Abstract
Kidney function highly depends on mitochondria, organelles that regulate different metabolic pathways. Mitochondria-altered function and structure are present during acute kidney injury (AKI) and chronic kidney disease (CKD). Targeting mitochondria using several strategies has been shown to improve kidney function. Here, we review some experimental mitochondria targeting strategies with clinical potential in kidney diseases encompassing cationic/lipophilic small molecules, peptides, nanocarriers, and even the entire organelle.
1. Introduction
Kidneys are among the most energy-demanding organs due to their filtration and reabsorption functions. In particular, the proximal tubular segment of the nephron consumes large amounts of energy in the form of adenosine triphosphate (ATP) provided by oxidative phosphorylation, a metabolic process performed in the mitochondria [1].
In addition to their energy-producing function, mitochondria also exert other metabolic processes such as glutaminolysis, the catabolism of branched-chain amino acids, fatty acid beta-oxidation, nucleotide biosynthesis, heme metabolism, redox balance, the management of metabolic by-products, cellular death regulation, calcium homeostasis, etc. [2,3].
Acute kidney injury (AKI) is characterized by an abrupt reduction in kidney function due to pre-renal, renal, and post-renal causes such as the reduction of blood supply, nephrotoxins, and obstruction, respectively [4]. On the other hand, chronic kidney disease (CKD) is characterized by the progressive and irreversible loss of kidney function and structure for more than three months and may be a consequence of other conditions such as diabetes, hypertension, or aging [5].
AKI and CKD are related to each other since the presence of one could predispose the development of the other [6,7,8]. In addition to their complicated pathophysiology, several mitochondrial alterations have been reported in both pathologies, contributing to their progression.
In different experimental AKI models, mitochondrial morphological alterations are prevalent in tubular segments, showing fragmentation, swelling, and the loss of cristae; moreover, functionality is also compromised, with reduced electron transport chain (ETC) activity, a loss of membrane potential, and increased reactive oxygen species (ROS) production as a consequence [9,10,11,12,13,14,15]. Interestingly, these alterations also persist during AKI to CKD progression [16,17,18]. Similarly, in established CKD, mitochondrial alterations are present, showing low membrane potential and consequently reduced ETC activity and overproduction of ROS [19,20,21]; on the other hand, morphological alterations such as mitochondrial fragmentation have been noticed, especially in podocytes [21,22,23,24].
ROS overproduction is present in AKI and CKD, representing a therapeutic target since their abrogation reduces tissue damage and improves kidney function [25,26,27,28,29,30,31,32,33,34]. ROS are well-known inducers of the inflammatory response through the activation of the transcription factor nuclear factor kappa B (NF-kB) [35]; moreover, mitochondria-derived ROS are activators of the NLR family pyrin domain containing 3 (NLRP3) inflammasome/interleukin (IL)-1β axis [36,37], which has been reported to promote kidney injury [38]. The specific blocking of mitochondria-derived ROS also reduces kidney damage and improves kidney function [15,23].
Hence, specific mitochondrial targeting in order to block excessive ROS production and restore some mitochondrial functions could be a suitable complementary therapeutic strategy for kidney diseases.
Here, we review some of the most promising strategies to improve mitochondrial function in kidney diseases. Many of these strategies have been proven in the treatment of other diseases; for this reason, drug repositioning may be advantageous in the context of regulatory procedures [39] for its implementation in kidney diseases.
Mitochondria targeting strategies include the use of small molecules, peptides, nanocarriers, and mitochondrial transplantation (Figure 1).
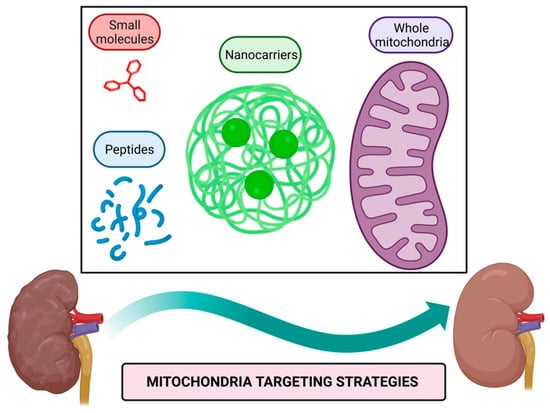
Figure 1.
Mitochondria targeting strategies. Small molecules, peptides, nanocarriers, and whole mitochondria transplantation represent therapeutic strategies targeted to mitochondria to alleviate their dysfunction in kidney diseases. Figure created with BioRender.com.
Mitochondria targeting compounds include lipophilic cationic small molecules and peptides that can be used alone or conjugated with other bioactive molecules [40,41]; additionally, nanocarriers of drugs harboring signals that direct them to mitochondria or even whole mitochondria transferred to target tissue could be used to alleviate mitochondrial dysfunction [42,43].
2. Lipophilic and Cationic Small Molecules
These molecules possess lipophilic characteristics which allow them to pass through membranes and positive charges that confer their affinity to mitochondrial membrane potential. They usually do not exert a biological activity by themselves; hence, they are used as carriers of other compounds.
2.1. Triphenylphosphonium (TPP) Conjugates
TPP is one of the small molecules that target mitochondria because it contains a central positively charged phosphorus atom linked to three phenyl rings [44]. This molecule has been extensively used in the famous molecular probe MitoSOXTM that detects mitochondrial-derived ROS since it contains a TPP moiety bound to hydroethidine [45]. TPP has also been extensively conjugated with other molecules to direct them to and act in mitochondria, such as antioxidants and chemotherapeutics that have been used in different disease models [46,47].
As mentioned above, TPP has been conjugated with a broad range of antioxidants such as ubiquinone, vitamin E, vitamin C, curcumin, and quercetin, among others [46]; here, we review some of the TPP–antioxidant conjugates with potential use in kidney diseases.
MitoQ is a TPP–ubiquinone conjugate that is already marketed as a nutritional supplement; in addition, clinical trials in healthy young and older adults using mitoQ are demonstrated to be safe and reduce oxidative stress markers in plasma and leukocytes [48,49,50]. Although this compound is not prescribed as a treatment for any diseases, it has been tested in clinical trials for chronic and degenerative diseases. In phase II clinical trials in patients with chronic liver damage due to hepatitis C viral (HCV) infection who cannot receive the standard treatment, the oral intake of mitoQ after 28 days reduces alanine aminotransferase (ALT) and aspartate aminotransferase (AST) serum levels, indicating a reduction in liver damage [51]. On the other hand, in patients with Parkinson’s disease, the oral intake of mitoQ for several months seems not to affect disease outcomes [52]. Moreover, antioxidant protective effects of mitoQ have been tested in a broad range of disease models, including AKI and CKD.
Regarding AKI, in a model of ischemia/reperfusion (I/R) in mice, a single intravenous (IV) administration of mitoQ preserves mitochondrial desoxyribonucleic acid (mtDNA) content and reduces functional kidney alterations [53]. More profound findings were reported in a cisplatin-AKI model in which mitoQ intraperitoneally (IP) administrated one hour before cisplatin administration results in kidney function improvement, less oxidative stress, less inflammation, and reduced mitochondrial structural alterations [54].
In terms of CKD, in a model of galactose-induced aging that leads to renal damage, the IP administration of mitoQ for two weeks IP reduces fibrotic markers in the kidney [55]; moreover, in a CKD model by angiotensin II infusion, the co-administration of mitoQ for four weeks ameliorates glomerular and podocyte injury by decreasing mitochondrial fission and ROS production [23].
Diabetic nephropathy are one of many complications of diabetes mellitus physiopathology and are one of the leading causes of CKD and end-stage renal disease (ESRD) [56]. In different diabetic nephropathy (DN) models, the use of mitoQ seems to ameliorate kidney damage and reduce fibrotic and inflammatory markers. In diabetic mice models, conversely to other CKD models, ATP and ADP levels are increased; interestingly, the oral administration of mitoQ for twelve weeks reduces their levels similar to control mice and mitigates structural and functional damage in kidneys without affecting glycemic levels [57,58]. Additionally, the IP administration of this compound for twelve weeks seems to have more in-depth effects, decreasing oxidative stress, diminishing NLRP3-derived IL-1β production and tubular damage, and slightly reducing glucose levels; at the mitochondrial level, mitoQ treatment preserves membrane potential and mtDNA content, reduces mitochondrial fragmentation and restores mitophagy [37,59].
Although mitoQ seems to have promising results in kidney diseases models, some in vitro findings are relevant to take into account, in which direct administration of mitoQ to proximal tubules causes mitochondrial swelling and depolarization due to TPP–ubiquinone linker, the alkyl chain [60]; hence, a modification of the linker could improve the effects of mitoQ.
SkQ1 is a TPP–plastoquinone conjugate that has been tested as an ophthalmic solution in clinical trials for dry eye syndrome reducing corneal damage and discomfort symptoms [61,62]; in fact, SkQ1 is already marketed in Russia as Visomitin drops (Mitotech LLC, Moscow, Russian Federation). Although it functions similarly to mitoQ and even has more potent antioxidant effects [63], this has not been tested in kidney diseases yet. However, its protective effects could be possible since, in an aging model in mice with mtDNA defects, SkQ1 treatment reduces oxidative damage in several organs, including kidneys, when administrated in drinking water for 150 days [64], suggesting that its use is suitable in AKI or CKD.
MitoTEMPO is a TPP-2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) conjugate that acts as a mimetic of the antioxidant enzyme superoxide dismutase (SOD) [65]. Although this compound has not been tested in clinical trials yet, this has been used in hepatic, cardiovascular, nervous system, infectious, and kidney disease models.
In different AKI models in rodents, the pre-treatment and treatment with mitoTEMPO reduce kidney and mitochondrial damage, as has been demonstrated in cisplatin-induced AKI in which seven days pre-treatment with mitoTEMPO administrated IP reduces oxidative and tubular damage in kidneys [66]; or in I/R-induced AKI, in which mitoTEMPO administration directly to the kidney during the ischemic induction and followed by four days of IP administration preserves mtDNA and ATP content, avoids mitochondrial swelling, and reduces oxidative damage [15]. Additionally, in septic shock-associated AKI, in which mitoTEMPO administration IP or IV after sepsis induction partially restores ETC function and ATP content in kidney mitochondria, whereas in whole tissue reduces oxidative damage and decreases IL-1β levels resulting in improved kidney function [67,68,69].
In CKD models, mitoTEMPO shows similar results improving mitochondrial function and reducing kidney damage. In 5/6 nephrectomy-induced CKD models in rodents, with or without aldosterone administration, the IP mitoTEMPO administration for four to twelve weeks maintains mitochondrial function and morphology; moreover, it reduces fibrotic, inflammatory, and oxidative markers in the kidney, preserving podocyte’s structure [70,71,72]. Interestingly, mitoTEMPO also impacts the skeletal muscle, promoting its regeneration and recovering ATP production [73]. In a model of kidney fibrosis by unilateral ureteral obstruction (UUO) in mice, the IP administration of mitoTEMPO for seven days reduced ROS levels and the fibrotic area in kidneys, as well as decreased gene expression of alpha-smooth muscle actin (α-SMA), collagen, transforming growth factor-beta (TGF-β), and fibronectin [74].
