The Pharmacogenetics of Treatment with Quetiapine
Abstract
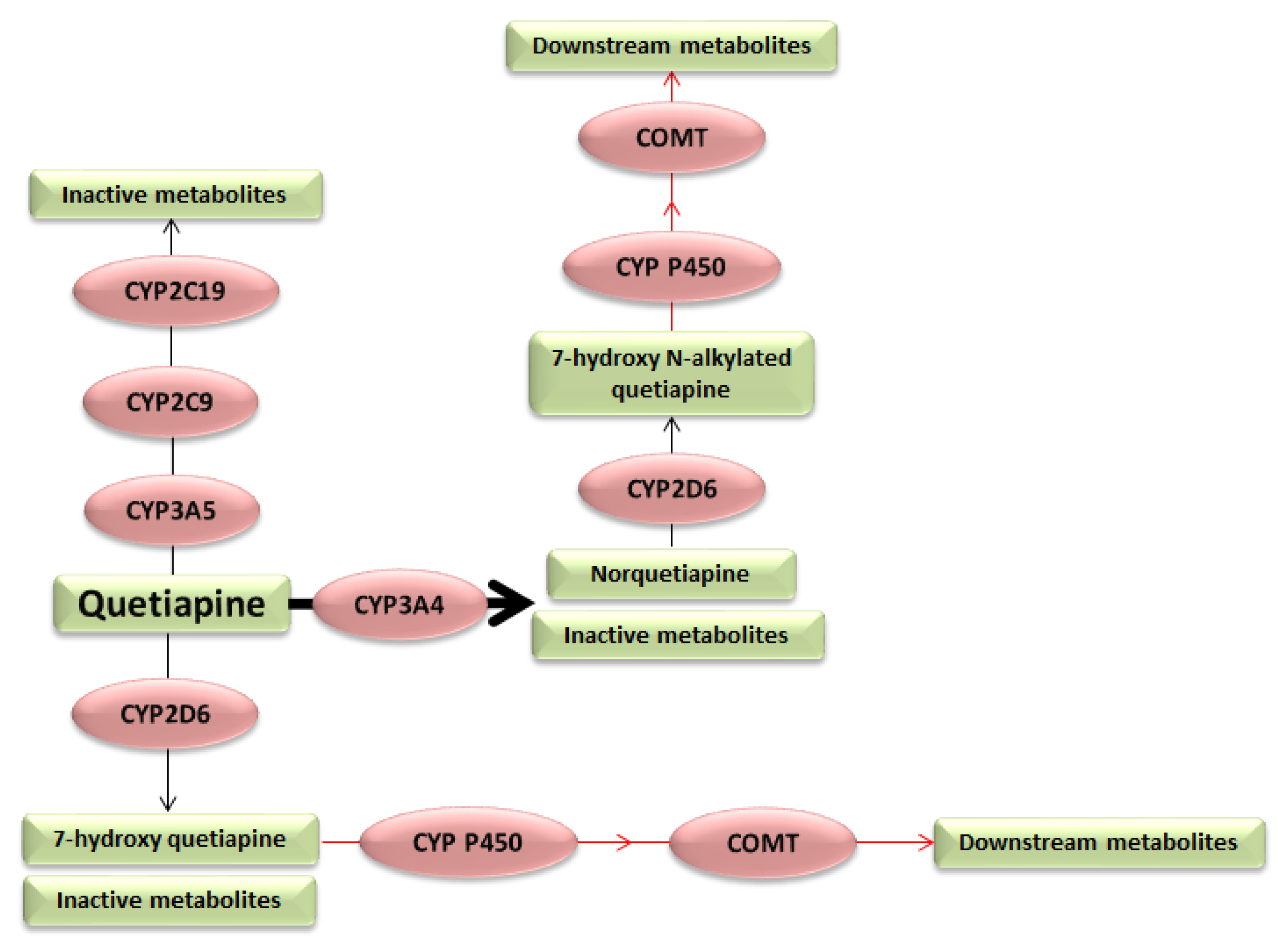
1. Introduction
2. Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA. Seroquel. Drug Label. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/020639s061lbl.pdf (accessed on 20 June 2022).
