Abstract
Fetal arrhythmias complicate 1% of pregnancies. Although most of them have a benign and intermittent course, sustained fetal tachyarrhythmias constitute an emerging situation, which is associated with high fetal morbidity and mortality. However, one of the major milestones in fetal therapy is the pharmacologic management of fetal arrhythmias by crossing the placental barrier. To date, there is no consensus on the first-line antiarrhythmic treatment for fetal tachyarrhythmias. The role of sotalol in therapeutic management, the use of flecainide versus digoxin as first line of treatment, the need for fetal intramuscular treatment administration, or the best treatment in case of fetal hydrops are situations whose application or management are controversial. The current paper is a scoping review of observational and experimental evidence, addressing the types of best management strategies for each type of tachyarrhythmia and the optimal pharmacological dose, considering precautions and safety elements. Finally, we will highlight new therapeutic perspectives and future diagnostic and therapeutic strategies.
1. Introduction
1.1. Epidemiology
Fetal arrhythmias are diagnosed in 1–3% of pregnancies [1]. Despite this, they account for up to 20% of consultations related to fetal congenital heart disease in referral units [1,2]. Among rhythm disorders, fetal tachyarrhythmias affect approximately 0.1% of pregnancies. However, this percentage may be underestimated because a large number of tachyarrhythmias are intermittent and may resolve spontaneously, so they are not diagnosed [3].
To date, there is no single etiology for the development of fetal arrhythmias. They have been related to mutations in the GATA4, NKX2-5, TBX3, and TBX5 genes, linked to cardiac structural development [1,4], ischemia, inflammation, and electrolyte disorders [5]. More rarely, fetal tachyarrhythmias are associated with cardiac structural abnormalities [5]. We must also differentiate tachyarrhythmias with irregular rhythm fromfetal sinus tachycardias caused by fetal conditions such as infection or hypoxia [6], or maternal conditions such as viral disease during pregnancy [7,8].
1.2. Classification
Although there are many types of classifications, supraventricular tachyarrhythmias can be divided into supraventricular tachyarrhythmias (SVT), ventricular tachyarrhythmias (VT), and sinus tachycardias. SVT are the most common diagnosis, accounting for 60% to 90% of cases [9]. Together with atrial flutter, these are the tachyarrhythmias associated with the greatest morbidity and mortality.
In SVT, tachycardia usually ranges between 210 and 240 beats per minute, with a 1:1 AV conduction ratio. SVT are divided into atrial tachycardia and conduction system tachycardia. They can be categorized into reentry ventriculoatrial tachycardia (orthodromic reentrant tachycardia and antidromic reentrant tachycardia), intranodal reentrant tachycardia, and intra-atrial reentrant tachycardia [1,10]. Other less frequent prenatal diagnoses include permanent junctional reciprocating tachycardia, atrial ectopic tachycardia, and VT [11]. The most frequent cause is the presence of an accessory pathway between the atrium and ventricle, allowing retrograde ventriculoatrial (VA) conduction. Because the accessory pathway usually has a shorter path than the normal atrioventricular (AV) conduction, in these cases, the VA interval is reduced. This is the most frequent mechanism, with a postnatal diagnosis of Wolf–Parkinson–White of up to 10% [12].
Atrial flutter is less common than SVT and tends to occur in the third trimester. It constitutes 20% of fetal arrhythmias [13] and is defined by a regular, rapid tachycardia of up to 600 beats per minute, accompanied by varying degrees of AV block, resulting in a ventricular rate of 210 to 240 beats per minute. This block is usually 2:1 [14]. In this case, the reentrant circuit is located within the atrium itself. Postnatal evidence with atrial flutter suggests that nearly 20% of fetuses will also have SVT. In both cases, when the arrhythmia persists, up to 40% of cases will develop fetal hydrops [14].
1.3. Hemodynamic Effects and Outcome
Following the diagnosis of a fetal tachyarrhythmia, we can expect a variety of outcomes, from spontaneous resolution to intrauterine fetal death. Although most fetal tachyarrhythmias are benign and transient, there are incessant forms that can result in low cardiac output, increased central venous pressure, fetal hydrops, fetoplacental circulatory failure, and fetal death. Therefore, the main prognostic factor is the presence of hydrops as a marker of heart failure due to fetal tachyarrhythmia [15].
This hemodynamic insult may also have an impact on long-term neurodevelopment [11], so an appropriate prenatal therapeutic strategy is essential to optimize outcomes. It is also necessary to assess long-term neurodevelopment in cases in which fetal arrhythmia has been maintained over time or has conditioned the appearance of hydrops.
The main risk factors for the prenatal development of heart failure are the onset of tachyarrhythmia before 32 weeks, arrhythmias with an incessant rhythm, and those associated with congenital heart disease [6]. Approximately 10% of arrhythmias meet these criteria [6].
Although an association between fetal growth and the presence of CHD, as well as preeclampsia and preterm birth, has been suggested, this association has not been established for fetal tachyarrhythmias without associated congenital heart disease [16,17].
1.4. Prenatal Diagnosis
Although there are many differences in obstetric care worldwide, in most countries, at least one ultrasound examination is performed during pregnancy. This allows screening for fetal arrhythmias, since fetal heart rate determination is one of the basic examinations of any fetal examination. All second- and third-trimester fetal scans include visualization and documentation of the four cardiac chambers, as well as the outflow tracts and fetal heart rate. For the complete second-trimester fetal cardiac examination, there are well-standardized protocols that include assessment of the visceral situs, cardiac position in the thorax and relative size, cardiac axis, four-chamber view, left and right ventricular outflow tracts, pulmonary artery, three-vessel view, short- and long-axis view, ductal and aortic arch, and superior and inferior venae cavae [18]. For the evaluation of fetal heart rhythm, pulsed-wave spectral Doppler ultrasound is a critical tool [18]. This allows the identification of any rhythm alteration (bradyarrhythmias, tachyarrhythmias or irregular rhythms) and referral to tertiary units for appropriate multidisciplinary management if necessary, since not all changes in fetal heart rate are pathological.