On the other hand, during DN using the db/db mice model, the oxidative stress induces an increase in apoptotic cell death and impaired mitophagy in the kidney, which could be partially reversed by antioxidant treatment, including IP administration of mitoTEMPO for four weeks, thus resulting in improved renal function [75]. Similar results in other models of diabetes using streptozotocin and Ins2+/−AkitaJ mice have been reported, in which subcutaneous administration of mitoTEMPO for three weeks improves kidney function, reduces glomerular injury, and partially avoids the loss of endothelial cells and podocytes [76].
Many antioxidants have been demonstrated to have beneficial effects on mitochondrial function during AKI and CKD models [25,26,27,28,29,30,31,32,33,34]; among these, curcumin and lipoic acid represent two molecules to potentially be conjugated with TPP to reach mitochondria and to explore in kidney diseases. A TPP–curcumin conjugate has been developed and tested in rotenone-induced liver damage in mice, reducing the lipid peroxidation and partially preserving the activity of the antioxidant enzymes superoxide dismutase and catalase [77]. On the other hand, a TPP–lipoic acid conjugate has also been developed; however, the conjugation with TPP seems to compromise the antioxidant effect [78].
Despite the great potential of TTP conjugates, there are some shortcomings, such as transportability, as TPP can only transport electroneutral and small molecules. Furthermore, some adverse effects on mitochondrial function of TTP conjugates have been reported, such as decreased ETC activity and reduced membrane potential [79]. For this reason, more research is needed to improve transportability and reduce the toxicity of TPP. For example, it has been proposed that phenyl rings of TPP could be modified by trifluoromethyl groups that function as electron withdrawers to abrogate adverse effects [44].
2.2. Rhodamine Conjugates
Rhodamines, such as fluorescein, are xanthene derivatives that have been extensively used as fluorescent probes. Depending on the chemical modifications, there are several rhodamine types, such as rhodamine B, 6G, 19, 101, 110, 116, 123, and tetramethyl rhodamine [80]. Among these, rhodamine 123, tetramethylrhodamine methyl ester (TMRM), and tetramethylrhodamine ethyl ester (TMRE) possess mitochondrial affinity and has been used as mitochondrial membrane potential probes [81,82].
Rhodamine 19 has also been demonstrated to target mitochondria, acting as a mild uncoupler, and has been used as a carrier of the antioxidant molecule plastoquinone, a compound named SkQR1 [83,84].
SkQR1 Has Been Proven in Neurological Disease, Kidney Disease, and Aging Models. In AKI models, the renoprotective effects of SkQR1 have been demonstrated in rhabdomyolysis and I/R-induced AKI in rats, in which its IP administration previous and after damage induction decreases oxidative stress markers, lowers tubular epithelial necrotic areas, and reduces tubular dilatation, thus resulting in improved renal function and increased animal survival rate [85,86]. Similarly, in a model of gentamicin nephrotoxicity-induced AKI in rats, SkQR1 administrated IP improves kidney function and increases animal survival rate; moreover, the hearing loss associated in this model is also abrogated by SkQR1 treatment [87]. In addition, in the sepsis-associated AKI model in rats, the pre-treatment with SkQR1 IP reduces the damage markers kidney injury molecule-1 (Kim-1) and neutrophil gelatinase-associated lipocalin 2 (NGAL) levels, improves kidney function, and reduces mortality [88].
Although pyelonephritis is not considered a cause of AKI, its presence is a risk factor for AKI development [89]. In an acute pyelonephritis model in rats, the IP administration of SkQR1 after bacterial inoculation and followed by four IP administrations every 12 h reduces neutrophil infiltration and oxidative damage in the kidney; moreover, it impacts systemic inflammatory status by reducing tumor necrosis factor-alpha (TNF-α) in serum, reducing neutrophil numbers in blood and increasing animal survival rate [90].
SkQR1 has not been tested in CKD and DN models, opening a new field to explore.
Rhodamine B also targets mitochondria and has been conjugated with the antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) [91] which have been proved in vitro, suggesting the potential use in kidney disease models.
A summary of proven and not proven mitochondria targeting small molecules in kidney disease models is shown in Figure 2.
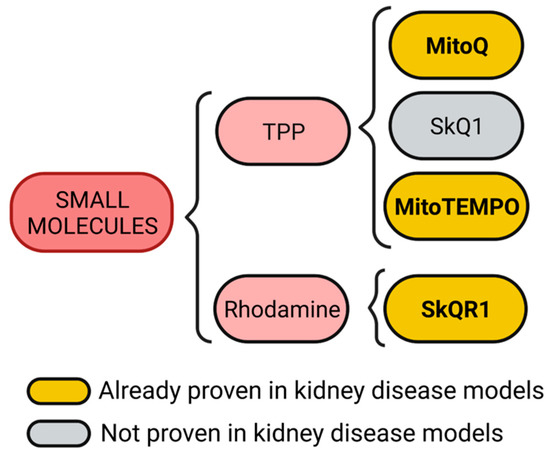
Figure 2.
Mitochondria targeting small molecules. Triphenylphosphonium (TPP) and rhodamine are small molecules with cationic and lipophilic characteristics. TPP can be conjugated with quinone, plastoquinone, or 2,2,6,6-tetramethylpiperidine-N-oxyl (TEMPO) to generate mitoQ, SkQ1, and mitoTEMPO molecules, respectively. On the other hand, rhodamine conjugated with plastoquinone generates the SkQR1 molecule. Figure created with BioRender.com.
3. Mitochondria Targeting Peptides
Peptides as therapeutics have emerged recently and show several advantages over other molecules, such as their chemical synthesis, selectivity, and minimal side effects. These could be used alone or conjugated with another bioactive compound [92,93].
Nowadays, the peptide peginesatide, an antagonist of the erythropoietin receptor, is used to treat CKD-associated anemia in humans [94]. Hence, other experimental approaches focused on mitochondria have been explored; for example, using a peptide to block the interaction of nucleophosmin with Bcl-2-associated X protein (Bax) inhibits apoptotic cell death; thus, resulting in decreased renal damage caused by ischemia [95] and suggesting that mitochondria targeting peptides could also be a potential therapy for kidney diseases.
Therapeutic peptides are classified as cell-targeting peptides (CTP) if they are directed specifically to a receptor or as cell-penetrating peptides (CPP) if they pass the plasma membrane to reach the cytoplasm [92,93]. Mitochondria targeting peptides require CPP characteristics to enter cells, and to reach mitochondria requires CTP characteristics harboring a mitochondria targeting sequence (MTS) or possessing cationic charges.
Although only one kind of cationic mitochondrial penetrating peptides named Szeto-Schiller (SS) peptides has been proven in kidney diseases, here we review some MTS-containing peptides and other cationic mitochondrial penetrating peptides with potential use.
3.1. MTS-Containing Peptides
Mitochondrial proteome mainly is constituted by nuclear-encoded proteins that once synthesized possess an MTS to reach mitochondria through the recognition by the mitochondrial TOM complex eliciting the integration to mitochondrial membranes. The conserved pattern residues on MTS are φχχφφ, where φ represents an aromatic or hydrophobic residue, whereas χ represents any kind of residue, for example, the pattern LSRLL; additionally, MTS acquires an alpha-helix conformation that facilitates the insertion to the mitochondrial outer membrane. Once inside, MTS is degraded by mitochondrial processing proteinases (MPP) [3,96,97]. Considering those mentioned above, synthetic MTS-containing peptides have been developed and used as carriers of other compounds to facilitate their delivery into mitochondria to exert biological functions. The construct of a CPP with an MTS improves cellular and mitochondrial uptake [98], as has been demonstrated in vitro with peptides conjugated with DNase, human metallothionein 1A (hMT1A), and manganese-porphyrin [99,100,101]. Moreover, the cell-penetrating artificial mitochondria targeting peptide (CAMP)-hMT1A conjugate has been tested in a Parkinson’s disease model in rats and demonstrated to restore tyrosine hydroxylase levels in striatum and substantia nigra resulting in improved motor coordination when it is administrated intracerebrally [100]; similarly, using a recombinant MTS-containing mitochondrial transcription factor A (TFAM) IV injected in mice also improves motor coordination, although it could be by the increase in complex I of the ETC [102]. MTS-containing TFAM has also been proven in a septic shock model, increasing animal survival and, in healthy mice, increasing the brain and muscle complex I level of the ETC [102,103].
Although for kidney diseases, there are no reports of the use of MTS-containing peptides, the conjugation of these with antioxidant molecules such as the mentioned metallothionein and manganese–porphyrin could have promising results, since both molecules have been reported to reduce renal damage in aristocholic acid-induced CKD and I/R-induced AKI, respectively [104,105]. Moreover, recombinant MTS-containing TFAM could also help maintain mitochondrial DNA and increase complex I levels in kidney tubular epithelial cells.
One advantage of MTS-containing peptides over other molecules that target mitochondria is that cationic charges are expendable to enter mitochondria; hence, their insertion mechanism is independent of mitochondrial membrane potential.
3.2. Cationic Mitochondrial Penetrating Peptides
Positive charges and alpha-helix structures are basal characteristics of these peptides, and they differ from each other due to other structural features. Among this category, we found the cationic amphiphilic polyproline helix (CAPH) peptides, the cationic cysteine-rich peptides, the hexapeptides, the structurally modified peptoids, and the SS peptides.
CAPH peptides possess the ability to enter the cell through endocytosis and reach mitochondria due to enriched proline residues in their structures, such as P11LRR and P14LRR peptides [106,107]; moreover, the addition of a dimethyl tyrosine (Dmt) residue to P11LRR structure exert antioxidant functions demonstrated in vitro [106,107].
As mentioned above, oxidative stress is a hallmark of kidney diseases, in which mitochondria are the primary sources of ROS [108]. Ergo, the use CAPH-Dmt has excellent potential to explore in AKI and CKD models.
The plant derivate roseltide rT1 is a cationic cysteine-rich peptide recognized by the TOM complex in an MTS-independent way; interestingly, roseltide rT1 by itself can bind ATP synthase and enhance ATP production in different cell lines [109]. During AKI and CKD, ATP production is compromised, as demonstrated in experimental models [11,16,17,18,26,31,110,111], and for this reason, roseltide rT1 by itself without conjugation with another bioactive compound could be helpful in the treatment of kidney disease.
Hexapeptides with delocalized lipophilic cations contain the modified residue cyclohexyl alanine in their structure to bring hydrophobicity and facilitate cellular uptake; positive charge residues such as lysine and arginine also are incorporated. In addition to these characteristics, cationic moieties of pyridyl salts in alanine residues bring mitochondrial selectivity [112]. Although these peptides are not proven in any disease model, it seems to have great potential as a drug delivery system to mitochondria. As described above, non-peptidic cationic molecules are a comprehensive system to target mitochondria due to the charge affinity.
Peptoids resemble backbone peptide structures but are resistant to proteolysis due to structural modifications, in which side chains are attached to the nitrogen atom instead of the alpha carbon [113]. These peptoids also require the lipophilic, cationic, and alpha-helix structure characteristics to enter the cell and mitochondria [114]. Although peptoids conjugated with any kind of drugs are not assessed in any disease models in animals, they represent a vast field to explore in kidney diseases, in which the conjugation with molecules that require more stability, such as transcription factors, bioactive lipids, or proteins involved in mitochondrial dynamics.