- Lalonde, C.D.; Van Lieshout, R.J. Treating Generalized Anxiety Disorder with Second Generation Antipsychotics: A Systematic Review and Meta-Analysis. J. Clin. Psychopharmacol. 2011, 31, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Poyurovsky, M.; Weizman, A. Quetiapine for Bipolar Depressive Episode in Obsessive-Compulsive Disorder Patients Maintained on Selective Serotonin Reuptake Inhibitor Treatment. Clin. Neuropharmacol. 2021, 44, 123–125. [Google Scholar] [CrossRef] [PubMed]
- DeVane, C.L.; Nemeroff, C.B. Clinical Pharmacokinetics of Quetiapine: An Atypical Antipsychotic. Clin. Pharmacokinet. 2001, 40, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Zubiaur, P.; Fernández-Campos, P.; Navares-Gómez, M.; Soria-Chacartegui, P.; Villapalos-García, G.; Román, M.; Mejía-Abril, G.; Ochoa, D.; Abad-Santos, F. Variants in COMT, CYP3A5, CYP2B6, and ABCG2 Alter Quetiapine Pharmacokinetics. Pharmaceutics 2021, 13, 1573. [Google Scholar] [CrossRef]
- Hasselstrøm, J.; Linnet, K. IN VITRO STUDIES ON QUETIAPINE METABOLISM USING THE SUBSTRATE DEPLETION APPROACH WITH FOCUS ON DRUG-DRUG INTERACTIONS. Drug Metabol. Drug Interact. 2006, 21, 187–211. [Google Scholar] [CrossRef]
- López-Muñoz, F.; Álamo, C. Active Metabolites as Antidepressant Drugs: The Role of Norquetiapine in the Mechanism of Action of Quetiapine in the Treatment of Mood Disorders. Front. Psychiatry 2013, 4, 102. [Google Scholar] [CrossRef]
- Björkholm, C.; Jardemark, K.; Marcus, M.M.; Malmerfelt, A.; Nyberg, S.; Schilström, B.; Svensson, T.H. Role of concomitant inhibition of the norepinephrine transporter for the antipsychotic effect of quetiapine. Eur. Neuropsychopharmacol. 2013, 23, 709–720. [Google Scholar] [CrossRef]
- Flores-Rojas, L.E.; Hernández, L.A.G.-Z. Efectos secundarios metabólicos de los antipsicóticos de segunda generación. Med. Interna México 2019, 35, 721–731. [Google Scholar]
- The Royal Dutch Society of Pharmacology (KNMP) Dutch Pharmacogenetics Working Group (DPWG). Pharmacogenetics Guidelines. CYP2D6: Aripiprazole 2022. Available online: https://www.g-standaard.nl/risicoanalyse/B0001542.PDF (accessed on 1 July 2022).
- The Royal Dutch Society of Pharmacology (KNMP) Dutch Pharmacogenetics Working Group (DPWG). Pharmacogenetics Guidelines; CYP3A4: Quetiapine; Netherlands, 2022. Available online: https://www.g-standaard.nl/risicoanalyse/B0005991.PDF (accessed on 1 July 2022).