Fetal tachyarrhythmias are defined by a persistent elevation of the fetal heart rate above 160 beats per minute. They are usually diagnosed from 20 weeks of gestation, since the cardiac conduction system is functionally mature from 16 weeks with a regular rhythm and rate between 110 and 160 beats per minute [19]. Specifically, accessory pathway reentrant tachycardias, which are among the most common SVT, are usually diagnosed between 24 and 32 weeks of gestation [6].
In most cases, they are an isolated finding, being associated with other congenital heart diseases in up 11% of cases [19,20].
Over the last decades, diagnostic techniques such as fetal electrocardiography and magnetocardiography have been developed for the diagnosis of arrhythmias. Nevertheless, the main tools currently used for prenatal diagnosis of tachyarrhythmias are two-dimensional ultrasound and M-mode and pulsed-wave Doppler [19]. Although fetal echocardiography is the prime tool for the detection of prenatal tachyarrhythmias, it only explains its mechanisms partially, so the distinction between different mechanisms of reentrant tachyarrhythmias is still a challenge during this period [3].
Pulsed-wave Doppler makes it possible to determine mechanical phenomena and evaluate atrial and ventricular contractility and their relationship. Although there are different windows for its acquisition, the left ventricular outflow tract is usually used. This allows us to capture the time it takes for the impulse to travel from the atrium to the ventricle (AV interval), which is the equivalent of the PR interval detected by the electrocardiogram (Figure 1).
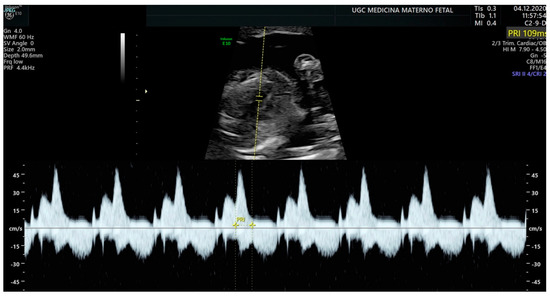
Figure 1.
Measurement of the AV interval (PR) by pulsed Doppler in the fetal left ventricular outflow tract.
To classify tachyarrhythmias, the efficacy of AV and VA intervals based on Doppler echocardiography is inquired [20,21,22,23]. Short VA SVT is the typical pattern in reentry tachycardia, while long VA SVT suggests atrial ectopic tachycardia or permanent junctional reciprocating tachycardia. Regarding this, it is important to highlight that the size of the fetal atrium is an important factor in the propagation of atrial flutter, achieving its critical size at around 27–30 weeks of GA [24,25].
Another very useful but not widely used ultrasound method is tissue Doppler echocardiography. This technique makes it possible to determine myocardial mobility and thus better pinpoint the origin of the arrhythmia, but it requires specialized software for its analysis [26].
1.5. Fetal Hidrops
Fetuses with sustained or severe tachyarrhythmia end up suffering heart failure and fetal hydrops as a pathophysiological representation [27,28], which is reached in 30–40% of fetuses with SVT and 7–43% in those with atrial flutter (AFL) [14,29] (Figure 2). In the absence of treatment, intrauterine death occurs in up to 9% of cases [30].
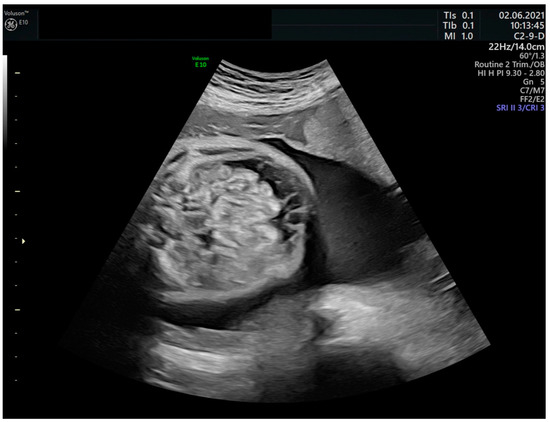
Figure 2.
Fetal ascites in the context of hydrops due to fetal tachyarrhythmia.
Prenatal conversion of arrhythmia, a relevant determinant of postnatal outcome, is more frequent in the absence of fetal hydrops, with fetal hydrops being considered one of the main factors affecting the effectiveness of treatment [14,31]. In fact, numerous studies have shown that fetal hydrops are an independent predictor of treatment failure, concerning less placental transfer and increased fetal volume of distribution due to the aforementioned mechanism [3].
As detailed below regarding treatment, digoxin presents poor transplacental transfer in presence of hydrops; however, when this resolves, a high dose of previously administered maternal digoxin may manifest paradoxically as fetal bradycardia [32,33]. Likewise, premature labor could be triggered due to increased fetal diuresis in the intra-amniotic space, causing the appearance of polyhydramnios [32].
2. Treatment of Fetal Tachyarrhythmias
2.1. Fetal Therapy
2.1.1. Time of Treatment
The main goal in the prenatal treatment of fetal tachyarrhythmias is not necessarily to reverse the arrhythmia but to slow the heart rate in order to improve cardiac output, reaching a >15% rate reduction [34,35]. Therefore, fetal treatment is usually initiated with a tachyarrhythmia of at least 12 h of evolution, with a fetal heart rate of 200 beats per minute or higher and at a gestational age of fewer than 36 weeks, since postnatal treatment may be considered above that week [36]. Whether the tachyarrhythmia is intermittent or sustained also influences the delay in initiating treatment, as sustained forms are less well-tolerated and often progress to cardiac dysfunction and fetal hydrops.