SS peptides are aromatic and cationic tetrapeptides able to enter mitochondria and, if they possess a tyrosine or Dmt residue in their structure, also function as antioxidants themselves. SS-01 and SS-20 that lack tyrosine or Dmt residues can enter mitochondria but lack antioxidant activity, whereas SS-02 and SS-31, which possess any of those two residues, enter mitochondria and are potent antioxidants.
Among SS peptides, SS-31 (also known as MTP-131, Bendavia, and elamipretide) has gained great attention for the potent antioxidant activity and safety demonstrated in experimental models; in fact, SS-31 has been proved in clinical trials for human mitochondrial myopathies, Barth syndrome, cardiovascular diseases, and renal arterial stenosis [115,116,117,118,119].
In AKI, SS-31 IP administration reduces structural and functional damage induced by cisplatin in mice; moreover, it decreases oxidative damage and NLPR3-derived IL-1β synthesis [120]. Similarly, in I/R-induced AKI in rats, SS-31 subcutaneous administration reaches a high concentration in kidneys and reduces epithelial and endothelial damage; at the subcellular level, it avoids mitochondrial swelling, maintains cristae structure by its binding with cardiolipin, and recover ATP levels [121,122,123]. SS-31 peptide has been modified with CPP characteristics or encapsulated in nanopolyplexes to increase its cellular uptake and mitochondrial accumulation, thus resulting in enhanced antioxidant capacity demonstrated in vitro [124,125]. Moreover, the efficiency of SS-31 encapsulated in nanopolyplexes has been demonstrated in lipopolysaccharide (LPS)-induced AKI model in mice, showing better results than SS-31 alone [125]. Although cisplatin, I/R, and LPS-induced AKI SS-31 have demonstrated promising results, for other models such as aristocholic acid (AA) and adriamycin-induced AKI, there are controversial results [126] that could be explained by the specific physiopathology induced by these compounds or even by their chemical interaction with SS-31. It is known that AKI predisposes to CKD development; as has been reported in CKD development induced I/R, in which the treatment with SS-31 for six weeks and started four weeks after ischemic injury reduces the structural damage of kidneys, fibrotic damage, and mitochondrial swelling; surprisingly, this protective effect persists even nine months after I/R induction [127].
On the other hand, during diabetic nephropathy, the IP or subcutaneous administration of SS-31for at least four weeks of SS-31 does not affect glycemic levels; however, it improves kidney function, preserves podocyte structure, diminishes inflammatory and fibrotic markers, and reduces oxidative stress [128,129,130,131,132,133].
SS-02 and SS-20 peptides in conjugation with deferoxamine have also been demonstrated to possess mitochondrial antioxidant properties in vitro [134], suggesting their potential use.
A summary of proven and not proven mitochondria targeting peptides in kidney disease models is shown in Figure 3.
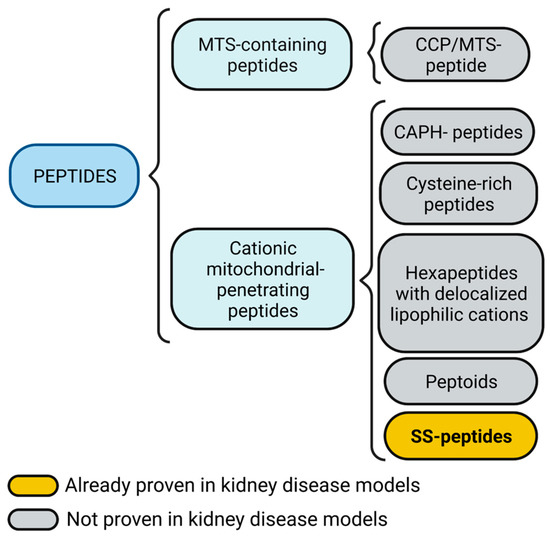
Figure 3.
Mitochondria targeting peptides. Mitochondria targeting sequence (MTS)-containing peptides also could include a cell-penetrating peptide (CPP) feature. Cationic mitochondrial penetrating peptides could be subdivided into cationic amphiphilic polyproline helix (CAPH) peptides, cysteine-rich peptides, and hexapeptides with delocalized lipophilic cations, peptoids, and Szeto-Schiller (SS) peptides. Figure created with BioRender.com.
4. Nanocarriers
Nanocarriers serve as a platform to deliver different compounds to the target tissue. Several nanocarrier systems targeting mitochondria have been developed and proven in disease models other than kidney diseases; among the most known are mitoPorter, DQAsomes, and PEG-based nanoparticles.
MitoPorter is a liposome-based system composed of 1,2-dioleoyl-sn-glycerol-3-phosphatidyl ethanolamine, phosphatidic acid, and sphingomyelin; its surface also contains octarginine moieties to facilitate its cellular uptake [135]. MitoPorter has been used for nucleic acids delivery [136,137,138,139] with potential use in mitochondrial diseases; however, has recently been used to deliver ubiquinone in an I/R model in the liver, showing protective effects even compared to ubiquinone treatment alone [140], suggesting that this delivery system of antioxidants also could be helpful in kidney diseases.
DQAsomes are made from dequalinium chloride molecules, which form a liposome-like structure in an aqueous solution [141], serving as a delivery system for anti-cancerogenic compounds [142,143,144]; DQAsomes have also been proven to deliver curcumin without toxic effects [145], a natural antioxidant that ameliorates damage in kidney diseases.
Polyethylene glycol (PEG)-based nanoparticles are molecules coated with PEG to improve their biodisponibility, a process called PEGylation [146]. Some strategies to use PEGylation and target mitochondria include the combination of PEG by itself with TPP moieties to improve doxorubicin delivery [147]; in addition; other strategies such as the using PEGylated nanoparticles of poly (lactic-co-glycolide acid) (PLGA) with TPP moieties has been proven as nanocarrier systems for curcumin, lodinamine, α-tocopheryl succinate, and dinitrophenol [148].
Some mitochondria targeting nanocarriers based on PEGylation incluiding nanoceria, PEG-polycaprolactone (PCL) nanoparticles; and the encapsulation of SS peptides in hyaluronic Acid (HA)-Chitosan nanoparticles have been proven in kidney disease models.
4.1. Nanoceria
Cerium oxide nanoparticles, also known as nanoceria, are metal-based nanoparticles sized from 5 to 36 nm with antioxidant capacity by themself [149]; in addition, the formulation of nanoceria with vitamin C has been demonstrating renoprotective effects in rhabdomyolysis-induced AKI model in mice [150]. In LPS-induced AKI in mice, nanoceria has been modified by adding a TPP moiety to target mitochondria, loaded with atorvastatin, a drug that improves kidney function, and covered with methoxy PEG-thioketal-PLGA as stabilizers, showing that its IV administration reduces oxidative damage and inflammation; moreover, preserves mitochondrial structure [151].
4.2. PEG-PCL Nanoparticles
PEG-PCL nanoparticles with TPP moieties and carrying ubiquinone molecules have been proven in I/R-induced AKI, demonstrating a marked reduction of tubular damage and inflammation compared to ubiquinone alone [152].
4.3. Hyaluronic Acid (HA)-Chitosan Nanoparticles
As mentioned above, SS-31 is a mitochondria targeting peptide with renoprotective functions; to increase its biodisponibility, this was encapsulated in nanoparticles made of HA and chitosan, demonstrating better results than SS-31 alone in an LPS-induced AKI model in mice [125].
A summary of proven and not proven mitochondria targeting nanocarriers in kidney disease models is shown in Figure 4.
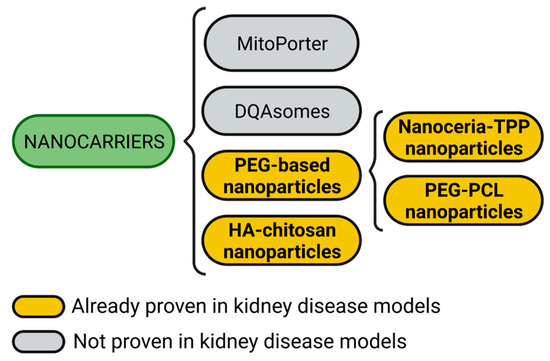
Figure 4.
Mitochondria targeting nanocarriers. Nanocarrier systems targeting mitochondria include mitoPorter, DQAsomes, hyaluronic acid (HA)-chitosan nanoparticles, and polyethylene glycol (PEG)-based nanoparticles. PEG-based nanoparticles could also be subdivided into cerium oxide nanoparticles (nanoceria) harboring triphenylphosphonium (TPP) moieties and PEG-polycaprolactone (PCL) nanoparticles. Figure created with BioRender.com.
5. Mitochondrial Replacement
Mitochondrial replacement, also known as mitochondrial transplantation, is a novel experimental therapeutic strategy to transfer healthy mitochondria to the target tissue to recover mitochondrial function (Figure 5). This strategy has already been used in pediatric patients after cardiogenic shock, in which mitochondria isolated from their muscles are directly injected into the myocardium, demonstrating that patients with mitochondrial transplantation do not suffer short adverse effects and show fewer cardiovascular events several months after the intervention [153,154].
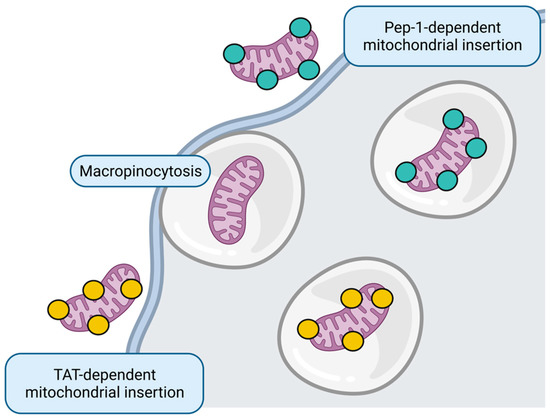
Figure 5.
Mitochondrial replacement. Whole healthy mitochondria insertion to target cell could occur through micropinocytosis or directed through Pep-1 and transactivator of transcription (TAT) peptides. Figure created with BioRender.com.
Only AKI models have explored the effect of mitochondrial replacement. In the doxorubicin-induced AKI model, the transplantation of mesenchymal stem cell (MSC)-derived mitochondria to the renal subcapsular region results in improved kidney function and increased antioxidant enzyme levels; however, although tubular regeneration was increased, tubular dilation persists [155]. Similarly, in I/R-induced AKI in rats and pigs, the intra-arterial administration of muscle-derived mitochondria improves renal function in the first 24 to 48 h [156,157] and even promotes proliferation of renal cells [156] and reduces inflammation [157]. However, for CKD, mitochondrial replacement remains unexplored.
Although the primary mechanism described for internalization of transferred mitochondria to the tissue is micropinocytosis [158], some strategies that improve the uptake in vitro include mitochondria harboring CPP, such as Pep-1 [159,160,161] and transactivators of transcription (TAT) peptides [162].
6. Concluding Remarks
Mitochondria targeting strategies have been explored in different diseases and represent a suitable additional therapeutic option for AKI and CKD. Taking advantage of some clinical trials that have tested some of these strategies, drug repositioning facilitates their scaling to be used in other diseases [39], including kidney diseases, based on the experimental result with the same molecules. In this context, mitoQ, SkQ1, SS-31, and mitochondrial replacement are the most suitable therapeutic strategies already proven in clinical trials (Table 1) with potential use in kidney diseases.