- Quetiapine. Available online: https://www.pharmgkb.org/chemical/PA451201/clinicalAnnotation (accessed on 5 July 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Xu, Q.; Wu, X.; Li, M.; Huang, H.; Minica, C.; Yi, Z.; Wang, G.; Shen, L.; Xing, Q.; Shi, Y.; et al. Association studies of genomic variants with treatment response to risperidone, clozapine, quetiapine and chlorpromazine in the Chinese Han population. Pharm. J. 2016, 16, 357–365. [Google Scholar] [CrossRef]
- Porcelli, S.; Balzarro, B.; Lee, S.-J.; Han, C.; Patkar, A.A.; Pae, C.-U.; Serretti, A. PDE7B, NMBR and EPM2A Variants and Schizophrenia: A Case-Control and Pharmacogenetics Study. Neuropsychobiology 2016, 73, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, C.; Chiesa, A.; Han, C.; Lee, S.-J.; Park, M.H.; Balzarro, B.; Andrisano, C.; Patkar, A.A.; Pae, C.-U.; Serretti, A. Case–control association study for 10 genes in patients with schizophrenia: Influence of 5HTR1A variation rs10042486 on schizophrenia and response to antipsychotics. Eur. Arch. Psychiatry Clin. Neurosci. 2012, 262, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.L.; Souza, R.P.; Adkins, D.E.; Åberg, K.; Bukszár, J.; McClay, J.L.; Sullivan, P.F.; van den Oord, E.J.C.G. Genome-wide association study of patient-rated and clinician-rated global impression of severity during antipsychotic treatment. Pharmacogenet. Genom. 2013, 23, 69–77. [Google Scholar] [CrossRef]
- Campbell, D.B.; Ebert, P.J.; Skelly, T.; Stroup, T.S.; Lieberman, J.; Levitt, P.; Sullivan, P.F. Ethnic Stratification of the Association of RGS4 Variants with Antipsychotic Treatment Response in Schizophrenia. Biol. Psychiatry 2008, 63, 32–41. [Google Scholar] [CrossRef]
- Monteleone, P.; Milano, W.; Petrella, C.; Canestrelli, B.; Maj, M. Endocannabinoid Pro129Thr FAAH Functional Polymorphism But Not 1359G/A CNR1 Polymorphism Is Associated With Antipsychotic-Induced Weight Gain. J. Clin. Psychopharmacol. 2010, 30, 441–445. [Google Scholar] [CrossRef]
- López-Rodríguez, R.; Cabaleiro, T.; Ochoa, D.; Román, M.; Borobia, A.M.; Carcas, A.J.; Ayuso, C.; Novalbos, J.; Abad-Santos, F. Pharmacodynamic genetic variants related to antipsychotic adverse reactions in healthy volunteers. Pharmacogenomics 2013, 14, 1203–1214. [Google Scholar] [CrossRef] [PubMed]
- Czerwensky, F.; Leucht, S.; Steimer, W. MC4R rs489693: A clinical risk factor for second generation antipsychotic-related weight gain? Int. J. Neuropsychopharmacol. 2013, 16, 2103–2109. [Google Scholar] [CrossRef][Green Version]
- Malhotra, A.K. Association Between Common Variants Near the Melanocortin 4 Receptor Gene and Severe Antipsychotic Drug–Induced Weight Gain. Arch. Gen. Psychiatry 2012, 69, 904. [Google Scholar] [CrossRef]
- Delacrétaz, A.; Zdralovic, A.; Vandenberghe, F.; Saigi-Morgui, N.; Glatard, A.; Quteineh, L.; Gholam-Rezaee, M.; Raffoul, W.; Applegate, L.A.; Jafari, P.; et al. Association of variants in SH2B1 and RABEP1 with worsening of low-density lipoprotein and glucose parameters in patients treated with psychotropic drugs. Gene 2017, 628, 8–15. [Google Scholar] [CrossRef]
- Cabaleiro, T.; López-Rodríguez, R.; Román, M.; Ochoa, D.; Novalbos, J.; Borobia, A.; Carcas, A.; Abad-Santos, F. Pharmacogenetics of quetiapine in healthy volunteers: Association with pharmacokinetics, pharmacodynamics, and adverse effects. Int. Clin. Psychopharmacol. 2015, 7, 82–88. [Google Scholar] [CrossRef]
- van der Weide, K.; van der Weide, J. The Influence of the CYP3A4*22 Polymorphism on Serum Concentration of Quetiapine in Psychiatric Patients. J. Clin. Psychopharmacol. 2014, 34, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Saiz-Rodríguez, M.; Almenara, S.; Navares-Gómez, M.; Ochoa, D.; Román, M.; Zubiaur, P.; Koller, D.; Santos, M.; Mejía, G.; Borobia, A.M.; et al. Effect of the Most Relevant CYP3A4 and CYP3A5 Polymorphisms on the Pharmacokinetic Parameters of 10 CYP3A Substrates. Biomedicines 2020, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-A.; Joo, H.-J.; Lee, H.-M.; Park, J.-Y. Influence of ABCB1 and CYP3A5 genetic polymorphisms on the pharmacokinetics of quetiapine in healthy volunteers. Pharmacogenet. Genom. 2014, 24, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Pharmacogenetics in Psychiatry: Misconceptions, Challenges, and Successes. Available online: https://www.pharmacytimes.com/view/pharmacogenetics-in-psychiatry-misconceptions-challenges-and-successes (accessed on 7 June 2022).
