Influence of Polyether Backbone PEO–PPO on the Drug Release Behavior of Polyurea Xerogels
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Polyureas
2.3. Synthesis of Polyureas Containing Drug
2.4. Characterization
3. Results and Discussion
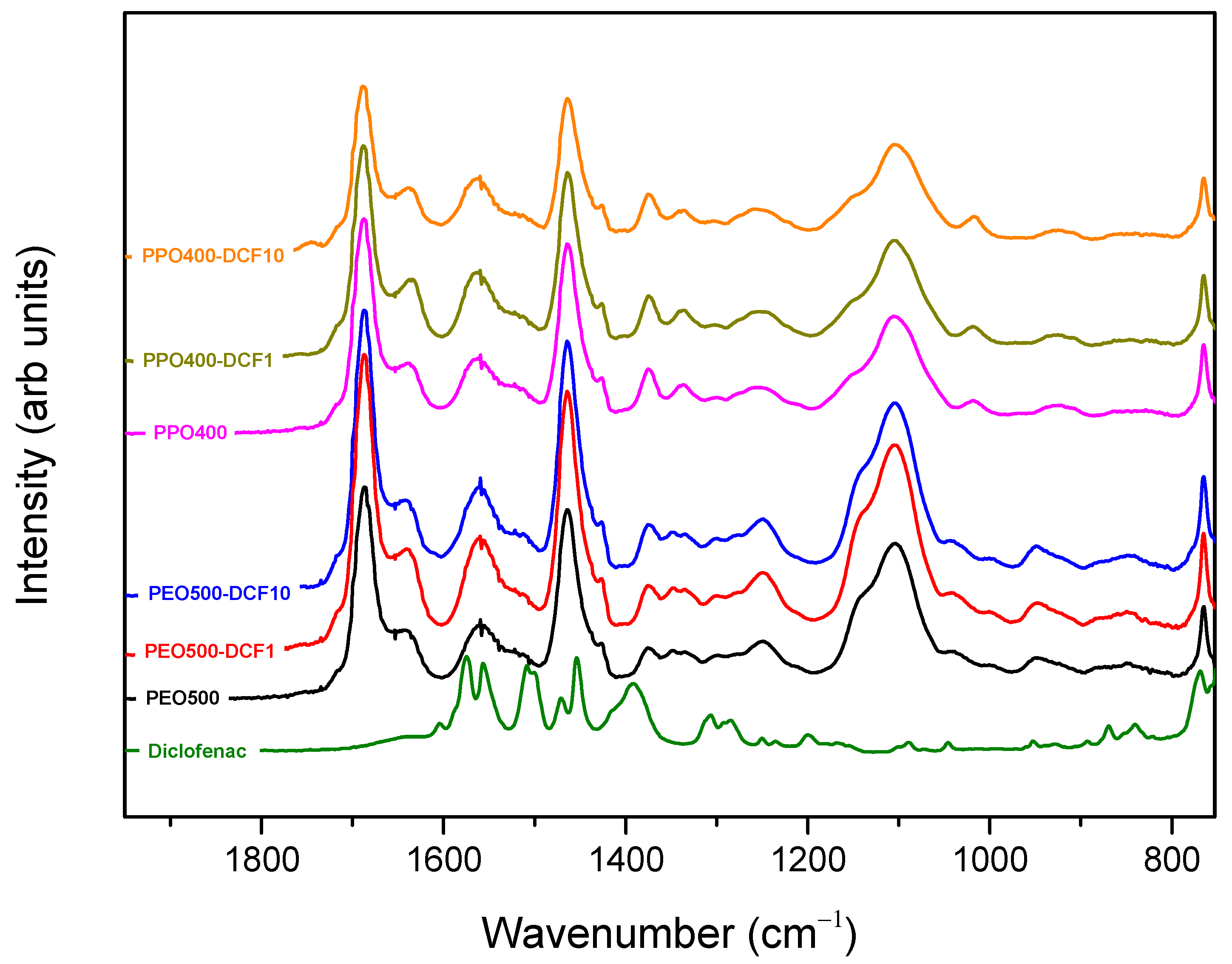
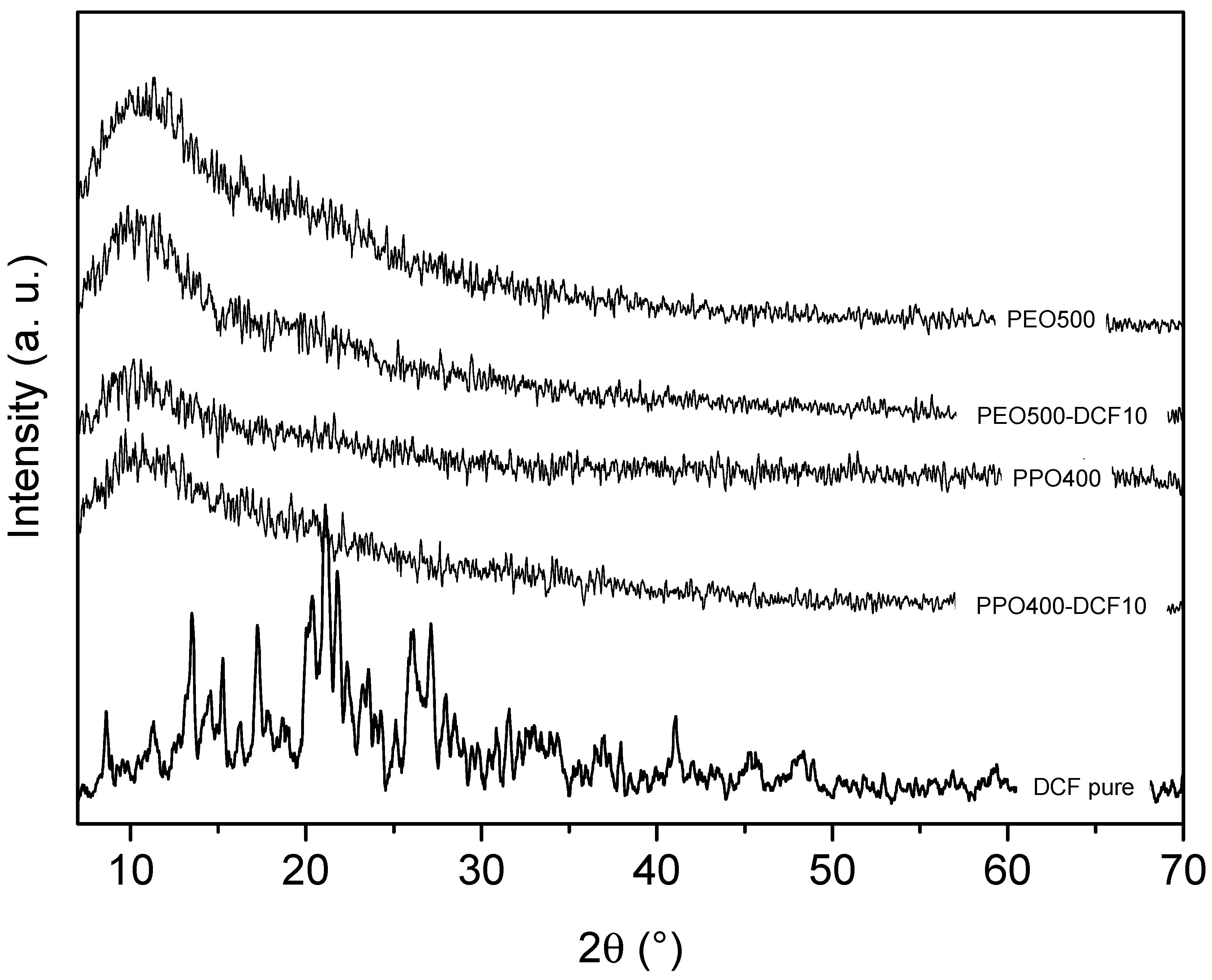
3.1. Structural Characterization
3.2. TGA Analysis
3.3. Swelling Behavior of Polyurea PEO/PPO Matrixes
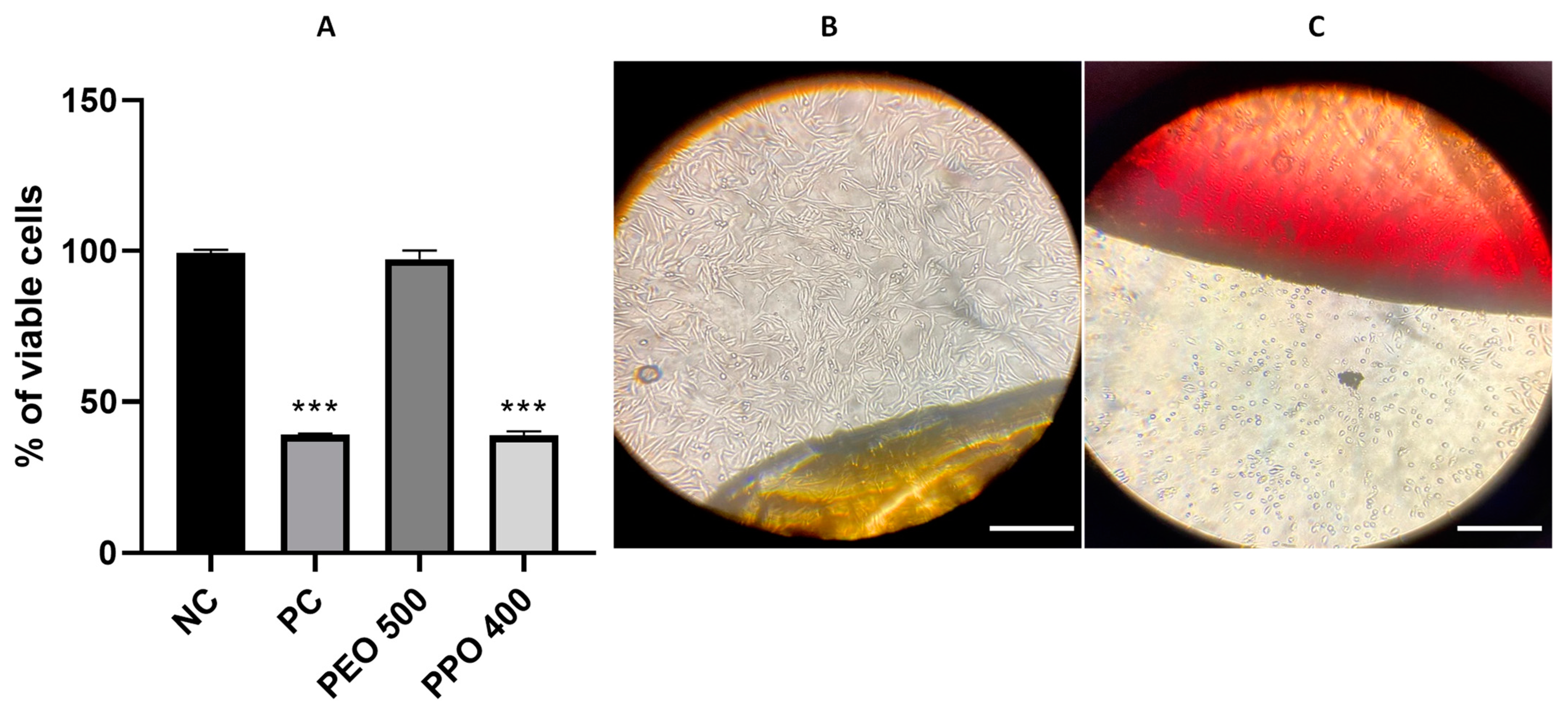
3.4. Cell Viability Assays
3.5. In Situ Diclofenac Release Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kaul, L.; Grundmann, C.E.; Köll-weber, M.; Löffler, H.; Weiz, A.; Zannettino, A.C.W.; Richter, K.; Süss, R. A Thermosensitive, Chitosan-Based Hydrogel as Delivery System for Antibacterial Liposomes to Surgical Site Infections. Pharmaceutics 2022, 14, 2841. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Cheng, W.; Tang, Q.; Pan, X.; Li, J.; Zhao, L.; Xi, Z.; Yuan, W. Multiblock Poly(Ether-b-Amide) Copolymers Comprised of PA1212 and PPO-PEO-PPO with Specific Moisture-Responsive and Antistatic Properties. Chin. J. Chem. Eng. 2022, 53, 421–430. [Google Scholar] [CrossRef]
- Sanopoulou, M.; Papadokostaki, K.G. Controlled Drug Release Systems: Mechanisms and Kinetics. Biomed. Membr. (Bio) Artif. Organs 2017, 2, 1–33. [Google Scholar] [CrossRef]
- Macha, I.J.; Ben-Nissan, B.; Vilchevskaya, E.N.; Morozova, A.S.; Abali, B.E.; Müller, W.H.; Rickert, W. Drug Delivery from Polymer-Based Nanopharmaceuticals-an Experimental Study Complemented by Simulations of Selected Diffusion Processes. Front. Bioeng. Biotechnol. 2019, 7, 37. [Google Scholar] [CrossRef]
- Rajabi, M.; Srinivasan, M.; Mousa, S.A. Nanobiomaterials in Drug Delivery; William Andrew Publishing: Norwich, NY, USA, 2016; Volume 9, pp. 1–37. ISBN 9780323428897. [Google Scholar] [CrossRef]
