Snake Venom and 3D Microenvironment Cell Culture: From Production to Drug Development
Abstract
:1. Introduction
2. Snake Venom Toxins
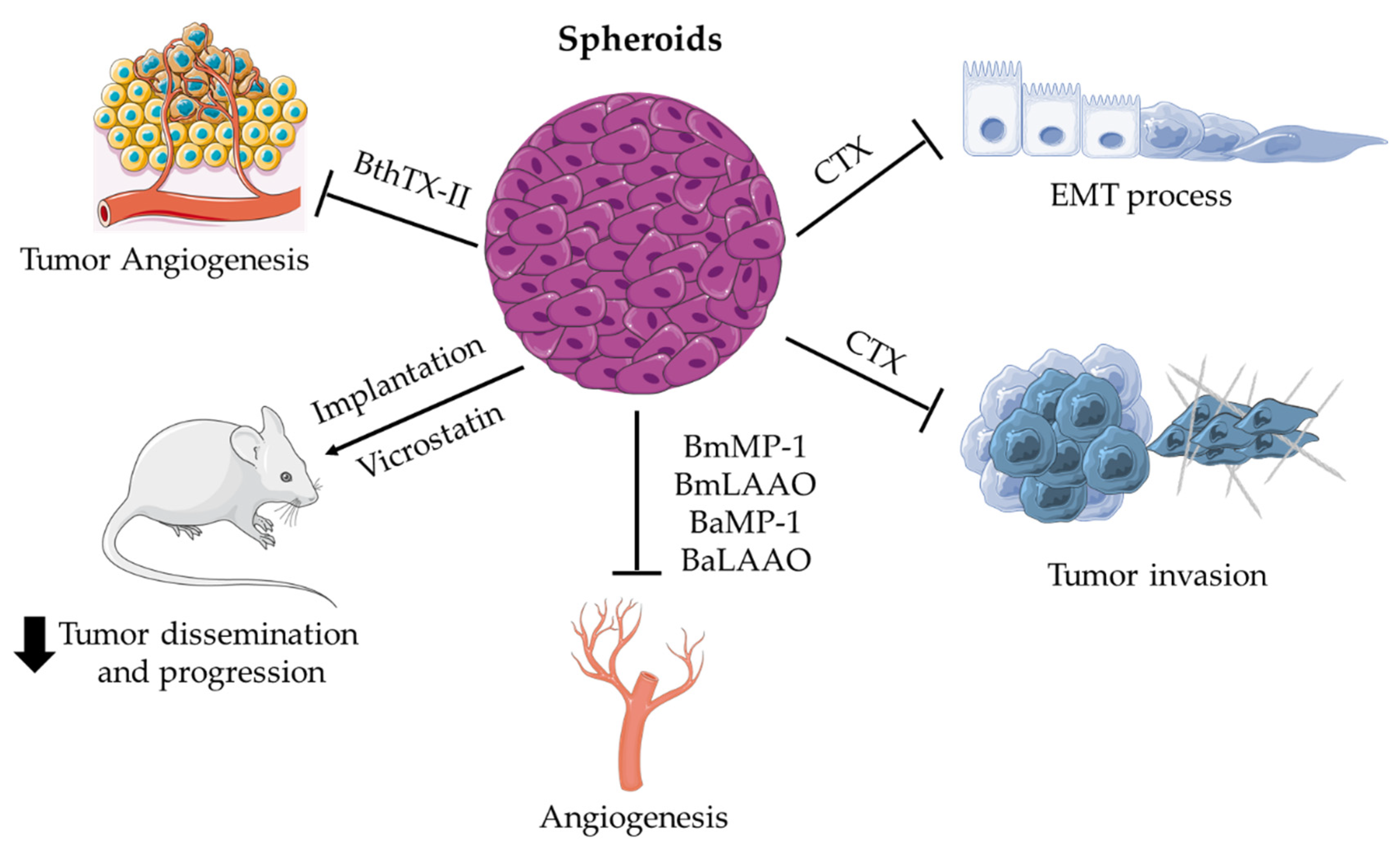
3. Spheroid Technology
Applications of Spheroids for Evaluating Snake Venom Components
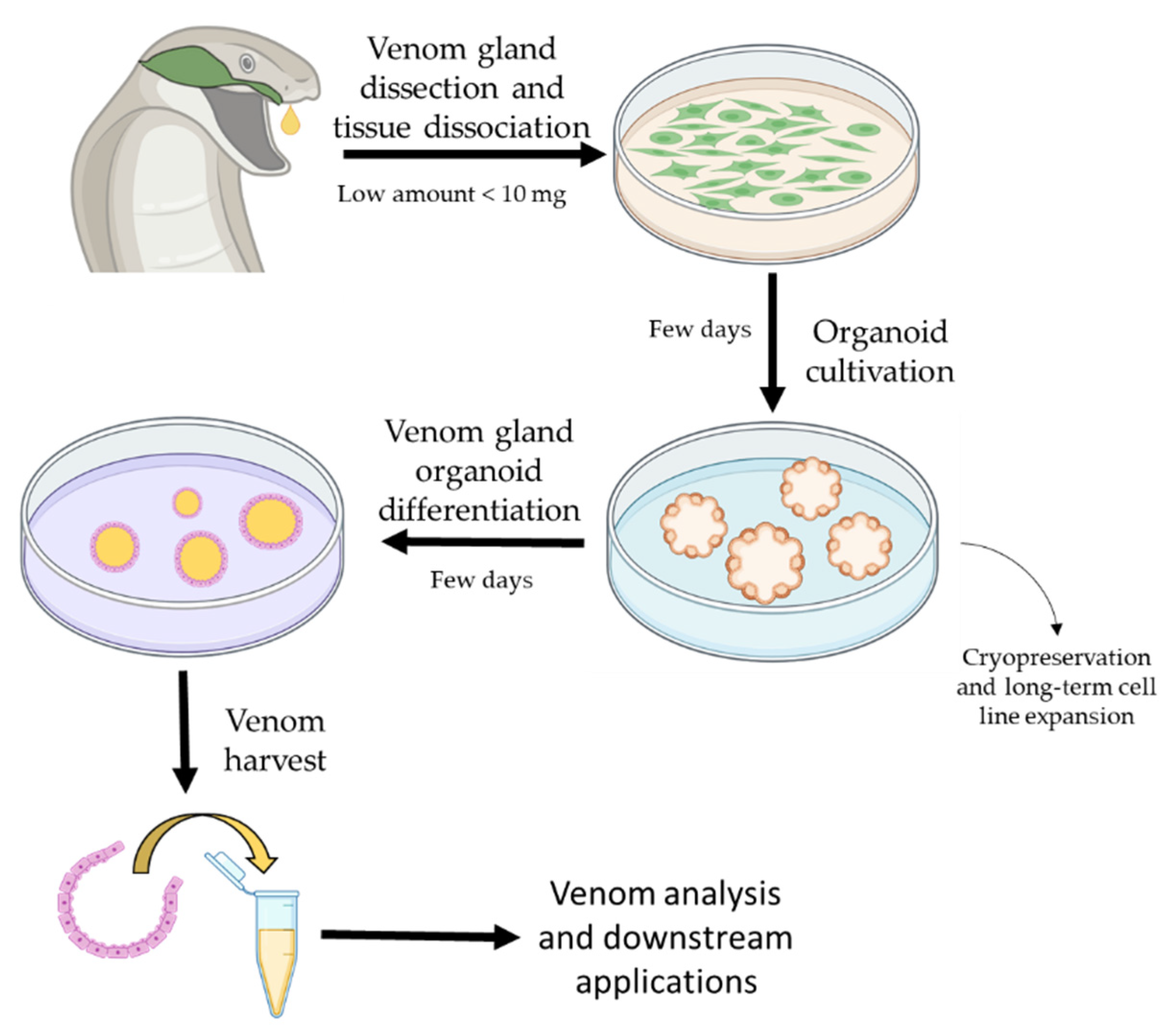
4. Organoid Technology
Application of Organoids in Snake Venom Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Ravi, M.; Paramesh, V.; Kaviya, S.R.; Anuradha, E.; Paul Solomon, F.D. 3D cell culture systems: Advantages and applications. J. Cell. Physiol. 2015, 230, 16–26. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef]
- Ravi, M.; Ramesh, A.; Pattabhi, A. Contributions of 3D Cell Cultures for Cancer Research. J. Cell. Physiol. 2017, 232, 2679–2697. [Google Scholar] [CrossRef]
- Fang, Y.; Eglen, R.M. Three-Dimensional Cell Cultures in Drug Discovery and Development. SLAS Discov. 2017, 22, 456–472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lv, D.; Hu, Z.; Lu, L.; Lu, H.; Xu, X. Three-dimensional cell culture: A powerful tool in tumor research and drug discovery. Oncol. Lett. 2017, 14, 6999–7010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decarli, M.C.; do Amaral, R.L.; Dos Santos, D.P.; Tofani, L.B.; Katayama, E.; Rezende, R.A.; da Silva, J.V.; Swiech, K.; Suazo, C.A.; Mota, C.; et al. Cell spheroids as a versatile research platform: Formation mechanisms, high throughput production, characterization and applications. Biofabrication 2021, 13, 032002. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, J.; Jiang, M.; Sang, L.; Hao, K.; He, H. The Combination of Cell Cultured Technology and In Silico Model to Inform the Drug Development. Pharmaceutics 2021, 13, 704. [Google Scholar] [CrossRef]
- Ong, C.S.; Zhou, X.; Han, J.; Huang, C.Y.; Nashed, A.; Khatri, S.; Mattson, G.; Fukunishi, T.; Zhang, H.; Hibino, N. In vivo therapeutic applications of cell spheroids. Biotechnol. Adv. 2018, 36, 494–505. [Google Scholar] [CrossRef]
- Bordon, K.C.F.; Cologna, C.T.; Fornari-Baldo, E.C.; Pinheiro-Júnior, E.L.; Cerni, F.A.; Amorim, F.G.; Anjolette, F.A.P.; Cordeiro, F.A.; Wiezel, G.A.; Cardoso, I.A.; et al. From Animal Poisons and Venoms to Medicines: Achievements, Challenges and Perspectives in Drug Discovery. Front. Pharmacol. 2020, 11, 1132. [Google Scholar] [CrossRef]
