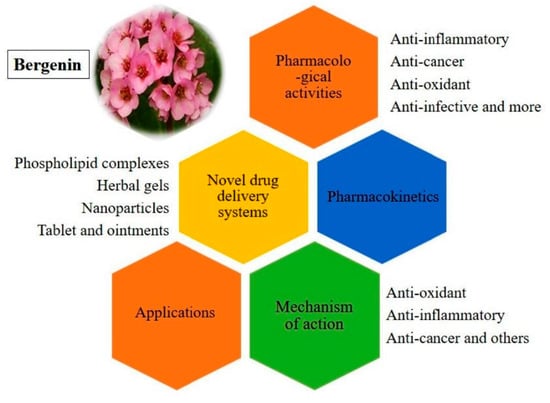
A Fresh Look on Bergenin: Vision of Its Novel Drug Delivery Systems and Pharmacological Activities
Abstract
1. Introduction
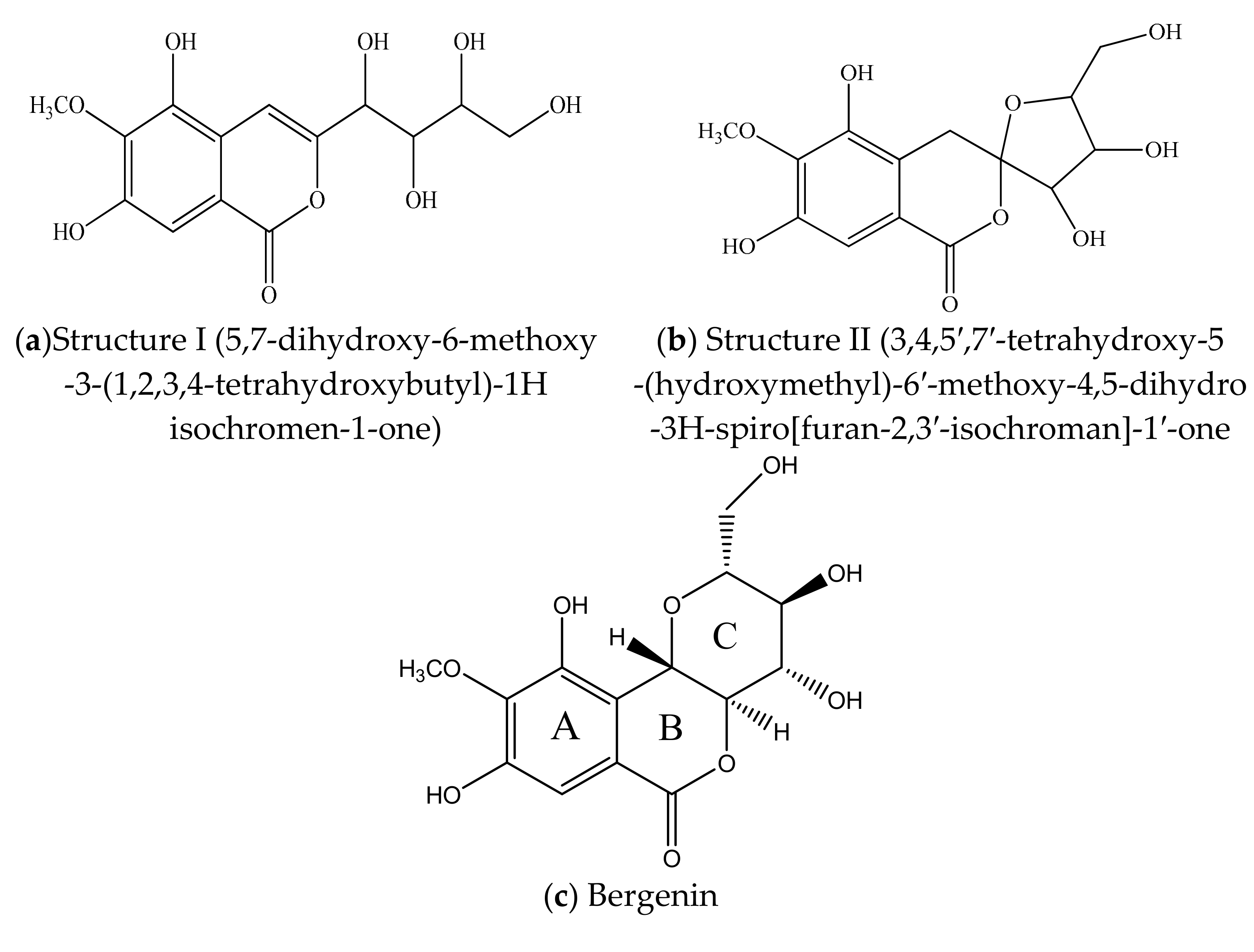
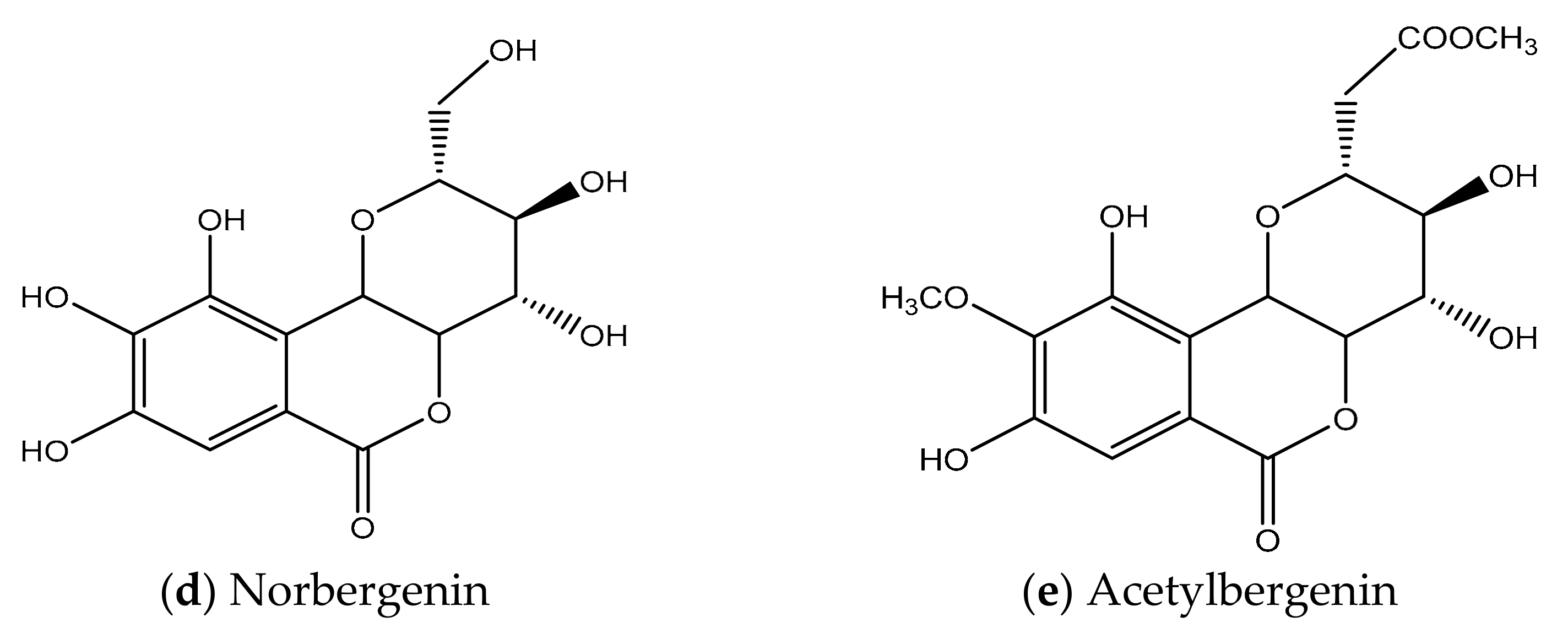
2. Chemical Structure & Physicochemical Properties of BER
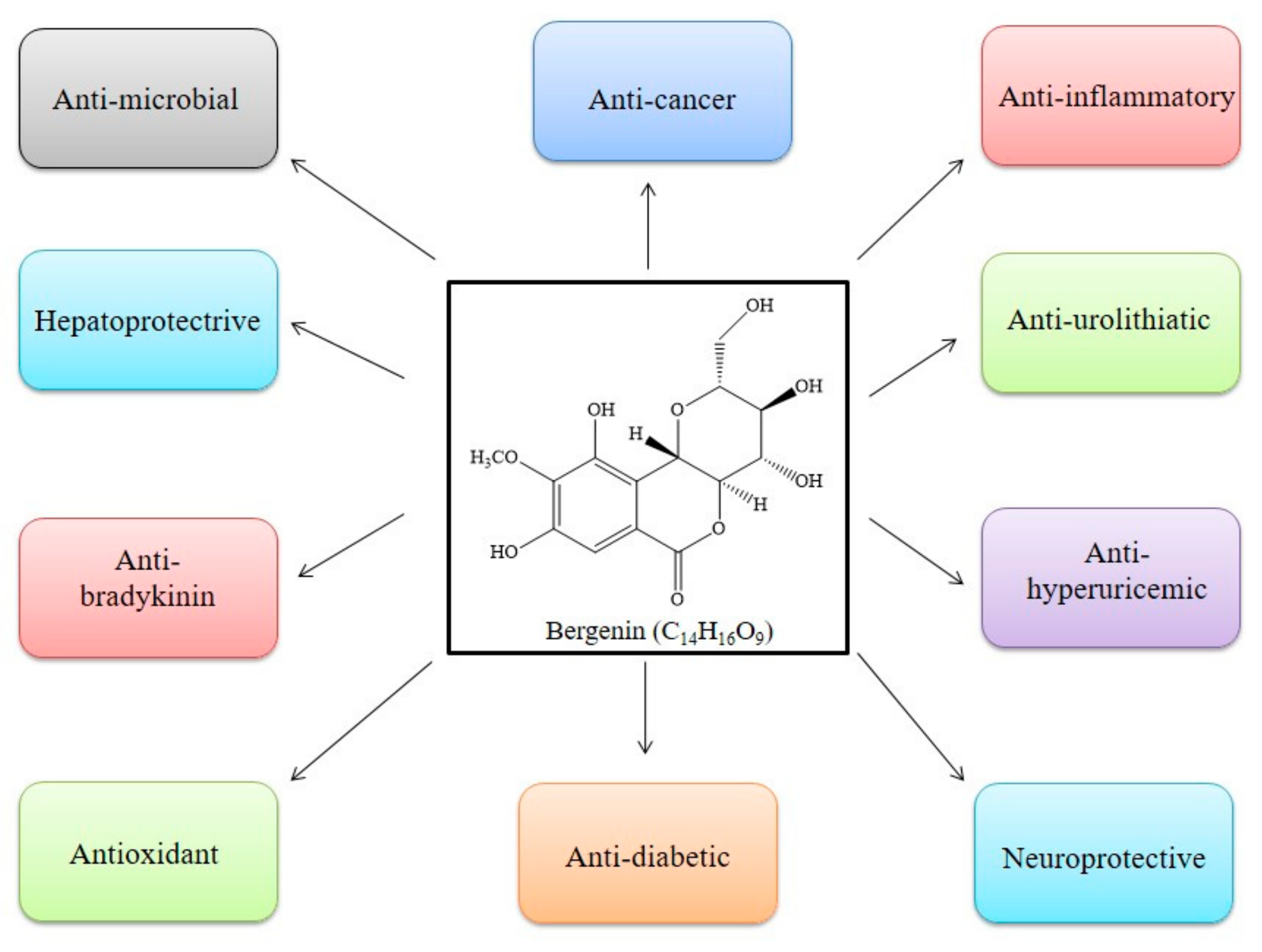
3. Mode of Action of BER
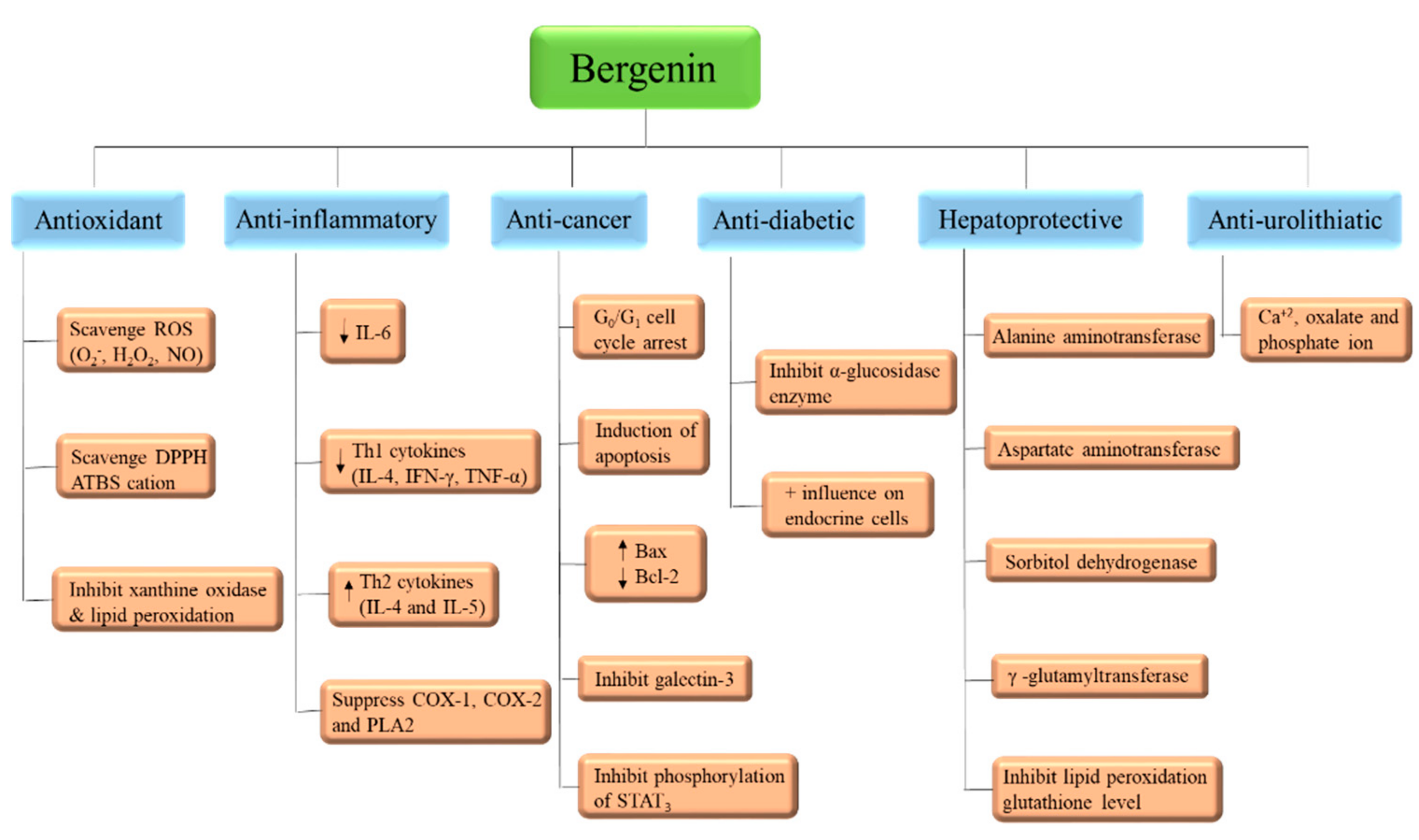
3.1. Antioxidant
3.2. Anti-Inflammatory
3.3. Anti-Microbial
3.4. Anti-Cancer
3.5. Anti-Diabetic
3.6. Neuroprotective
3.7. Hepatoprotective
3.8. Anti-Hyperuricemic
3.9. Immunomodulatory
4. Applications
4.1. Anxiolytic
4.2. Antimalarial
4.3. Antituberculosis
4.4. Antiplatelet Aggregation
4.5. Antihyperlipidemic
4.6. Anti-Arrhythmic
4.7. Anti-Ulcer
4.8. Wound Healing
4.9. Bone Healing
4.10. Anti-Bronchitis
4.11. Antileishmania
5. Bergenin Novel Formulations
5.1. Phospholipid Complex
5.2. Phospholipid Complex Solid Dispersion
5.3. Coated Floating Tablets
5.4. Prodrug
5.5. Herbal Gel
5.6. Nanoparticles
5.7. Poly (Lactic Acid) Polymer
5.8. Sustained Release Capsule
6. Pharmacokinetics
7. Conclusions: The Road Ahead
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.; Xu, F.-R.; Wang, Y.-Z. Traditional Uses, Chemical Diversity and Biological Activities of Panax L.(Araliaceae): A Review. J. Ethnopharmacol. 2020, 263, 112792. [Google Scholar] [CrossRef] [PubMed]
- Nazir, N.; Koul, S.; Qurishi, M.A.; Najar, M.H.; Zargar, M.I. Evaluation of Antioxidant and Antimicrobial Activities of Bergenin and Its Derivatives Obtained by Chemoenzymatic Synthesis. Eur. J. Med. Chem. 2011, 46, 2415–2420. [Google Scholar] [CrossRef] [PubMed]
- Alkadi, K.A.; Adam, A.; Taha, M.; Hasan, M.H.; Shah, S.A.A. Antiplatelet Aggregation Activity of 5-Hydroxyflavone, 2′-Hydroxyflavanone, Paeonol and Bergenin Isolated from Stem Bark of Garcinia Malaccensis in Human Whole Blood. Orient. J. Chem. 2013, 29, 871. [Google Scholar] [CrossRef][Green Version]
- Mehta, P.; Pawar, A.; Mahadik, K.; Bothiraja, C. Emerging Novel Drug Delivery Strategies for Bioactive Flavonol Fisetin in Biomedicine. Biomed. Pharmacother. 2018, 106, 1282–1291. [Google Scholar] [CrossRef]
- Bajracharya, G.B. Diversity, Pharmacology and Synthesis of Bergenin and Its Derivatives: Potential Materials for Therapeutic Usages. Fitoterapia 2015, 101, 133–152. [Google Scholar] [CrossRef]
- Kour, H.; Raina, R.; Verma, P.K.; Pankaj, N.K.; Singh, S.P. Phytochemical Ingredients and Pharmacological Properties of Bergenia Ciliata. J. Vet. Pharmacol. Toxicol. 2019, 18, 1–10. [Google Scholar]
- Singh, M.; Pandey, N.; Agnihotri, V.; Singh, K.K.; Pandey, A. Antioxidant, Antimicrobial Activity and Bioactive Compounds of Bergenia Ciliata Sternb.: A Valuable Medicinal Herb of Sikkim Himalaya. J. Tradit. Complement. Med. 2017, 7, 152–157. [Google Scholar] [CrossRef]
- Ren, Y.; Wan, C.; Liao, M.; Zhang, X.; Cheng, X.; Yuan, L.; Zhang, L. Pharmacokinetics and Excretion Study of Bergenin and Its Phase II Metabolite in Rats by Liquid Chromatography Tandem Mass Spectrometry. Biomed. Chromatogr. 2019, 33, e4513. [Google Scholar] [CrossRef]
- Patel, D.K.; Patel, K.; Kumar, R.; Gadewar, M.; Tahilyani, V. Pharmacological and Analytical Aspects of Bergenin: A Concise Report. Asian Pac. J. Trop. Dis. 2012, 2, 163–167. [Google Scholar] [CrossRef]