Benign arrhythmias, such as sinus tachycardia or supraventricular extrasystoles, do not require prenatal pharmacological treatment. On the contrary, in about 1 in 2500 pregnancies, it is necessary to administer antiarrhythmic therapy [37].
2.1.2. Route of Administration
First reported in 1980 [38,39], prenatal therapy is based on the transplacental transfer of the drug after maternal administration, alone or in combination. This pathway allows the passage of small molecules of the drug through the placenta. Fetal membranes may also play an important role here, having enzymes similar to those of the placenta for drug transfer [40]. Drugs transferring from maternal to fetal blood cross the intervillous space and pass a barrier of syncytiotrophoblast, fetal connective tissue, and the endothelium of fetal capillaries [41]. The ability of transplacental transfer depends on blood pH values (fetal and maternal), placental perfusion, and the properties of the drug (size, protein binding, etc.).
This raises safety concerns, since a drug is being administered to a healthy mother for the sole purpose of treating the fetus, subjecting her to the side effects of the drug. For this reason, we must take the drug’s safety profile into account [42], and we must be extremely vigilant with regard to controls and follow-up. Nevertheless, it is a safe and effective strategy for the treatment of fetal tachyarrhythmias, improving survival rate [1], since conversion to sinus rhythm can be achieved in more than 80% of cases using different drugs. We must also maintain an optimal level of the drug in maternal blood to avoid recurrences of fetal tachyarrhythmia.
In case the transplacental route is not effective, there are other alternative therapeutic routes, such as fetal intramuscular injection and intraumbilical or intraperitoneal instillation (especially in case of hydrops).
2.1.3. Adverse Effects
Before initiating pharmacological therapy, as we have commented, a physical assessment must be carried out together with a maternal electrocardiogram, as well as evaluation of concomitant treatments due to possible interactions and a family history of heart disease, among other relevant aspects [6]. It is important to give the most efficient drug at the lowest possible dose, avoiding as much as possible the risk of maternal morbidity. Adverse effects frequently appear when the serum digoxin level is greater than 2 ng/mL, but they are usually mild and self-limited, especially at the gastrointestinal level [42]. However, it is still recommended to monitor electrocardiographic changes and digoxinemia throughout treatment to prevent serious complications related to its toxicity.
Once the target therapeutic levels are reached together with the control of the fetal heart rate, weekly fetal echocardiograms are recommended to evaluate treatment failure due to the possibility of recurrence between 8–15% having been described [6]. However, there is also no homogeneous consensus on the follow-up protocol. Flecainide and sotalol do not require monitoring of serum levels. Digoxin is contraindicated in mothers with AV block, hypertrophic cardiomyopathy, or Wolff–Parkinson–White syndrome.
To our knowledge, no maternal deaths due to pharmacological treatment of fetal tachyarrhythmias have been reported.
2.1.4. Drug Selection
Prenatal reversion to sinus rhythm before birth improves the prognosis of arrhythmia [3]. Recent multicenter studies show that with adequate prenatal treatment, survival occurs in 96% of cases [43]. The first-line treatments used for the treatment of fetal arrhythmias are digoxin, flecainide, and sotalol. Despite the growing evidence in this regard, there are currently no randomized studies that define the superiority of one treatment over another [19]. Although there are no standards for drug dosing or the need for loading doses, the doses and characteristics of the most commonly used drugs for prenatal treatment of fetal arrhythmias are summarized in Table 1.

Table 1.
First- and second-line treatments for fetal arrhythmias [37,44].
Management of SVT should be individualized considering maternal and fetal factors. The decision should be made jointly by the fetal cardiology team (usually consisting of fetal medicine specialists and pediatric cardiologists) and the obstetrics team to determine the best treatment strategy, as well as the timing and route of delivery. A pediatric cardiologist should be an integral part of the management team. This also allows a better transition from fetal life to the postnatal stage, ensuring adequate continuity of care.
The choice of antiarrhythmic therapy, as well as the criteria for management in case of failure of the initial medical treatment, are controversial. Even though digoxin is the most widely used antiarrhythmic drug and has a better-known safety profile, administered both orally and intravenously, since 2002, there has been discussion about its use as first-line treatment, since it has been proven that drugs such as flecainide seem to have greater efficacy in the control of fetal STV [38], as reported in the meta-analyses by Hill et al. (2017) and Alsaied et al. (2017). However, sotalol, flecainide, and amiodarone have been used as second-line therapy [14,31], taking into account that amiodarone has a more significant maternal and fetal toxicity profile [37]. A recent meta-analysis compared the antiarrhythmic effect of transplacental administration of digoxin, flecainide, and sotalol as first-line drugs. In this study, flecainide (OR: 1.4, 95% CI: 1.1–2.0, I2 = 60%, p = 0.03) and sotalol (OR: 1.4, 95% CI: 1.1–2.0, I2 = 30%, p = 0.02) were superior to digoxin for the conversion of fetal tachyarrhythmia. In fetuses with hydrops, the benefit over digoxin was more notable for both flecainide (OR: 5.0, 95% CI: 2.5–10.0, I2 = 0%, p < 0.001) and sotalol (OR: 2.5, 95% CI: 1.7–5.0, I2 = 0%, p < 0.001) [45].