- Prioritization of CPIC Guidelines. Available online: https://cpicpgx.org/prioritization-of-cpic-guidelines/ (accessed on 17 May 2022).
- The Royal Dutch Society of Pharmacology (KNMP) Dutch Pharmacogenetics Working Group (DPWG). Pharmacogenetics guidelines. CYP2D6: Quetiapine 2022. Available online: https://www.g-standaard.nl/risicoanalyse/B0002394.PDF (accessed on 1 July 2022).
- Annotation of DPWG Guideline for Quetiapine and CYP3A4, PharmGKB. Available online: https://www.pharmgkb.org/guidelineAnnotation/PA166265421 (accessed on 9 June 2022).
- Annotation of DPWG Guideline for Risperidone and CYP2D6, PharmGKB. Available online: https://www.pharmgkb.org/guidelineAnnotation/PA166104943 (accessed on 9 June 2022).
- Annotation of DPWG Guideline for Aripiprazole and CYP2D6, PharmGKB. Available online: https://www.pharmgkb.org/guidelineAnnotation/PA166104937/annotation (accessed on 9 June 2022).
- Annotation of DPWG Guideline for Haloperidol and CYP2D6, PharmGKB. Available online: https://www.pharmgkb.org/guidelineAnnotation/PA166104988 (accessed on 9 June 2022).
- Annotation of DPWG Guideline for Zuclopenthixol and CYP2D6, PharmGKB. Available online: https://www.pharmgkb.org/guidelineAnnotation/PA166104992 (accessed on 9 June 2022).
- Annotation of DPWG Guideline for Pimozide and CYP2D6, PharmGKB. Available online: https://www.pharmgkb.org/guidelineAnnotation/PA166182819 (accessed on 9 June 2022).
- CPIC® Guideline for Tacrolimus and CYP3A5. Available online: https://cpicpgx.org/guidelines/guideline-for-tacrolimus-and-CYP3A5/ (accessed on 17 May 2022).
- González Jiménez, E.; Aguilar Cordero, M.J.; Padilla López, C.A.; García García, I. Obesidad monogénica humana: Papel del sistema leptina-melanocortina en la regulación de la ingesta de alimentos y el peso corporal en humanos. An. Sist. Sanit. Navar. 2012, 35, 285–293. [Google Scholar] [CrossRef]

| Receptor | Norquetiapine Activity | Quetiapine Activity | Effect |
|---|---|---|---|
| Serotonin 5-HT1A receptor | Partial agonism (high affinity *) | Partial agonism | Antidepressant and anxiolytic effects. |
| Serotonin 5-HT2A receptor | Antagonism (high affinity *) $ | Antagonism $ | Treatment of negative and cognitive symptoms, antidepressant activity. |
| Serotonin 5-HT2C receptor | Antagonism (high affinity *) | Antagonism | Possibly related to weight gain. |
| Serotonin 5-HT7 receptor | Antagonism (high affinity *) | Antagonism | Possibly related to antidepressant activity. |
| Dopamine D2 receptor | Antagonism (moderate affinity) | Antagonism (moderate affinity) | Treatment of negative and positive symptoms. |
| Histamine H1 receptor | Antagonism (high affinity *) | Antagonism | Possibly related to weight gain and sedative effects. |
| α1 receptor | Antagonism | Antagonism | Possibly related to orthostatic hypotension. |
| α2 receptor | Antagonism | Antagonism | Antidepressant activity. |
| Norepinephrine transporter (NET) | Antagonism | Antidepressant activity. | |
| Muscarinic receptors | Antagonism (high affinity) | Anticolinergic effects, possibly related to adverse atropinic effects. |
| Gene | Variant | Population | Association | p Value | References |
|---|---|---|---|---|---|
| COMT | rs4818 G>C | 995 Patients with schizophrenia. | C allele was associated with a worse response (PANSS score) compared to G allele carriers. | 0.007 | Xu et al. (2016) [14] |
| rs6269 G>A | A allele was associated with a worse response (PANSS score) compared to G allele. | 0.008 | |||
| rs5993883 G>T | T allele was associated with a worse response (PANSS score) compared to G allele. | 0.007 | |||