- Waheed, H.; Moin, S.F.; Choudhary, M.I. Snake Venom: From Deadly Toxins to Life-saving Therapeutics. Curr. Med. Chem. 2017, 24, 1874–1891. [Google Scholar] [CrossRef]
- Camargo, A.C.M.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M.T. Bradykinin-potentiating peptides: Beyond captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H.; Rocha e Silva, M. Potentiation of bradykinin and eledoisin by BPF (bradykinin potentiating factor) fromBothrops jararaca venom. Experientia 1965, 21, 347–349. [Google Scholar] [CrossRef]
- Kochva, E. The origin of snakes and evolution of the venom apparatus. Toxicon 1987, 25, 65–106. [Google Scholar] [CrossRef]
- Casewell, N.R.; Wüster, W.; Vonk, F.J.; Harrison, R.A.; Fry, B.G. Complex cocktails: The evolutionary novelty of venoms. Trends Ecol. Evol. 2013, 28, 219–229. [Google Scholar] [CrossRef]
- Fry, B.G.; Vidal, N.; Norman, J.A.; Vonk, F.J.; Scheib, H.; Ramjan, S.F.R.; Kuruppu, S.; Fung, K.; Hedges, S.B.; Richardson, M.K.; et al. Early evolution of the venom system in lizards and snakes. Nature 2005, 439, 584–588. [Google Scholar] [CrossRef]
- Underwood, G. An overview of venomous snake evolution. In Venomous Snakes: Ecology, Evolution and Snakebite; Clarendon Press: Oxford, UK, 1997; pp. 1–13. [Google Scholar]
- Chippaux, J.P.; Williams, V.; White, J. Snake venom variability: Methods of study, results and interpretation. Toxicon 1991, 29, 1279–1303. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Calvete, J.J.; Habib, A.G.; Harrison, R.A.; Williams, D.J.; Warrell, D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Fox, J.W.; Serrano, S.M.T. Timeline of key events in snake venom metalloproteinase research. J. Proteom. 2009, 72, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, J.; Lin, Y. Snake Venoms in Cancer Therapy: Past, Present and Future. Toxins 2018, 10, 346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calderon, L.A.; Sobrinho, J.C.; Zaqueo, K.D.; de Moura, A.A.; Grabner, A.N.; Mazzi, M.V.; Marcussi, S.; Nomizo, A.; Fernandes, C.F.C.; Zuliani, J.P.; et al. Antitumoral Activity of Snake Venom Proteins: New Trends in Cancer Therapy. Biomed Res. Int. 2014, 2014, 203639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, D.C.I.; Armugam, A.; Jeyaseelan, K. Snake venom components and their applications in biomedicine. Cell. Mol. Life Sci. 2006, 63, 3030–3041. [Google Scholar] [CrossRef]