Due to the familiarity with its use, and considering not the current evasion but a historical perspective, digoxin continues to be used as the first line of treatment in many centers, adding other treatments to the protocol in case of poor control [43]. Serum digoxin levels in the fetus are between 60% and 80% of maternal serum levels in a fetus without hydrops [46,47]. Serum digoxin levels in the nonhydrops fetus are usually much lower and do not reach the optimal therapeutic level [47]. Thus, while in the nonhydrotropic fetus it may be considered the treatment of choice, conversion rates in hydrotropic fetuses with SVT are low [14]. Furthermore, digoxin is not effective in the case of ectopic atrial tachycardia and permanent junctional tachycardia [32]. It has also been described that the conversion time to sinus rhythm can be up to 14 days, being ineffective due to its poor placental transfer to the fetus in a higher percentage in the presence of hydrops. The bioavailability from the gastrointestinal tract is 70–80% and is metabolized in the liver and excreted by the kidneys (50–70% unchanged) [48,49].
Its success rate increases notably in the case of associating direct fetal therapy through fetal intramuscular administration of digoxin under ultrasound control [11]. Faced with this, in the presence of hydrops, both sotalol and flecainide are considered to have a good placental transfer capacity, which is why they should be used as first-line treatment [34]. Some authors use flecainide in combination with digoxin in cases of nonresponse to digoxin monotherapy [14]. Furthermore, unlike digoxin, both sotalol and flecainide accumulate in amniotic space, increasing the efficacy of long-term treatment [50,51]. Fetal levels of flecainide are ~50% of maternal levels in the absence of fetal hydrops [52].
Sotalol is the first-line treatment in many centers [23]. Transplacental transfer of the drug is superior to digoxin, reaching fetal serum levels similar to maternal levels 2–4 h after administration. It is considered the treatment of choice in cases of ectopic atrial tachycardia and permanent junctional tachycardia, as well as in the hydropic fetus. In addition, its combination with digoxin can be very effective in the treatment of atrial flutter [31]. The administration of sotalol is accompanied by monitoring of the maternal QT interval. Oral bioavailability of sotalol ranges from 89 to 100%. The drug is not metabolized by the liver and is excreted by the kidneys (80–90% unchanged) [48,49]. For sotalol, the ratio of fetus-to-maternal blood level was found to range from 1.07 to 1.11 [43,51].
Direct access through the umbilical cord carries an added risk to treatment. Some studies estimate a risk of death up to 50% higher in direct treatment in the umbilical cord versus transplacental treatment [53]. Transplacental administration does not always achieve adequate efficacy, requiring higher maternal doses or even the addition of several simultaneous drugs to control fetal tachyarrhythmia. Direct intramuscular injection can be repeated every 12 h until three doses are reached, in association with maternal treatment [30].
In case of failure to achieve reversal with first-line drugs, we should consider the use of other drugs. There is no clear consensus in the literature on how long we have to wait to explore the possibility of using second-line drugs, although in general it is advisable to wait a minimum of 48 to 72 h. There is also no consensus when deciding whether to add this second treatment to digoxin or to discontinue treatment with digoxin to start with the second-line drug. Finally, the decision will depend on whether or not there has been an initial response to digoxin, even if only partial. Although some authors consider flecainide, sotalol, and amiodarone to be second-line drugs at the same level, the current evidence obliges us to consider only amiodarone in this subgroup.
Amiodarone is a highly useful drug in cases of tachyarrhythmia refractory to first-line drugs [53,54]. However, its use should be limited and very controlled, since due to its long half-life, cases of neonatal hypothyroidism linked to its use have been detected [55,56]. Moreover, unlike other much faster-acting drugs, such as sotalol, amiodarone can take six days to convert.
In our review, we found no restriction on the use of first-line drugs in relation to fetal weight or gestational age. Due to the prolonged half-life of amiodarone, tapering before delivery has limited benefits. Before 30 weeks, when first-line therapy fails, the risk associated with the use of amiodarone to prevent the birth of a preterm hydrotropic fetus may be accepted.
It is always advisable to suspend maternal intake of factors known to be linked to the onset of fetal arrhythmias, such as smoking, excessive caffeine consumption, and beta-mimetics, if possible.
2.2. Mode and Time of Delivery
When tachyarrhythmias produce significant hemodynamic fluctuations refractory to prenatal medical treatment, it can sometimes lead to preterm delivery for postnatal treatment, although prenatal drug treatment is usually attempted up to 36 weeks. Difficulty in fetal monitoring due to fetal tachyarrhythmia requires a cesarean section in cases where it has not been controlled by fetal treatment. This leads some groups to consider not instituting prenatal therapy when the diagnosis occurs above 36.0 weeks of pregnancy. However, other authors consider the initiation of prenatal treatment even at term gestation, since control of fetal arrhythmia allows vaginal delivery. In addition, in the event of birth, the effective antiarrhythmic drug processing capacity of the placenta and maternal circulation is exchanged for drug processing by the infant’s liver and kidneys [57].
Therefore, based on current evidence, we consider that in the absence of fetal hydrops, if the arrhythmia has been controlled with transplacental medical treatment, it is advisable to attempt to go to term to avoid prematurity complicating the condition. In the case of term diagnosis, initiation of treatment prior to delivery could improve neonatal care and the possibility of faster cardioversion.
The presence of hydrops, despite being an ominous marker of fetal heart failure, invites us to consider deferring delivery planning until the arrhythmia is controlled, even beyond 36.0 weeks. If the arrhythmia is resistant to treatment, assessing the gestational age, the evolution of the hydrops, and the degree of cardiac involvement, it is considered that if the secondary risk to prematurity is lower than carrying out a new line of treatment, it would be indicated to perform a cesarean section [3]. This is because control of arrhythmia and a reduction in hydrops in fetal life allows better postnatal respiratory management [37].