Table 1.
Mitochondrial targeting strategies have already been tested in clinical trials.
On the other hand, here, we present some other mitochondria targeting strategies already proven in kidney disease models with great potential to be used in clinics (Table 2), such as the case of mitoTEMPO and SkQR1. Additionally, some other mitochondrial targeting strategies not explored already in kidney disease models, such as peptides (other than SS peptides) and the nanocarriers mitoPorter and DQAsomes, have been tested in vitro or other disease models, opening a field to explore them in kidney diseases models.

Table 2.
Mitochondria targeting strategies tested in kidney disease models.
Most of the mitochondria targeting strategies used in AKI and CKD models rely on antioxidant function by the conjugation with scavengers; however, most of the described mitochondria targeting molecules possess the ability to carry more complex compounds such as enzymes or transcription factors, as the mentioned metallothionein and TFAM [100,102]; hence, we open the possibility that mitochondria targeting molecules could exert other functions, such as promoting the mitochondrial biogenesis, stimulating ETC components transcription, and enhancing enzymatic reactions, among others.
Despite all the beneficial effects described above, some points must be considered. For example, the efficiency of many of these molecules depends on mitochondrial membrane potentials, such as small molecules, cationic peptides, and some nanocarriers; in this context, only MTS-containing peptides, some nanocarriers, and whole mitochondria could be helpful in the loss of membrane potential during kidney diseases.
For the case of safety in terms of immunogenicity, small molecules and peptides represent the strategies with lower risk; conversely, nanocarriers have immunogenicity potential [163] to take into consideration; similarly, if the whole mitochondrial for transplantation is damaged this could induce a proinflammatory response due to the exposure of danger-associated molecular patterns (DAMPs) [164].
In terms of obtention, small molecules, peptides, and whole mitochondria isolation seem to be the more suitable options, followed by nanocarrier systems.
Additionally, for each one, pharmacokinetic and pharmacodynamic studies are necessary; in this context, SS-31 is the most advanced option for AKI and CKD treatment.
Author Contributions
Conceptualization, A.P.J.-U. and J.P.-C.; investigation, A.P.J.-U.; resources, J.P.-C.; writing—original draft preparation, A.P.J.-U.; writing—review and editing, J.P.-C.; funding acquisition, J.P.-C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Consejo Nacional de Ciencia y Tecnología (CONACYT) México, Grants Numbers A1-S-7495; by Programa de Apoyo a Proyectos de Investigación e Innovación Tecnológica (PAPIIT), Grant Number IN200922 of the Universidad Nacional Autónoma de México (UNAM); by Programa de Apoyo a la Investigación y el Posgrado (PAIP), Grant Number 5000-9105.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
To Ariadna Ortega-Lozano and Estefani Yaquelin Hernández-Cruz for their valuable comments and suggestions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.B.; Haigis, M.C. The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 2018, 20, 745–754. [Google Scholar] [CrossRef] [PubMed]
- Orrenius, S.; Gogvadze, V.; Zhivotovsky, B. Calcium and mitochondria in the regulation of cell death. Biochem. Biophys. Res. Commun. 2015, 460, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Shad, F.; Smith, M.C. Acute kidney injury: A guide to diagnosis and management. Am. Fam. Physician 2012, 86, 631–639. [Google Scholar]
- Charles, C.; Ferris, A.H. Chronic Kidney Disease. Prim. Care Clin. Off. Pract. 2020, 47, 585–595. [Google Scholar] [CrossRef]
- Sato, Y.; Takahashi, M.; Yanagita, M. Pathophysiology of AKI to CKD progression. Semin. Nephrol. 2020, 40, 206–215. [Google Scholar] [CrossRef]
- Singh, P.; Rifkin, D.E.; Blantz, R.C. Chronic kidney disease: An inherent risk factor for acute kidney injury? Clin. J. Am. Soc. Nephrol. 2010, 5, 1690–1695. [Google Scholar] [CrossRef]
- Fiorentino, M.; Grandaliano, G.; Gesualdo, L.; Castellano, G. Acute Kidney Injury to Chronic Kidney Disease Transition. Contrib. Nephrol. 2018, 193, 45–54. [Google Scholar] [CrossRef]
- Li, M.; Li, C.M.; Ye, Z.C.; Huang, J.; Li, Y.; Lai, W.; Peng, H.; Lou, T.Q. Sirt3 modulates fatty acid oxidation and attenuates cisplatin-induced AKI in mice. J. Cell. Mol. Med. 2020, 24, 5109–5121. [Google Scholar] [CrossRef]
- Liang, N.N.; Zhao, Y.; Guo, Y.Y.; Zhang, Z.H.; Gao, L.; Yu, D.X.; Xu, D.X.; Xu, S. Mitochondria-derived reactive oxygen species are involved in renal cell ferroptosis during lipopolysaccharide-induced acute kidney injury. Int. Immunopharmacol. 2022, 107, 108687. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Reyes-Fermin, L.M.; Briones-Herrera, A.; Tapia, E.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Pedraza-Chaverri, J. Protective effects of N-acetyl-cysteine in mitochondria bioenergetics, oxidative stress, dynamics and S-glutathionylation alterations in acute kidney damage induced by folic acid. Free Radic. Biol. Med. 2019, 130, 379–396. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.A.; Schnellmann, R.G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am J Physiol.-Ren. Physiol. 2012, 302, F853–F864. [Google Scholar] [CrossRef] [PubMed]
- Adil, M.; Kandhare, A.D.; Dalvi, G.; Ghosh, P.; Venkata, S.; Raygude, K.S.; Bodhankar, S.L. Ameliorative effect of berberine against gentamicin-induced nephrotoxicity in rats via attenuation of oxidative stress, inflammation, apoptosis and mitochondrial dysfunction. Ren. Fail. 2016, 38, 996–1006. [Google Scholar] [CrossRef]
- Hall, A.M.; Rhodes, G.J.; Sandoval, R.M.; Corridon, P.R.; Molitoris, B.A. In vivo multiphoton imaging of mitochondrial structure and function during acute kidney injury. Kidney Int. 2013, 83, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Wang, Y.; Li, L.; Liu, S.; Wang, C.; Yuan, Y.; Yang, G.; Chen, Y.; Cheng, J.; Lu, Y.; et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics 2021, 11, 1845–1863. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Uribe, A.P.; Bellido, B.; Aparicio-Trejo, O.E.; Tapia, E.; Sanchez-Lozada, L.G.; Hernandez-Santos, J.A.; Fernandez-Valverde, F.; Hernandez-Cruz, E.Y.; Orozco-Ibarra, M.; Pedraza-Chaverri, J. Temporal characterization of mitochondrial impairment in the unilateral ureteral obstruction model in rats. Free Radic. Biol. Med. 2021, 172, 358–371. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Avila-Rojas, S.H.; Tapia, E.; Rojas-Morales, P.; Leon-Contreras, J.C.; Martinez-Klimova, E.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Pedraza-Chaverri, J. Chronic impairment of mitochondrial bioenergetics and beta-oxidation promotes experimental AKI-to-CKD transition induced by folic acid. Free Radic. Biol. Med. 2020, 154, 18–32. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Rojas-Morales, P.; Avila-Rojas, S.H.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Jimenez-Uribe, A.P.; Prieto-Carrasco, R.; Sanchez-Lozada, L.G.; Pedraza-Chaverri, J.; Tapia, E. Temporal Alterations in Mitochondrial beta-Oxidation and Oxidative Stress Aggravate Chronic Kidney Disease Development in 5/6 Nephrectomy Induced Renal Damage. Int. J. Mol. Sci. 2020, 21, 6512. [Google Scholar] [CrossRef]
- Thome, T.; Coleman, M.D.; Ryan, T.E. Mitochondrial Bioenergetic and Proteomic Phenotyping Reveals Organ-Specific Consequences of Chronic Kidney Disease in Mice. Cells 2021, 10, 3282. [Google Scholar] [CrossRef]
- Liu, X.; Huang, S.; Wang, F.; Zheng, L.; Lu, J.; Chen, J.; Li, S. Huangqi-Danshen Decoction Ameliorates Adenine-Induced Chronic Kidney Disease by Modulating Mitochondrial Dynamics. Evid.-Based Complement. Altern. Med. 2019, 2019, 9574045. [Google Scholar] [CrossRef]
- Qin, X.; Jiang, M.; Zhao, Y.; Gong, J.; Su, H.; Yuan, F.; Fang, K.; Yuan, X.; Yu, X.; Dong, H.; et al. Berberine protects against diabetic kidney disease via promoting PGC-1alpha-regulated mitochondrial energy homeostasis. Br. J. Pharmacol. 2020, 177, 3646–3661. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Yang, Q.; Yang, Y.; Gao, Z.; Ma, Y.; Zhang, L.; Liang, W.; Ding, G. Sirt6 Suppresses High Glucose-Induced Mitochondrial Dysfunction and Apoptosis in Podocytes through AMPK Activation. Int. J. Biol. Sci. 2019, 15, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Liang, W.; Chen, Z.; Hu, J.; Feng, J.; Cao, Y.; Ma, Y.; Ding, G. Mitoquinone Protects Podocytes from Angiotensin II-Induced Mitochondrial Dysfunction and Injury via the Keap1-Nrf2 Signaling Pathway. Oxidative Med. Cell. Longev. 2021, 2021, 1394486. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Chen, Z.; Tao, Y.; Zhu, J.; Yang, H.; Liang, W.; Ding, G. Increased mitochondrial fission of glomerular podocytes in diabetic nephropathy. Endocr. Connect. 2019, 8, 1206–1212. [Google Scholar] [CrossRef]
- Ortega-Dominguez, B.; Aparicio-Trejo, O.E.; Garcia-Arroyo, F.E.; Leon-Contreras, J.C.; Tapia, E.; Molina-Jijon, E.; Hernandez-Pando, R.; Sanchez-Lozada, L.G.; Barrera-Oviedo, D.; Pedraza-Chaverri, J. Curcumin prevents cisplatin-induced renal alterations in mitochondrial bioenergetics and dynamic. Food Chem. Toxicol. 2017, 107, 373–385. [Google Scholar] [CrossRef]