- Stylos, E.; Chatziathanasiadou, M.V.; Syriopoulou, A.; Tzakos, A.G. Liquid Chromatography Coupled with Tandem Mass Spectrometry (LC–MS/MS) Based Bioavailability Determination of the Major Classes of Phytochemicals. J. Chromatogr. B 2017, 1047, 15–38. [Google Scholar] [CrossRef]
- Pradhan, B.K.; Badola, H.K. Ethnomedicinal Plant Use by Lepcha Tribe of Dzongu Valley, Bordering Khangchendzonga Biosphere Reserve, in North Sikkim, India. J. Ethnobiol. Ethnomed. 2008, 4, 22. [Google Scholar] [CrossRef] [PubMed]
- Rai, L.K.; Prasad, P.; Sharma, E. Conservation Threats to Some Important Medicinal Plants of the Sikkim Himalaya. Biol. Conserv. 2000, 93, 27–33. [Google Scholar] [CrossRef]
- Sajad, T.; Zargar, A.; Ahmad, T.; Bader, G.N.; Naime, M.; Ali, S. Antibacterial and Anti-Inflammatory Potential Bergenia Ligulata. Am. J. Biomed. Sci 2010, 2, 313–321. [Google Scholar] [CrossRef]
- Walter, N.S.; Bagai, U.; Kalia, S. Antimalarial Activity of Bergenia Ciliata (Haw.) Sternb. against Plasmodium Berghei. Parasitol. Res. 2013, 112, 3123–3128. [Google Scholar] [CrossRef]
- Shakeel, F.; AlAjmi, M.F.; Haq, N.; Siddiqui, N.A.; Alam, P.; Al-Rehaily, A.J. Solubility and Thermodynamic Function of a Bioactive Compound Bergenin in Various Pharmaceutically Acceptable Neat Solvents at Different Temperatures. J. Chem. Thermodyn. 2016, 101, 19–24. [Google Scholar] [CrossRef]
- Malik, P.; Bhatia, V. Comparative Testing of Antibacterial Activity of Aqueous Extract of Bergenia Ligulata Rhizomes and Ethanolic Extract of Butea Monosperma Flowers for Herbal Gel Formulation. IOSRJPBS 2017, 12, 89–94. [Google Scholar] [CrossRef]
- Shi, X.; Xu, M.; Luo, K.; Huang, W.; Yu, H.; Zhou, T. Anticancer Activity of Bergenin against Cervical Cancer Cells Involves Apoptosis, Cell Cycle Arrest, Inhibition of Cell Migration and the STAT3 Signalling Pathway. Exp. Ther. Med. 2019, 17, 3525–3529. [Google Scholar] [CrossRef]
- Bharate, S.B.; Kumar, V.; Bharate, S.S.; Singh, B.; Singh, G.; Singh, A.; Gupta, M.; Singh, D.; Kumar, A.; Singh, S. Discovery and Preclinical Development of IIIM-160, a Bergenia Ciliata-Based Anti-Inflammatory and Anti-Arthritic Botanical Drug Candidate. J. Integr. Med. 2019, 17, 192–204. [Google Scholar] [CrossRef]
- Rao, K.; Roome, T.; Aziz, S.; Razzak, A.; Abbas, G.; Imran, M.; Jabri, T.; Gul, J.; Hussain, M.; Sikandar, B. Bergenin Loaded Gum Xanthan Stabilized Silver Nanoparticles Suppress Synovial Inflammation through Modulation of the Immune Response and Oxidative Stress in Adjuvant Induced Arthritic Rats. J. Mater. Chem. B 2018, 6, 4486–4501. [Google Scholar] [CrossRef]
- Nazir, N.; Koul, S.; Qurishi, M.A.; Taneja, S.C.; Ahmad, S.F.; Bani, S.; Qazi, G.N. Immunomodulatory Effect of Bergenin and Norbergenin against Adjuvant-Induced Arthritis—A Flow Cytometric Study. J. Ethnopharmacol. 2007, 112, 401–405. [Google Scholar] [CrossRef]
- Uddin, G.; Sadat, A.; Siddiqui, B.S. Comparative Antioxidant and Antiplasmodial Activities of 11-O-Galloylbergenin and Bergenin Isolated from Bergenia Ligulata. Trop. Biomed. 2014, 31, 143–148. [Google Scholar] [PubMed]