2.3. Future Perspectives in Fetal Therapy
In drug refractory cases, and especially in the presence of hydrops, direct fetal therapy should be considered in most of the cases associated with the previous one. Intraumbilical and intracardiac injections, as well as intraperitoneal, intra-amniotic, and intramuscular injections, are already known and used. The first group can achieve a rapid therapeutic response by directly accessing the fetal circulation, which has a risk of fetal traumatic injury, taking into account that several studies have reported increased mortality (up to 25–50%) in fetuses treated directly through the cord versus intramuscular injection [5,33]. The second group is considered safer: while the intraumbilical administration of antiarrhythmic agents can be performed under ultrasound control, with the drawback of the technical difficulty presented by the fetal position, direct intramuscular administration has more usage experience, but it has been associated with sciatic nerve injury in 1 in 20 cases when the drug is injected into the thigh or buttocks. There are cases describing the use of intraperitoneal and intra-amniotic amiodarone injections in the treatment of AF [58].
Intravenous administration of digoxin, as well as the combination of fetal intramuscular and maternal intravenous routes, have also been tested for reversal of fetal tachyarrhythmia. Although there is no strong evidence in this regard, the data indicate that there may be a shorter time to reversion to sinus rhythm [33].
On the other hand, intrauterine transesophageal stimulation [3,59] has been described through the placement of a fetal asynchronous esophageal pacemaker with a bipolar esophageal pacing electrode (FIAB Esokid 4S, Florence, Italy) placed behind the left atrium for the treatment of fetal atrial fibrillation, performed fetoscopically. Immediate cardioversion to sinus rhythm was shown [34], without recurrence.
Likewise, the use of the percutaneously implantable fetal pacemaker is under investigation, with successful results during the animal experimentation process [1,60,61]. It is a miniaturized and self-contained cardiac pacemaker based on the following mechanism described by Loeb et al. [61]: “A corkscrew electrode made from activated iridium can be screwed into the myocardium, followed by release of the pacemaker and a short, flexible lead entirely within the chest of the fetus to avoid dislodgement from fetal movement”. It has produced successful results during the animal experimentation process.
With all this, there are open lines of research regarding fetal therapy for fetal tachyarrhythmias, with the aim of improving the prognosis and reducing the morbidity and mortality associated with them, especially in the presence of hydrops, refractoriness of arrhythmia, and development of fetal heart failure. In addition, more research related to drug pharmacokinetics and the role of ion channels is needed to improve prenatal treatment protocols.
3. Conclusions
In fetal tachyarrhythmias, transplacental treatment is usually initiated with an arrhythmia of at least 12 h of evolution, fetal heart rate of 200 beats per minute or higher, and gestational age of fewer than 36 weeks, since postnatal treatment may be considered above that week. The first-line treatments used for the treatment of fetal arrhythmias are digoxin, flecainide, and sotalol through maternal administration (transplacental therapy), but there are no randomized studies that define the superiority of one treatment over another. Likewise, in cases refractory to said therapy and/or in the presence of hydrops, direct fetal therapy can be chosen, particularly highlighting the administration of fetal intramuscular digoxin in combination with transplacental therapy, which seems to improve perinatal outcomes. However, more studies are needed to determine the efficacy of other routes of administration.
The choice of management protocol provided depends of the type of arrhythmia, the time of evolution, the presence of hydrops fetalis, maternal comorbidities, and the experience of the team and the center with the drug. With all this, it is necessary to continue progressing in the lines of research mentioned, as well as to try to standardize the treatment and follow-up protocols for this fetal pathology, to improve its prognosis and perinatal results.
Author Contributions
Conceptualization, Á.C., C.V.-R., L.G.-D. and G.A.; data curation, Á.C. and C.V.-R.; formal analysis, Á.C. and G.A.; investigation, Á.C., C.V.-R., L.G.-D. and G.A.; methodology, Á.C. and G.A.; project administration, Á.C.; resources, Á.C.; software, Á.C.; supervision, L.G.-D. and G.A.; visualization, Á.C., C.V.-R., L.G.-D. and G.A.; writing—original draft, Á.C.; writing—review and editing, Á.C., C.V.-R., L.G.-D. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
All images included have the patients’ informed consent for publication.