- Bruschi, M.L. Strategies to Modify the Drug Release from Pharmaceutical Systems/Edited by Marcos Luciano Bruschi; Woodhead Publishing Series in Biomedicine; Elsevier: Cambridge, UK, 2015; p. 85. ISBN 0-08-100112-6. [Google Scholar]
- Kamaly, N.; Yameen, B.; Wu, J.; Farokhzad, O.C. Degradable Controlled-Release Polymers and Polymeric Nanoparticles: Mechanisms of Controlling Drug Release. Chem. Rev. 2016, 116, 2602–2663. [Google Scholar] [CrossRef]
- Malekjani, N.; Jafari, S.M. Modeling the Release of Food Bioactive Ingredients from Carriers/Nanocarriers by the Empirical, Semiempirical, and Mechanistic Models. Compr. Rev. Food Sci. Food Saf. 2021, 20, 3–47. [Google Scholar] [CrossRef]
- Anghel, N.; Melinte, V.; Spiridon, I.; Pertea, M. Antioxidant, Antimicrobial, and Kinetic Studies of Β-Cyclodextrin Crosslinked with Lignin for Drug Delivery. Pharmaceutics 2022, 14, 2260. [Google Scholar] [CrossRef]
- Uboldi, M.; Pasini, C.; Pandini, S.; Baldi, F.; Briatico-vangosa, F.; Inverardi, N.; Maroni, A.; Moutaharrik, S.; Melocchi, A.; Gazzaniga, A.; et al. Expandable Drug Delivery Systems Based on Shape Memory Polymers: Impact of Film Coating on Mechanical Properties and Release and Recovery Performance. Pharmaceutics 2022, 14, 2814. [Google Scholar] [CrossRef]
- Ferrero, C.; Casas, M.; Caraballo, I. Redox-Responsive Polymersomes as Smart Doxorubicin Delivery Systems. Pharmaceutics 2022, 14, 1724. [Google Scholar] [CrossRef]
- Huang, Y.; Yan, H.; Cai, M.; Song, S.; He, C.; Fan, X.; Zhu, M. Polyurea as a Reinforcing Filler for the Anti-Corrosion and Wear-Resistant Application of Epoxy Resin. Prog. Org. Coat. 2022, 171, 107049. [Google Scholar] [CrossRef]
