguminosae), a plant native to southern China, Malaysia, and south-central India [62]. In addition to its antiviral, anti-allergic, antioxidant, and anti-inflammatory activities [63], 3-deoxysappanchalcone also exhibits anti-ESCC activities against KYSE 410 (IC50 = 12.2 µM), KYSE 30 (IC50 = 19.8 µM), KYSE 70 (IC50 = 20 µM), KYSE 450 (IC50 = 24.7 µM), and KYSE 510 (IC50 = 24.8 µM) cell lines [23]. The mechanisms by which this compound acts against ESCC cells include induction of apoptosis via the JNK/p38/MAPK signaling pathway, cell cycle arrest in the G2/M phase, and production of ROS [23]. The work of Fu et al. [64] demonstrated in vitro safety on healthy cells HaCaT, JB6CI41-5a (JB6), and Normal Human Dermal Fibroblasts (NHDF), as well as in vivo safety following acute administration in mice at concentrations ≤ 20 µM, even after 74 h of exposure.
3.2.4. Osthole
Osthole is a natural coumarin extracted from several medicinal plants, such as Angelica pubescens and Cnidium monnieri [65]. This compound exhibits a diverse range of biological activities, including antitumor, anti-inflammatory, neuroprotective, immunomodulatory, and hepatitis-suppressive effects [66]. Among its antitumor activities, osthole demonstrates activity against the ESCC phenotype of esophageal cancer with IC50 of 102.51 μM and 114.02 μM for KYSE150 and KYSE410, respectively [24]. Osthole acts by inducing G2/M phase arrest and apoptosis in ESCC cells through decreased expression of cyclin B1, Bcl-2, Cdc2, PARP1, PI3K, Survivin, and phosphorylated AKT (p-AKT); as well as increased expression of PTEN, cleaved PARP1, BAX, and cleaved Caspases 3 and 9 [24]. Osthole shows no adverse effects on acute oral administration at 2000 mg/kg in mice [67]. However, its LD50 in acute intraperitoneal administration is 750 mg/kg. Only doses below 25 mg/kg are without adverse effect in subchronic oral administration for 45 days [68]. Osthole is cytotoxic in vitro on normal liver cell lines (L-02) or induces cell apoptosis [69], and significantly affects the immune response negatively [70], although it has no adverse effect on the kidney [71].
3.2.5. Quercetin
An organic compound from the flavonoid family, specifically flavonols, quercetin is found in a diverse range of food plants (grapes, onions, berries, broccoli, cherries, and citrus fruits) [72]. It is mainly known for its antioxidant activities, as well as for the treatment of diseases caused by stress and oxidants [72]. It also possesses anti-ESCC activities and acts by significantly inhibiting the proliferation, migration, and invasion of Eca-109 cells at 10 μg/mL concentration [25]. Their anti-ESCC activity is based on reduced expression of MMP2, VEGF-A, and MMP9 proteins involved in angiogenesis and tumorigenesis [25,73]. Toxicologically, quercetin is non-toxic when administered subchronically (98 days) at doses of 250 mg/kg in CD2F1 mice [74]. High doses of quercetin cause mutagenic, hepatic, prooxidant, and renal complications [75]. Acutely, 3807 mg/kg is non-toxic in BALB/c mice [76]. In vitro, quercetin exhibited cytotoxicity against normal MRC-5 (human lung fibroblasts) cells with an IC50 > 80 μM, in contrast to doxorubicin (IC50 = 0.9 μM) commonly used against cancer in the same cell line [77].
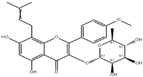
3.2.6. Icariin
Icariin, a flavonol glycoside, is the primary bioactive constituent isolated from Epimedium (Berberidaceae), a plant native to China [78]. Among its multiple biological properties is anti-ESCC activity against KYSE70 (IC50 = 40 μM) [26], Eca-109 (IC50 = 38.59 μM) and TE1 (IC50 = 42.21 μM) lines [27]. Icariin exerts its action by inducing G2/M-phase cell cycle arrest, apoptosis via ROS production, and Caspase 9 activity, and reducing intracellular glutathione (GSH) levels and NADPH oxidase activity, as well as cell migration and viability, through suppression of the STAT3 and PI3K/AKT pathways [26,27]. In vitro toxicology studies have reported that this molecule is non-toxic against HEK-293 human kidney cells [79]. Other authors, such as Zhu et al. [80] and Song et al. [81], have also reported the safety of Icariin on normal cell lines.
3.2.7. Purpurogallin
Purpurogallin is a natural aglycone isolated from plants of the genus Quercus spp., with the chemical formula C11H4O(OH)4 [82]. Currently, its activities against esophageal cancer have only been reported on ESCC types, specifically KYSE30, KYSE70, KYSE410, KYSE450, and KYSE510, with IC50 ≈ 7 µM [28,29]. In vivo in mice, purpurogallin at 100 mg/kg suppressed the growth of patient-derived ESCC tumors [28]. Its anti-ESCC mechanism relies on inhibition of the MEK1/2, ERK1/2 signaling pathways, as well as inhibition of cell cycle arrest at the G2/S phase via reduction of cyclin A2 and B1 expression [28]. Purpurogallin at a dose of 100 mg/kg has no adverse effects when administered consecutively over 14 days, and is harmless to the liver, kidney, and spleen after repeated administration over 31 days [28]. This effect on the liver is supported by the work of Wu et al. [83], who demonstrated its hepatoprotective effect in vitro and in vivo. It also plays a preventive role against the onset of hypercholesterolemic atherosclerosis [84]. Thanks to its strong antioxidant capacity, purpurogallin protects normal cells (HaCaT keratinocytes) from damage and apoptosis induced by UVB radiation [85].
3.2.8. (6,7,4′-THIF) or 6,7,4′-Trihydroxyisoflavone
6,7,4′-Trihydroxyisoflavone is a natural hydroxyisoflavone found in the Capsicum annuum plant of the Solanaceae family [86]. It inhibits proliferation and increases apoptosis by targeting the Pin1 protein that controls the cell cycle (G0-G1/S transition) [30]. 6,7,4′-THIF has neuroprotective effects against SH-SY5y neuroblastoma cells alone and exposed to CoCl2 [87].
3.2.9. Genistein
Genistein is a phytoestrogen belonging to the isoflavone class (7-hydroxyisoflavone) commonly found in variety food vegetables, such as soybeans and broad beans [88]. Its structure resembles that of endogenous estrogens [89]. Its anti-ESCC activity in vitro revealed IC50 of 5 μM, 12 μM, and 15 μM, respectively, against Eca-109, CaES-17, and EC9706 cell lines [31]. In vivo, genistein at a dose of 10 mg/kg significantly reduced the size of artificially induced ESCC tumors in mice over 42 days. Moreover, genistein potentiates the in vivo effect of GLPG0634 and MK-2206, two molecules with antiproliferative effects [31]. It exerts its action by promoting apoptosis, preventing cell proliferation, and arresting the cell cycle at the G0/G1 phase by inhibiting expression of EGFR signaling pathways (MDM2/AKT/p53 and STAT3-JAK1/2) [31]. Acute administration in mice of doses below 250 mg/kg genistein does not produce toxic effects but rather has a beneficial effect on biochemical and antioxidant markers [90]. Okazaki et al. [91] reported that this molecule had no adverse biochemical, hormonal, or reproductive effects in animals of either sex when administered subacutely for 28 days at a dose of 120 mg/kg. Similarly, genistein is harmless at 50 mg/kg when administered chronically, and is non-clastogenic in vivo [92,93]. Genistein has no carcinogenic activity in rats exposed for two years to 5, 100, or 500 ppm for males and 5 or 100 ppm for females. The 500 ppm dose induced carcinogenic effects in female rats with severe estrogenic disruption [94]. Genistein has good intestinal absorption properties [95].
3.2.10. Hesperetin
Hesperetin is a flavanone, a subgroup of Dihydroflavonoids, found in several citrus juices [96]. It possesses anti-inflammatory, antioxidant, antiviral, antihyperglycemic, blood lipid-modulating, antiallergic, and cholesterol-lowering anticancer properties [97]. In vitro, Hesperetin also possesses anti-ESCC activity against the Eca109 cell line with an IC50 > 200 μM [32,33]. This compound acts by inducing an increase in ROS, cleaved caspase-3 and -9, Bax protein, Apaf-1, and SuFu, and decreasing levels of Survivin and intracellular Bcl-2, which are involved in apoptosis [32]. It also suppressed the expression of cyclin D1, phosphorylated PI3K/AKT, MMP-9, and MMP-2, and increased phosphorylated p21 and PTEN, with consequent inhibition of proliferation and cell cycle arrest at the G0/G1 phase [33]. In vivo, in xenograft mice, the combination of hesperetin and 5-fluorouracil has a synergistic anticancer effect on an Eca-109-induced cancer model after 21 days [33]. In vitro, hesperetin is non-cytotoxic to GES-1 gastric epithelial cells and has a better effect than cisplatin [98]. Despite demonstrating cardioprotective effects [99], testicular protection, anti-apoptotic spermatogonia stem cells [100], anti-hyperuricemia [101], anti-lipotoxicity [102], protection against heavy metal toxicity [103], real-life toxicological studies remain virtually absent.
3.2.11. Baohuoside-I
Baohuoside I, also known as Icariside II, is a prenylated flavonoid (glycosyloxyflavone) from Epimedium koreanum, a medicinal plant distributed from southern Russia to eastern China and from Korea to Japan [104,105]. It is renowned for its anticancer properties, including anti-ESCC activities with an IC50 of 24.8 µg/mL on the Eca-109 cell line. Wang et al. [34] reported that 25 mg/kg of this compound significantly reduced the size of tumors induced with the Eca109 cell line in nude Balb/c mice. Its mechanism of action in vitro and in vivo is based on the induction of apoptosis through decreased expression of β-catenin, cyclin D1, and survivin [34]. Subacute toxicity studies lasting 15 days at doses ≤ 60 mg/kg revealed no signs of toxicity in mice [106]. In vitro, cytotoxicity studies revealed that Baohuoside I has an IC50 of 51 µM on normal pancreas cell lines hTERT-HPNE [107].