- Sanhajariya, S.; Duffull, S.B.; Isbister, G.K. Pharmacokinetics of snake venom. Toxins 2018, 10, 73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, H.; Pan, H.; Liao, K.; Yang, M.; Huang, C. Snake Venom PLA2, a Promising Target for Broad-Spectrum Antivenom Drug Development. Biomed Res. Int. 2017, 2017, 6592820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gutiérrez, J.M.; Lomonte, B. Phospholipases A2: Unveiling the secrets of a functionally versatile group of snake venom toxins. Toxicon 2013, 62, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Ferraz, C.R.; Arrahman, A.; Xie, C.; Casewell, N.R.; Lewis, R.J.; Kool, J.; Cardoso, F.C. Multifunctional toxins in snake venoms and therapeutic implications: From pain to hemorrhage and necrosis. Front. Ecol. Evol. 2019, 7, 218. [Google Scholar] [CrossRef] [Green Version]
- El-Aziz, T.M.A.; Soares, A.G.; Stockand, J.D. Snake Venoms in Drug Discovery: Valuable Therapeutic Tools for Life Saving. Toxins 2019, 11, 564. [Google Scholar] [CrossRef] [Green Version]
- Mackessy, S.P. Thrombin-Like Enzymes in Snake Venoms. In Toxins and Hemostasis; Springer: Heidelberg, Germany, 2010; pp. 519–557. [Google Scholar] [CrossRef]
- Slagboom, J.; Kool, J.; Harrison, R.A.; Casewell, N.R. Haemotoxic snake venoms: Their functional activity, impact on snakebite victims and pharmaceutical promise. Br. J. Haematol. 2017, 177, 947–959. [Google Scholar] [CrossRef] [Green Version]
- Izidoro, L.F.M.; Sobrinho, J.C.; Mendes, M.M.; Costa, T.R.; Grabner, A.N.; Rodrigues, V.M.; Da Silva, S.L.; Zanchi, F.B.; Zuliani, J.P.; Fernandes, C.F.C.; et al. Snake venom L-amino acid oxidases: Trends in pharmacology and biochemistry. Biomed Res. Int. 2014, 2014, 196754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bickler, P.E. Amplification of Snake Venom Toxicity by Endogenous Signaling Pathways. Toxins 2020, 12, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rivas-Mercado, E.A.; Garza-Ocañas, L. Disintegrins obtained from snake venom and their pharmacological potential. Med. Univ. 2017, 19, 32–37. [Google Scholar] [CrossRef]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Eble, J.A. Structurally Robust and Functionally Highly Versatile—C-Type Lectin (-Related) Proteins in Snake Venoms. Toxins 2019, 11, 136. [Google Scholar] [CrossRef] [Green Version]