Data Availability Statement
Data sharing is not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yuan, S.-M. Fetal arrhythmias: Surveillance and management. Hell. J. Cardiol. 2019, 60, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Saileela, R.; Sachdeva, S.; Saggu, D.K.; Koneti, N.R. Ventricular tachycardia in a fetus: Benign course of a malignant arrhythmia. J. Obstet. Gynaecol. India 2019, 69, 383–386. [Google Scholar] [CrossRef] [PubMed]
- Bartin, R.; Maltret, A.; Nicloux, M.; Ville, Y.; Bonnet, D.; Stirnemann, J. Outcomes of sustained fetal tachyarrhythmias after transplacental treatment. Heart Rhythm. O2 2021, 2, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Tsuji, Y.; Makita, N. Inherited bradyarrhythmia: A diverse genetic background. J. Arrhythmia 2016, 32, 352–358. [Google Scholar] [CrossRef]
- Strasburger, J.F.; Wakai, R.T. Fetal cardiac arrhythmia detection and in utero therapy. Nat. Rev. Cardiol. 2010, 7, 277–290. [Google Scholar] [CrossRef]
- Purkayastha, S.; Weinreich, M.; Fontes, J.D.; Lau, J.F.; Wolfe, D.S.; Bortnick, A.E. Fetal supraventricular tachycardia: What the adult cardiologist needs to know. Cardiol. Rev. 2022, 30, 31–37. [Google Scholar] [CrossRef]
- Starodubtseva, N.; Kindysheva, S.; Potapova, A.; Kukaev, E.; Khodzhaeva, Z.; Bockeria, E.; Chagovets, V.; Frankevich, V.; Sukhikh, G. Transplacental therapeutic drug monitoring in pregnant women with fetal tachyarrhythmia using HPLC-MS/MS. Int. J. Mol. Sci. 2017, 3, 1848. [Google Scholar] [CrossRef]
- Dejong, S.; Salmanian, B.; Shamshirsaz, A.A.; Ruano, R. Perinatal management of fetal supraventricular tachycardia complicated by maternal pertussis. BMJ Case Rep. 2015, 2015, bcr2015209909. [Google Scholar] [CrossRef]
- Simpson, J.; Silverman, N.H. Diagnosis of cardiac arrhythmias during fetal life. In Fetal Cardiology; Yagel, S., Silverman, N.H., Gembruch, U., Eds.; Martin Dunitz: London, UK, 2003; pp. 333–344. [Google Scholar]
- Naheed, Z.J.; Strasburger, J.F.; Deal, B.J.; Benson, D.W.; Gidding, S.S. Fetal tachycardia: Mechanisms and predictors of hydrops fetalis. J. Am. Coll. Cardiol. 1996, 27, 1736–1740. [Google Scholar] [CrossRef]
- Karmegeraj, B.; Namdeo, S.; Sudhakar, A.; Krishnan, V.; Kunjukutty, R.; Vaidyanathan, B. Clinical presentation, management, and postnatal outcomes of fetal tachyarrhythmias: A 10-year single-center experience. Ann. Pediatr. Cardiol. 2018, 11, 34–39. [Google Scholar]
- Kleinman, C.S.; Nehgme, R.; Copel, J.A. Fetal cardiac arrhythmias: Diagnosis and therapy (fourth edition). Matern.-Fetal Med. 1999, 301–318. [Google Scholar]
- Van Engelen, A.D.; Weijtens, O.; Brenner, J.I.; Kleinman, C.S.; Copel, J.A.; Stoutenbeek, P.; Meijboom, E.J. Management outcome and follow-up of fetal tachycardia. J. Am. Coll. Cardiol. 1994, 24, 1371–1375. [Google Scholar] [CrossRef] [PubMed]
- Krapp, M.; Kohl, T.; Simpson, J.M.; Sharland, G.K.; Katalinic, A.; Gembruch, U. Review of diagnosis, treatment, and outcome of fetal atrial flutter compared with supraventricular tachycardia. Heart 2003, 89, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Veduta, A.; Panaitescu, A.M.; Ciobanu, A.M.; Neculcea, D.; Popescu, M.R.; Peltecu, G.; Cavoretto, P. Treatment of fetal arrhythmias. J. Clin. Med. 2021, 10, 2510. [Google Scholar] [CrossRef]
- Inversetti, A.; Fesslova, V.; Deprest, J.; Candiani, M.; Giorgione, V.; Cavoretto, P. Prenatal Growth in Fetuses with Isolated Cyanotic and Non-Cyanotic Congenital Heart Defects. Fetal Diagn. Ther. 2020, 47, 411–419. [Google Scholar] [CrossRef]
- Giorgione, V.; Fesslova, V.; Boveri, S.; Candiani, M.; Khalil, A.; Cavoretto, P. Adverse perinatal outcome and placental abnormalities in pregnancies with major fetal congenital heart defects: A retrospective case-control study. Prenat. Diagn. 2020, 40, 1390–1397. [Google Scholar] [CrossRef]
- Quaresima, P.; Fesslova, V.; Farina, A.; Kagan, K.O.; Candiani, M.; Morelli, M.; Crispi, F.; Cavoretto, P.I. How to do a fetal cardiac scan. Arch. Gynecol. Obstet. 2023, 307, 1269–1276. [Google Scholar] [CrossRef]
- Ekici, H.; Ökmen, F.; İmamoğlu, M.; İmamoğlu, A.G.; Ergenoğlu, A.M. Fetal arrhythmias: Ten years’ experience and review of the literature. Turk. J. Obstet. Gynecol. 2022, 19, 302–307. [Google Scholar] [CrossRef]
- Kleinman, C.S.; Nehgme, R.A. Cardiac arrhythmias in the human fetus. Pediatr. Cardiol. 2004, 25, 234–251. [Google Scholar] [CrossRef]
- Fouron, J.C.; Fournier, A.; Proulx, F.; Lamarche, J.; Bigras, J.L.; Boutin, C.; Brassard, M.; Gamache, S. Management of fetal tachyarrhythmia based on superior vena cava/aorta Doppler flow recordings. Heart 2003, 89, 1211–12116. [Google Scholar] [CrossRef]