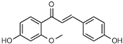
3.2.12. Curcumin
Curcumin, or diferuloylmethane, is a polyphenolic pigment of the diarylheptanoid group isolated from Curcuma longa (Zingiberaceae), a food and medicinal plant with multiple biological properties [108,109]. Its anti-ESCC effect was reported by Alibeiki et al. [36] on KYSE30 lines (IC50 = 5.42 µg/mL); Almanaa et al. [37] on KY-10, TE-1, KY-5, YES-1, TE-8, and YES-2 lines; and Mizumoto et al. [35] on TE-1 (IC50 = 19.23 μM), TE-5 (IC50 = 19.45 μM), TE-6 (IC50 = 7.03 μM), TE-8 (IC50 = 8.88 μM), TE-10 (IC50 = 12.91 μM), TE-11(IC50 = 8.98 μM), TE-11R (IC50 = 34.98 μM), T.Tn (IC50 = 19.66 μM), and HCE-4 (IC50 = 8.94 μM). It reduces the size of the ESCC tumor artificially induced in mice by 72.6% at a dose of 10,000 ppm. Curcumin induces cell cycle arrest at G2/M phase [35] and G1 phase [36] and increases SABG-positive senescence, and apoptosis by increasing caspase and Poly (ADP-ribose) polymerase (PARP) cleavage activity. It also exerts its effect on cancer stem cells [37]. Acute, repeated, and mutagenic toxicology studies have revealed that curcumin is considered safe and non-toxic [110]. However, controversy remains over its bioavailability [35,111].
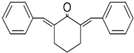
3.2.13. 2,6-Bis-Benzylidenocyclohexanone (BBCH)
2,6-Bis-benzylidenocyclohexanone (BBCH) is a compound that has been investigated for its anticancer properties. Available data demonstrate its effectiveness against ESCC cell lines such as KYSE30 (IC50 = 1.5 µM) [36]. BBCH exerts its anticancer effect by inducing apoptosis in KYSE30 cells and causing cell cycle arrest, particularly in the G2/M or G1 phases, thereby preventing cancer cells from dividing and proliferating. Preclinical studies in animal models, such as mice, show that BBCH reduces tumor size and weight by approximately 50% in xenograft mouse models infected with the KYSE30 cell line, indicating potential for cancer treatment in vivo [36]. Data on BBCH toxicity in mammals and humans are limited. However, preclinical studies suggest that BBCH is relatively safe at therapeutic doses, though further research is needed to fully assess its safety profile.
3.2.14. Proanthocyanidin
Proanthocyanidins are polyphenolic flavonoids (oligo- or polymers of flavan-3-ols) widely present in the nuts, fruits, seeds, vegetables, flowers, and bark of many plants [112]. They enable plants to defend themselves against abiotic and biotic stress factors [113]. The anticancer activities of proanthocyanidins have been demonstrated on both EAC (JHAD1, OE33, OE19) and ESCC (Eca-109 (IC50 = 37.15 μg/mL)) phenotypes [20]. In vivo, this molecule inhibits OE19 tumor proliferation via modulation of mTOR/AKT/MAPK signaling and induction of the autophagic form of LC3B [20]. G2/M cell cycle arrest, inactivation of AKT/PI3K/mTOR, and induction of pro-apoptotic proteins (BAX, BAK1, Cytochrome C, PARP, deamidated BCL-xL), modulation of MAPKs (P-P38/P-JNK) are other mechanisms used by proanthocyanidins against ESCC cells. Mechanisms involving caspase-3 activation and attenuation of NF-κB signaling pathway activation have also been reported by Guo et al. [37]. Yamakoshi et al. [114] reported that proanthocyanidins are harmless at 4000 mg/kg in acute oral administration, at 1410 mg/kg in subchronic administration (90 days), and have no mutagenic effect according to the Ames test. Clinical studies in human subjects revealed no signs of toxicity in subjects receiving doses of 2500 mg/day for 4 weeks [115].
3.2.15. Gallic Acid
Gallic acid (3,4,5-trihydroxybenzoic acid) is a natural polyphenolic compound found in vegetables, fruits, and medicinal plants [116] and is renowned for its interesting antioxidant activities. Faried et al. [39] reported that gallic acid induced apoptosis and inhibited cell proliferation in ESCC (TE-2) cells. Gallic acid is without adverse effects when administered for 13 weeks at 128 mg/kg in F344 rats [117]. A dose of 430 mg/kg administered subacutely for 21 days had no toxic effect on the reproductive organs of rats [118]. In vitro, gallic acid is non-toxic to normal CHEK-1 esophageal cells.
3.2.16. Sesamin
Sesamin is a lignan isolated from sesame oil and seeds, as well as from the bark of Fagara plants [119]. TRIM44 levels were significantly increased in ESCC cells and tissues. In vitro, sesamin inhibits cell proliferation in ESCC cells by inhibiting NF-kB, TLR4 signaling, and TRIM44 expression. In vivo, the 150 mg/kg dose reduces tumor size and TRIM44 expression levels by 50% [40]. Regarding sesamin toxicity, Hori et al. [120] reported that it is non-genotoxic in vitro and in vivo in mice. Hepatoprotective [121], neuroprotective [122], and nephroprotective [123] effects have also been observed with sesamin. Sesamin is non-toxic in vitro against normal Vero (ATCC#CCL-81) [124] and immortalized human small airway epithelial (SAEC) cells [125].
3.2.17. (-)-Epigallocatechin-3-Gallate
Formally obtained by the condensation of gallic acid with the (3R)-hydroxy group of (-)-epigallocatechin, (-)-epigallocatechin 3-gallate is a gallate ester [126]. It is the most abundant flavanol in green tea [127]. In addition to its significant antioxidant potential, this compound possesses anti-ESCC activity with an IC50 of 17 µM against the KYSE 510 cell line [29]. Although the mechanism of action of (-)-epigallocatechin-3-gallate on esophageal cancer cells is not yet fully elucidated, the MAPK signaling pathway appears to play a key role in this process. Anticancer mechanisms involving Pin1 inhibition have been reported in other cancer types [128].
3.2.18. Theaflavin-3-3′-Digallate
Theaflavin-3-3′-digallate is one of the natural polyphenolic theaflavins isolated from black tea and belonging to the catechin class [129]. In addition to its significant antioxidant potential, this compound has anti-ESCC activity with an IC50 of 18 µM on the KYSE 510 cell line [29]. Although the mechanism of action of theaflavin-3-3′-digallate on esophageal cancer cells is not yet fully elucidated, the MAPK signaling pathway appears to play a key role in this process. Anticancer mechanisms involving inhibition of cell proliferation, aromatase, and tyrosine kinase activity have been reported in other cancer types [130].
3.2.19. Theaflavate A
Theaflavate A, or theaflavic acid A, is a natural polyphenolic theaflavin isolated from black tea and belonging to the catechin class [131]. Initially known for its significant antioxidant potential, this compound also possesses anti-ESCC activity with an IC50 of 18 µM on the KYSE 510 cell line [29]. Although the mechanism by which Theaflavate A acts on esophageal cancer cells has yet to be fully elucidated, numerous anticancer mechanisms (e.g., induction of apoptosis, cell cycle arrest, activation of caspase 9, 8, and 3) have been demonstrated with other theaflavins in various types of cancer [132]. This compound protects normal PC12 cells against ROS-induced mitochondrial apoptosis via activation of the Nrf2/ARE signaling pathway [131].
3.2.20. Lapachol
Lapachol is a naturally occurring 1,4-naphthoquinone first isolated in 1882 by E. Paterno from the plant Tabebuia avellanedae (Bignoniaceae) [133]. Lapachol exhibits notable activity against ESCC phenotypes, including KYSE30, KYSE450, and KYSE 510 (IC50 ≈ 2 µM) [43], and WHCO1 (IC50 = 24.1 µM) [42]. This molecule acts by inhibiting the RSK2 protein [43]. Lapachol is non-toxic to NIH3T3 normal fibroblast cells [42]. This compound is non-toxic upon acute oral administration, with LD50 values ≥ 0.621 g/kg in mice, >2.4 g/kg in albino rats, and >0.5 g/kg/day in monkeys [134]. Guerra et al. [135] reported embryotoxicity, and Sá and Guerra [136] reported the reprotoxicity of lapachol in Wistar rats.
3.2.21. β-Lapachone
β-Lapachone is a natural ortho-naphthoquinone compound, originally found in the bark of Tabebuia avellanedae L. [137]. β-Lapachone exhibits notable activity against ESCC phenotypes, including WHCO1 (IC50 = 1.6 mM) [42]. This compound induces apoptosis in cancer cells by activating the c-Jun signaling pathway and is harmless to normal NIH3T3 [42] and HDF fibroblast cells [138]. Toxicity tests reveal that the 80 mg/kg dose of β-Lapachone is harmless upon acute administration in mice [139]. Although harmless to the kidney and liver, doses ≥ 40 mg/kg in repeated administration are abortive, teratogenic, hematotoxic, and splenotoxic in rats [140].
3.2.22. Pristimerin
Prestimerin is a natural quinonemethide triterpenoid found in Celastraceae and Hippocrateaceae species [141]. It exhibits a diverse range of biological activity, including anti-ESCC activities on EC9706 (IC50 = 1.98 µM), EC109 (IC50 = 1.76 µM), KYSE30 (IC50 = 1.13 µM) lines [44,45]. In vivo, pristimerin at a dose of 1.5 mg/kg/day reduced tumor size and weight by 71% in nude mice artificially infected with the Eca-109 cancer line after 2 weeks of treatment [44,45]. The anti-ESCC effect of this compound is based on its ability to inhibit the NF-κB pathway (inhibits TNFα activity) and induce G0/G1 phase arrest (through decreased protein expression of CDK4, CDK2, BCL-2, and cyclin E and increased CDKN1B expression and LC3-II/LC3-I ratio) [44,45]. Despite its promising activities against ESCC, the toxicity of pristimerin remains unknown, although it has no mutagenic activity [142].