- Roselli, M.; Lentini, G.; Habtemariam, S. Phytochemical, Antioxidant and Anti-α-Glucosidase Activity Evaluations of Bergenia Cordifolia. Phytother. Res. 2012, 26, 908–914. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, D.K.; Prasad, S.K.; Laloo, D.; Krishnamurthy, S.; Hemalatha, S. Type 2 Antidiabetic Activity of Bergenin from the Roots of Caesalpinia Digyna Rottler. Fitoterapia 2012, 83, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Kosaka, M.; Watanabe, Y.; Nakade, K.; Fukuyama, Y. Synthesis and Neuroprotective Activity of Bergenin Derivatives with Antioxidant Activity. Bioorganic. Med. Chem. 2003, 11, 1781–1788. [Google Scholar] [CrossRef]
- Lim, H.-K.; Kim, H.-S.; Choi, H.-S.; Oh, S.; Choi, J. Hepatoprotective Effects of Bergenin, a Major Constituent of Mallotus Japonicus, on Carbon Tetrachloride-Intoxicated Rats. J. Ethnopharmacol. 2000, 72, 469–474. [Google Scholar] [CrossRef]
- Patel, T.B.; Golwala, D.K.; Vaidya, S.K. Antiurolithiatic Activity of Alcoholic Leaf Extract of Mallotus Philippinensis Lam. against Ethylene Glycol Induced Urolithiasis in Rats. Indian J. Pharmacol 2016, 48, 270–274. [Google Scholar]
- Liang, J.; Li, Y.; Liu, X.; Huang, Y.; Shen, Y.; Wang, J.; Liu, Z.; Zhao, Y. In vivo and in vitro Antimalarial Activity of Bergenin. Biomed. Rep. 2014, 2, 260–264. [Google Scholar] [CrossRef]
- Dwivedi, V.P.; Bhattacharya, D.; Yadav, V.; Singh, D.K.; Kumar, S.; Singh, M.; Ojha, D.; Ranganathan, A.; Van Kaer, L.; Chattopadhyay, D. The Phytochemical Bergenin Enhances T Helper 1 Responses and Anti-Mycobacterial Immunity by Activating the MAP Kinase Pathway in Macrophages. Front. Cell. Infect. Microbiol. 2017, 7, 149. [Google Scholar] [CrossRef]
- Jahromi, M.F.; Chansouria, J.P.N.; Ray, A.B. Hypolipidaemic Activity in Rats of Bergenin, the Major Constituent of Flueggea Microcarpa. Phytother. Res. 1992, 6, 180–183. [Google Scholar] [CrossRef]
- Piacente, S.; Pizza, C.; De Tommasi, N.; Mahmood, N. Constituents of Ardisia Japonica and Their in Vitro Anti-HIV Activity. J. Nat. Prod. 1996, 59, 565–569. [Google Scholar] [CrossRef]
- Pu, H.-L.; Huang, X.; Zhao, J.-H.; Hong, A. Bergenin Is the Antiarrhythmic Principle of Fluggea Virosa. Planta Med. 2002, 68, 372–374. [Google Scholar] [CrossRef] [PubMed]
- Goel, R.K.; Maiti, R.N.; Manickam, M.; Ray, A.B. Antiulcer Activity of Naturally Occurring Pyrano-Coumarin and Isocoumarins and Their Effect on Prostanoid Synthesis Using Human Colonic Mucosa. Indian J. Exp. Biol. 1997, 35, 1080–1083. [Google Scholar] [PubMed]
- Magaji, M.G.; Musa, A.M.; Abdullahi, M.I.; Ya’u, J.; Hussaini, I.M. Isolation of Bergenin from the Root Bark of Securinega Virosa and Evaluation of Its Potential Sleep Promoting Effect. Avicenna J. Phytomed. 2015, 5, 587. [Google Scholar] [PubMed]
- Rajbhandari, M.; Mentel, R.; Jha, P.K.; Chaudhary, R.P.; Bhattarai, S.; Gewali, M.B.; Karmacharya, N.; Hipper, M.; Lindequist, U. Antiviral Activity of Some Plants Used in Nepalese Traditional Medicine. Evid.-Based Complement. Altern. Med. 2009, 6, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Murugesan, T.; Pal, M.; Saha, B.P. Evaluation of Anti-Tussive Activity of Bergenia Ciliata Sternb. Rhizome Extract in Mice. Phytomedicine 2001, 8, 298–301. [Google Scholar] [CrossRef]