| EPM2A | rs1415744 T>C | 573 Patients with schizophrenia. | C allele was associated with an increased response (PANSS score) compared to T allele. | 0.004 | Porcelli et al. (2016) [15] |
| HTR1A | rs10042486 T>C | 221 Patients with schizophrenia and 171 healthy controls. | CT and CC were associated with a worse response (PANSS score) compared to TT allele diplotype. | 1.0 × 10−6 | Crisafulli et al. (2012) [16] |
| PDE4D | rs17742120 A>G | 738 Patients with schizophrenia. | G allele was associated with a decreased response (PANSS score) compared to A allele. | 1.58 × 10−7 | Clark et al. (2013) [17] |
| rs2164660 G>A | A allele was associated with an increased response (PANSS score) compared to G allele. | 1.87 × 10−7 | |||
| rs17382202 C>T | T allele was associated with an increased response (PANSS score) compared to C allele. | 4.21 × 10−8 | |||
| RGS4 | rs951439 C>T | 678 Patients with schizophrenia. | CC diplotype was associated with an increased response (PANSS score) compared to CT and TT allele. | 0.010 | Campbell et al. (2008) [18] |
| Gene | Variant | Population | Association | p Value | References |
|---|---|---|---|---|---|
| FAAH | rs324420 C>A | 83 Patients with psychotic episodes. | A allele was associated with increased weight gain compared to C allele carriers. | 0.002 | Monteleone, P. et al. (2010) [19] |
| GRIN2B | rs1806201 G>A | 211 healthy volunteers treated with a single dose of risperidone olanzapine or quetiapine. | AA diplotype was associated with an increased likelihood of adverse neuronal reactions compared to AG and GG diplotypes. | 0.025 | López-Rodríguez, R et al. (2013) [20] |
| MC4R | rs17782313 T>C | 345 patients with psychiatric episodes. | CC diplotype was associated with an increased likelihood of weight gain compared to CT and TT diplotypes. | 0.005 | Czerwensky, F et al. (2013) [21] |
| rs489693 A>C | 139 patients with psychiatric episodes. | AA diplotype was associated with a decreased likelihood of ADRs compared to AC and CC diplotypes. | <0.01 | Malhotra, A. K (2012) [22] | |
| 345 patients with psychiatric episodes. | 0.017 | Czerwensky, F et al. (2013) [21] | |||
| SH2B1 | rs388819 A>C | 357 patients with psychiatric episodes. | CC diplotype was associated with increased LDL levels compared to AA and AC diplotypes. | 0.005 | Delacrétaz, A et al. (2017) [23] |
| RABEP1 | rs1000940 A>G | 357 patients with psychiatric episodes. | AG and GG diplotypes were associated with lower glucose concentrations compared to AA diplotype. | <0.001 | |
| CYP2C19 | *2, and *4 | 79 healthy volunteers receiving a single dose of each quetiapine formulation. | *1/*2, *2/*2 and *2/*4 diplotypes were associated with higher prolactin plasma concentrations compared to *1/*1 diplotype. | 0.012 | Cabaleiro et al. (2015) [24] |
| CYP2C9 | *2 and *3 | *1/*2, *1/*3 and *2/*3 diplotypes showed somnolence. | 0.015 | ||
| CYP1A1 | *2 | *1/*2 diplotype showed somnolence. | 0.020 | ||
| CYP1A1 | *2 | *1/*2 diplotype showed neurological events. | 0.024 |
| Gene | Variant | Population | Association | p Value | References |
|---|---|---|---|---|---|
| DRD3 | rs6280 C>T | 79 healthy volunteers receiving a single dose of each quetiapine formulation. | TT diplotype was associated with increased clearance compared to CC and CT diplotypes. | 0.030 | Cabaleiro et al. (2015) [24] |
| CYP1A2 | *1,*1C | *1C/*1C diplotype was associated with higher exposure to quetiapine compared to *1/*1 diplotype. | 0.067 | ||