3.2.23. Plumbagin
Plumbagin, or 5-hydroxy-2-methyl-1,4-naphthoquinone (C11H8O3), is a natural naphthoquinone isolated from Plumbago zeylanica L. roots, a medicinal plant native to Florida and belonging to the Plumbaginaceae family [143]. It is renowned for its antioxidant, anti-inflammatory, antibacterial, antifungal, and anticancer properties [144]. Recently, Cao et al. [46] reported its efficacy against the ESCC phenotype with IC50 of 6.4 and 8.0 μM on KYSE150 and KYSE450 cell lines, respectively. In vivo, in mice, plumbagin inhibits cell proliferation, reducing tumor size and mass by over 80% after 3 weeks of treatment at a dose of 2 mg/kg administered 5 times a week [46]. It acts by inducing cell cycle arrest and apoptosis through inhibition of STAT3-PLK1-AKT expression. Plumbagin is non-toxic at 150 mg/kg acute and 25 mg/kg subacute for 28 days in rats. Its bioavailability is 9.63 and its half-life is 5.0 h in rats [145]. This molecule is mutagenic in normal cells undergoing exponential growth [146].
3.2.24. Corilagin
Corilagin is an ellagitanin (gallotannin) isolated from Dividivi and Caesalpinia coriaria in 1951 and is now found in numerous plants, such as Phmllanthi Fructus [147]. This molecule has preventive and curative properties against several types of cancer [148]. Wu et al. [47] reported that corilagin is also effective against ESCC cancer cell lines, including Eca-109 (IC50 = 28.58 μM) and KYSE150 (IC50 = 35.05 μM). The 20 mg/kg dose significantly reduces tumor size in nude mice. It acts by arresting the cell cycle in the G0/G1 phase, resulting in apoptosis [47]. Toxicological studies available to date reveal that corilagin is non-mutagenic according to the Ames test [149] and non-cytotoxic in vitro to normal Vero [150], RAW264.7, BV-2 [151], MHCC97-H, SKOv3ip, and ovarian (OSE01, OSE02, and OSE03) and hepatic (Chang-liver) surface epithelial cells [152]. Corilagin is non-toxic at doses ≥ 3500 mg/kg in acute oral administration and at 1000 mg/kg for four weeks in subacute administration in mice [153].
3.2.25. Griffipavixanthone
Griffipavixanthone is a natural bixanthone found in Garcinia spp. [154,155,156]. At a concentration of 10 µM, this molecule completely inhibits the proliferation of TE1 and KYSE150 cells [48]. In vivo, the 20 mg/kg dose of griffipavixanthone prevented and reduced tumor sizes and lung metastases in mice infected with the KYSE150 cancer cell line, outperforming 5-Fluorouracil at the same dose. Griffipavixanthone acts by inhibiting AKT, decreasing cyclin B1 protein expression levels, the RAS-RAF-MEK-ERK cascade, and inducing G2/M cell cycle arrest [48]. Despite its promising activities against ESCC cells, toxicological studies on griffipavixanthone remain limited and are a future research priority.
A summary of the mechanisms of action of the various phenolic compounds is shown in Figure 2.
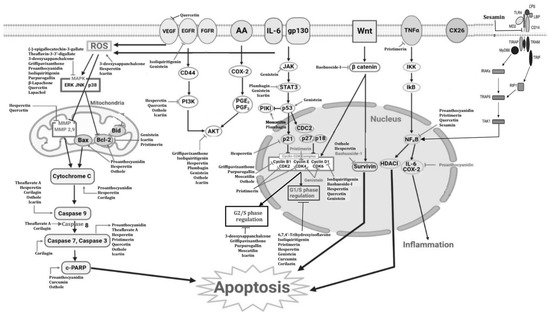
Figure 2.
Schematic overview of how phenolic compounds act against esophageal cancer [157].
4. Discussion
This study, which aimed to identify phenolic compounds with effects on esophageal cancer, assess their toxicological profile, and explore future perspectives, identified 25 compounds belonging to the group of phenolic compounds with anti-ESCC properties. This large number is justified by the fact that this group of compounds is the most abundant in plants, unlike the alkaloid and terpene groups [3]. Except for osthole and hesperetin, which have moderately significant cytotoxic activities (IC50 > 100 μM), all other compounds exhibit significant cytotoxic activity (IC50 < 100 μM) against all ESCC lines to which they were exposed [158]. However, it is important to note that compounds such as moscatilin, genistein, lapachol, curcumin, β-lapachone, pristimerin, and plumbagin have very significant cytotoxic activities (IC50 < 10 μM) against ESCC cells, approaching the activities of doxorubicin (0.9 μM). These compounds demonstrate efficacy at very low concentrations, often indicative of potent anticancer activity. This suggests they possess a significant capacity to inhibit the growth of ESCC and EAC cells at relatively low doses, offering promising prospects for future development as potential therapeutic agents against cancer. These compounds need to be thoroughly investigated, as they could revolutionize esophageal cancer therapy.
Similarly, individual studies conducted in vivo with these various compounds revealed that they reduced tumor sizes in mice more effectively than conventional anticancer drugs, a phenomenon that contrasts with the results observed in vitro. This can be explained by the fact that these compounds are potentiated by in vivo metabolism, resulting in more pronounced activity. According to Herman and Santos [159], secondary metabolites can exhibit enhanced activity during their passage through a living organism, leading to more pronounced effects. A substance may be metabolized into a toxic, inactive, less active, or active form as it passes through a living organism [160].
Phenolic compounds exhibit various mechanisms of action against esophageal cancer, providing promising alternatives to conventional treatments. These compounds induce apoptosis by increasing caspase activity and causing cell cycle arrest at different phases, such as isoliquiritigenin, hesperetin, moscatilin, and icariin, while also acting through ROS production and modulation of signaling pathways such as STAT3 and PI3K/AKT. Unlike chemotherapy (e.g., cisplatin) and radiotherapy, which cause severe side effects and can lead to resistance, phenolic compounds might offer less toxic approaches while targeting pathways such as EGFR, MAPK, and PI3K/AKT. Additionally, their ability to inhibit cell proliferation and angiogenesis (e.g., quercetin and proanthocyanidins), as well as induce oxidative stress (with hesperetin, icariin, and 3-deoxysappanchalcone), represents a potential alternative that could reduce the side effects of traditional therapies, such as those observed with bevacizumab [161,162]. Furthermore, due to their less severe side effects, phenolic compounds might help overcome some treatment resistance mechanisms [163]. While current treatments are effective, they are often limited by significant side effects and the development of resistance, which can compromise their long-term efficacy [164]. These compounds could offer interesting therapeutic alternatives to drugs such as gefitinib, cisplatin, and bevacizumab, against which several esophageal cancer cell lines have already developed resistance. The specific modulation of signaling pathways and the induction of oxidative stress could be developed to overcome the limitations of current therapies and pave the way for new therapeutic strategies.
The available toxicological studies for most of these substances, although limited, reveal that they have selective toxicity towards cancer cells, with the exception of osthole, which induces liver and immune toxicity [69,70]; lapachol and β-lapachone, which are embryotoxic [135], reprotoxic [136], teratogenic, hematotoxic, and splenotoxic [140] in rodents. The adverse effects observed following direct exposure to normal cells or administration of these compounds in animals have strong predictive value for human toxicity [38,165]. To date, only proanthocyanidin, with significant activities on EAC and ESCC phenotypes, has demonstrated its safety in human subjects in a preclinical study [115], confirming that many of these substances could offer therapeutic hope against esophageal cancer following comprehensive toxicological studies.
5. Limitations and Futures Perspectives
The compounds studied show promising potential as anticancer treatments for esophageal cancer. They exhibit various mechanisms of action and demonstrate encouraging results in inhibiting tumor growth both in vitro and in vivo. However, several challenges remain. The primary limitation of this study is the limited toxicological data available for many compounds, highlighting the need for further investigation into their safety. Additionally, the lack of in vivo studies for several substances means that the impact of metabolism on their efficacy remains unconfirmed. Future research should prioritize in vivo studies in animals and subsequently in humans through clinical trials to verify that the beneficial effects observed in vitro are sustained or enhanced. Another critical issue is the absence of bioavailability and formulation studies, which are necessary to identify the optimal administration route for these compounds. For instance, although curcumin has low oral bioavailability, it remains effective in vivo, suggesting that optimizing its dosage could improve its efficacy. Furthermore, the lack of positive controls, such as established anticancer agents, in both in vitro and in vivo experiments complicates the evaluation of the phenolic compounds’ effectiveness, as there is no direct comparison to standard treatments. Despite this, some phenolic compounds (hesperetin, moscatilin, isoliquiritigenin, and 3-deoxysappanchalcione) exhibit unique mechanisms, such as specific modulation of signaling pathways and induction of oxidative stress, which may offer novel therapeutic strategies not fully explored by traditional EC treatments. Overall, gaps in information regarding bioavailability, pharmacokinetics, toxicology, and clinical trials present significant barriers to the swift and successful clinical application of these compounds. Addressing these limitations is crucial for advancing their development into effective therapeutic options.
6. Conclusions
At the conclusion of this work, which aimed to present the phenolic compounds that could constitute alternative treatments for esophageal cancer and future research prospects for their development, it was found that proanthocyanidin and curcumin represent immediate therapeutic avenues due to their significant in vitro and in vivo activity, and their safety in human clinical trials. However, moscatilin, genistein, and pristimerin exhibit anticancer activities (≤10 µM) very close to those of doxorubicin, 5-FU, etc., although their safety has not yet been fully established. In vivo studies with these various compounds have demonstrated highly significant activity compared with the results obtained in vitro and sometimes exceed the effectiveness of conventional molecules used in esophageal cancer. Generally speaking, except for plumbagin, lapachol, and β-lapachone, all other molecules are relatively non-toxic to normal human cells and represent a promising therapeutic avenue for exploration by pharmaceutical companies in the fight against esophageal cancer. Future research should focus on aspects such as bioavailability, pharmacokinetics, toxicity, and clinical trials to facilitate a successful transition to clinical applications.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/futurepharmacol4030034/s1, File S1: PRISMA 2020 Checklist.
Author Contributions
G.T.K. and E.J.N. have contributed equally to the conceptualization, writing, and preparation of this manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was co-funded by the South African Medical Research Council (MRC) Strategic Health Innovation Partnerships and the National Research Foundation (NRF) Competitive Support for Unrated Researchers, awarded to Eugene Jamot Ndebia (Grants number: SRUG200512521370).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
Correction Statement
This article has been republished with a minor correction to the order of some references. This change does not affect the scientific content of the article.