- Xu, L.-J.; Liu, A.-L.; Du, G.-H. Bergenin. In Natural Small Molecule Drugs from Plants; Du, G.-H., Ed.; Springer: Singapore, 2018; pp. 379–384. ISBN 978-981-10-8022-7. [Google Scholar]
- Zhou, D.; Qin, X.; Zhang, Z.-R.; Huang, Y. Physicochemical Properties of Bergenin. Available online: https://www.ingentaconnect.com/content/govi/pharmaz/2008/00000063/00000005/art00008 (accessed on 29 December 2021).
- Tschitschibabin, A.E.; Kirssanow, A.W.; Korolew, A.J.; Woroschzow, N.N. Über Nichtgerbende Substanzen Des Extraktes Aus Dem Wurzelstock Des Badans (Saxifraga Crassifolia). I. Bergenin. Justus Liebigs Ann. Chem. 1929, 469, 93–127. [Google Scholar] [CrossRef]
- Hay, J.E.; Haynes, L.J. 453. Bergenin, a C-Glycopyranosyl Derivative of 4-O-Methylgallic Acid. J. Chem. Soc. Resumed 1958, 2231–2238. [Google Scholar] [CrossRef]
- Posternak, T.; Dürr, K. Sur La Constitution de La Bergénine. Helv. Chim. Acta 1958, 41, 1159–1162. [Google Scholar] [CrossRef]
- Fujise, S.; Suzuki, M.; Watanabe, Y.; Matsueda, S. Studies of the Structure of Bergenin. Bull. Chem. Soc. Jpn. 1959, 32, 97–98. [Google Scholar] [CrossRef]
- Caldas, C.S.; De Simone, C.A.; Pereira, M.A.; Malta, V.R.S.; Carvalho, R.L.P.; Da Silva, T.B.C.; Sant’ana, A.E.G.; Conserva, L.M. Bergenin Monohydrate, a Constituent of Hurmiria Balsamifera, at 120 K. Acta Cryst. E 2002, 58, o609–o611. [Google Scholar] [CrossRef]
- Rastogi, S.; RAwAt, A.K.S. A Comprehensive Review on Bergenin, a Potential Hepatoprotective and Antioxidative Phytoconstituent. Herba Pol. 2008, 54, 66–79. [Google Scholar]
- Ye, Y.-P.; Sun, H.-X.; Pan, Y.-J. Bergenin Monohydrate from the Rhizomae of Astilbe Chinensis. Acta Cryst. C 2004, 60, o397–o398. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhong, Y.; Duan, Y.; Chen, Q.; Li, F. Antioxidant Mechanism of Tea Polyphenols and Its Impact on Health Benefits. Anim. Nutr. 2020, 6, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Luo, Q.; Sun, M.; Corke, H. Antioxidant Activity and Phenolic Compounds of 112 Traditional Chinese Medicinal Plants Associated with Anticancer. Life Sci. 2004, 74, 2157–2184. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From Enhanced Biosynthesis and Bioavailability to Multitargeting Chronic Diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Tian, B.; Xingke, J.U.; Tian, L.; Li, J.; Ruan, S.; Jia, M.; Tian, D.; Li, H.; Qianqian, Z.; Wang, X. Bergenin Lipoic Acid Ester with Antioxidant Activity and a Method of Preparing the Same. U.S. Patent 10,494,377, 3 December 2019. [Google Scholar]
- Mitra, S.K.; Saxena, E.; Babu, U.V. Herbal Composition for Maintaining/Caring the Skin around the Eye, Methods of Preparing the Same and Uses Thereof 2011. U.S. Patent 20100285162A1, 11 November 2010. [Google Scholar]
- Mitra, S.K.; Saxena, E.; Babu, U.V. Herbal Composition for Maintaining/Caring the Skin around the Eye, Methods of Preparing the Same and Uses Thereof 2008. U.S. Patent 2008/0081085A1, 3 April 2008. [Google Scholar]
- A Pharmaceutical Composition Useful as an Antioxidant—Patent Details. Available online: https://www.quickcompany.in/patents/a-pharmaceutical-composition-useful-as-an-antioxidant (accessed on 30 December 2021).