- Tang, S.; Shi, Z.; Cao, Y.; He, W. Facile Aqueous-Phase Synthesis of Multi-Responsive Nanogels Based on Polyetheramines and Bisepoxide. J. Mater. Chem. B 2013, 1, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Lv, Y.; Xu, C.; Li, J.; Yin, L.; He, W.; Lv, Y.; Zhao, Y.; Xu, C.; Jin, Z.; et al. Compositional Tuning of Epoxide-Polyetheramine “Click” Reaction toward Cytocompatible, Cationic Hydrogel Particles with Antimicrobial and DNA Binding Activities. Int. J. Pharm. 2020, 484, 15–40. [Google Scholar] [CrossRef]
- Bar, H.; Bianco-Peled, H. Modification of Shellac Coating Using Jeffamine® for Enhanced Mechanical Properties and Stability. Prog. Org. Coat. 2020, 141, 105559. [Google Scholar] [CrossRef]
- Huang, H.; Wei, H.; Huang, L.; Fan, T.; Li, X.; Zhang, Z.; Shi, T. Spontaneous Alternating Copolymerization of Aziridines with Tosyl Isocyanate toward Polyureas. Eur. Polym. J. 2023, 182, 111731. [Google Scholar] [CrossRef]
- Ren, G.; Zhou, C.; Fan, X.; Zheng, M.; Wang, S. Investigating the Rheological and Tribological Properties of Polyurea Grease via Regulating Ureido Amount. Tribol. Int. 2022, 173, 107643. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Rogez, D.; Martinoty, P. Synthesis and Characterization of New Polyurea Elastomers by Sol/Gel Chemistry. Macromol. Chem. Phys. 2010, 211, 1712–1721. [Google Scholar] [CrossRef]
- Zhang, C.; Lu, L.; Ouyang, R.; Zhou, C. Polyurethane/Liquid Crystal Microfibers with PDNA Polyplex Loadings for the Optimal Release and Promotion of HUVEC Proliferation. Pharmaceutics 2022, 14, 2489. [Google Scholar] [CrossRef]
- Du, J.; Ibaseta, N.; Guichardon, P. Characterization of Polyurea Microcapsules Synthesized with an Isocyanate of Low Toxicity and Eco-Friendly Esters via Microfluidics: Shape, Shell Thickness, Morphology and Encapsulation Efficiency. Chem. Eng. Res. Des. 2022, 182, 256–272. [Google Scholar] [CrossRef]