References
- Ndebia, E.J.; Kamsu, G.T. Natural Alkaloids as Potential Treatments for Esophageal Squamous-Cell Cancer: A Comprehensive Review. Gastroenterol. Endosc. 2024, 2, 131–136. [Google Scholar] [CrossRef]
- Ntemafack, A.; Ayoub, M.; Hassan, Q.P.; Gandhi, S.G. A systematic review of pharmacological potential of phytochemicals from Rumex abyssinicus Jacq. S. Afr. J. Bot. 2023, 154, 11–25. [Google Scholar] [CrossRef]
- Ayad, R.; Akkal, S. Chapter 12—Phytochemistry and biological activities of algerian Centaurea and related genera. Stud. Nat. Prod. Chem. 2019, 63, 357–414. [Google Scholar]
- Randhir, R.; Lin, Y.T.; Shetty, K. Stimulation of phenolics, antioxidant and antimicrobial activities in dark germinated mung bean sprouts in response to peptide and phytochemical elicitors. Process Biochem. 2004, 39, 637–646. [Google Scholar] [CrossRef]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; AyalaZavala, J.F.; Chen, C.-Y.O.; Robles-Sanchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Xu, C.C.; Wang, B.; Pu, Y.Q.; Tao, J.S.; Zhang, T. Advances in extraction and analysis of phenolic compounds from plant materials. Chin. J. Nat. Med. 2017, 15, 721–731. [Google Scholar] [CrossRef]
- Alu’datt, M.H.; Rababah, T.; Alhamad, M.N.; Al-Mahasneh, M.A.; Almajwal, A.; Gammoh, S. A review of phenolic compounds in oil-bearing plants: Distribution, identification and occurrence of phenolic compounds. Food Chem. 2017, 218, 99–106. [Google Scholar] [CrossRef]
- Husain, N.; Gupta, S.A. critical study on chemistry and distribution of phenolic compounds in plants, and their role in human health. IOSR J. Environ. Sci. Toxicol. Food Technol. 2015, 1, 57–60. [Google Scholar]
- Šamec, D.; Karalija, E.; Šola, I.; Vujčić Bok, V.; Salopek-Sondi, B. The Role of Polyphenols in Abiotic Stress Response: The Influence of Molecular Structure. Plants 2021, 10, 118. [Google Scholar] [CrossRef]
- Leonhardt, S.D.; Chui, S.X.; Kuba, K. The role of non-volatile chemicals of floral rewards in plant-pollinator interactions. Basic Appl. Ecol. 2024, 75, 31–43. [Google Scholar] [CrossRef]
- Rahman, M.M.; Rahaman, M.S.; Islam, M.R.; Rahman, F.; Mithi, F.M.; Alqahtani, T. Role of Phenolic Compounds in Human Disease: Current Knowledge and Future Prospects. Molecules 2021, 27, 233. [Google Scholar] [CrossRef] [PubMed]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of Phenolic Compounds in the Reduction of Risk Factors of Cardiovascular Diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Li, J.; Xu, J.; Zheng, Y.; Gao, Y.; He, S.; Li, H.; Zou, K.; Li, N.; Tian, J.; Chen, W.; et al. Esophageal cancer: Epidemiology, risk factors and screening. Chin. J. Cancer Res. 2021, 33, 535–547. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Ndebia, E.J.; Kamsu, G.T. A Comprehensive Meta-Analysis of Dietary and Culinary Practices on Esophageal Cancer Incidence in the East African Corridor. SVU-Int. J. Med. Sci. 2024, 7, 207–222. [Google Scholar] [CrossRef]
- Chen, C.A.; Chen, C.C.; Shen, C.C.; Chang, H.H.; Chen, Y.J. Moscatilin induces apoptosis and mitotic catastrophe in human esophageal cancer cells. J. Med. Food 2013, 16, 869–877. [Google Scholar] [CrossRef]
- Kresty, L.A.; Weh, K.M.; Zeyzus-Johns, B.; Perez, L.N.; Howell, A.B. Cranberry proanthocyanidins inhibit esophageal adenocarcinoma in vitro and in vivo through pleiotropic cell death induction and PI3K/AKT/mTOR inactivation. Oncotarget 2015, 6, 33438–33455. [Google Scholar] [CrossRef]
- Chen, W.K.; Chen, C.A.; Chi, C.W.; Li, L.H.; Lin, C.P.; Shieh, H.R.; Hsu, M.L.; Ko, C.C.; Hwang, J.J.; Chen, Y.J. Moscatilin Inhibits Growth of Human Esophageal Cancer Xenograft and Sensitizes Cancer Cells to Radiotherapy. J. Clin Med. 2019, 8, 187. [Google Scholar] [CrossRef] [PubMed]
- Ye, L.; Zhang, J.; Zhang, Y.; Gu, B.; Zhu, H.; Mao, X. Isoliquiritigenin Suppressed Esophageal Squamous Carcinoma Growth by Blocking EGFR Activation and Inducing Cell Cycle Arrest. Biomed. Res. Int. 2020, 2020, 9259852. [Google Scholar] [CrossRef] [PubMed]
- Kwak, A.W.; Lee, M.J.; Lee, M.H.; Yoon, G.; Cho, S.S.; Chae, J.I.; Shim, J.H. The 3-deoxysappanchalcone induces ROS-mediated apoptosis and cell cycle arrest via JNK/p38 MAPKs signaling pathway in human esophageal cancer cells. Phytomedicine 2021, 86, 153564. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Z.; Li, T.; Long, F.; Lv, Y.; Liu, L.; Liu, X.; Zhan, Q. Osthole inhibits the PI3K/AKT signaling pathway via activation of PTEN and induces cell cycle arrest and apoptosis in esophageal squamous cell carcinoma. Biomed. Pharmacother. 2018, 102, 502–509. [Google Scholar] [CrossRef]
- Liu, Y.; Li, C.L.; Xu, Q.Q.; Cheng, D.; Liu, K.D.; Sun, Z.Q. Quercetin inhibits invasion and angiogenesis of esophageal cancer cells. Pathol. Res. Pract. 2021, 222, 153455. [Google Scholar] [CrossRef]
- Gu, Z.F.; Zhang, Z.T.; Wang, J.Y.; Xu, B.B. Icariin exerts inhibitory effects on the growth and metastasis of KYSE70 human esophageal carcinoma cells via PI3K/AKT and STAT3 pathways. Environ. Toxicol. Pharmacol. 2017, 54, 7–13. [Google Scholar] [CrossRef]
- Fan, C.; Yang, Y.; Liu, Y.; Jiang, S.; Di, S.; Hu, W.; Ma, Z.; Li, T.; Zhu, Y.; Xin, Z.; et al. Icariin displays anticancer activity against human esophageal cancer cells via regulating endoplasmic reticulum stress-mediated apoptotic signaling. Sci. Rep. 2016, 6, 21145. [Google Scholar] [CrossRef]
- Xie, X.; Zu, X.; Liu, F.; Wang, T.; Wang, X.; Chen, H.; Liu, K.; Wang, P.; Liu, F.; Zheng, Y.; et al. Purpurogallin is a novel mitogen-activated protein kinase kinase 1/2 inhibitor that suppresses esophageal squamous cell carcinoma growth in vitro and in vivo. Mol. Carcinog. 2019, 58, 1248–1259. [Google Scholar] [CrossRef]
- Sang, S.; Lambert, J.D.; Tian, S.; Hong, J.; Hou, Z.; Ryu, J.H.; Stark, R.E.; Rosen, R.T.; Huang, M.T.; Yang, C.S.; et al. Enzymatic synthesis of tea theaflavin derivatives and their anti-inflammatory and cytotoxic activities. Bioorg. Med. Chem. 2004, 12, 459–467. [Google Scholar] [CrossRef]
- Lim, T.-G.; Lee, S.-Y.; Duan, Z.; Lee, M.-H.; Chen, H.; Liu, F.; Liu, K.; Jung, S.K.; Kim, D.J.; Bode, A.M.; et al. The Prolyl Isomerase Pin1 Is a Novel Target of 6, 7, 40-Trihydroxyisoflavone for Suppressing Esophageal Cancer Growth. Cancer Prev. Res. 2017, 10, 308–318. [Google Scholar] [CrossRef]
- Gao, J.; Xia, R.; Chen, J.; Gao, J.; Luo, X.; Ke, C.; Ren, C.; Li, J.; Mi, Y. Inhibition of esophageal-carcinoma cell proliferation by genistein via suppression of JAK1/2-STAT3 and AKT/MDM2/p53 signaling pathways. Aging 2020, 12, 6240–6259. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, J.; Wang, J.; Li, J.; Liao, F.; Dong, W. Hesperetin induces apoptosis of esophageal cancer cells via mitochondrial pathway mediated by the increased intracellular reactive oxygen species. Tumour Biol. 2016, 37, 3451–3459. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, J.; Hu, X.; Ma, J.; Dong, W. Hesperetin inhibits Eca-109 cell proliferation and invasion by suppressing the PI3K/AKT signaling pathway and synergistically enhances the anti-tumor effect of 5-fluorouracil on esophageal cancer in vitro and in vivo. RSC Adv. 2018, 8, 24434–24443. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, A.; Liu, X.; Sang, M.; Shan, B.; Meng, F.; Cao, Q.; Ji, X. The flavonoid Baohuoside-I inhibits cell growth and downregulates survivin and cyclin D1 expression in esophageal carcinoma via β-catenin-dependent signaling. Oncol. Rep. 2011, 26, 1149–1156. [Google Scholar]
- Mizumoto, A.; Ohashi, S.; Kamada, M.; Saito, T.; Nakai, Y.; Baba, K.; Hirohashi, K.; Mitani, Y.; Kikuchi, O.; Matsubara, J.; et al. Combination treatment with highly bioavailable curcumin and NQO1 inhibitor exhibits potent antitumor effects on esophageal squamous cell carcinoma. J. Gastroenterol. 2019, 54, 687–698. [Google Scholar] [CrossRef]
- Alibeiki, F.; Jafari, N.; Karimi, M.; Peeri-Dogaheh, H. Potent anti-cancer effects of less polar Curcumin analogues on gastric adenocarcinoma and esophageal squamous cell carcinoma cells. Sci. Rep. 2017, 7, 2559. [Google Scholar] [CrossRef]