- Adade, C.M.; Carvalho, A.L.O.; Tomaz, M.A.; Costa, T.F.R.; Godinho, J.L.; Melo, P.A.; Lima, A.P.C.A.; Rodrigues, J.C.F.; Zingali, R.B.; Souto-Padrón, T. Crovirin, a Snake Venom Cysteine-Rich Secretory Protein (CRISP) with Promising Activity against Trypanosomes and Leishmania. PLoS Negl. Trop. Dis. 2014, 8, e3252. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Morita, T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon 2004, 44, 227–231. [Google Scholar] [CrossRef]
- Péterfi, O.; Boda, F.; Szabó, Z.; Ferencz, E.; Bába, L. Hypotensive Snake Venom Components—A Mini-Review. Molecules 2019, 24, 2778. [Google Scholar] [CrossRef] [Green Version]
- Murayama, N.; Hayashi, M.A.F.; Ohi, H.; Ferreira, L.A.F.; Hermann, V.V.; Saito, H.; Fujita, Y.; Higuchi, S.; Fernandes, B.L.; Yamane, T.; et al. Cloning and sequence analysis of a Bothrops jararaca cDNA encoding a precursor of seven bradykinin-potentiating peptides and a C-type natriuretic peptide. Proc. Natl. Acad. Sci. USA 1997, 94, 1189–1193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef]
- Kronemberger, G.S.; Carneiro, F.A.; Rezende, D.F.; Baptista, L.S. Spheroids and organoids as humanized 3D scaffold-free engineered tissues for SARS-CoV-2 viral infection and drug screening. Artif. Organs 2021, 45, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, E.M.; Yamamoto, M.; Park, H.; Shin, H. Engineering Multi-Cellular Spheroids for Tissue Engineering and Regenerative Medicine. Adv. Healthc. Mater. 2020, 9, 2000608. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2018, 116, 206–226. [Google Scholar] [CrossRef] [Green Version]
- Kim, J. Bin Three-dimensional tissue culture models in cancer biology. Semin. Cancer Biol. 2005, 15, 365–377. [Google Scholar] [CrossRef]
- Bazaa, A.; Luis, J.; Srairi-Abid, N.; Kallech-Ziri, O.; Kessentini-Zouari, R.; Defilles, C.; Lissitzky, J.-C.; El Ayeb, M.; Marrakchi, N. MVL-PLA2, a phospholipase A2 from Macrovipera lebetina transmediterranea venom, inhibits tumor cells adhesion and migration. Matrix Biol. 2009, 28, 188–193. [Google Scholar] [CrossRef]
- Bazaa, A.; Pasquier, E.; Defilles, C.; Limam, I.; Kessentini-Zouari, R.; Kallech-Ziri, O.; El Battari, A.; Braguer, D.; El Ayeb, M.; Marrakchi, N.; et al. MVL-PLA2, a snake venom phospholipase A2, inhibits angiogenesis through an increase in microtubule dynamics and disorganization of focal adhesions. PLoS ONE 2010, 5, e10124. [Google Scholar] [CrossRef] [Green Version]