- Resende, M.A.; Pedroza, G.A.; Macêdo, L.H.G.M.C.; Oliveira, R.; Amela-Cortes, M.; Molard, Y.; Molina, E.F. Design of Polyurea Networks Containing Anticancer and Anti-inflammatory Drugs for Dual Drug Delivery Purposes. J. Appl. Polym. Sci. 2022, 139, 51970. [Google Scholar] [CrossRef]
- Caravieri, B.B.; de Jesus, N.A.M.; de Oliveira, L.K.; Araujo, M.D.; Andrade, G.P.; Molina, E.F. Ureasil Organic–Inorganic Hybrid as a Potential Carrier for Combined Delivery of Anti-Inflammatory and Anticancer Drugs. ACS Appl. Bio Mater. 2019, 2, 1875–1883. [Google Scholar] [CrossRef]
- Bonattini, V.H.; Paula, L.A.L.; Jesus, N.A.; Tavares, D.C.; Nicolella, H.D.; Magalhães, L.G.; Molina, E.F. One-step Formation of Polyurea Gel as a Multifunctional Approach for Biological and Environmental Applications. Polym. Int. 2020, 69, 476–484. [Google Scholar] [CrossRef]
- Pedroza, G.A.; Macêdo, L.H.G.M.C.; de Oliveira, R.; Silveira, N.N.; Orenha, R.P.; Parreira, R.L.T.; dos Santos, R.A.; Molard, Y.; Amela-Cortes, M.; Molina, E.F. Cost-Efficient Polyurea Carrier for Precise Control of an Anti-Inflammatory Drug Loading and Release. J. Drug Deliv. Sci. Technol. 2022, 76, 103744. [Google Scholar] [CrossRef]
- Lowinger, M.B.; Barrett, S.E.; Zhang, F.; Williams, R.O. Sustained Release Drug Delivery Applications of Polyurethanes. Pharmaceutics 2018, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Knight, P.T.; Mather, P.T. Tailored Drug Release from Biodegradable Stent Coatings Based on Hybrid Polyurethanes. J. Control. Release 2009, 137, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Liu, W.; Zhao, Y.; Jiang, L.; Xu, H.; Yang, X. Preparation and Characterization of PEG-Modified Polyurethane Pressure-Sensitive Adhesives for Transdermal Drug Delivery. Drug Dev. Ind. Pharm. 2009, 35, 704–711. [Google Scholar] [CrossRef]