- D’Alto, M.; Russo, M.G.; Paladini, D.; Di Salvo, G.; Romeo, E.; Ricci, C.; Felicetti, M.; Tartaglione, A.; Cardaropoli, D.; Pacileo, G.; et al. The challenge of fetal dysrhythmias: Echocardiographic diagnosis and clinical management. J. Cardiovasc. Med. 2008, 9, 153–160. [Google Scholar] [CrossRef]
- Jaeggi, E.T.; Nii, M. Fetal brady- and tachyarrhythmias: New and accepted diagnostic and treatment methods. Semin. Fetal Neonatal. Med. 2005, 10, 504–514. [Google Scholar] [CrossRef]
- Boineau, J.P. Atrial flutter: A synthesis of concepts. Circulation 1985, 72, 249–257. [Google Scholar] [CrossRef]
- Pickoff, A.S.; Singh, S.; Flinn, C.J.; McCormack, J.; Stolfi, A.; Gelband, H. Atrial vulnerability in the immature canine heart. Am. J. Cardiol. 1985, 55, 1402–1406. [Google Scholar] [CrossRef]
- Rein, A.J.; O’Donnell, C.; Geva, T.; Nir, A.; Perles, Z.; Hashimoto, I.; Li, X.K.; Sahn, D.J. Use of tissue velocity imaging in the diagnosis of fetal cardiac arrhythmias. Circulation 2002, 106, 1827. [Google Scholar] [CrossRef]
- Gembruch, U.; Yagel, S. Fetal Cardiology: Embryology, Genetics, Physiology, Echocardiographic Evaluation, Diagnosis and Perinatal Management of Cardiac Diseases, 3rd ed.; Taylor and Francis: London, UK, 2005. [Google Scholar]
- Vergani, P.; Mariani, E.; Ciriello, E.; Locatelli, A.; Strobelt, N.; Galli, M.; Ghidini, A. Fetal arrhythmias: Natural history and management. Ultrasound Med. Biol. 2005, 31, 1–6. [Google Scholar] [CrossRef]
- Cuneo, B.F.; Strasburger, J.F. Management strategy for fetal tachycardia. Obstet. Gynecol. 2000, 96, 575–581. [Google Scholar]
- Alsaied, T.; Baskar, S.; Fares, M.; Alahdab, F.; Czosek, R.J.; Murad, M.H.; Prokop, L.J.; Divanovic, A.A. First-line antiarrhythmic transplacental treatment for fetal tachyarrhythmia: A systematic review and meta-analysis. J. Am. Heart Assoc. 2017, 6, e007164. [Google Scholar] [CrossRef]
- Jaeggi, E.T.; Carvalho, J.S.; De Groot, E.; Api, O.; Clur, S.A.; Rammeloo, L.; McCrindle, B.W.; Ryan, G.; Manlhiot, C.; Blom, N.A. Comparison of transplacental treatment of fetal supraventricular tachyarrhythmias with digoxin, flecainide, and sotalol: Results of a nonrandomized multicenter study. Circulation 2011, 124, 1747–1754. [Google Scholar] [CrossRef]
- Strasburger, J.F. Prenatal diagnosis of fetal arrhythmias. Clin. Perinatol. 2005, 32, 891–912. [Google Scholar] [CrossRef]
- Parilla, B.; Strasburger, J.; Socol, M. Fetal supraventricular tachycardia complicated by hydrops fetalis: A role for direct fetal intramuscular therapy. Am. J. Perinatol. 1996, 13, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.-M.; Xu, Z.Y. Fetal arrhythmias: Prenatal evaluation and intrauterine therapeutics. Ital. J. Pediatr. 2020, 46, 21. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Sullivan, I.; Tomek, V.; Wolfenden, J.; Škovránek, J.; Yates, R.; Janoušek, J.; Dominguez, T.E.; Marek, J. Flecainide versus digoxin for fetal supraventricular tachycardia: Comparison of two drug treatment protocols. Heart Rhythm. 2016, 13, 1913–1919. [Google Scholar] [CrossRef] [PubMed]
- Donofrio, M.T.; Moon-Grady, A.J.; Hornberger, L.K.; Copel, J.A.; Sklansky, M.S.; Abuhamad, A.; Cuneo, B.F.; Huhta, J.C.; Jonas, R.A.; Krishnan, A.; et al. Diagnosis and treatment of fetal cardiac disease: A scientific statement from the American Heart Association. Circulation 2014, 129, 2183–2242. [Google Scholar] [CrossRef]
- Strasburger, J.F.; Eckstein, G.; Butler, M.; Noffke, P.; Wacker-Gussmann, A. Fetal arrhythmia diagnosis and pharmacologic management. J. Clin. Pharmacol. 2022, 62, S53–S66. [Google Scholar] [CrossRef]
- Qin, J.; Deng, Z.; Tang, C.; Zhang, Y.; Hu, R.; Li, J.; Hua, Y.; Li, Y. Efficacy and safety of various first-line therapeutic strategies for fetal tachycardias: A network meta-analysis and systematic review. Front. Pharmacol. 2022, 13, 935455. [Google Scholar] [CrossRef]
- Kerenyi, T.; Gleicher, N.; Meller, J.; Brown, E.; Steinfeld, L.; Chitkara, U.; Raucher, H. Transplancental cardioversion of intrauterine supraventricular tachycardia with digitalis. Lancet 1980, 2, 393–394. [Google Scholar] [CrossRef]
- Menon, R. Human fetal membranes at term: Dead tissue or signalers of parturition? Placenta 2016, 44, 1–5. [Google Scholar] [CrossRef]
- Griffiths, S.; Campbell, J. Placental structure, function and drug transfer. Contin. Educ. Anaesth. Crit. Care Pain 2015, 15, 84–89. [Google Scholar] [CrossRef]
- Chimenea, Á.; García-Díaz, L.; Méndez, A.; Antiñolo, G. Maternal effects induced by oral digoxin during treatment of fetal tachyarrhythmia: Case series and literature review. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 256, 354–357. [Google Scholar] [CrossRef]