- Zouari-Kessentini, R.; Srairi-Abid, N.; Bazaa, A.; El Ayeb, M.; Luis, J.; Marrakchi, N. Antitumoral potential of Tunisian snake venoms secreted phospholipases A2. Biomed Res. Int. 2013, 2013, 391389. [Google Scholar] [CrossRef] [Green Version]
- Kato, E.E.; Pimenta, L.A.; de Almeida, M.E.S.; Zambelli, V.O.; Santos, M.F.; Sampaio, S.C. Crotoxin Inhibits Endothelial Cell Functions in Two- and Three-dimensional Tumor Microenvironment. Front. Pharmacol. 2021, 12, 1. [Google Scholar] [CrossRef]
- De Vasconcelos Azevedo, F.V.P.; Zóia, M.A.P.; Lopes, D.S.; Gimenes, S.N.; Vecchi, L.; Alves, P.T.; Rodrigues, R.S.; Silva, A.C.A.; Yoneyama, K.A.G.; Goulart, L.R.; et al. Antitumor and antimetastatic effects of PLA2-BthTX-II from Bothrops jararacussu venom on human breast cancer cells. Int. J. Biol. Macromol. 2019, 135, 261–273. [Google Scholar] [CrossRef]
- Van Petten de Vasconcelos Azevedo, F.; Lopes, D.S.; Zóia, M.A.P.; Correia, L.I.V.; Saito, N.; Fonseca, B.B.; Polloni, L.; Teixeira, S.C.; Goulart, L.R.; de Melo Rodrigues Ávila, V. A New Approach to Inhibiting Triple-Negative Breast Cancer: In Vitro, Ex Vivo and In Vivo Antiangiogenic Effect of BthTx-II, a PLA2-Asp-49 from Bothrops jararacussu Venom. Biomolecules 2022, 12, 258. [Google Scholar] [CrossRef]
- Kato, E.E.; Sampaio, S.C. Crotoxin Modulates Events Involved in Epithelial–Mesenchymal Transition in 3D Spheroid Model. Toxins 2021, 13, 830. [Google Scholar] [CrossRef] [PubMed]
- Mambelli-Lisboa, N.C.; Sciani, J.M.; da Silva, A.R.B.P.; Kerkis, I. Co-Localization of Crotamine with Internal Membranes and Accentuated Accumulation in Tumor Cells. Molecules 2018, 23, 968. [Google Scholar] [CrossRef] [Green Version]
- Arruda Macêdo, J.K.; Fox, J.W.; de Souza Castro, M. Disintegrins from snake venoms and their applications in cancer research and therapy. Curr. Protein Pept. Sci. 2015, 16, 532–548. [Google Scholar] [CrossRef] [Green Version]
- Calvete, J.J.; Marcinkiewicz, C.; Monleón, D.; Esteve, V.; Celda, B.; Juárez, P.; Sanz, L. Snake venom disintegrins: Evolution of structure and function. Toxicon 2005, 45, 1063–1074. [Google Scholar] [CrossRef]
- Swenson, S.D.; Markland, F.S.; Minea, R. A Novel, Non-Cytotoxic, Anti-Invasive Therapeutic Agent for Ovarian Cancer. In Advances in Biological Sciences Research; Atlantis Press: Paris, France, 2016; pp. 159–165. [Google Scholar] [CrossRef] [Green Version]