- Li, B.; Yoshii, T.; Hafeman, A.E.; Nyman, J.S.; Wenke, J.C.; Guelcher, S.A. The Effects of RhBMP-2 Released from Biodegradable Polyurethane/Microsphere Composite Scaffolds on New Bone Formation in Rat Femora. Biomaterials 2009, 30, 6768–6779. [Google Scholar] [CrossRef]
- Martinelli, A.; D’Ilario, L.; Francolini, I.; Piozzi, A. Water State Effect on Drug Release from an Antibiotic Loaded Polyurethane Matrix Containing Albumin Nanoparticles. Int. J. Pharm. 2011, 407, 197–206. [Google Scholar] [CrossRef]
- Castagna, A.M.; Pangon, A.; Choi, T.; Dillon, G.P.; Runt, J. The Role of Soft Segment Molecular Weight on Microphase Separation and Dynamics of Bulk Polymerized Polyureas. Macromolecules 2012, 45, 8438–8444. [Google Scholar] [CrossRef]
- Choi, T.; Fragiadakis, D.; Roland, C.M.; Runt, J. Microstructure and Segmental Dynamics of Polyurea under Uniaxial Deformation. Macromolecules 2012, 45, 3581–3589. [Google Scholar] [CrossRef]
- Zheng, T.; Li, T.; Shi, J.; Wu, T.; Zhuang, Z.; Xu, J.; Guo, B. Molecular Insight into the Toughness of Polyureas: A Hybrid All-Atom/Coarse-Grained Molecular Dynamics Study. Macromolecules 2022, 55, 3020–3029. [Google Scholar] [CrossRef]
- Sánchez-Ferrer, A.; Soprunyuk, V.; Engelhardt, M.; Stehle, R.; Gilg, H.A.; Schranz, W.; Richter, K. Polyurea Networks from Moisture-Cure, Reaction-Setting, Aliphatic Polyisocyanates with Tunable Mechanical and Thermal Properties. ACS Appl. Polym. Mater. 2021, 3, 4070–4078. [Google Scholar] [CrossRef]