- Almanaa, T.N.; Geusz, M.E.; Jamasbi, R.J. Effects of curcumin on stem-like cells in human esophageal squamous carcinoma cell lines. BMC Complement. Altern. Med. 2012, 12, 195. [Google Scholar]
- Guo, F.; Hu, Y.; Niu, Q.; Li, Y.; Ding, Y.; Ma, R.; Wang, X.; Li, S.; Xie, J. Grape Seed Proanthocyanidin Extract Inhibits Human Esophageal Squamous Cancerous Cell Line ECA109 via the NF-κB Signaling Pathway. Mediators Inflamm. 2018, 2018, 3403972. [Google Scholar] [CrossRef]
- Faried, A.; Kurnia, D.; Faried, L.S.; Usman, N.; Miyazaki, T.; Kato, H.; Kuwano, H. Anticancer effects of gallic acid isolated from Indonesian herbal medicine, Phaleria macrocarpa (Scheff.) Boerl, on human cancer cell lines. Int. J. Oncol. 2007, 30, 605–613. [Google Scholar] [CrossRef]
- Wen, L.; Mao, W.; Xu, L.; Cai, B.; Gu, L. Sesamin exerts anti-tumor activity in esophageal squamous cell carcinoma via inhibition of TRIM44 and NF-κB signaling. Chem. Biol. Drug Des. 2022, 99, 118–125. [Google Scholar] [CrossRef]
- Gao, Y.; Li, W.; Jia, L.; Li, B.; Chen, Y.C.; Tu, Y. Enhancement of (-)-epigallocatechin-3-gallate and theaflavin-3-3′-digallate induced apoptosis by ascorbic acid in human lung adenocarcinoma SPC-A-1 cells and esophageal carcinoma Eca-109 cells via MAPK pathways. Biochem. Biophys. Res. Commun. 2013, 438, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Sunassee, S.N.; Veale, C.G.; Shunmoogam, G.N.; Osoniyi, O.; Hendricks, D.T.; Caira, M.R.; de la Mare, J.A.; Edkins, A.L.; Pinto, A.V.; da Silva Júnior, E.N.; et al. Cytotoxicity of lapachol, β-lapachone and related synthetic 1, 4-naphthoquinones against oesophageal cancer cells. Eur. J. Med. Chem. 2013, 62, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Zu, X.; Xie, X.; Zhang, Y.; Liu, K.; Bode, A.M.; Dong, Z.; Kim, D.J. Lapachol is a novel ribosomal protein S6 kinase 2 inhibitor that suppresses growth and induces intrinsic apoptosis in esophageal squamous cell carcinoma cells. Phytother. Res. 2019, 33, 2337–2346. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Sun, L.Y.; Zhang, Y.Q. A Hopeful Natural Product, Pristimerin, Induces Apoptosis, Cell Cycle Arrest, and Autophagy in Esophageal Cancer Cells. Anal. Cell Pathol. 2019, 2019, 6127169. [Google Scholar] [CrossRef]
- Tu, Y.; Tan, F.; Zhou, J.; Pan, J. Pristimerin targeting NF-κB pathway inhibits proliferation, migration, and invasion in esophageal squamous cell carcinoma cells. Cell Biochem. Funct. 2018, 36, 228–240. [Google Scholar] [CrossRef]
- Cao, Y.Y.; Yu, J.; Liu, T.T.; Yang, K.X.; Yang, L.Y.; Chen, Q.; Shi, F.; Hao, J.J.; Cai, Y.; Wang, M.R.; et al. Plumbagin inhibits the proliferation and survival of esophageal cancer cells by blocking STAT3-PLK1-AKT signaling. Cell Death Dis. 2018, 9, 17. [Google Scholar] [CrossRef]
- Wu, C.; Huang, H.; Choi, H.-Y.; Ma, Y.; Zhou, T.; Peng, Y.; Pang, K.; Shu, G.; Yang, X. Anti-esophageal Cancer Effect of Corilagin Extracted from Phmllanthi Fructus via the Mitochondrial and Endoplasmic Reticulum Stress Pathways. J. Ethnopharmacol. 2021, 269, 113700. [Google Scholar] [CrossRef]
- Ding, Z.; Lao, Y.; Zhang, H.; Fu, W.; Zhu, L.; Tan, H.; Xu, H. Griffipavixanthone, a dimeric xanthone extracted from edible plants, inhibits tumor metastasis and proliferation via downregulation of the RAF pathway in esophageal cancer. Oncotarget 2016, 7, 1826–1837. [Google Scholar] [CrossRef]
- Cardile, V.; Avola, R.; Graziano, A.C.E.; Russo, A. Moscatilin, a bibenzyl derivative from the orchid Dendrobium loddigesii, induces apoptosis in melanoma cells. Chem. Biol. Interact. 2020, 323, 109075. [Google Scholar] [CrossRef]
- Aljeldah, M.M. Evaluation of the anticancer and antibacterial activities of moscatilin. Heliyon 2024, 10, e31131. [Google Scholar] [CrossRef]
- Zhang, Z.; Yung, K.K.; Ko, J.K. Therapeutic Intervention in Cancer by Isoliquiritigenin from Licorice: A Natural Antioxidant and Redox Regulator. Antioxidants 2022, 11, 1349. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, Z.; Peng, C.; You, J.; Shen, J.; Han, S.; Chen, J. Dietary compound isoliquiritigenin targets GRP78 to chemosensitize breast cancer stem cells via beta-catenin/ABCG2 signaling. Carcinogenesis 2014, 35, 2544–2554. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, N.; Liu, P.; Chen, Q.; Situ, H.; Xie, T.; Zhang, J.; Peng, C.; Lin, Y.; Chen, J. MicroRNA-25 regulates chemoresistance associated autophagy in breast cancer cells, a process modulated by the natural autophagy inducer isoliquiritigenin. Oncotarget 2014, 5, 7013–7026. [Google Scholar] [CrossRef] [PubMed]
- Qiao, F.; Zhao, Y.; Mai, Y.; Guo, J.; Dong, L.; Zhang, W.; Yang, J. Isoliquiritigenin Nanosuspension Enhances Cytostatic Effects in A549 Lung Cancer Cells. Planta Med. 2020, 86, 538–547. [Google Scholar] [CrossRef] [PubMed]
- Na, A.-Y.; Yang, E.-J.; Jeon, J.M.; Ki, S.H.; Song, K.-S.; Lee, S. Protective Effect of Isoliquiritigenin against Ethanol-Induced Hepatic Steatosis by Regulating the SIRT1-AMPK Pathway. Toxicol. Res. 2018, 34, 23–29. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, H.-Y.; Wang, C.-W.; Shieh, T.-M.; Huang, T.-C.; Lin, L.-C.; Wang, K.L.; Hsia, S.M. Isoliquiritigenin induces apoptosis and autophagy and inhibits endometrial cancer growth in mice. Oncotarget 2016, 7, 73432–73447. [Google Scholar] [CrossRef]
- Huang, F.; Wang, J.; Xu, Y.; Zhang, Y.; Xu, N.; Yin, L. Discovery of novel isoliquiritigenin analogue ISL-17 as a potential anti-gastric cancer agent. Biosci. Rep. 2020, 40, 20201199. [Google Scholar]
- Kwon, H.M.; Choi, Y.J.; Choi, J.S.; Kang, S.W.; Bae, J.Y.; Kang, I.J.; Jun, J.G.; Lee, S.S.; Lim, S.S.; Kang, Y.H. Blockade of cytokine induced endothelial cell adhesion molecule expression by licorice isoliquiritigenin through NF-kappaB signal disruption. Exp. Biol. Med. 2007, 232, 235–245. [Google Scholar]
- Hu, F.-W.; Yu, C.-C.; Hsieh, P.-L.; Liao, Y.-W.; Lu, M.-Y.; Chu, P.-M. Targeting oral cancer stemness and chemoresistance by isoliquiritigenin-mediated GRP78 regulation. Oncotarget 2017, 8, 93912–93923. [Google Scholar] [CrossRef]
- Zhang, X.; Yeung, E.D.; Wang, J.; Panzhinskiy, E.E.; Tong, C.; Li, W.; Li, J. Isoliquiritigenin, a natural anti-oxidant, selectively inhibits the proliferation of prostate cancer cells. Clin. Exp. Pharmacol. Physiol. 2010, 37, 841–847. [Google Scholar]
- Selvaraj, B.; Kim, D.W.; Huh, G.; Lee, H.; Kang, K.; Lee, J.W. Synthesis and biological evaluation of isoliquiritigenin derivatives as a neuroprotective agent against glutamate mediated neurotoxicity in HT22 cells. Bioorg. Med. Chem. Lett. 2020, 30, 127058. [Google Scholar] [CrossRef] [PubMed]
- Vij, T.; Anil, P.P.; Shams, R.; Dash, K.K.; Kalsi, R.; Pandey, V.K.; Harsányi, E.; Kovács, B.; Shaikh, A.M. A Comprehensive Review on Bioactive Compounds Found in Caesalpinia sappan. Molecules 2023, 28, 6247. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.E.; Choi, H.C.; Lee, I.C.; Yuk, D.Y.; Lee, H.; Choi, B.Y. 3-Deoxysappanchalcone Promotes Proliferation of Human Hair Follicle Dermal Papilla Cells and Hair Growth in C57BL/6 Mice by Modulating WNT/β-Catenin and STAT Signaling. Biomol. Ther. 2016, 24, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Zhao, R.; Yoon, G.; Shim, J.-H.; Choi, B.Y.; Yin, F.; Xu, B.; Laster, K.V.; Liu, K.; Dong, Z.; et al. 3-Deoxysappanchalcone Inhibits Skin Cancer Proliferation by Regulating T-Lymphokine-Activated Killer Cell-Originated Protein Kinase in vitro and in vivo. Front. Cell Dev. Biol. 2021, 9, 638174. [Google Scholar] [CrossRef]
- Sun, M.; Sun, M.; Zhang, J. Osthole: An overview of its sources, biological activities, and modification development. Med. Chem. Res. 2021, 30, 1767–1794. [Google Scholar] [CrossRef]
- Zhang, Z.R.; Leung, W.N.; Cheung, H.Y.; Chan, C.W. Osthole: A review on its bioactivities, pharmacological properties, and potential as alternative medicine. Evid. Based Complement. Altern. Med. 2015, 2015, 919616. [Google Scholar] [CrossRef]