- Bhat, S.K.; Joshi, M.B.; Vasishta, S.; Jagadale, R.N.; Biligiri, S.G.; Coronado, M.A.; Arni, R.K.; Satyamoorthy, K. P-I metalloproteinases and L-amino acid oxidases from Bothrops species inhibit angiogenesis. J. Venom. Anim. Toxins Incl. Trop. Dis. 2021, 27, 1–15. [Google Scholar] [CrossRef]
- Takeda, S.; Takeya, H.; Iwanaga, S. Snake venom metalloproteinases: Structure, function and relevance to the mammalian ADAM/ADAMTS family proteins. Biochim. Biophys. Acta-Proteins Proteom. 2012, 1824, 164–176. [Google Scholar] [CrossRef]
- Drost, J.; Clevers, H. Organoids in cancer research. Nat. Rev. Cancer 2018, 18, 407–418. [Google Scholar] [CrossRef]
- Kretzschmar, K. Cancer research using organoid technology. J. Mol. Med. 2021, 99, 501–515. [Google Scholar] [CrossRef] [PubMed]
- Post, Y.; Puschhof, J.; Beumer, J.; Kerkkamp, H.M.; de Bakker, M.A.G.; Slagboom, J.; de Barbanson, B.; Wevers, N.R.; Spijkers, X.M.; Olivier, T.; et al. Snake Venom Gland Organoids. Cell 2020, 180, 233–247.e21. [Google Scholar] [CrossRef]
- Puschhof, J.; Post, Y.; Beumer, J.; Kerkkamp, H.M.; Bittenbinder, M.; Vonk, F.J.; Casewell, N.R.; Richardson, M.K.; Clevers, H. Derivation of snake venom gland organoids for in vitro venom production. Nat. Protoc. 2021, 16, 1494–1510. [Google Scholar] [CrossRef]
- Vogt, N. Venomous organoids. Nat. Methods 2020, 17, 360. [Google Scholar] [CrossRef]
- Carneiro, S.M.; Zablith, M.B.; Kerchove, C.M.; Moura-Da-Silva, A.M.; Quissell, D.O.; Markus, R.P.; Yamanouye, N. Venom production in long-term primary culture of secretory cells of the Bothrops jararaca venom gland. Toxicon 2006, 47, 87–94. [Google Scholar] [CrossRef]
- Yamanouye, N.; Kerchove, C.M.; Moura-da-Silva, A.M.; Carneiro, S.M.; Markus, R.P. Long-term primary culture of secretory cells of Bothrops jararaca venom gland for venom production in vitro. Nat. Protoc. 2007, 1, 2763–2766. [Google Scholar] [CrossRef]
- Viana, L.G.; Valente, R.H.; Heluany, C.S.; Souza-Imberg, A.; Luna, M.S.; Perales, J.; Yamanouye, N. Bothrops jararaca venom gland secretory cells in culture: Effects of noradrenaline on toxin production and secretion. Toxicon 2017, 133, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.S.; Valente, R.H.; Perales, J.; Vieira, M.L.; Yamanouye, N. Activation of Bothrops jararaca snake venom gland and venom production: A proteomic approach. J. Proteom. 2013, 94, 460–472. [Google Scholar] [CrossRef] [PubMed]