- Lee, K.; Lee, S.; Lee, K.; Jeong, J. Cosmetic Composition for Remedying Skin Wrinkles Comprising Bergenia Emeiensis Extract as Active Ingredient. U.S. Patent US20040115286A1, 17 June 2004. [Google Scholar]
- Bharate, S.S.; Singh, R.; Gupta, M.; Singh, B.; Katare, A.K.; Kumar, A.; Bharate, S.B.; Vishwakarma, R. Gastroretentive Sustained Release Formulations of Bergenia Ciliata. U.S. Patent US20200316150A1, 8 October 2020. [Google Scholar]
- Venkatadri, R.; Guha, G.; Kumar, R.; Mathew, L. Evaluation of Antioxidant Activities of Bergenia Ciliata Rhizome. Rec. Nat. Prod. 2010, 4, 38–48. [Google Scholar]
- Oliveira, G.A.; Araujo, A.K.; Pacheco, G.; Oliveira, A.P.; Carvalho, J.L.; Chaves, L.S.; Sousa, G.C.; Lopes, A.L.; Silva, P.C.; Leódido, A.C.M. Anti-Inflammatory Properties of Bergenin in Mice. J. Appl. Pharm. Sci. 2019, 9, 69–77. [Google Scholar]
- Luo, C.; Zou, L.; Sun, H.; Peng, J.; Gao, C.; Bao, L.; Ji, R.; Jin, Y.; Sun, S. A Review of the Anti-Inflammatory Effects of Rosmarinic Acid on Inflammatory Diseases. Front. Pharmacol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Ren, X.; Ma, S.; Wang, J.; Tian, S.; Fu, X.; Liu, X.; Li, Z.; Zhao, B.; Wang, X. Comparative Effects of Dexamethasone and Bergenin on Chronic Bronchitis and Their Anti-Inflammatory Mechanisms Based on NMR Metabolomics. Mol. Bio Syst. 2016, 12, 1938–1947. [Google Scholar] [CrossRef]
- Yang, S.; Yu, Z.; Wang, L.; Yuan, T.; Wang, X.; Zhang, X.; Wang, J.; Lv, Y.; Du, G. The Natural Product Bergenin Ameliorates Lipopolysaccharide-Induced Acute Lung Injury by Inhibiting NF-KappaB Activition. J. Ethnopharmacol. 2017, 200, 147–155. [Google Scholar] [CrossRef]
- Chen, M.; Chen, C.; Gao, Y.; Li, D.; Huang, D.; Chen, Z.; Zhao, X.; Huang, Q.; Wu, D.; Lai, T.; et al. Bergenin-Activated SIRT1 Inhibits TNF-α-Induced Proinflammatory Response by Blocking the NF-ΚB Signaling Pathway. Pulm. Pharmacol. Ther. 2020, 62, 101921. [Google Scholar] [CrossRef] [PubMed]
- Joshi, M.; Prabhakar, B. Phytoconstituents and Pharmaco-Therapeutic Benefits of Pitaya: A Wonder Fruit. J. Food Biochem. 2020, 44, e13260. [Google Scholar] [CrossRef] [PubMed]
- Raj, M.K.; Duraipandiyan, V.; Agustin, P.; Ignacimuthu, S. Antimicrobial Activity of Bergenin Isolated from Peltophorum Pterocarpum DC. Flowers. Asian Pac. J. Trop. Biomed. 2012, 2, S901–S904. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, Y.; Yu, C.; Zhang, P.; Gu, S.; Wang, G.; Xiao, H.; Li, S. Bergenin Inhibits Bladder Cancer Progression via Activating the PPARγ/PTEN/Akt Signal Pathway. Drug Dev. Res. 2021, 82, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Jayakody, R.S.; Wijewardhane, P.; Herath, C.; Perera, S. Bergenin: A Computationally Proven Promising Scaffold for Novel Galectin-3 Inhibitors. J. Mol. Modeling 2018, 24, 302. [Google Scholar] [CrossRef]
- Wang, Z.; Zhao, X.; Liu, X.; Lu, W.; Jia, S.; Hong, T.; Li, R.; Zhang, H.; Peng, L.; Zhan, X. Anti-Diabetic Activity Evaluation of a Polysaccharide Extracted from Gynostemma Pentaphyllum. Int. J. Biol. Macromol. 2019, 126, 209–214. [Google Scholar] [CrossRef]
- Rajput, S.A.; Mirza, M.R.; Choudhary, M.I. Bergenin Protects Pancreatic Beta Cells against Cytokine-Induced Apoptosis in INS-1E Cells. PLoS ONE 2020, 15, e0241349. [Google Scholar] [CrossRef]
- Ji, Y.; Wang, D.; Zhang, B.; Lu, H. Bergenin Ameliorates MPTP-Induced Parkinson’s Disease by Activating PI3K/Akt Signaling Pathway. J. Alzheimer’s Dis. 2019, 72, 823–833. [Google Scholar] [CrossRef]
- Barai, P.; Raval, N.; Acharya, S.; Borisa, A.; Bhatt, H.; Acharya, N. Neuroprotective Effects of Bergenin in Alzheimer’s Disease: Investigation through Molecular Docking, in Vitro and in Vivo Studies. Behav. Brain Res. 2019, 356, 18–40. [Google Scholar] [CrossRef]
- Qu, H.; Gao, X.; Zhao, H.-T.; Wang, Z.-Y.; Yi, J.-J. Structural Characterization and in Vitro Hepatoprotective Activity of Polysaccharide from Pine Nut (Pinus Koraiensis Sieb. et Zucc.). Carbohydr. Polym. 2019, 223, 115056. [Google Scholar] [CrossRef]
- Xiang, S.; Chen, K.; Xu, L.; Wang, T.; Guo, C. Bergenin Exerts Hepatoprotective Effects by Inhibiting the Release of Inflammatory Factors, Apoptosis and Autophagy via the PPAR-γ Pathway. Drug Des. Devel. 2020, 14, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, J.; Chen, K.; Feng, J.; Guo, C. Bergenin Attenuates Hepatic Fibrosis by Regulating Autophagy Mediated by the Ppar-Γ/Tgf-B Pathway. PPAR Res. 2020, 2020, 6694214. [Google Scholar] [CrossRef] [PubMed]
- Sriset, Y.; Chatuphonprasert, W.; Jarukamjorn, K. Hepatoprotective Activity of Bergenin against Xenobiotics-Induced Oxidative Stress in Human Hepatoma (HepG2) Cells. CMUJ. Nat. Sci. 2021, 20, e2021011. [Google Scholar]
- Ibrahim, N.; Nuryanti, S.; Hasanuddin, A.; Zubair, M.S. Phytochemical Analysis and Antihyperuricemic Activity of Ethanolic Extract of Moringa Oleifera Seeds. Pharmacogn. J. 2020, 12, 1698–1704. [Google Scholar] [CrossRef]
- Qi, Q.; Dong, Z.; Sun, Y.; Li, S.; Zhao, Z. Protective Effect of Bergenin against Cyclophosphamide-Induced Immunosuppression by Immunomodulatory Effect and Antioxidation in Balb/c Mice. Molecules 2018, 23, 2668. [Google Scholar] [CrossRef]
- Singh, J.; Kumar, A.; Sharma, A. Antianxiety Activity Guided Isolation and Characterization of Bergenin from Caesalpinia Digyna Rottler Roots. J. Ethnopharmacol. 2017, 195, 182–187. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, Z.; Kong, F.; Gao, F. Current Scenario of Ferrocene-Containing Hybrids for Antimalarial Activity. Eur. J. Med. Chem. 2020, 185, 111791. [Google Scholar] [CrossRef]
- Singh, S.V.; Manhas, A.; Kumar, Y.; Mishra, S.; Shanker, K.; Khan, F.; Srivastava, K.; Pal, A. Antimalarial Activity and Safety Assessment of Flueggea Virosa Leaves and Its Major Constituent with Special Emphasis on Their Mode of Action. Biomed. Pharmacother. 2017, 89, 761–771. [Google Scholar] [CrossRef]
- Kumar, S.; Sharma, C.; Kaushik, S.R.; Kulshreshtha, A.; Chaturvedi, S.; Nanda, R.K.; Bhaskar, A.; Chattopadhyay, D.; Das, G.; Dwivedi, V.P. The Phytochemical Bergenin as an Adjunct Immunotherapy for Tuberculosis in Mice. J. Biol. Chem. 2019, 294, 8555–8563. [Google Scholar] [CrossRef]
- Kim, K.-M.; Kim, J.; Baek, M.-C.; Bae, J.-S. Novel Factor Xa Inhibitor, Maslinic Acid, with Antiplatelet Aggregation Activity. J. Cell. Physiol. 2020, 235, 9445–9456. [Google Scholar] [CrossRef]
- Varsha, D.; Shubhangi, S.; Mangesh, P.; Naikwade, N.S. Antihyperlipidemic Activity of Cinnamomum Tamala Nees. on High Cholesterol Diet Induced Hyperlipidemia. Int. J. PharmTech Res. 2010, 2, 2517–2521. [Google Scholar]