- Singh, G.; Bhatti, R.; Mannan, R.; Singh, D.; Kesavan, A.; Singh, P. Osthole ameliorates neurogenic and inflammatory hyperalgesia by modulation of iNOS, COX-2, and inflammatory cytokines in mice. Inflammopharmacology 2019, 27, 949–960. [Google Scholar] [CrossRef]
- Shokoohinia, Y.; Bazargan, S.; Miraghaee, S.; Javadirad, E.; Farahani, F.; Hosseinzadeh, L. Safety Assessment of Osthole Isolated from Prangos ferulacea: Acute and Subchronic Toxicities and Modulation of Cytochrome P450. Jundishapur J. Nat. Pharm. Prod. 2017, 12, e63764. [Google Scholar] [CrossRef]
- Shen, Z.; Chen, J.; Lu, H. Osthole induced apoptosis in human normal liver cells by regulating cell proliferation and endoplasmic reticulum stress. Environ. Toxicol. 2019, 34, 768–776. [Google Scholar] [CrossRef]
- Callahan, B.N.; Kammala, A.K.; Syed, M.; Yang, C.; Occhiuto, C.J.; Nellutla, R.; Chumanevich, A.P.; Oskeritzian, C.A.; Das, R.; Subramanian, H. Osthole, a natural plant derivative inhibits mrgprx2 induced mast cell responses. Front. Immunol. 2020, 11, 703. [Google Scholar] [CrossRef]
- Kan, W.C.; Hwang, J.Y.; Chuang, L.Y.; Guh, J.Y.; Ye, Y.L.; Yang, Y.L.; Huang, J.S. Effect of osthole on advanced glycation end products-induced renal tubular hypertrophy and role of klotho in its mechanism of action. Phytomedicine 2019, 53, 205–212. [Google Scholar] [CrossRef] [PubMed]
- Anand-David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of Biological Importance of Quercetin: A Bioactive Flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed]
- Augoff, K.; Hryniewicz, J.A.; Tabola, R.; Stach, K. MMP9: A Tough Target for Targeted Therapy for Cancer. Cancers 2022, 14, 1847. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, P.; Patton, E.; VanderVeen, B.N.; Unger, C.; Aladhami, A.; Enos, R.T.; Madero, S.; Chatzistamou, I.; Fan, D.; Murphy, E.A.; et al. Sub-chronic oral toxicity screening of quercetin in mice. BMC Complement. Med. Ther. 2022, 22, 279. [Google Scholar]
- Chen, R.; Lin, J.; Hong, J.; Han, D.; Zhang, A.D.; Lan, R.; Fu, L.; Wu, Z.; Lin, J.; Zhang, W.; et al. Potential toxicity of quercetin: The repression of mitochondrial copy number via decreased POLG expression and excessive TFAM expression in irradiated murine bone marrow. Toxicol. Rep. 2014, 1, 450–458. [Google Scholar] [CrossRef]
- Dibal, N.I.; Hyedima, G.S.; Watson, J.T. Acute Toxicity of Quercetin from Onion Skin in Mice. Pharm. Biomed. Res. 2020, 6, 269–276. [Google Scholar] [CrossRef]
- da Silva, S.V.S.; Barboza, O.M.; Souza, J.T.; Soares, É.N.; dos Santos, C.C.; Pacheco, L.V.; Santos, I.P.; Magalhães, T.B.d.S.; Soares, M.B.P.; Guimarães, E.T.; et al. Structural design, synthesis and antioxidant, antileishmania, anti-inflammatory and anticancer activities of a novel quercetin acetylated derivative. Molecules 2021, 26, 6923. [Google Scholar] [CrossRef]
- Shen, R.; Deng, W.; Li, C.; Zeng, G. A natural flavonoid glucoside icariin inhibits Th1 and Th17 cell differentiation and ameliorates experimental autoimmune encephalomyelitis. Int. Immunopharmacol. 2015, 24, 224–231. [Google Scholar] [CrossRef]
- Zhou, Y.D.; Hou, J.G.; Yang, G.; Jiang, S.; Chen, C.; Wang, Z.; Liu, Y.Y.; Ren, S.; Li, W. Icariin ameliorates cisplatin-induced cytotoxicity in human embryonic kidney 293 cells by suppressing ROS-mediated PI3K/Akt pathway. Biomed. Pharmacother. 2019, 109, 2309–2317. [Google Scholar] [CrossRef]
- Zhu, F.; Ren, Z. Icariin inhibits the malignant progression of lung cancer by affecting the PI3K/Akt pathway through the miR-205-5p/PTEN axis. Oncol. Rep. 2022, 47, 115. [Google Scholar] [CrossRef]
- Song, L.; Chen, X.; Mi, L.; Liu, C.; Zhu, S.; Yang, T.; Luo, X.; Zhang, Q.; Lu, H.; Liang, X. Icariin-induced inhibition of SIRT6/NF-κB triggers redox mediated apoptosis and enhances anti-tumor immunity in triple-negative breast cancer. Cancer Sci. 2020, 111, 4242–4256. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.C.; Kim, T.; Silva, J.L.; Chen, B.Y. A New Approach for Quantifying Purpurogallin in Brewed Beverages Using LC-MS in Combination with Solid Phase Extraction. Foods 2022, 11, 1429. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.W.; Zeng, L.H.; Wu, J.; Carey, D. Purpurogallin--a natural and effective hepatoprotector in vitro and in vivo. Biochem. Cell Biol. 1991, 69, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Mantha, S.V.; Kalra, J.; Kapoor, R.; Kamalarajan, B.R.C. Purpurogallin in the prevention of hypercholesterolemic atherosclerosis. Int. J. Angiol. 1997, 6, 157–166. [Google Scholar] [CrossRef]
- Zhen, A.X.; Piao, M.J.; Hyun, Y.J.; Kang, K.A.; Ryu, Y.S.; Cho, S.J.; Kang, H.K.; Koh, Y.S.; Ahn, M.J.; Kim, T.H.; et al. Purpurogallin protects keratinocytes from damage and apoptosis induced by Ultraviolet B radiation and particulate matter 2.5. Biomol. Ther. 2019, 27, 395–403. [Google Scholar] [CrossRef]
- NCBI (National Center for Biotechnology Information). PubChem Compound Summary for CID 5284649, 6, 7, 4′-Trihydroxyisoflavone. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6_7_4_-Trihydroxyisoflavone (accessed on 27 June 2024).
- Lee, H.-S.; Jeong, G.-S. Protective Effects of 6, 7, 4′ -Trihydroxyflavanone on Hypoxia-Induced Neurotoxicity by Enhancement of HO-1 through Nrf2 Signaling Pathway. Antioxidants 2021, 10, 341. [Google Scholar] [CrossRef]
- Sohel, M.; Biswas, P.; Al Amin, M.; Hossain, M.A.; Sultana, H.; Dey, D.; Aktar, S.; Setu, A.; Khan, M.S.; Paul, P.; et al. Genistein, a potential phytochemical against breast cancer treatment-insight into the molecular mechanisms. Processes 2022, 10, 415. [Google Scholar] [CrossRef]
- Yu, L.; Rios, E.; Castro, L.; Liu, J.; Yan, Y.; Dixon, D. Genistein: Dual Role in Women’s Health. Nutrients 2021, 13, 3048. [Google Scholar] [CrossRef]
- Singh, P.; Sharma, S.; Rath, S.K. Genistein induces deleterious effects during its acute exposure in Swiss mice. Biomed. Res. Int. 2014, 2014, 619617. [Google Scholar] [CrossRef]
- Okazaki, K.; Okazaki, S.; Nakamura, H.; Kitamura, Y.; Hatayama, K.; Wakabayashi, S.; Tsuda, T.; Katsumata, T.; Nishikawa, A.; Hirose, M. A repeated 28-day oral dose toxicity study of genistein in rats, based on the ‘Enhanced OECD Test Guideline 407′ for screening endocrine-disrupting chemicals. Arch. Toxicol. 2002, 76, 553–559. [Google Scholar] [CrossRef]
- McClain, R.M.; Wolz, E.; Davidovich, A.; Bausch, J. Genetic toxicity studies with genistein. Food Chem. Toxicol. 2006, 44, 42–55. [Google Scholar] [CrossRef] [PubMed]
- McClain, R.M.; Wolz, E.; Davidovich, A.; Pfannkuch, F.; Edwards, J.A.; Bausch, J. Acute, subchronic and chronic safety studies with genistein in rats. Food Chem. Toxicol. 2006, 44, 56–80. [Google Scholar] [CrossRef] [PubMed]
- NTP (National Toxicology Program). Toxicology and carcinogenesis studies of genistein (Cas No. 446-72-0) in Sprague-Dawley rats (feed study). Natl. Toxicol. Program Tech. Rep. Ser. 2008, 545, 1–240. [Google Scholar]
- Yang, Z.; Kulkarni, K.; Zhu, W.; Hu, M. Bioavailability and pharmacokinetics of genistein: Mechanistic studies on its ADME. Anticancer Agents Med. Chem. 2012, 12, 1264–1280. [Google Scholar] [CrossRef]
- Alipour, M.; Sharifi, S.; Samiei, M.; Shahi, S.; Aghazadeh, M.; Dizaj, S.M. Synthesis, characterization, and evaluation of Hesperetin nanocrystals for regenerative dentistry. Sci. Rep. 2023, 13, 2076. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Xu, S.; Ren, J.; Tang, L.; Gong, J.; Lin, Y.; Fang, H.; Su, D. Hesperetin, a promising treatment option for diabetes and related complications: A literature review. J. Agric. Food Chem. 2022, 70, 8582–8592. [Google Scholar] [CrossRef]
- He, P.; Ma, J.; Liu, Y.; Deng, H.; Dong, W. Hesperetin Promotes Cisplatin−Induced Apoptosis of Gastric Cancer In Vitro and In Vivo by Upregulating PTEN Expression. Front. Pharmacol. 2020, 11, 1326. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Kushwaha, S.; Tripathi, D.N.; Jena, G.B. Cardioprotective Effects of Hesperetin against Doxorubicin-Induced Oxidative Stress and DNA Damage in Rat. Cardiovasc. Toxicol. 2011, 11, 215–225. [Google Scholar] [CrossRef]