- Avila-Rojas, S.H.; Aparicio-Trejo, O.E.; Briones-Herrera, A.; Medina-Campos, O.N.; Reyes-Fermin, L.M.; Martinez-Klimova, E.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Tapia, E.; Pedraza-Chaverri, J. Alterations in mitochondrial homeostasis in a potassium dichromate model of acute kidney injury and their mitigation by curcumin. Food Chem. Toxicol. 2020, 145, 111774. [Google Scholar] [CrossRef]
- Molina-Jijon, E.; Tapia, E.; Zazueta, C.; El Hafidi, M.; Zatarain-Barron, Z.L.; Hernandez-Pando, R.; Medina-Campos, O.N.; Zarco-Marquez, G.; Torres, I.; Pedraza-Chaverri, J. Curcumin prevents Cr(VI)-induced renal oxidant damage by a mitochondrial pathway. Free Radic. Biol. Med. 2011, 51, 1543–1557. [Google Scholar] [CrossRef]
- Molina-Jijon, E.; Aparicio-Trejo, O.E.; Rodriguez-Munoz, R.; Leon-Contreras, J.C.; Del Carmen Cardenas-Aguayo, M.; Medina-Campos, O.N.; Tapia, E.; Sanchez-Lozada, L.G.; Hernandez-Pando, R.; Reyes, J.L.; et al. The nephroprotection exerted by curcumin in maleate-induced renal damage is associated with decreased mitochondrial fission and autophagy. Biofactors 2016, 42, 686–702. [Google Scholar] [CrossRef]
- Negrette-Guzman, M.; Garcia-Nino, W.R.; Tapia, E.; Zazueta, C.; Huerta-Yepez, S.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Aparicio-Trejo, O.E.; Madero, M.; Pedraza-Chaverri, J. Curcumin Attenuates Gentamicin-Induced Kidney Mitochondrial Alterations: Possible Role of a Mitochondrial Biogenesis Mechanism. Evid.-Based Complement. Altern. Med. 2015, 2015, 917435. [Google Scholar] [CrossRef]
- Tapia, E.; Sanchez-Lozada, L.G.; Garcia-Nino, W.R.; Garcia, E.; Cerecedo, A.; Garcia-Arroyo, F.E.; Osorio, H.; Arellano, A.; Cristobal-Garcia, M.; Loredo, M.L.; et al. Curcumin prevents maleate-induced nephrotoxicity: Relation to hemodynamic alterations, oxidative stress, mitochondrial oxygen consumption and activity of respiratory complex I. Free Radic. Res. 2014, 48, 1342–1354. [Google Scholar] [CrossRef]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijon, E.; Medina-Campos, O.N.; Macias-Ruvalcaba, N.A.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Garcia-Arroyo, F.E.; Cristobal, M.; Sanchez-Lozada, L.G.; et al. Curcumin prevents mitochondrial dynamics disturbances in early 5/6 nephrectomy: Relation to oxidative stress and mitochondrial bioenergetics. Biofactors 2017, 43, 293–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yang, Y.; Zou, X.; Zheng, Z.; Zhang, J. Curcumin ameliorates CKD-induced mitochondrial dysfunction and oxidative stress through inhibiting GSK-3beta activity. J. Nutr. Biochem. 2020, 83, 108404. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Liu, G.; Long, M.; Zou, H.; Cui, H. Alpha lipoic acid attenuates cadmium-induced nephrotoxicity via the mitochondrial apoptotic pathways in rat. J. Inorg. Biochem. 2018, 184, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, C.G.; Fang, C.Q.; Gao, J.; Liu, Y.Z.; Chen, Y.; Chen, Y.N.; Xu, Z.G. The protective effect of alpha-Lipoic acid on mitochondria in the kidney of diabetic rats. Int. J. Clin. Exp. Med. 2013, 6, 90–97. [Google Scholar] [PubMed]
- Lingappan, K. NF-kappaB in Oxidative Stress. Curr. Opin. Toxicol. 2018, 7, 81–86. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, Q.; Han, B.; Chen, Y.; Qiao, X.; Wang, L. CD36 promotes NLRP3 inflammasome activation via the mtROS pathway in renal tubular epithelial cells of diabetic kidneys. Cell Death Dis. 2021, 12, 523. [Google Scholar] [CrossRef]
- Han, Y.; Xu, X.; Tang, C.; Gao, P.; Chen, X.; Xiong, X.; Yang, M.; Yang, S.; Zhu, X.; Yuan, S.; et al. Reactive oxygen species promote tubular injury in diabetic nephropathy: The role of the mitochondrial ros-txnip-nlrp3 biological axis. Redox Biol. 2018, 16, 32–46. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Bi, X.; Hu, C.; Ding, W. NLRP3 Deletion Attenuated Angiotensin II-Induced Renal Fibrosis by Improving Mitochondrial Dysfunction and Endoplasmic Reticulum Stress. Nephron 2021, 145, 518–527. [Google Scholar] [CrossRef]
- Jourdan, J.P.; Bureau, R.; Rochais, C.; Dallemagne, P. Drug repositioning: A brief overview. J. Pharm. Pharm. 2020, 72, 1145–1151. [Google Scholar] [CrossRef]
- Battogtokh, G.; Cho, Y.Y.; Lee, J.Y.; Lee, H.S.; Kang, H.C. Mitochondrial-Targeting Anticancer Agent Conjugates and Nanocarrier Systems for Cancer Treatment. Front. Pharm. 2018, 9, 922. [Google Scholar] [CrossRef]
- Zinovkin, R.A.; Zamyatnin, A.A. Mitochondria-Targeted Drugs. Curr. Mol. Pharm. 2019, 12, 202–214. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.K.; Kolishetti, N.; Dhar, S. Targeted nanoparticles in mitochondrial medicine. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2015, 7, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Yamada, Y.; Ito, M.; Arai, M.; Hibino, M.; Tsujioka, T.; Harashima, H. Challenges in Promoting Mitochondrial Transplantation Therapy. Int. J. Mol. Sci. 2020, 21, 6365. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, C.A.; Fink, B.D.; Gibbs, B.E.; Chheda, P.R.; Wu, M.; Sivitz, W.I.; Kerns, R.J. A Novel Triphenylphosphonium Carrier to Target Mitochondria without Uncoupling Oxidative Phosphorylation. J. Med. Chem. 2021, 64, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Mailloux, R.J. Teaching the fundamentals of electron transfer reactions in mitochondria and the production and detection of reactive oxygen species. Redox Biol. 2015, 4, 381–398. [Google Scholar] [CrossRef]
- Wang, J.Y.; Li, J.Q.; Xiao, Y.M.; Fu, B.; Qin, Z.H. Triphenylphosphonium (TPP)-Based Antioxidants: A New Perspective on Antioxidant Design. Chem. Med. Chem. 2020, 15, 404–410. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Xiao, Y.; Fu, B.; Qin, Z. TPP-based mitocans: A potent strategy for anticancer drug design. RSC Med. Chem. 2020, 11, 858–875. [Google Scholar] [CrossRef]
- Rossman, M.J.; Santos-Parker, J.R.; Steward, C.A.C.; Bispham, N.Z.; Cuevas, L.M.; Rosenberg, H.L.; Woodward, K.A.; Chonchol, M.; Gioscia-Ryan, R.A.; Murphy, M.P.; et al. Chronic Supplementation With a Mitochondrial Antioxidant (MitoQ) Improves Vascular Function in Healthy Older Adults. Hypertension 2018, 71, 1056–1063. [Google Scholar] [CrossRef]
- Shill, D.D.; Southern, W.M.; Willingham, T.B.; Lansford, K.A.; McCully, K.K.; Jenkins, N.T. Mitochondria-specific antioxidant supplementation does not influence endurance exercise training-induced adaptations in circulating angiogenic cells, skeletal muscle oxidative capacity or maximal oxygen uptake. J. Physiol. 2016, 594, 7005–7014. [Google Scholar] [CrossRef]
- Williamson, J.; Hughes, C.M.; Cobley, J.N.; Davison, G.W. The mitochondria-targeted antioxidant MitoQ, attenuates exercise-induced mitochondrial DNA damage. Redox Biol. 2020, 36, 101673. [Google Scholar] [CrossRef]
- Gane, E.J.; Weilert, F.; Orr, D.W.; Keogh, G.F.; Gibson, M.; Lockhart, M.M.; Frampton, C.M.; Taylor, K.M.; Smith, R.A.; Murphy, M.P. The mitochondria-targeted anti-oxidant mitoquinone decreases liver damage in a phase II study of hepatitis C patients. Liver. Int. 2010, 30, 1019–1026. [Google Scholar] [CrossRef] [PubMed]
- Snow, B.J.; Rolfe, F.L.; Lockhart, M.M.; Frampton, C.M.; O’Sullivan, J.D.; Fung, V.; Smith, R.A.; Murphy, M.P.; Taylor, K.M.; Protect Study, G. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov. Disord. 2010, 25, 1670–1674. [Google Scholar] [CrossRef] [PubMed]
- Dare, A.J.; Bolton, E.A.; Pettigrew, G.J.; Bradley, J.A.; Saeb-Parsy, K.; Murphy, M.P. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015, 5, 163–168. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Horvath, B.; Zsengeller, Z.; Zielonka, J.; Tanchian, G.; Holovac, E.; Kechrid, M.; Patel, V.; Stillman, I.E.; Parikh, S.M.; et al. Mitochondrial-targeted antioxidants represent a promising approach for prevention of cisplatin-induced nephropathy. Free Radic. Biol. Med. 2012, 52, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Liu, J.; Niu, J.; Zhang, Y.; Shen, W.; Luo, C.; Liu, Y.; Li, C.; Li, H.; Yang, P.; et al. Wnt/beta-catenin/RAS signaling mediates age-related renal fibrosis and is associated with mitochondrial dysfunction. Aging Cell 2019, 18, e13004. [Google Scholar] [CrossRef]
- Koye, D.N.; Magliano, D.J.; Nelson, R.G.; Pavkov, M.E. The Global Epidemiology of Diabetes and Kidney Disease. Adv. Chronic. Kidney Dis. 2018, 25, 121–132. [Google Scholar] [CrossRef]
- Ward, M.S.; Flemming, N.B.; Gallo, L.A.; Fotheringham, A.K.; McCarthy, D.A.; Zhuang, A.; Tang, P.H.; Borg, D.J.; Shaw, H.; Harvie, B.; et al. Targeted mitochondrial therapy using MitoQ shows equivalent renoprotection to angiotensin converting enzyme inhibition but no combined synergy in diabetes. Sci. Rep. 2017, 7, 15190. [Google Scholar] [CrossRef]
- Chacko, B.K.; Reily, C.; Srivastava, A.; Johnson, M.S.; Ye, Y.; Ulasova, E.; Agarwal, A.; Zinn, K.R.; Murphy, M.P.; Kalyanaraman, B.; et al. Prevention of diabetic nephropathy in Ins2(+/)(-)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem. J. 2010, 432, 9–19. [Google Scholar] [CrossRef]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Gottwald, E.M.; Duss, M.; Bugarski, M.; Haenni, D.; Schuh, C.D.; Landau, E.M.; Hall, A.M. The targeted anti-oxidant MitoQ causes mitochondrial swelling and depolarization in kidney tissue. Physiol. Rep. 2018, 6, e13667. [Google Scholar] [CrossRef]