- Beik, A.; Joukar, S.; Najafipour, H. A Review on Plants and Herbal Components with Antiarrhythmic Activities and Their Interaction with Current Cardiac Drugs. J. Tradit. Complement. Med. 2020, 10, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.K.; Sahoo, H.B.; Priyadarshini, D.; Soundarya, G.; Kumar, C.K.; Rani, K.U. Antiulcer Activity of Ethanolic Extract of Salvadora Indica (W.) Leaves on Albino Rats. J. Clin. Diagn. Res. JCDR 2016, 10, FF07. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Li, Y.; Lv, Q.; Li, X.; Dai, Y.; Wei, Z. Bergenin, Acting as an Agonist of PPARγ, Ameliorates Experimental Colitis in Mice through Improving Expression of SIRT1, and Therefore Inhibiting NF-ΚB-Mediated Macrophage Activation. Front. Pharmacol. 2018, 8, 981. [Google Scholar] [CrossRef]
- Mukherjee, H.; Ojha, D.; Bharitkar, Y.P.; Ghosh, S.; Mondal, S.; Kaity, S.; Dutta, S.; Samanta, A.; Chatterjee, T.K.; Chakrabarti, S. Evaluation of the Wound Healing Activity of Shorea Robusta, an Indian Ethnomedicine, and Its Isolated Constituent (s) in Topical Formulation. J. Ethnopharmacol. 2013, 149, 335–343. [Google Scholar] [CrossRef]
- Suh, K.S.; Chon, S.; Choi, E.M. Bergenin Increases Osteogenic Differentiation and Prevents Methylglyoxal-Induced Cytotoxicity in MC3T3-E1 Osteoblasts. Cytotechnology 2018, 70, 215–224. [Google Scholar] [CrossRef]
- Lee, K.H.; Choi, E.M. Effects of Bergenin on Methylglyoxal-Induced Damage in Osteoblastic MC3T3-E1 Cells. J. Appl. Toxicol. 2018, 38, 585–593. [Google Scholar] [CrossRef]
- Hou, W.; Ye, C.; Chen, M.; Li, W.; Gao, X.; He, R.; Zheng, Q.; Zhang, W. Bergenin Activates SIRT1 as a Novel Therapeutic Agent for Osteogenesis of Bone Mesenchymal Stem Cells. Front. Pharmacol. 2019, 10, 618. [Google Scholar] [CrossRef]
- Zhang, C.; Zhao, B.; Zhang, C.; Qiu, M.; Ma, S.; Jin, X.; Shao, Y.; Wang, M.; Wang, X. Mechanisms of Bergenin Treatment on Chronic Bronchitis Analyzed by Liquid Chromatography-Tandem Mass Spectrometry Based on Metabolomics. Biomed. Pharmacother. 2019, 109, 2270–2277. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, S. Evaluation of in Vitro and in Vivo Antileishmanial Potential of Bergenin Rich Bergenia Ligulata (Wall.) Engl. Root Extract against Visceral Leishmaniasis in Inbred BALB/c Mice through Immunomodulation. J. Tradit. Complement. Med. 2018, 8, 251–260. [Google Scholar] [CrossRef]
- Qin, X.; Yang, Y.; Fan, T.; Gong, T.; Zhang, X.; Huang, Y. Preparation, Characterization and in Vivo Evaluation of Bergenin-Phospholipid Complex. Acta Pharmacol. Sin. 2010, 31, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Thu, H.E.; Amjad, M.W.; Hussain, F.; Ahmed, T.A.; Khan, S. Exploring Recent Developments to Improve Antioxidant, Anti-Inflammatory and Antimicrobial Efficacy of Curcumin: A Review of New Trends and Future Perspectives. Mater. Sci. Eng. C 2017, 77, 1316–1326. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Qin, X.; Zhou, Z.; Zhang, Q.; Huang, Y. Investigation of the Mechanisms of Improved Oral Bioavailability of Bergenin Using Bergenin–Phospholipid Complex. Drug Dev. Ind. Pharm. 2014, 40, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Wei, Y.; Xi, L.; Sun, Y.; Zhang, T. Evaluation of Intestinal Absorption and Bioavailability of a Bergenin–Phospholipid Complex Solid Dispersion in Rats. AAPS PharmSciTech 2018, 19, 1720–1729. [Google Scholar] [CrossRef]
- He, S.; Li, F.; Zhou, D.; Du, J.; Huang, Y. Formulation and Evaluation of Novel Coated Floating Tablets of Bergenin and Cetirizine Dihydrochloride for Gastric Delivery. Drug Dev. Ind. Pharm. 2012, 38, 1280–1288. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, V.; Bharate, S.S.; Vishwakarma, R.A. Synthesis, PH Dependent, Plasma and Enzymatic Stability of Bergenin Prodrugs for Potential Use against Rheumatoid Arthritis. Bioorganic. Med. Chem. 2017, 25, 5513–5521. [Google Scholar] [CrossRef]
- Ren, Y.; Shen, M.; Ding, Y.; Yuan, M.; Jiang, L.; Li, H.; Yuan, M. Study on Preparation and Controlled Release in Vitro of Bergenin-Amino Polylactic Acid Polymer. Int. J. Biol. Macromol. 2020, 153, 650–660. [Google Scholar] [CrossRef]
- Shen, M.; Li, H.; Yuan, M.; Jiang, L.; Zheng, X.; Zhang, S.; Yuan, M. Preparation of Bergenin-Poly (Lactic Acid) Polymers and in Vitro Controlled Release Studies. Int. J. Biol. Macromol. 2018, 116, 354–363. [Google Scholar] [CrossRef]
- Qin, X.; Zhou, D.; Zhang, Z.-R.; Huang, Y. Determination of Bergenin in Rat Plasma by High-Performance Liquid Chromatography. Die Pharm.-Int. J. Pharm. Sci. 2007, 62, 323–326. [Google Scholar]
- Shi, Y.-B.; Shi, Y.-P.; Meng, Q.-G. Determination and Pharmacokinetic Study of Bergenin in Rat Plasma by RP-HPLC Method. Biomed. Chromatogr. 2006, 20, 1065–1070. [Google Scholar] [CrossRef]
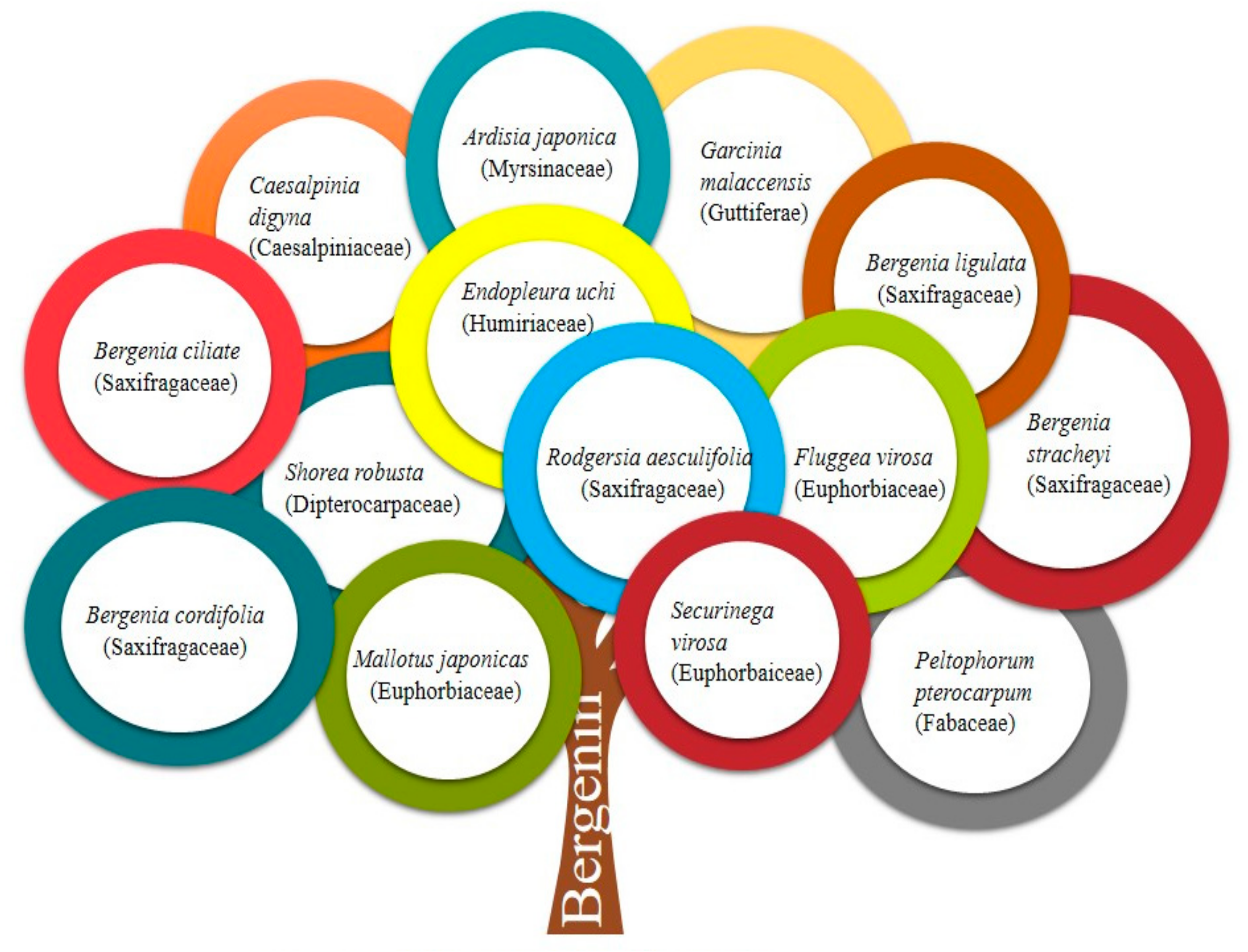
| Sr. No. | Plants (Families) | Part Used | Pharmacological/Biological Activities | Mechanism(s) of Action | Study Models | References |
|---|---|---|---|---|---|---|
| 1. | Bergenia ligulata | Rhizome | Anti-microbial | Inhibits anaerobic glycosis and aerobic respiration | Agar well-diffusion assay | [16] |
| 2. | Bergenia spp. | - | Anti-cancer (cervical cancer) | Inaugural of apoptosis and cell cycle inhibition in the G0/G1 phase. Inhibit phosphorylation of STAT3 proteins. | Cervical cancer cell line HeLa | [17] |
| 3. | Bergenia ciliata, Bergenia spp. Bergenia stracheyi | Aerial parts - Rhizome | Anti-inflammatory, immunomodulatory | Inhibition of IL-6 and TNF-α; Targeting cytokine (IL-1b, IL-6 and TNF-α) and reactive oxygen species (ROS), Prevent the development of proinflammatory Th1 cytokines (IFN-γ, IL-2 and TNF-α) whereas potentiate anti-inflammatory Th2 cytokines (IL-5 and IL-4) | Human monocyte leukemia THP-1 cells, Carrageenan-induced paw edema and Mycobacterium-induced arthritis in rats; CFA-induced arthritis model | [18,19,20] |
| 4. | Bergenia stracheyi, Bergenia ligulata, Bergenia cordifolia, Endopleura uchi, Peltophorum pterocarpum | Rhizome Bark, Flowers | Antioxidant | Free radical scavenging activity | DPPH assay, Agar well diffusion method, Disc diffusion method | [2,21,22] |
| 5. | Bergenia cordifolia, Caesalpinia digyna Rottler | Rhizome Roots | Anti-diabetic | Inhibition of α-glucosidase enzyme. Positive effect on endocrine cells of pancreas results in enhanced development of insulin. | Microtitre-based assay. Streptozotocin-nicotinamide induced diabetic rats. | [22,23] |
| 6. | Mallotus japonicus | Dried bark | Neuroprotective | Inhibit generation of ROS in brain | Culture of rat cortical neurons in DMEM supplemented with Nitrogen | [24] |