- Trivedi, P.P.; Tripathi, D.N.; Jena, G. Hesperetin protects testicular toxicity of doxorubicin in rat: Role of NFκB, p38 and caspase-3. Food Chem. Toxicol. 2011, 49, 838–847. [Google Scholar] [CrossRef]
- An, M.-F.; Shen, C.; Zhang, S.-S.; Wang, M.-Y.; Sun, Z.-R.; Fan, M.-S.; Zhang, L.J.; Zhao, Y.L.; Sheng, J.; Wang, X.J. Anti-hyperuricemia effect of hesperetin is mediated by inhibiting the activity of xanthine oxidase and promoting excretion of uric acid. Front. Pharmacol. 2023, 14, 1128699. [Google Scholar] [CrossRef]
- Geng, Y.; Wu, Z.; Buist-Homan, M.; Blokzijl, H.; Moshage, H. Hesperetin protects against palmitate-induced cellular toxicity via induction of GRP78 in hepatocytes. Toxicol. Appl. Pharmacol. 2020, 404, 115183. [Google Scholar] [CrossRef] [PubMed]
- Famurewa, A.C.; Renu, K.; Eladl, M.A.; Chakraborty, R.; Myakala, H.; El-Sherbiny, M.; Elsherbini, D.M.A.; Vellingiri, B.; Madhyastha, H.; Ramesh-Wanjari, U.; et al. Hesperidin and hesperetin against heavy metal toxicity: Insight on the molecular mechanism of mitigation. Biomed. Pharmacother. 2022, 149, 112914. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; He, X.; Yang, Y.; Li, M.; Hao, D.; Jia, Z. The genus Epimedium: An ethnopharmacological and phytochemical review. J. Ethnopharmacol. 2011, 134, 519–541. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Zhang, Z.-h.; Sun, E.; Qian, Q.; Tan, X.-b.; Jia, X.-b. Preparation of a nanoscale baohuoside I-phospholipid complex and determination of its absorption: In vivo and in vitro evaluations. Int. J. Nanomed. 2012, 7, 4907–4916. [Google Scholar]
- Li, S.Y.; Ping, G.; Geng, L.; Seow, W.K.; Thong, Y.H. Immunopharmacology and toxicology of the plant flavonoid baohuoside-1 in mice. Int. J. Immunopharmacol. 1994, 16, 227–231. [Google Scholar] [CrossRef]
- Ni, F.; Tang, H.; Wang, C.; Zhang, H.; Zheng, C.; Zhang, N.; Chen, B.; Sun, L. Baohuoside I Inhibits the Proliferation of Pancreatic Cancer Cells via mTOR/S6K1-Caspases/Bcl2/Bax Apoptotic Signaling. Cancer Manag. Res. 2019, 11, 10609–10621. [Google Scholar] [CrossRef]
- Lestari, M.L.A.D.; Indrayanto, G. Curcumin. In Profiles of Drug Substances, Excipients, and Related Methodology; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 39, pp. 113–204. [Google Scholar]
- Kamsu, G.T.; Fodouop, S.P.; Tagne, R.S.; Kodjio, N.; Fakam, A.L.; Gatsing, D. Evaluation of the acute and sub-chronic toxicity of the ethanolic extract of Curcuma longa (Zingiberaceae) in wistar albino rats. Mod. Chem. Appl. 2019, 7, 267. [Google Scholar]
- Tiwari, R.; Siddiqui, M.H.; Mahmood, T.; Farooqui, A.; Bagga, P.; Ahsan, F.; Shamim, A. An exploratory analysis on the toxicity & safety profile of Polyherbal combination of curcumin, quercetin and rutin. Clin. Phytosci. 2020, 6, 82. [Google Scholar]
- Vitaglione, P.; Lumaga, R.B.; Ferracane, R.; Radetsky, I.; Mennella, I.; Schettino, R.; Koder, S.; Shimoni, E.; Fogliano, V. Curcumin bioavailability from enriched bread: The effect of microencapsulated ingredients. J. Agric. Food Chem. 2012, 60, 3357–3366. [Google Scholar] [CrossRef]
- Mannino, G.; Chinigò, G.; Serio, G.; Genova, T.; Gentile, C.; Munaron, L.; Bertea, C.M. Proanthocyanidins and where to find them: A meta-analytic approach to investigate their chemistry, biosynthesis, distribution, and effect on human health. Antioxidants 2021, 10, 1229. [Google Scholar] [CrossRef]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahtisham, U.-H.; Patel, S.; Pan, X.; Naz, S.; Sanches-Silva, A.; Saeed, F.; Rasul-Suleria, H.A. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- Yamakoshi, J.; Saito, M.; Kataoka, S.; Kikuchi, M. Safety evaluation of proanthocyanidin-rich extract from grape seeds. Food Chem. Toxicol. 2002, 40, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Sano, A. Safety assessment of 4-week oral intake of proanthocyanidin-rich grape seed extract in healthy subjects. Food Chem. Toxicol. 2017, 108, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Yang, K.; Zhang, L.; Liao, P.; Xiao, Z.; Zhang, F.; Sindaye, D.; Xin, Z.; Tan, C.; Deng, J.; Yin, Y.; et al. Impact of Gallic Acid on Gut Health: Focus on the Gut Microbiome, Immune Response, and Mechanisms of Action. Front. Immunol. 2020, 11, 580208. [Google Scholar] [CrossRef]
- Niho, N.; Shibutani, M.; Tamura, T.; Toyoda, K.; Uneyama, C.; Takahashi, N.; Hirose, M. Subchronic toxicity study of gallic acid by oral administration in F344 rats. Food Chem. Toxicol. 2001, 39, 1063–1070. [Google Scholar] [CrossRef]
- Booth, A.; Amen, R.J.; Scott, M.; Greenway, F.L. Oral dose-ranging developmental toxicity study of an herbal supplement (NT) and gallic acid in rats. Adv. Ther. 2010, 27, 250–255. [Google Scholar] [CrossRef]
- Dalibalta, S.; Majdalawieh, A.F.; Manjikian, H. Health benefits of sesamin on cardiovascular disease and its associated risk factors. Saudi Pharm. J. 2020, 28, 1276–1289. [Google Scholar] [CrossRef]
- Hori, H.; Takayanagi, T.; Kamada, Y.; Shimoyoshi, S.; Ono, Y.; Kitagawa, Y.; Shibata, H.; Nagao, M.; Fujii, W.; Sakakibara, Y. Genotoxicity evaluation of sesamin and episesamin. Mutat. Res. 2011, 719, 21–28. [Google Scholar] [CrossRef]
- Liu, C.-M.; Zheng, G.-H.; Ming, Q.-L.; Chao, C.; Sun, J.-M. Sesamin protects mouse liver against nickel-induced oxidative dna damage and apoptosis by the PI3K-Akt Pathway. J. Agric. Food Chem. 2013, 61, 1146–1154. [Google Scholar] [CrossRef]
- Cheng, F.C.; Jinn, T.R.; Hou, R.C.; Tzen, J.T. Neuroprotective effects of sesamin and sesamolin on gerbil brain in cerebral ischemia. Int. J. Biomed. Sci. 2006, 2, 284–288. [Google Scholar]
- Guo, H.; Liu, Y.; Wang, L.; Zhang, G.; Su, S.; Zhang, R.; Zhang, J.; Li, A.; Shang, C.; Bi, B.; et al. Alleviation of doxorubicin-induced hepatorenal toxicities with sesamin via the suppression of oxidative stress. Hum. Exp. Toxicol. 2016, 35, 1183–1193. [Google Scholar] [CrossRef] [PubMed]
- Srisongkram, T.; Weerapreeyakul, N. Route of intracellular uptake and cytotoxicity of sesamol, sesamin, and sesamolin in human melanoma SK-MEL-2 cells. Biomed. Pharmacother. 2022, 146, 112528. [Google Scholar] [CrossRef] [PubMed]
- Salas, F.; Rojas, J.; Morales, A.; Ramos-Nino, M.E.; Colmenares, N.G. In vitro Cytotoxic Activity of Sesamin Isolated from Vismia baccifera var. dealbata Triana & Planch (Guttiferae) Collected from Venezuela. Nat. Prod. Commun. 2008, 3, 1705–1708. [Google Scholar]
- Nagle, D.G.; Ferreira, D.; Zhou, Y.D. Epigallocatechin-3-gallate (EGCG): Chemical and biomedical perspectives. Phytochemistry 2006, 67, 1849–1855. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Brausi, M.; Rizzi, F.; Castagnetti, G.; Peracchia, G.; Corti, A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: A preliminary report from a one-year proof-of-principle study. Cancer Res. 2006, 66, 1234–1240. [Google Scholar] [CrossRef]
- Urusova, D.V.; Shim, J.H.; Kim, D.J.; Jung, S.K.; Zykova, T.A.; Carper, A.; Bode, A.M.; Dong, Z. Epigallocatechin-gallate suppresses tumorigenesis by directly targeting Pin1. Cancer Prev. Res. 2011, 4, 1366–1377. [Google Scholar] [CrossRef]
- Liu, Z.; de Bruijn, W.J.C.; Bruins, M.E.; Vincken, J.-P. Microbial metabolism of Theaflavin-3, 3′-digallate and its gut microbiota composition modulatory effects. J. Agric. Food Chem. 2021, 69, 232–245. [Google Scholar] [CrossRef]
- Way, T.-D.; Lee, H.-H.; Kao, M.-C.; Lin, J.-K. Black tea polyphenol Theaflavins inhibit aromatase activity and attenuate tamoxifen resistance in HER2/Neu-Transfected human breast cancer cells through tyrosine kinase suppression. Eur. J. Cancer 2004, 40, 2165–2174. [Google Scholar] [CrossRef]
- Li, Y.; Shi, J.; Sun, X.; Li, Y.; Duan, Y.; Yao, H. Theaflavic acid from black tea protects PC12 cells against ROS-mediated mitochondrial apoptosis induced by OGD/R via activating Nrf2/ARE signaling pathway. J. Nat. Med. 2020, 74, 238–246. [Google Scholar] [CrossRef]