- Brzheskiy, V.V.; Efimova, E.L.; Vorontsova, T.N.; Alekseev, V.N.; Gusarevich, O.G.; Shaidurova, K.N.; Ryabtseva, A.A.; Andryukhina, O.M.; Kamenskikh, T.G.; Sumarokova, E.S.; et al. Results of a Multicenter, Randomized, Double-Masked, Placebo-Controlled Clinical Study of the Efficacy and Safety of Visomitin Eye Drops in Patients with Dry Eye Syndrome. Adv. Ther. 2015, 32, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.; Perekhvatova, N.; Skulachev, M.; Stein, L.; Ousler, G. SkQ1 Ophthalmic Solution for Dry Eye Treatment: Results of a Phase 2 Safety and Efficacy Clinical Study in the Environment and During Challenge in the Controlled Adverse Environment Model. Adv. Ther. 2016, 33, 96–115. [Google Scholar] [CrossRef] [PubMed]
- Skulachev, V.P.; Anisimov, V.N.; Antonenko, Y.N.; Bakeeva, L.E.; Chernyak, B.V.; Erichev, V.P.; Filenko, O.F.; Kalinina, N.I.; Kapelko, V.I.; Kolosova, N.G.; et al. An attempt to prevent senescence: A mitochondrial approach. Biochim. Biophys. Acta 2009, 1787, 437–461. [Google Scholar] [CrossRef] [PubMed]
- Shabalina, I.G.; Vyssokikh, M.Y.; Gibanova, N.; Csikasz, R.I.; Edgar, D.; Hallden-Waldemarson, A.; Rozhdestvenskaya, Z.; Bakeeva, L.E.; Vays, V.B.; Pustovidko, A.V.; et al. Improved health-span and lifespan in mtDNA mutator mice treated with the mitochondrially targeted antioxidant SkQ1. Aging 2017, 9, 315–339. [Google Scholar] [CrossRef]
- Shetty, S.; Kumar, R.; Bharati, S. Mito-TEMPO, a mitochondria-targeted antioxidant, prevents N-nitrosodiethylamine-induced hepatocarcinogenesis in mice. Free Radic. Biol. Med. 2019, 136, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.J.; Bak, S.H.; Han, K.H.; Kim, J.I.; Park, J.W.; Park, K.M. Fragmentation of kidney epithelial cell primary cilia occurs by cisplatin and these cilia fragments are excreted into the urine. Redox Biol. 2019, 20, 38–45. [Google Scholar] [CrossRef]
- Sims, C.R.; MacMillan-Crow, L.A.; Mayeux, P.R. Targeting mitochondrial oxidants may facilitate recovery of renal function during infant sepsis. Clin. Pharmacol. Ther. 2014, 96, 662–664. [Google Scholar] [CrossRef]
- Patil, N.K.; Parajuli, N.; MacMillan-Crow, L.A.; Mayeux, P.R. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: Mitochondria-targeted antioxidant mitigates injury. Am. J. Physiol. Ren. Physiol. 2014, 306, F734–F743. [Google Scholar] [CrossRef]
- Arulkumaran, N.; Pollen, S.J.; Tidswell, R.; Gaupp, C.; Peters, V.B.M.; Stanzani, G.; Snow, T.A.C.; Duchen, M.R.; Singer, M. Selective mitochondrial antioxidant MitoTEMPO reduces renal dysfunction and systemic inflammation in experimental sepsis in rats. Br. J. Anaesth. 2021, 127, 577–586. [Google Scholar] [CrossRef]
- Ding, W.; Liu, T.; Bi, X.; Zhang, Z. Mitochondria-Targeted Antioxidant Mito-Tempo Protects Against Aldosterone-Induced Renal Injury In Vivo. Cell Physiol. Biochem. 2017, 44, 741–750. [Google Scholar] [CrossRef]
- Chu, S.; Mao, X.; Guo, H.; Wang, L.; Li, Z.; Zhang, Y.; Wang, Y.; Wang, H.; Zhang, X.; Peng, W. Indoxyl sulfate potentiates endothelial dysfunction via reciprocal role for reactive oxygen species and RhoA/ROCK signaling in 5/6 nephrectomized rats. Free Radic. Res. 2017, 51, 237–252. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Y.; Ding, W.; Wang, Y. Mito-TEMPO Alleviates Renal Fibrosis by Reducing Inflammation, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Oxidative Med. Cell Longev. 2018, 2018, 5828120. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Perumal, E.; Bi, X.; Wang, Y.; Ding, W. Potential mechanisms of uremic muscle wasting and the protective role of the mitochondria-targeted antioxidant Mito-TEMPO. Int. Urol. Nephrol. 2020, 52, 1551–1561. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lin, Q.; Shao, X.; Zhu, X.; Wu, J.; Wu, B.; Zhang, M.; Zhou, W.; Zhou, Y.; Jin, H.; et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic. Biol. Med. 2020, 152, 632–649. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Zhu, H.; Wang, X.; Gao, Q.; Li, Z.; Huang, H. CoQ10 ameliorates mitochondrial dysfunction in diabetic nephropathy through mitophagy. J. Endocrinol. 2019, 240, 445–465. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Casalena, G.; Shi, S.; Yu, L.; Ebefors, K.; Sun, Y.; Zhang, W.; D’Agati, V.; Schlondorff, D.; Haraldsson, B.; et al. Glomerular Endothelial Mitochondrial Dysfunction Is Essential and Characteristic of Diabetic Kidney Disease Susceptibility. Diabetes 2017, 66, 763–778. [Google Scholar] [CrossRef]
- Hasan, W.; Kori, R.K.; Thakre, K.; Yadav, R.S.; Jat, D. Synthesis, characterization and efficacy of mitochondrial targeted delivery of TPP-curcumin in rotenone-induced toxicity. DARU J. Pharm. Sci. 2019, 27, 557–570. [Google Scholar] [CrossRef]
- Brown, S.E.; Ross, M.F.; Sanjuan-Pla, A.; Manas, A.R.; Smith, R.A.; Murphy, M.P. Targeting lipoic acid to mitochondria: Synthesis and characterization of a triphenylphosphonium-conjugated alpha-lipoyl derivative. Free Radic. Biol. Med. 2007, 42, 1766–1780. [Google Scholar] [CrossRef]
- Trnka, J.; Elkalaf, M.; Andel, M. Lipophilic triphenylphosphonium cations inhibit mitochondrial electron transport chain and induce mitochondrial proton leak. PLoS ONE 2015, 10, e0121837. [Google Scholar] [CrossRef]
- Beija, M.; Afonso, C.A.; Martinho, J.M. Synthesis and applications of Rhodamine derivatives as fluorescent probes. Chem. Soc. Rev. 2009, 38, 2410–2433. [Google Scholar] [CrossRef]
- Perry, S.W.; Norman, J.P.; Barbieri, J.; Brown, E.B.; Gelbard, H.A. Mitochondrial membrane potential probes and the proton gradient: A practical usage guide. Biotechniques 2011, 50, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Scaduto, R.C., Jr.; Grotyohann, L.W. Measurement of mitochondrial membrane potential using fluorescent rhodamine derivatives. Biophys. J. 1999, 76, 469–477. [Google Scholar] [CrossRef]
- Rogov, A.G.; Trendeleva, T.A.; Aliverdieva, D.A.; Zvyagilskaya, R.A. More about Interactions of Rhodamine 19 Butyl Ester with Rat Liver Mitochondria. Biochemistry 2016, 81, 432–438. [Google Scholar] [CrossRef] [PubMed]
- Antonenko, Y.N.; Avetisyan, A.V.; Cherepanov, D.A.; Knorre, D.A.; Korshunova, G.A.; Markova, O.V.; Ojovan, S.M.; Perevoshchikova, I.V.; Pustovidko, A.V.; Rokitskaya, T.I.; et al. Derivatives of rhodamine 19 as mild mitochondria-targeted cationic uncouplers. J. Biol. Chem. 2011, 286, 17831–17840. [Google Scholar] [CrossRef] [PubMed]
- Plotnikov, E.Y.; Chupyrkina, A.A.; Jankauskas, S.S.; Pevzner, I.B.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mechanisms of nephroprotective effect of mitochondria-targeted antioxidants under rhabdomyolysis and ischemia/reperfusion. Biochim. Biophys. Acta 2011, 1812, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Jankauskas, S.S.; Andrianova, N.V.; Alieva, I.B.; Prusov, A.N.; Matsievsky, D.D.; Zorova, L.D.; Pevzner, I.B.; Savchenko, E.S.; Pirogov, Y.A.; Silachev, D.N.; et al. Dysfunction of Kidney Endothelium after Ischemia/Reperfusion and Its Prevention by Mitochondria-Targeted Antioxidant. Biochemistry 2016, 81, 1538–1548. [Google Scholar] [CrossRef]
- Jankauskas, S.S.; Plotnikov, E.Y.; Morosanova, M.A.; Pevzner, I.B.; Zorova, L.D.; Skulachev, V.P.; Zorov, D.B. Mitochondria-targeted antioxidant SkQR1 ameliorates gentamycin-induced renal failure and hearing loss. Biochemistry 2012, 77, 666–670. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Pevzner, I.B.; Zorova, L.D.; Chernikov, V.P.; Prusov, A.N.; Kireev, I.I.; Silachev, D.N.; Skulachev, V.P.; Zorov, D.B. Mitochondrial Damage and Mitochondria-Targeted Antioxidant Protection in LPS-Induced Acute Kidney Injury. Antioxidants 2019, 8, 176. [Google Scholar] [CrossRef]
- Jeon, D.H.; Jang, H.N.; Cho, H.S.; Lee, T.W.; Bae, E.; Chang, S.H.; Park, D.J. Incidence, risk factors, and clinical outcomes of acute kidney injury associated with acute pyelonephritis in patients attending a tertiary care referral center. Ren. Fail. 2019, 41, 204–210. [Google Scholar] [CrossRef]
- Plotnikov, E.Y.; Morosanova, M.A.; Pevzner, I.B.; Zorova, L.D.; Manskikh, V.N.; Pulkova, N.V.; Galkina, S.I.; Skulachev, V.P.; Zorov, D.B. Protective effect of mitochondria-targeted antioxidants in an acute bacterial infection. Proc. Natl. Acad. Sci. USA 2013, 110, E3100–E3108. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, D.; Li, X.; Ding, F.; Tang, X.; Liu, N.; Huang, H.; Liu, C. The conjugation of rhodamine B enables carrier-free mitochondrial delivery of functional proteins. Org. Biomol. Chem. 2020, 18, 6829–6839. [Google Scholar] [CrossRef] [PubMed]
- Erak, M.; Bellmann-Sickert, K.; Els-Heindl, S.; Beck-Sickinger, A.G. Peptide chemistry toolbox—Transforming natural peptides into peptide therapeutics. Bioorg. Med. Chem. 2018, 26, 2759–2765. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Wang, C.; He, Z.; Cheng, B.; Zheng, L.; Huang, K. Peptide-Drug Conjugate: A Novel Drug Design Approach. Curr. Med. Chem. 2017, 24, 3373–3396. [Google Scholar] [CrossRef] [PubMed]
- Fishbane, S.; Schiller, B.; Locatelli, F.; Covic, A.C.; Provenzano, R.; Wiecek, A.; Levin, N.W.; Kaplan, M.; Macdougall, I.C.; Francisco, C.; et al. Peginesatide in patients with anemia undergoing hemodialysis. N. Engl. J. Med. 2013, 368, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gall, J.M.; Bonegio, R.; Havasi, A.; Illanes, K.; Schwartz, J.H.; Borkan, S.C. Nucleophosmin, a critical Bax cofactor in ischemia-induced cell death. Mol. Cell Biol. 2013, 33, 1916–1924. [Google Scholar] [CrossRef]