- Scudiero, D.A.; Shoemaker, R.H.; Paull, K.D.; Monks, A.; Tierney, S.; Nofziger, T.H.; Currens, M.J.; Seniff, D.; Boyd, M.R. Evaluation of a Soluble Tetrazolium/Formazan Assay for Cell Growth and Drug Sensitivity in Culture Using Human and Other Tumor Cell Lines. Cancer Res. 1988, 48, 4827–4833. [Google Scholar] [PubMed]
- Chaker, J.A.; Santilli, C.V.; Pulcinelli, S.H.; Dahmouche, K.; Briois, V.; Judeinstein, P. Multi-Scale Structural Description of Siloxane-PPO Hybrid Ionic Conductors Doped by Sodium Salts. J. Mater. Chem. 2007, 17, 744–757. [Google Scholar] [CrossRef]
- Xu, D.; Yu, K.; Qian, K. Thermal Degradation Study of Rigid Polyurethane Foams Containing Tris(1-Chloro-2-Propyl)Phosphate and Modified Aramid Fiber. Polym. Test. 2018, 67, 159–168. [Google Scholar] [CrossRef]
- Levchik, S.V.; Weil, E.D. Thermal Decomposition, Combustion and Fire-Retardancy of Polyurethanes—A Review of the Recent Literature. Polym. Int. 2004, 53, 1585–1610. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of Solute Release from Porous Hydrophilic Polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Higuchi, T. Rate of release of medicaments from ointment bases containing drugs in suspension. J. Pharm. Sci. 1961, 50, 874–875. [Google Scholar] [CrossRef]
- Chen, L.; Kuang, L.; Ross, A.E.; Farhat, W.; Boychev, N.; Sharfi, S.; Kanu, L.N.; Liu, L.; Kohane, D.S.; Ciolino, J.B. Topical Sustained Delivery of Miltefosine Via Drug-Eluting Contact Lenses to Treat Acanthamoeba Keratitis. Pharmaceutics 2022, 14, 2750. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Qi, Z.; Tao, X.; Kundu, S.C.; Lu, S. Sustained Release of Insulin from Silk Microneedles. J. Drug Deliv. Sci. Technol. 2022, 74, 103611. [Google Scholar] [CrossRef]




| Polyurea Sample | ||||||
|---|---|---|---|---|---|---|