- Lahiry, L.; Saha, B.; Chakraborty, J.; Adhikary, A.; Mohanty, S.; Hossain, D.M.S.; Banerjee, S.; Das, K.; Sa, G.; Das, T. Theaflavins Target Fas/Caspase-8 and Akt/PBad Pathways to Induce Apoptosis in P53-Mutated Human Breast Cancer Cells. Carcinogenesis 2010, 31, 259–268. [Google Scholar] [CrossRef]
- Epifano, F.; Genovese, S.; Fiorito, S.; Mathieu, V.; Kiss, R. Lapachol and its congeners as anticancer agents: A review. Phytochem. Rev. 2014, 13, 37–49. [Google Scholar] [CrossRef]
- Morrison, R.K.; Brown, D.E.; Oleson, J.J.; Cooney, D.A. Oral toxicology studies with lapachol. Toxicol. Appl. Pharmacol. 1970, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Guerra, M.O.; Mazoni, A.S.; Brandão, M.A.; Peters, V.M. Toxicology of Lapachol in rats: Embryolethality. Braz. J. Biol. 2001, 61, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Sá, R.-D.-C.-d.-S.E.; Guerra, M.O. Reproductive toxicity of lapachol in adult male Wistar rats submitted to short-term treatment. Phytother. Res. 2007, 21, 658–662. [Google Scholar][Green Version]
- Gomes, C.L.; de Albuquerque, W.S.V.; Gomes-de-Melo, C.; Ferreira-da-Silva, R.M.; Vicente, N.R.H.; Rolim, L.A.; Rolim, N.P.J. Beta-lapachone: Natural occurrence, physicochemical properties, biological activities, toxicity and synthesis. Phytochemistry 2021, 186, 112713. [Google Scholar] [CrossRef]
- Kim, K.H.; Park, S.H.; Adhikary, P.; Cho, J.H.; Kang, N.G.; Jeong, S.H. Stability of β-Lapachone upon Exposure to Various Stress Conditions: Resultant Efficacy and Cytotoxicity. Chem. Pharm. Bull. 2016, 64, 381–389. [Google Scholar]
- Oliveira, M.E.F.A.G.; Silva, É.C.G.M.; Câmara, C.A.; de Souza, I.A.; Amorim, R.V.S. Evaluation of acute toxicity of β-lapachone associated with chitosan as a cytoprotective agent. J. Bras. Patol. Med. Lab. 2018, 54, 279–287. [Google Scholar]
- de Almeida, E.R.; Lucena, F.R.S.; Silva, C.V.N.S.; da Silva, C.-J.W.; Cavalcanti, J.B.; Couto, G.B.L.; da Silva, L.L.; da Mota, D.L.; da Silveira, A.B.; de Sousa-Filho, S.D.; et al. Toxicological assessment of beta-lapachone on organs from pregnant and non-pregnant rats. Phyther. Res. 2009, 23, 1276–1280. [Google Scholar]
- Li, J.J.; Yan, Y.Y.; Sun, H.M.; Liu, Y.; Su, C.Y.; Chen, H.B.; Zhang, J.Y. Anti-Cancer Effects of Pristimerin and the Mechanisms: A Critical Review. Front. Pharmacol. 2019, 12, 746. [Google Scholar] [CrossRef]
- Gomes, J.P.M.; Cardoso, C.R.P.; Varanda, E.A.; Molina, J.-M.; Fernandez, M.F.; Olea, N.; Arlos, I.Z.; Vilegas, W. Antitumoral, mutagenic and (anti)estrogenic activities of tingenone and pristimerin. Rev. Bras. Farmacogn. 2011, 21, 963–971. [Google Scholar]
- Aziz, M.H.; Dreckschmidt, N.E.; Verma, A.K. Plumbagin, a medicinal plant-derived naphthoquinone, is a novel inhibitor of the growth and invasion of hormone-refractory prostate cancer. Cancer Res. 2008, 68, 9024–9032. [Google Scholar] [CrossRef] [PubMed]
- Padhye, S.; Dandawate, P.; Yusufi, M.; Ahmad, A.; Sarkar, F.H. Perspectives on medicinal properties of plumbagin and its analogs. Med. Res. Rev. 2012, 32, 1131–1158. [Google Scholar] [CrossRef] [PubMed]
- Sumsakul, W.; Plengsuriyakarn, T.; Na-Bangchang, K. Pharmacokinetics, toxicity, and cytochrome P450 modulatory activity of plumbagin. BMC Pharmacol. Toxicol. 2016, 17, 50. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.B.; Natvig, D.O.; Kogoma, T. Toxicity and mutagenicity of plumbagin and the induction of a possible new DNA repair pathway in Escherichia coli. J. Bacteriol. 1985, 164, 1309–1316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jia, B.; Velu, P.; Wu, H. Corilagin induces apoptosis and inhibits HMBG1/PI3K/AKT signaling pathways in a rat model of gastric carcinogenesis induced by methylnitronitrosoguanidine. Environ. Toxicol. 2022, 37, 1222–1230. [Google Scholar] [CrossRef]
- Li, X.; Deng, Y.; Zheng, Z.; Huang, W.; Chen, L.; Tong, Q.; Ming, Y. Corilagin, a promising medicinal herbal agent. Biomed. Pharmacother. 2018, 99, 43–50. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, D.; Chen, Y.; Ren, L.; Xu, J.; Li, K.; Wang, Q.; Zhang, W. Studies on anti-tumor activity, mutagenicity action by corilagin. China J. Cancer Prevent. Treat. 2003, 10, 469–472. [Google Scholar]
- Yang, C.M.; Cheng, H.Y.; Lin, T.C.; Chiang, L.C.; Lin, C.C. Hippomanin A from acetone extract of Phyllanthus urinaria inhibited HSV-2 but not HSV-1 infection in vitro. Phytother. Res. 2007, 21, 1182–1186. [Google Scholar] [CrossRef]
- Dong, X.R.; Luo, M.; Fan, L.; Zhang, T.; Liu, L.; Dong, J.H.; Wu, G. Corilagin inhibits the double strand break-triggered NF-κB pathway in irradiated microglial cells. Int. J. Mol. Med. 2010, 25, 531–536. [Google Scholar]
- Ming, Y.L.; Zheng, Z.Z.; Chen, L.H.; Zheng, G.H.; Liu, S.S.; Yu, Y.; Tong, Q.X. Corilagin inhibits hepatocellular carcinoma cell proliferation by inducing G2/M phase arrest. Cell. Biol. Int. 2013, 37, 1046–1054. [Google Scholar] [CrossRef]
- Reddy, B.U.; Mullick, R.; Kumar, A.; Sharma, G.; Bag, P.; Roy, C.L.; Sudha, G.; Tandon, H.; Dave, P.; Shukla, A.; et al. A natural small molecule inhibitor corilagin blocks HCV replication and modulates oxidative stress to reduce liver damage. Antivir. Res. 2018, 150, 47–59. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cao, S.; Wu, X.; Lai, Y.H.; Tan, B.K.; Pereira, J.T.; Goh, S.H.; Venkatraman, G.; Harrison, L.J.; Sim, K.-Y. Griffipavixanthone, a novel cytotoxic bixanthone from Garcinia griffithii and G. pavifolia. Tetrahedron Lett. 1998, 39, 9103–9106. [Google Scholar] [CrossRef]
- Feng, S.; Jiang, Y.; Li, J.; Qiu, S.; Chen, T. A new bixanthone derivative from the bark of Garcinia oblongifolia. Nat. Prod. Res. 2013, 28, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Tshisekedi, P.T.; Kapepula, P.M.; Muganza, D.M.; Ngombe, N.K.; Kalenda, D.T.; Tchinda, A.T.; Jansen, O.; Frédérich, M. Antiplasmodial Activity of Griffipavixanthone and Morelloflavone the Main Compounds from Garcinia Chromocarpa Engl. (Clusiaceae). Int. J. Pharmacogn. Chin. Med. 2022, 6, 000225. [Google Scholar] [CrossRef]
- An, J.; An, S.; Choi, M.; Jung, J.H.; Kim, B. Natural Products for Esophageal Cancer Therapy: From Traditional Medicine to Modern Drug Discovery. Int. J. Mol. Sci. 2022, 23, 13558. [Google Scholar] [CrossRef]
- Kuete, V.; Efferth, T. African flora has the potential to fight multidrug resistance of cancer. Biomed. Res. Int. 2015, 2015, 914813. [Google Scholar] [CrossRef]
- 1Herman, T.F.; Santos, C. First-Pass Effect. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Susa, S.T.; Hussain, A.; Preuss, C.V. Drug Metabolism. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Koeberle, D.; Betticher, D.C.; von Moos, R.; Dietrich, D.; Brauchli, P.; Baertschi, D.; Matter, K.; Winterhalder, R.; Borner, M.; Anchisi, S.; et al. Bevacizumab continuation versus no continuation after first-line chemotherapy plus bevacizumab in patients with metastatic colorectal cancer: A randomized phase III non-inferiority trial (SAKK 41/06). Ann. Oncol. 2015, 26, 709–714. [Google Scholar] [CrossRef]
- Maleki-Dana, P.; Sadoughi, F.; Asemi, Z.; Yousefi, B. The role of polyphenols in overcoming cancer drug resistance: A comprehensive review. Cell. Mol. Biol. Lett. 2022, 27, 1. [Google Scholar] [CrossRef]
- Bonta; Ramesh, K. Anti-Cancer Agents. Med. Chem. 2020, 20, 29–48. [Google Scholar]
- He, S.; Xu, J.; Liu, X.; Zhen, Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm. Sin. B 2021, 11, 3379–3392. [Google Scholar] [CrossRef]
- Kamsu, G.T.; Chuisseu, D.P.D.; Fodouop, C.S.P.; Feudjio, H.B.L.; Famen, L.-C.N.; Kodjio, N.; Sokoudjou, J.B.; Gatsing, D. Toxicological profile of the aqueous extract of Tectona grandis L.F. (Verbenaceae) leaves: A medicinal plant used in the treatment of typhoid fever in traditional Cameroonian medicine. J. Toxicol. 2021, 2021, 6646771. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).