- Saitoh, T.; Igura, M.; Obita, T.; Ose, T.; Kojima, R.; Maenaka, K.; Endo, T.; Kohda, D. Tom20 recognizes mitochondrial presequences through dynamic equilibrium among multiple bound states. EMBO J. 2007, 26, 4777–4787. [Google Scholar] [CrossRef]
- Wiedemann, N.; Pfanner, N. Mitochondrial Machineries for Protein Import and Assembly. Annu. Rev. Biochem. 2017, 86, 685–714. [Google Scholar] [CrossRef]
- Lin, R.; Zhang, P.; Cheetham, A.G.; Walston, J.; Abadir, P.; Cui, H. Dual peptide conjugation strategy for improved cellular uptake and mitochondria targeting. Bioconjug. Chem. 2015, 26, 71–77. [Google Scholar] [CrossRef]
- Jain, A.; Chugh, A. Mitochondrial transit peptide exhibits cell penetration ability and efficiently delivers macromolecules to mitochondria. FEBS Lett. 2016, 590, 2896–2905. [Google Scholar] [CrossRef]
- Kang, Y.C.; Son, M.; Kang, S.; Im, S.; Piao, Y.; Lim, K.S.; Song, M.Y.; Park, K.S.; Kim, Y.H.; Pak, Y.K. Cell-penetrating artificial mitochondria-targeting peptide-conjugated metallothionein 1A alleviates mitochondrial damage in Parkinson’s disease models. Exp. Mol. Med. 2018, 50, 1–13. [Google Scholar] [CrossRef]
- Asayama, S.; Kawamura, E.; Nagaoka, S.; Kawakami, H. Design of manganese porphyrin modified with mitochondrial signal peptide for a new antioxidant. Mol. Pharm. 2006, 3, 468–470. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.R.; Khan, S.M.; Portell, F.R.; Smigrodzki, R.M.; Bennett, J.P., Jr. Recombinant human mitochondrial transcription factor A stimulates mitochondrial biogenesis and ATP synthesis, improves motor function after MPTP, reduces oxidative stress and increases survival after endotoxin. Mitochondrion 2011, 11, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Thomas, R.R.; Portell, F.R.; Dunham, L.D.; Quigley, C.K.; Bennett, J.P., Jr. Recombinant mitochondrial transcription factor A with N-terminal mitochondrial transduction domain increases respiration and mitochondrial gene expression. Mitochondrion 2009, 9, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.J.; Wu, Y.J.; Chen, L.J.; Ko, B.S.; Chang, T.C.; Wu, Y.J.; Liang, S.M.; Jan, Y.J.; Liou, J.Y. Reduced Expression of Metallothionein-I/II in Renal Proximal Tubules Is Associated with Advanced Chronic Kidney Disease. Toxins 2021, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Saba, H.; Batinic-Haberle, I.; Munusamy, S.; Mitchell, T.; Lichti, C.; Megyesi, J.; MacMillan-Crow, L.A. Manganese porphyrin reduces renal injury and mitochondrial damage during ischemia/reperfusion. Free Radic. Biol. Med. 2007, 42, 1571–1578. [Google Scholar] [CrossRef]
- Li, L.; Geisler, I.; Chmielewski, J.; Cheng, J.X. Cationic amphiphilic polyproline helix P11LRR targets intracellular mitochondria. J. Control. Release 2010, 142, 259–266. [Google Scholar] [CrossRef]
- Kalafut, D.; Anderson, T.N.; Chmielewski, J. Mitochondrial targeting of a cationic amphiphilic polyproline helix. Bioorg. Med. Chem. Lett. 2012, 22, 561–563. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Pedraza-Chaverri, J. Mitochondrial Redox Signaling and Oxidative Stress in Kidney Diseases. Biomolecules 2021, 11, 1144. [Google Scholar] [CrossRef]
- Kam, A.; Loo, S.; Dutta, B.; Sze, S.K.; Tam, J.P. Plant-derived mitochondria-targeting cysteine-rich peptide modulates cellular bioenergetics. J. Biol. Chem. 2019, 294, 4000–4011. [Google Scholar] [CrossRef]
- Rojas-Morales, P.; Leon-Contreras, J.C.; Aparicio-Trejo, O.E.; Reyes-Ocampo, J.G.; Medina-Campos, O.N.; Jimenez-Osorio, A.S.; Gonzalez-Reyes, S.; Marquina-Castillo, B.; Hernandez-Pando, R.; Barrera-Oviedo, D.; et al. Fasting reduces oxidative stress, mitochondrial dysfunction and fibrosis induced by renal ischemia-reperfusion injury. Free Radic. Biol. Med. 2019, 135, 60–67. [Google Scholar] [CrossRef]
- Briones-Herrera, A.; Avila-Rojas, S.H.; Aparicio-Trejo, O.E.; Cristobal, M.; Leon-Contreras, J.C.; Hernandez-Pando, R.; Pinzon, E.; Pedraza-Chaverri, J.; Sanchez-Lozada, L.G.; Tapia, E. Sulforaphane prevents maleic acid-induced nephropathy by modulating renal hemodynamics, mitochondrial bioenergetics and oxidative stress. Food Chem. Toxicol. 2018, 115, 185–197. [Google Scholar] [CrossRef] [PubMed]
- Kelley, S.O.; Stewart, K.M.; Mourtada, R. Development of novel peptides for mitochondrial drug delivery: Amino acids featuring delocalized lipophilic cations. Pharm. Res. 2011, 28, 2808–2819. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.; Kirshenbaum, K. Peptoid architectures: Elaboration, actuation, and application. Curr. Opin. Chem. Biol. 2008, 12, 714–721. [Google Scholar] [CrossRef] [PubMed]
- Nam, H.Y.; Hong, J.A.; Choi, J.; Shin, S.; Cho, S.K.; Seo, J.; Lee, J. Mitochondria-Targeting Peptoids. Bioconjug. Chem. 2018, 29, 1669–1676. [Google Scholar] [CrossRef]
- Karaa, A.; Haas, R.; Goldstein, A.; Vockley, J.; Weaver, W.D.; Cohen, B.H. Randomized dose-escalation trial of elamipretide in adults with primary mitochondrial myopathy. Neurology 2018, 90, e1212–e1221. [Google Scholar] [CrossRef]
- Reid Thompson, W.; Hornby, B.; Manuel, R.; Bradley, E.; Laux, J.; Carr, J.; Vernon, H.J. A phase 2/3 randomized clinical trial followed by an open-label extension to evaluate the effectiveness of elamipretide in Barth syndrome, a genetic disorder of mitochondrial cardiolipin metabolism. Genet. Med. 2021, 23, 471–478. [Google Scholar] [CrossRef]
- Chakrabarti, A.K.; Feeney, K.; Abueg, C.; Brown, D.A.; Czyz, E.; Tendera, M.; Janosi, A.; Giugliano, R.P.; Kloner, R.A.; Weaver, W.D.; et al. Rationale and design of the EMBRACE STEMI study: A phase 2a, randomized, double-blind, placebo-controlled trial to evaluate the safety, tolerability and efficacy of intravenous Bendavia on reperfusion injury in patients treated with standard therapy including primary percutaneous coronary intervention and stenting for ST-segment elevation myocardial infarction. Am. Heart. J. 2013, 165, 509–514 e507. [Google Scholar] [CrossRef]
- Butler, J.; Khan, M.S.; Anker, S.D.; Fonarow, G.C.; Kim, R.J.; Nodari, S.; O’Connor, C.M.; Pieske, B.; Pieske-Kraigher, E.; Sabbah, H.N.; et al. Effects of Elamipretide on Left Ventricular Function in Patients With Heart Failure With Reduced Ejection Fraction: The PROGRESS-HF Phase 2 Trial. J. Card. Fail. 2020, 26, 429–437. [Google Scholar] [CrossRef]
- Saad, A.; Herrmann, S.M.S.; Eirin, A.; Ferguson, C.M.; Glockner, J.F.; Bjarnason, H.; McKusick, M.A.; Misra, S.; Lerman, L.O.; Textor, S.C. Phase 2a Clinical Trial of Mitochondrial Protection (Elamipretide) During Stent Revascularization in Patients With Atherosclerotic Renal Artery Stenosis. Circ. Cardiovasc. Interv. 2017, 10, e005487. [Google Scholar] [CrossRef]
- Yang, S.K.; Han, Y.C.; He, J.R.; Yang, M.; Zhang, W.; Zhan, M.; Li, A.M.; Li, L.; Na, S.; Liu, Y.T.; et al. Mitochondria targeted peptide SS-31 prevent on cisplatin-induced acute kidney injury via regulating mitochondrial ROS-NLRP3 pathway. Biomed. Pharm. 2020, 130, 110521. [Google Scholar] [CrossRef]
- Szeto, H.H.; Liu, S.; Soong, Y.; Wu, D.; Darrah, S.F.; Cheng, F.Y.; Zhao, Z.; Ganger, M.; Tow, C.Y.; Seshan, S.V. Mitochondria-targeted peptide accelerates ATP recovery and reduces ischemic kidney injury. J. Am. Soc. Nephrol. 2011, 22, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Soong, Y.; Seshan, S.V.; Szeto, H.H. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am. J. Physiol. Ren. Physiol. 2014, 306, F970–F980. [Google Scholar] [CrossRef] [PubMed]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Cerrato, C.P.; Pirisinu, M.; Vlachos, E.N.; Langel, U. Novel cell-penetrating peptide targeting mitochondria. FASEB J. 2015, 29, 4589–4599. [Google Scholar] [CrossRef]
- Liu, D.; Jin, F.; Shu, G.; Xu, X.; Qi, J.; Kang, X.; Yu, H.; Lu, K.; Jiang, S.; Han, F.; et al. Enhanced efficiency of mitochondria-targeted peptide SS-31 for acute kidney injury by pH-responsive and AKI-kidney targeted nanopolyplexes. Biomaterials 2019, 211, 57–67. [Google Scholar] [CrossRef]
- Wyss, J.C.; Kumar, R.; Mikulic, J.; Schneider, M.; Mary, J.L.; Aebi, J.D.; Juillerat-Jeanneret, L.; Golshayan, D. Differential Effects of the Mitochondria-Active Tetrapeptide SS-31 (D-Arg-dimethylTyr-Lys-Phe-NH2) and Its Peptidase-Targeted Prodrugs in Experimental Acute Kidney Injury. Front. Pharm. 2019, 10, 1209. [Google Scholar] [CrossRef]
- Szeto, H.H.; Liu, S.; Soong, Y.; Seshan, S.V.; Cohen-Gould, L.; Manichev, V.; Feldman, L.C.; Gustafsson, T. Mitochondria Protection after Acute Ischemia Prevents Prolonged Upregulation of IL-1beta and IL-18 and Arrests CKD. J. Am. Soc. Nephrol. 2017, 28, 1437–1449. [Google Scholar] [CrossRef]
- Hou, Y.; Shi, Y.; Han, B.; Liu, X.; Qiao, X.; Qi, Y.; Wang, L. The antioxidant peptide SS31 prevents oxidative stress, downregulates CD36 and improves renal function in diabetic nephropathy. Nephrol. Dial. Transpl. 2018, 33, 1908–1918. [Google Scholar] [CrossRef]
- Yang, S.K.; Li, A.M.; Han, Y.C.; Peng, C.H.; Song, N.; Yang, M.; Zhan, M.; Zeng, L.F.; Song, P.A.; Zhang, W.; et al. Mitochondria-Targeted Peptide SS31 Attenuates Renal Tubulointerstitial Injury via Inhibiting Mitochondrial Fission in Diabetic Mice. Oxidative Med. Cell. Longev. 2019, 2019, 2346580. [Google Scholar] [CrossRef]