- Miyoshi, T.; Maeno, Y.; Sago, H.; Inamura, N.; Yasukochi, S.; Kawataki, M.; Horigome, H.; Yoda, H.; Taketazu, M.; Shozu, M.; et al. Antenatal antiarrhythmic treatment for fetal tachyarrhythmias: A study protocol for a prospective multicentre trial. BMJ Open 2017, 7, e016597. [Google Scholar] [CrossRef]
- Abuhamad, A.; Chaoui, R. A Practical Guide to Fetal Echocardiography. Normal and Abdnormal Hearts, 4th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2022. [Google Scholar]
- Hill, G.D.; Kovach, J.R.; Saudek, D.E.; Singh, A.K.; Wehrheim, K.; Frommelt, M.A. Transplacental treatment of fetal tachycardia: Asystematic review and meta-analysis. Prenat. Diagn. 2017, 37, 1076–1083. [Google Scholar] [CrossRef]
- Syme, M.R.; Paxton, J.W.; Keelan, J.A. Drug transfer and metabolism by the human placenta. Clin. Pharmacokinet. 2004, 43, 487–514. [Google Scholar] [CrossRef]
- Api, O.; Carvalho, J.S. Fetal dysrhythmias. Best. Pract. Res. Clin. Obstet. Gynaecol. 2008, 22, 31–48. [Google Scholar] [CrossRef]
- Suarez, A.M.; Sanchez-Hernandez, J.G.; Barajas, F.M.; Pérez-Blanco, J.S.; Lanao, J.M.; Alvarez, L.G.-C.; Calvo, M.V. Pharmacokinetics and dosing requirements of digoxin in pregnant women treated for fetal supraventricular tachycardia. Expert. Rev. Clin. Pharmacol. 2017, 10, 911–917. [Google Scholar] [CrossRef]
- Hebert, M.F.; Easterling, T.R.; Kirby, B.; Carr, D.B.; Buchanan, M.L.; Rutherford, T.; Thummel, K.E.; Fishbein, D.P.; Unadkat, J.D. Effects of Pregnancy on CYP3A and P-glycoprotein Activities as Measured by Disposition of Midazolam and Digoxin: A University of Washington Specialized Center of Research Study. Clin. Pharmacol. Ther. 2008, 84, 248–253. [Google Scholar] [CrossRef]
- Bourget, P.; Pons, J.C.; Delouis, C.; Fermont, L.; Frydman, R. Flecainide distribution, transplacental passage, and accumulation in the amniotic fluid during the third trimester of pregnancy. Ann. Pharmacother. 1994, 28, 1031–1034. [Google Scholar] [CrossRef]
- Oudijk, M.A.; Ruskamp, J.M.; Ververs, F. Treatment of fetal tachycardia with sotalol: Transplacental pharmacokinetics and pharmacodynamics. J. Am. Coll. Cardiol. 2003, 42, 765–770. [Google Scholar] [CrossRef]
- Dimasc, V.; Taylor, M.; Cunnyngham, C.; Overholt, E.D.; Bourne, D.W.; Stanely, J.R.; Sheikh, A.; Wolf, R.; Valentine, B.; Ward, K.E. Transplacental Pharmacokinetics of Flecainide in the Gravid Baboon and Fetus. Pediatr. Cardiol. 2005, 26, 815–820. [Google Scholar] [CrossRef]
- Strasburger, J.F.; Cuneo, B.F.; Michon, M.M.; Gotteiner, N.L.; Deal, B.J.; McGregor, S.N.; Oudijk, M.A.; Meijboom, E.J.; Feinkind, L.; Hussey, M.; et al. Amiodarone therapy for drug-refractory fetal tachycardia. Circulation 2004, 109, 375–379. [Google Scholar] [CrossRef]
- Jouannic, J.M.; Delahaye, S.; Fermont, L.; Le Bidois, J.; Villain, E.; Dumez, Y.; Dommergues, M. Fetal supraventricular tachycardia: A role for amiodarone as second-line therapy? Prenat. Diagn. 2003, 23, 152. [Google Scholar] [CrossRef] [PubMed]
- Lomenick, J.P.; Jackson, W.A.; Backeljauw, P.F. Amiodarone-induced neonatal hypothyroidism: A unique form of transient early-onset hypothyroidism. J. Perinatol. 2004, 24, 397. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Grosso, S.; Berardi, R.; Cioni, M.; Morgese, G. Transient neonatal hypothyroidism after gestational exposure to amiodarone: A follow-up of two cases. J. Endocrinol. Investig. 1998, 21, 699. [Google Scholar] [CrossRef] [PubMed]
- Pinsky, W.W.; Jacobsen, J.R.; Gillette, P.C.; Adams, J.; Monroe, L.; McNamara, D.G. Dosage of digoxin in premature infants. J. Pediatr. 1979, 94, 639–642. [Google Scholar] [CrossRef]
- Lin, P.-H.; Wu, H.-H.; Tsai, H.-D.; Hsieh, C.T.-C. Successful treatment of atrial flutter by repeated intraperitoneal and intra-amniotic injections of amiodarone in a fetus with hydrops. Taiwan. J. Obstet. Gynecol. 2016, 55, 434–436. [Google Scholar] [CrossRef]
- Stirnemann, J.; Maltret, A.; Haydar, A.; Stos, B.; Bonnet, D.; Ville, Y. Successful in utero transesophageal pacing for severe drug-resistant tachyarrhythmia. Am. J. Obstet. Gynecol. 2018, 219, 320–325. [Google Scholar] [CrossRef]
- Loeb, G.E.; Zhou, L.; Zheng, K.; Nicholson, A.; Peck, R.A.; Krishnan, A.; Silka, M.; Pruetz, J.; Chmait, R.; Bar-Cohen, Y. Design and testing of a percutaneously implantable fetal pacemaker. Ann. Biomed. Eng. 2013, 41, 17–27. [Google Scholar] [CrossRef][Green Version]
- Zhou, L.; Vest, A.N.; Chmait, R.H.; Bar-Cohen, Y.; Pruetz, J.; Silka, M.; Zheng, K.; Peck, R.; Loeb, G.E. A Percutaneously Implantable Fetal Pacemaker. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; pp. 4459–4463. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).