| 7. | Mallotus japonicas | Cortex | Hepatoprotective | Attenuated the increase in the activities of alanine aminotransferase, sorbitol dehydrogenase, aspartate aminotransferase, γ-glutamyltransferase and also inhibit lipid peroxidation and recover the reduced hepatic glutathione level | CCl4-induced hepatic damage in rats | [25] |
| 8. | Mallotus philippinensis | Leaf | Anti-urolithiatic | Significantly reduction in calcium, oxalate and phosphate concentration in urine | Ethylene glycol-induced urolithiasis in wistar rats | [26] |
| 9. | Rodgersia aesculifolia Batal, Bergenia ligulata | Rhizome | Anti-malarial | Inhibition of heme polymerization pathway of malaria parasite | In vitro and In vivo assessment of antimalarial activity using Plasmodium falciparum and Plasmodium berghei infected BALB/c mice | [7,21,27] |
| 10. | Caesalpinia digyna Rottler | Root | Anxiolytic | - | EPM (mice) | [7] |
| 11. | Shorea robusta | Leaves | Anti-tubercular | Induces the production of TNF-α, NO, IFN-γ, IL-17 and IL-12 from both CD4 and CD8 T-cells | Murine model of Mycobacterim tuberculosis infection | [28] |
| 12. | Bergenia stracheyi | Rhizome | Anti-gout | Inhibition of xanthine oxidase enzyme | Assayed spectrophotometrically | [2] |
| 13. | Garcinia malaccensis | Stembark | Antiplatelet aggregation | Inhibition of platelet aggregation induced by arachidonic acid, adenosine diphosphate and collagen | Platelet aggregation test measured by ANOVA | [3] |
| 14. | Flueggea microcarpa | Leaves | Antihyperlipidemic | Reduced level of CH, LDL, VLDL, TG and increased proportion of HDL were reported via, increasing reverse cholesterol transport from arterial tissue to the liver | Albino rats of Charles Foster strain given hyperlipidaemic diet of arachis oil | [29] |
| 15. | Ardisia japonica | Aerial parts | Anti-HIV | Inhibition of antibody ADP358 binding to gp120 and interfere with gp120-CD4 interaction | C8166 cells infected with HIV-1 | [30] |
| 16. | Fluggea virosa | Aerial parts | Anti-arrhythmic | Coronary artery ligation and blood reperfusion | BaCl2 induced arrhythmia in rats | [31] |
| 17. | Flueggea microcarpa | Leaves and roots | Antiulcer | Protection against pylorus-ligated and gastric ulcers induced by aspirin | Gastric ulcers induced by cold restraint stress-induced in guinea pigs and rats. | [32] |
| 18. | Securinega virosa | Root bark | Soporific | - | Beam walking test and Diazepam-induced sleeping time assay in mice. | [33] |
| 19. | Bergenia ciliata | Rhizome | Anti-tussive | Bronchodilator action, inhibited the histamine and acetylcholine induced contractions | Cough model induced by sulphur dioxide gas in mice | [34,35] |
| Sr. No. | Commercial Herbal Formulations | Name and Amount of Extract Containing Bergenin | Therapeutic Dose Required | Potential Uses/Indications | Manufacturers |
|---|---|---|---|---|---|
| 1. | Albestone Capsule | Pashanbhed (Bergenia ligulata) 200 mg | As per directed by doctor | Kidney calculi, Bladder calculi, Ureter calculi, Retention of urine, Calculi induced UTI | Sanify Healthcare Pvt. Ltd. (Punjab) |
| 2. | Phytone Capsule | Pashanbhed Extract (Saxifrage lingulate) 100 mg | 1–2 Capsules twice daily | Medical Management of Urinary Calculi, for the prevention of recurrent calculi. | Abhinav Healthcare |
| 3. | Stonvil Capsule | Pashanbhed, (Saxifraga ligulata) 30 mg | U. T. I.: 2 b. d. for 2 weeks. Renal calculi: 2 b.d. upto 3 weeks. Burning Micturition: 1 b.d. upto 2 weeks. | Burning micturition, Grit, Calculi problems and Urinary tract infections | S.G Phyto Pharma Pvt. Ltd. |
| 4. | Cystone Tablet | Saxifraga ligulata (98 mg/tab.) | 2 Tabs. twice daily | Gritty kidney, Ureter, bladder and urethra Sialolithiasi, Urinary tract infection (UTI), Colic ureter, Glomerulonephritis crystalluria—fosfatouria Heart-Renal Edema, Bed wetting-urinary incontinence, Hyperuricemia Enlarged prostate: in concomitant use with speman or Himplasia prevents surgery. | The HimalayaTM Drug Company |
| 5. | Nefrotec~ DS VET Tablet | Pashanbhed (Saxifraga ligulata 30 mg) | Dogs: 1 Tablet two times daily for small breeds. 2 Tablets two times daily for large breeds Cats: 1 Tablet one time a day. | Nephrolithiasis, Recurrent urinary tract infections, Cystitis, Non-specific Urethritis, kidney dysfunction. | The HimalayaTM Drug Company |
| 6. | Neeri Tablet | Bergenia ligulata (60 mg/tab.) | Children: 1–2 Tabs. twice a day. Adults: 2–3 Tabs. thrice a day. | Dysuria, Burning Micturition, Crystalluria, Oedema, Anasarca, Non-specific UTIs. | Aimil Pharmaceuticals Ltd. |
| 7. | Patharina Tablet | Pashanbhed - | 2 Tablets twice a day orally with water or as directed by the physician. | Kidney Stones, Painful Urination | Shree Baidyanath Ayurved Bhawan Pvt. Ltd. |
| 8. | Cystone Syrup | Saxifraga ligulata (53 mg/5 mL) | Children: ½-1 Teaspoonful (2.5–5 mL) twice daily after meals. Adults: 1–2 Teaspoonful (5–10 mL) twice daily after meals. | Kidney stones, Crystalluria, crystals in the urine, Dysuria, Hyperuricemia, high amount of uric acid in the blood, Burning while urination, Non-specific Urethritis, i.e., irritation or swelling of the urethra. | The HimalayaTM Drug Company |
| 9. | Neeri Syrup | Bergenia ligulata (100 mg/10 mL) | Children: ½–1 Teaspoonful thrice daily. Adults: 2 teaspoonful thrice daily. | Dysuria, Burning Micturition, Oedema, Anasarca, Non-specific UTIs. | Aimil Pharmaceuticals Ltd. |
| 10. | StonDab Syrup | Pashanbheda - | 1–2 Teaspoonful of the syrup 3 times a day. | kidney stones, burning sensation while urination, non-specific urinary tract infection, urinary calculus | Dabar India limted |
| 11. | Ashmarihar kwath Powder | Saxifraga ligulata (15 g/100 g) | Mix 5–10 gm of kwath in around 400 mL water and boil it, till residue is 100 mL. | Kidney stone, gall stone problem. | Divya Pharmacy |
| 12. | Pashan Bhed Root Powder | (Saxifraga ligulata powder roots) 3 mg | 1–2 Tablespoon mix with water, blend in a smoothie drink/sprinkle over salad. | Urinary tract infection (UTI) burning and painful micturition, spleen related swelling. | Bixa botanical |
| 13. | Pushyanug Churna | (Saxifraga ligulata) 5 mg | 2–3 mg, twice a day. | leucorrhoea, menorrhagia, metrorrhagia, prolapse of uterus and also useful in diarrhea, dysentery and bleeding piles | Deep Ayurveda |
| 14. | Prakriti Pashanbhed Ark | Pashanbhed - | 10–15 mL of Pashanbhed ark, Twice a day with equal amount of warm water before meals. | Kidney Stones and Liver related problems | Prakriti Nutann Gausadan |