- Yang, Q.; Xie, W.; Wang, X.; Luo, J.; Zhou, Y.; Cao, H.; Sun, Q.; Jiang, L.; Yang, J. SS31 Ameliorates Podocyte Injury via Inhibiting OMA1-Mediated Hydrolysis of OPA1 in Diabetic Kidney Disease. Front. Pharmacol. 2022, 12, 707006. [Google Scholar] [CrossRef]
- Hou, Y.; Li, S.; Wu, M.; Wei, J.; Ren, Y.; Du, C.; Wu, H.; Han, C.; Duan, H.; Shi, Y. Mitochondria-targeted peptide SS-31 attenuates renal injury via an antioxidant effect in diabetic nephropathy. Am. J. Physiol. Ren. Physiol. 2016, 310, F547–F559. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, S.; Zhang, G.; Hall, D.; Oates, P.J.; Maity, S.; Madesh, M.; Han, X.; Sharma, K. Restoring mitochondrial superoxide levels with elamipretide (MTP-131) protects db/db mice against progression of diabetic kidney diease. J. Biol. Chem. 2020, 295, 7249–7260. [Google Scholar] [CrossRef] [PubMed]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef]
- Alta, R.Y.; Vitorino, H.A.; Goswami, D.; Liria, C.W.; Wisnovsky, S.P.; Kelley, S.O.; Machini, M.T.; Esposito, B.P. Mitochondria-penetrating peptides conjugated to desferrioxamine as chelators for mitochondrial labile iron. PLoS ONE 2017, 12, e0171729. [Google Scholar] [CrossRef]
- Yamada, Y.; Akita, H.; Kamiya, H.; Kogure, K.; Yamamoto, T.; Shinohara, Y.; Yamashita, K.; Kobayashi, H.; Kikuchi, H.; Harashima, H. MITO-Porter: A liposome-based carrier system for delivery of macromolecules into mitochondria via membrane fusion. Biochim. Biophys. Acta 2008, 1778, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, R.; Yamada, Y.; Kawamura, E.; Harashima, H. Mitochondrial delivery of antisense RNA by MITO-Porter results in mitochondrial RNA knockdown, and has a functional impact on mitochondria. Biomaterials 2015, 57, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Kawamura, E.; Maruyama, M.; Abe, J.; Sudo, A.; Takeda, A.; Takada, S.; Yokota, T.; Kinugawa, S.; Harashima, H.; Yamada, Y. Validation of Gene Therapy for Mutant Mitochondria by Delivering Mitochondrial RNA Using a MITO-Porter. Mol. Ther.—Nucleic Acids 2020, 20, 687–698. [Google Scholar] [CrossRef]
- Yamada, Y.; Somiya, K.; Miyauchi, A.; Osaka, H.; Harashima, H. Validation of a mitochondrial RNA therapeutic strategy using fibroblasts from a Leigh syndrome patient with a mutation in the mitochondrial ND3 gene. Sci. Rep. 2020, 10, 7511. [Google Scholar] [CrossRef]
- Yamada, Y.; Maruyama, M.; Kita, T.; Usami, S.I.; Kitajiri, S.I.; Harashima, H. The use of a MITO-Porter to deliver exogenous therapeutic RNA to a mitochondrial disease’s cell with a A1555G mutation in the mitochondrial 12S rRNA gene results in an increase in mitochondrial respiratory activity. Mitochondrion 2020, 55, 134–144. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, K.; Abe, J.; Hyodo, M.; Haga, S.; Ozaki, M.; Harashima, H. Mitochondrial delivery of Coenzyme Q10 via systemic administration using a MITO-Porter prevents ischemia/reperfusion injury in the mouse liver. J. Control. Release 2015, 213, 86–95. [Google Scholar] [CrossRef]
- Bailly, C. Medicinal applications and molecular targets of dequalinium chloride. Biochem. Pharm. 2021, 186, 114467. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Zhang, J.; Li, X.; Pan, S.; Li, J.; Yang, C.; Hu, H.; Qiao, M.; Chen, D.; Zhao, X. Mitochondria-targeted delivery of doxorubicin to enhance antitumor activity with HER-2 peptide-mediated multifunctional pH-sensitive DQAsomes. Int. J. Nanomed. 2018, 13, 4209–4226. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, B.; Vyas, S.P. Transferrin coupled vesicular system for intracellular drug delivery for the treatment of cancer: Development and characterization. J. Drug Target. 2012, 20, 372–380. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, G.G.; Cheng, S.M.; Boddapati, S.V.; Horobin, R.W.; Weissig, V. Nanocarrier-assisted sub-cellular targeting to the site of mitochondria improves the pro-apoptotic activity of paclitaxel. J. Drug Target. 2008, 16, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Zupancic, S.; Kocbek, P.; Zariwala, M.G.; Renshaw, D.; Gul, M.O.; Elsaid, Z.; Taylor, K.M.; Somavarapu, S. Design and development of novel mitochondrial targeted nanocarriers, DQAsomes for curcumin inhalation. Mol. Pharm. 2014, 11, 2334–2345. [Google Scholar] [CrossRef]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Khatun, Z.; Choi, Y.S.; Kim, Y.G.; Yoon, K.; Nurunnabi, M.; Li, L.; Lee, E.; Kang, H.C.; Huh, K.M. Bioreducible Poly(ethylene glycol)-Triphenylphosphonium Conjugate as a Bioactivable Mitochondria-Targeting Nanocarrier. Biomacromolecules 2017, 18, 1074–1085. [Google Scholar] [CrossRef]
- Marrache, S.; Dhar, S. Engineering of blended nanoparticle platform for delivery of mitochondria-acting therapeutics. Proc. Natl. Acad. Sci. USA 2012, 109, 16288–16293. [Google Scholar] [CrossRef]
- Dhall, A.; Self, W. Cerium Oxide Nanoparticles: A Brief Review of Their Synthesis Methods and Biomedical Applications. Antioxidants 2018, 7, 97. [Google Scholar] [CrossRef]
- Zhang, D.Y.; Liu, H.; Li, C.; Younis, M.R.; Lei, S.; Yang, C.; Lin, J.; Li, Z.; Huang, P. Ceria Nanozymes with Preferential Renal Uptake for Acute Kidney Injury Alleviation. ACS Appl. Mater. Interfaces 2020, 12, 56830–56838. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Jin, F.; Liu, D.; Shu, G.; Wang, X.; Qi, J.; Sun, M.; Yang, P.; Jiang, S.; Ying, X.; et al. ROS-responsive nano-drug delivery system combining mitochondria-targeting ceria nanoparticles with atorvastatin for acute kidney injury. Theranostics 2020, 10, 2342–2357. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, Y.; Li, C.; Yu, L.; Chang, Y.; Qu, M. Delivery of coenzyme Q10 with mitochondria-targeted nanocarrier attenuates renal ischemia-reperfusion injury in mice. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 131, 112536. [Google Scholar] [CrossRef] [PubMed]
- Guariento, A.; Piekarski, B.L.; Doulamis, I.P.; Blitzer, D.; Ferraro, A.M.; Harrild, D.M.; Zurakowski, D.; Del Nido, P.J.; McCully, J.D.; Emani, S.M. Autologous mitochondrial transplantation for cardiogenic shock in pediatric patients following ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2021, 162, 992–1001. [Google Scholar] [CrossRef] [PubMed]
- Emani, S.M.; Piekarski, B.L.; Harrild, D.; Del Nido, P.J.; McCully, J.D. Autologous mitochondrial transplantation for dysfunction after ischemia-reperfusion injury. J. Thorac. Cardiovasc. Surg. 2017, 154, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Kubat, G.B.; Ozler, M.; Ulger, O.; Ekinci, O.; Atalay, O.; Celik, E.; Safali, M.; Budak, M.T. The effects of mesenchymal stem cell mitochondrial transplantation on doxorubicin-mediated nephrotoxicity in rats. J. Biochem. Mol. Toxicol. 2021, 35, e22612. [Google Scholar] [CrossRef] [PubMed]
- Jabbari, H.; Roushandeh, A.M.; Rostami, M.K.; Razavi-Toosi, M.T.; Shokrgozar, M.A.; Jahanian-Najafabadi, A.; Kuwahara, Y.; Roudkenar, M.H. Mitochondrial transplantation ameliorates ischemia/reperfusion-induced kidney injury in rat. Biochim. Biophys. Acta Mol. Basis. Dis. 2020, 1866, 165809. [Google Scholar] [CrossRef]
- Doulamis, I.P.; Guariento, A.; Duignan, T.; Kido, T.; Orfany, A.; Saeed, M.Y.; Weixler, V.H.; Blitzer, D.; Shin, B.; Snay, E.R.; et al. Mitochondrial transplantation by intra-arterial injection for acute kidney injury. Am. J. Physiol. Ren. Physiol. 2020, 319, F403–F413. [Google Scholar] [CrossRef]
- Kitani, T.; Kami, D.; Matoba, S.; Gojo, S. Internalization of isolated functional mitochondria: Involvement of macropinocytosis. J. Cell Mol. Med. 2014, 18, 1694–1703. [Google Scholar] [CrossRef]
- Chang, J.C.; Hoel, F.; Liu, K.H.; Wei, Y.H.; Cheng, F.C.; Kuo, S.J.; Tronstad, K.J.; Liu, C.S. Peptide-mediated delivery of donor mitochondria improves mitochondrial function and cell viability in human cybrid cells with the MELAS A3243G mutation. Sci. Rep. 2017, 7, 10710. [Google Scholar] [CrossRef]
- Chang, J.C.; Liu, K.H.; Li, Y.C.; Kou, S.J.; Wei, Y.H.; Chuang, C.S.; Hsieh, M.; Liu, C.S. Functional recovery of human cells harbouring the mitochondrial DNA mutation MERRF A8344G via peptide-mediated mitochondrial delivery. Neurosignals 2013, 21, 160–173. [Google Scholar] [CrossRef]
- Chang, J.C.; Chang, H.S.; Wu, Y.C.; Cheng, W.L.; Lin, T.T.; Chang, H.J.; Kuo, S.J.; Chen, S.T.; Liu, C.S. Mitochondrial transplantation regulates antitumour activity, chemoresistance and mitochondrial dynamics in breast cancer. J. Exp. Clin. Cancer. Res. 2019, 38, 30. [Google Scholar] [CrossRef] [PubMed]
- Maeda, H.; Kami, D.; Maeda, R.; Murata, Y.; Jo, J.I.; Kitani, T.; Tabata, Y.; Matoba, S.; Gojo, S. TAT-dextran-mediated mitochondrial transfer enhances recovery from models of reperfusion injury in cultured cardiomyocytes. J. Cell Mol. Med. 2020, 24, 5007–5020. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yan, X.; Xia, M.; Shen, B.; Cao, Y.; Wu, X.; Sun, J.; Zhang, Y.; Zhang, M. Nanoparticle/Nanocarrier Formulation as an Antigen: The Immunogenicity and Antigenicity of Itself. Mol. Pharm. 2022, 19, 148–159. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Nuevo, A.; Zorzano, A. The sensing of mitochondrial DAMPs by non-immune cells. Cell Stress 2019, 3, 195–207. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).