| Snake Venom Toxin Family | Snake Family | Toxicological Effects | Pharmacological Effects | Reviewed by |
|---|---|---|---|---|
| PLA2 | Viperidae, Elapidae, Colubridae. | Neurotoxicity, Myotoxicity, Cytotoxicity, Cardiotoxicity, Hemolysis, Edema, Hyperalgesia | Antiinflammatory Analgesic, Antitumoral, Antiangiogenic, Antibacterial, Antiviral | [26,27] |
| SVMP | Viperidae, Elapidae, Atractaspididae, Colubridae. | Hemorrhage, Myonecrosis, Coagulopathy, Tissue Damage | - | [28,29] |
| SVSP | Viperidae, Elapidae, Colubridae. | Hemotoxic, Rupture Capillary Vessels, Pro-coagulant or Anti-coagulant, Fibrinolysis, Platelet Aggregation | Thrombolytic | [30,31] |
| LAAO | Viperidae, Elapidae. | Apoptosis, Hemorrhage, Cytotoxicity, Edema | Antibacterial, Antitumoral, Antiprotozoan, Antiviral | [32] |
| 3FTx | Elapidae, Colubridae. | Neurotoxicity, Paralysis | Analgesic | [28] |
| DIS | Viperidae, Atractaspididae, Elapidae, Colubridae. | Inhibit Cell-ECM, Loosen Anchoring Tissue | Antitumor, Anti-platelet | [33,34] |
| CTL | Viperidae, Elapidae. | Induction or inhibition of platelet aggregation | Antimetastatic, Antiangiogenic | [35,36] |
| CRISP | Viperidae, Elapidae, Colubridae. | Myotoxicity, Inhibition of smooth muscle contraction | Antiparasitary | [37,38] |
| BPP | Viperidae. | Hypotension | Anti-hypertensive | [39,40] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kato, E.E.; Viala, V.L.; Sampaio, S.C. Snake Venom and 3D Microenvironment Cell Culture: From Production to Drug Development. Future Pharmacol. 2022, 2, 117-125. https://doi.org/10.3390/futurepharmacol2020009
Kato EE, Viala VL, Sampaio SC. Snake Venom and 3D Microenvironment Cell Culture: From Production to Drug Development. Future Pharmacology. 2022; 2(2):117-125. https://doi.org/10.3390/futurepharmacol2020009
Chicago/Turabian StyleKato, Ellen Emi, Vincent Louis Viala, and Sandra Coccuzzo Sampaio. 2022. "Snake Venom and 3D Microenvironment Cell Culture: From Production to Drug Development" Future Pharmacology 2, no. 2: 117-125. https://doi.org/10.3390/futurepharmacol2020009
APA StyleKato, E. E., Viala, V. L., & Sampaio, S. C. (2022). Snake Venom and 3D Microenvironment Cell Culture: From Production to Drug Development. Future Pharmacology, 2(2), 117-125. https://doi.org/10.3390/futurepharmacol2020009