| COMT | rs13306278 C>T | 49 healthy volunteers treated with two doses of quetiapine. | T allele was associated with higher exposure to quetiapine compared to C allele. | 0.008 | Zubiaur et al. (2021) [5] |
| CYP2B6 | PM | PM showed higher t1/2 compared to RM, NM or IM. | 0.005 | ||
| ABCG2 | rs2231142 G>T | T allele was associated with quetiapine accumulation compared to G allele. | 0.027 | ||
| CYP3A5 | *3 | *3/*3 showed higher t1/2 compared to *1/*1 or *1/*3. | 0.018 | ||
| CYP3A4 | *22 | 238 patients treated with quetiapine. | *22/*22 or *22/*1 diplotypes were associated with increased exposure to quetiapine compared to *1/*1 diplotype. | 0.007 | Weide Karen et al. (2014) [25] |
| CYP3A4 | *3,*20,*22 | 19 patients treated with a single dose of quetiapine. | *3,*20,*22 alleles were associated with increased exposure to quetiapine compared to *1 allele. | 0.099 | Saiz Rodriguez et al. (2020) [26] |
| CYP3A5 | *3 | 40 healthy volunteers treated with a single dose of quetiapine. | *3/*3 diplotype was associated with increased exposure to quetiapine compared to *1/*3 or *1/*1 diplotypes. | 0.0017 | Kim, K.-A. et al. (2014) [27] |
| Drug | Phenotype | Implications | Recommendations | Authority |
|---|---|---|---|---|
| Quetiapine (2022) [31] | CYP3A4 PM | Reduced CYP3A4 activity. | Use 30% of the standard dose or choose another alternative that is not metabolized by CYP3A4. | DPWG |
| Risperidone (2020) [32] | CYP2D6 UM | Higher ratio of the active metabolite. | Choose another antipsychotic or titrate the dose according to the maximum dose for the active metabolite. | DPWG |
| CYP2D6 PM | Increased risperidone plasma concentration. | Reduce to 67% of the standard dose. | DPWG | |
| Aripiprazol (2021) [33] | CYP2D6 PM | Increased risk of ADRs. | Administer no more than 10 mg/day or 300 mg/month. | DPWG |
| Haloperidol (2021) [34] | CYP2D6 UM | Increased conversion of haloperidol. | Administer 1.5 times the standard dose or choose an alternative drug. | DPWG |
| CYP2D6 PM | Decreased conversion of haloperidol. | Administer 60% of the standard dose. | DPWG | |
| Zuclopenthixol (2022) [35] | CYP2D6 IM | Decreased conversion of zuclopentixol. | Administer 75% of the standard dose. | DPWG |
| CYP2D6 PM | Decreased conversion of zuclopentixol. | Administer 50% of the standard dose. | DPWG | |
| Pimozide (2021) [36] | CYP2D6 IM | Increased plasma concentration of pimozide. | Administer no more than 80% of the standard maximum dose. | DPWG |
| CYP2D6 PM | Increased plasma concentration of pimozide. | Administer no more than 50% of the standard maximum dose. | DPWG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Ruiz, M.; Soria-Chacartegui, P.; Villapalos-García, G.; Abad-Santos, F.; Zubiaur, P. The Pharmacogenetics of Treatment with Quetiapine. Future Pharmacol. 2022, 2, 276-286. https://doi.org/10.3390/futurepharmacol2030018
Ortega-Ruiz M, Soria-Chacartegui P, Villapalos-García G, Abad-Santos F, Zubiaur P. The Pharmacogenetics of Treatment with Quetiapine. Future Pharmacology. 2022; 2(3):276-286. https://doi.org/10.3390/futurepharmacol2030018
Chicago/Turabian StyleOrtega-Ruiz, María, Paula Soria-Chacartegui, Gonzalo Villapalos-García, Francisco Abad-Santos, and Pablo Zubiaur. 2022. "The Pharmacogenetics of Treatment with Quetiapine" Future Pharmacology 2, no. 3: 276-286. https://doi.org/10.3390/futurepharmacol2030018
APA StyleOrtega-Ruiz, M., Soria-Chacartegui, P., Villapalos-García, G., Abad-Santos, F., & Zubiaur, P. (2022). The Pharmacogenetics of Treatment with Quetiapine. Future Pharmacology, 2(3), 276-286. https://doi.org/10.3390/futurepharmacol2030018