| 15. | Pashan Bhed transdermal Cream | Pashanbhed - | Whole spine Swiping downwards 7 times in the morning and evening. | Autoimmune toxins, gall stone, inflammation, kidney stones | Prabhava Ayurvedic herbals |
| Sr. No. | Patents No./Patent Publication No. | Title | Invention | References |
|---|---|---|---|---|
| 1. | US 10,494,377 B1 | Bergenin lipoic acid ester with antioxidant activity and a method of preparing the same | Invented BER lipoic acid ester having excellent antioxidant potential. | [48] |
| 2. | US 8,007,837 B2 | Herbal composition for maintaining/caring the skin around the eye, methods of preparing the same and uses thereof | Invented a novel herbal skinceutical composition to maintain and improve skin health especially for delicate skin around the eyes comprising the extracts of Saxifraga ligulata, Cipadessa baccifera and Emblica officinalis, method for preparing the same and use | [49] |
| 3. | US 7,785,637 B2 | Herbal composition for other publications maintaining/caring the skin around the eye, methods of preparing the same and uses thereof | Invented a novel herbal skinceutical composition to improve and maintain skin health especially for delicate skin around the eyes comprising the extracts of Saxifraga ligulata, Cipadessa baccifera and Emblica officinalis, method for preparing the same and their uses. | [50] |
| 4. | 217147 | A Pharmaceutical Composition Useful as an Antioxidant | Invented the process of isolation of BER from Tinospora crispa. | [51] |
| 5. | US 2004/O115286A1 | Cosmetic composition of Remedying skin wrinkles comprising bergenia Emeensis extract as active ingredient | Invented a cosmetic composition having Bergenia emeiensisextract, for skin wrinkles owing to its potential inhibition of collagenase and elastase. | [52] |
| 6. | WO2019077620A1 | Gastroretentive sustained release formulations of Bergenia ciliata | Invented novel gastroretentive swellable oral formulations for sustained or delayed release of BER-rich Bergenia ciliata extract/fraction and a process for preparing the same. The novel formulations were found to be retained in the stomach, which avoids intestinal degradation of BER resulting in its sustained release in stomach over a time period of 16–24 h. | [53] |
| 7. | US 10,494,377 B1 | Bergenin lipoic acid ester with antioxidant activity and a method of preparing the same | Invented BER lipoic acid ester having excellent antioxidant potential. | [48] |
| 8. | US 8,007,837 B2 | Herbal composition for maintaining/caring the skin around the eye, methods of preparing the same and uses thereof | Invented a novel herbal skinceutical composition to maintain and improve skin health especially for delicate skin around the eyes comprising the extracts of Saxifraga ligulata, Cipadessa baccifera and Emblica officinalis, method for preparing the same and use | [49] |
| 9. | US 7,785,637 B2 | Herbal composition for other publications maintaining/caring the skin around the eye, methods of preparing the same and uses thereof | Invented a novel herbal skinceutical composition to improve and maintain skin health especially for delicate skin around the eyes comprising the extracts of Saxifraga ligulata, Cipadessa baccifera and Emblica officinalis, method for preparing the same and their uses. | [50] |
| 10. | 217147 | A Pharmaceutical Composition Useful as an Antioxidant | Invented the process of isolation of BER from Tinospora crispa. | [51] |
| 11. | US 2004/O115286A1 | Cosmetic composition of Remedying skin wrinkles comprising bergenia Emeensis extract as active ingredient | Invented a cosmetic composition having Bergenia emeiensis extract, for skin wrinkles owing to its potential inhibition of collagenase and elastase. | [52] |
| 12. | WO2019077620A1 | Gastroretentive sustained release formulations of Bergenia ciliata | Invented novel gastroretentive swellable oral formulations for sustained or delayed release of BER-rich Bergenia ciliata extract/fraction and a process for preparing the same. The novel formulations were found to be retained in the stomach, which avoids intestinal degradation of BER resulting in its sustained release in stomach over a time period of 16–24 h. | [53] |
| Sr. No. | Formulations | BER Concentration | Techniques | Applications | Assay | References |
|---|---|---|---|---|---|---|
| 1. | Phospholipid complex | - | Solvent evaporation method | Increased oral absorption | In vitro and in vivo | [89] |
| 2. | Coated floating tablets | 187.5 mg | Single-punch machine | Prolonged gastric retention time | In vitro and in vivo | [93] |
| 3. | Prodrug | - | - | Increased stability | [94] | |
| 4. | Herbal gel (Aqueous Extract of Bergenia Ligulata Rhizomes and Ethanolic Extract of Butea Monosperma Flowers) | - | Soxhlet extraction, blending | Wound healing by exhibiting antibacterial activity at the site of wound infection | In vitro | [16] |
| 5. | Phospholipid complex solid dispersion | - | Solvent evaporation | Increase the oral BCS IV drugs bioavailability | In vitro and in vivo | [92] |
| 6. | Poly herbal ointment (Bergenia Ciliata) | - | - | Excision and incision wounds | - | [6] |
| 7. | Nanoparticles | 0.5 mg/mL | - | Enhancing physiochemical properties and anti-arthritic activity | In vivo | [19] |
| 8. | Sustained release capsules (Bergenia Ciliata) | - | - | Sustained release | - | [18] |
| 9. | Poly lactic acid polymers | - | Sustained release | In vitro | [95] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehta, S.; Kadian, V.; Dalal, S.; Dalal, P.; Kumar, S.; Garg, M.; Rao, R. A Fresh Look on Bergenin: Vision of Its Novel Drug Delivery Systems and Pharmacological Activities. Future Pharmacol. 2022, 2, 64-91. https://doi.org/10.3390/futurepharmacol2010006
Mehta S, Kadian V, Dalal S, Dalal P, Kumar S, Garg M, Rao R. A Fresh Look on Bergenin: Vision of Its Novel Drug Delivery Systems and Pharmacological Activities. Future Pharmacology. 2022; 2(1):64-91. https://doi.org/10.3390/futurepharmacol2010006
Chicago/Turabian StyleMehta, Sidharth, Varsha Kadian, Sweta Dalal, Pooja Dalal, Sunil Kumar, Minakshi Garg, and Rekha Rao. 2022. "A Fresh Look on Bergenin: Vision of Its Novel Drug Delivery Systems and Pharmacological Activities" Future Pharmacology 2, no. 1: 64-91. https://doi.org/10.3390/futurepharmacol2010006
APA StyleMehta, S., Kadian, V., Dalal, S., Dalal, P., Kumar, S., Garg, M., & Rao, R. (2022). A Fresh Look on Bergenin: Vision of Its Novel Drug Delivery Systems and Pharmacological Activities. Future Pharmacology, 2(1), 64-91. https://doi.org/10.3390/futurepharmacol2010006