| Mass Loss | PEO500 | PEO500-DCF1 | PEO500-DCF10 | PPO400 | PPO400-DCF1 | PPO400-DCF10 |
| at first stage (%) | 54 | 55 | 63 | 61 | 66 | 59 |
| at second stage (%) | 36 | 35 | 26 | 30 | 25 | 30 |
| Char residue (%) | 4 | 4 | 5 | 3 | 3 | 4 |
| Amount Released (%) | Korsmeyer–Peppas Parameters | Release Mechanism | ||
|---|---|---|---|---|
| Sample | n | R2 | ||
| PEO500-DCF1 | 67.0 | 1.00 | 0.98 | Fickian diffusion |
| PEO500-DCF10 | 69.0 | 0.59 | 0.99 | anomalous transport |
| PPO400-DCF10 | 1.83 | 0.62 | 0.95 | anomalous transport |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vargas, J.G.; Andrada, H.E.; Fico, B.A.; Paulino, J.M.; Silveira, N.N.; dos Santos, R.A.; Molina, E.F. Influence of Polyether Backbone PEO–PPO on the Drug Release Behavior of Polyurea Xerogels. Future Pharmacol. 2023, 3, 426-439. https://doi.org/10.3390/futurepharmacol3020026
Vargas JG, Andrada HE, Fico BA, Paulino JM, Silveira NN, dos Santos RA, Molina EF. Influence of Polyether Backbone PEO–PPO on the Drug Release Behavior of Polyurea Xerogels. Future Pharmacology. 2023; 3(2):426-439. https://doi.org/10.3390/futurepharmacol3020026
Chicago/Turabian StyleVargas, Julia G., Heber E. Andrada, Bruno A. Fico, Julia M. Paulino, Natália N. Silveira, Raquel A. dos Santos, and Eduardo F. Molina. 2023. "Influence of Polyether Backbone PEO–PPO on the Drug Release Behavior of Polyurea Xerogels" Future Pharmacology 3, no. 2: 426-439. https://doi.org/10.3390/futurepharmacol3020026
APA StyleVargas, J. G., Andrada, H. E., Fico, B. A., Paulino, J. M., Silveira, N. N., dos Santos, R. A., & Molina, E. F. (2023). Influence of Polyether Backbone PEO–PPO on the Drug Release Behavior of Polyurea Xerogels. Future Pharmacology, 3(2), 426-439. https://doi.org/10.3390/futurepharmacol3020026








